Published online Apr 20, 2012. doi: 10.5493/wjem.v2.i2.26
Revised: April 1, 2012
Accepted: April 10, 2012
Published online: April 20, 2012
The common goal within the overwhelming interests in stem cell research is to safely translate the science to patients. Although there are various methods by which this goal can be reached, this editorial emphasizes the safety of mesenchymal stem cell (MSC) transplant and possible confounds by the growing information on cancer stem cells (CSCs). There are several ongoing clinical trials with MSCs and their interactions with CSCs need to be examined. The rapid knowledge on MSCs and CSCs has now collided with regards to the safe treatment of MSCs. The information discussed on MSCs can be extrapolated to other stem cells with similar phenotype and functions such as placenta stem cells. MSCs are attractive for cell therapy, mainly due to reduced ethical concerns, ease in expansion and reduced ability to be transformed. Also, MSCs can exert both immune suppressor and tissue regeneration simultaneously. It is expected that any clinical trial with MSCs will take precaution to ensure that the cells are not transformed. However, going forward, the different centers should be aware that MSCs might undergo oncogenic events, especially as undifferentiated cells or early differentiated cells. Another major concern for MSC therapy is their ability to promote tumor growth and perhaps, to protect CSCs by altered immune responses. These issues are discussed in light of a large number of undiagnosed cancers.
- Citation: Rameshwar P. Would cancer stem cells affect the future investment in stem cell therapy. World J Exp Med 2012; 2(2): 26-29
- URL: https://www.wjgnet.com/2220-315X/full/v2/i2/26.htm
- DOI: https://dx.doi.org/10.5493/wjem.v2.i2.26
There are ongoing experimental studies and clinical trials to use stem cells, including mesenchymal stem cells (MSCs), to deliver drugs to tumors[1-7]. There are several advantages of using MSCs but mostly, their ability to be delivered across allogeneic barrier[8]. MSCs can be available as “off-the-shelf” source for immediate delivery and this is mostly due to the cells’ immune suppressor functions[9]. However, a subset of MSCs expresses the major histocompatibility antigen, which can mediate the cells’ ability to function as antigen presenting cells, and also to cross-present antigens[10-12]. These immune functions of MSCs could also compromise their safety. However, this editorial is focused on the potential confound of cancer stem cells (CSCs) and will therefore not discuss possible confounds of MSCs in the event of switch to immune enhancer role. Similarly, although the literature indicates that MSCs, unlike embryonic stem cells, are less likely to form tumors, this topic will not be focused in this editorial, although it is an important issue for safety of MSCs as these stem cells move towards the clinic.
The definition of CSC is not different from any other stem cells. Thus, it is expected that a CSC will be present at low frequency, self-renew and initiate tumors[13]. The reports indicate that CSCs could be radioresistant[14]. Unlike the detailed hierarchy for hematopoietic stem cells (HSCs), similar steps in lineage commitment have not been elucidated for other stem cells, including CSCs. This gap in the field of stem cell is however not a surprise since the field is considered as relatively new, as compared to the HSCs, which has been studied for decades. To explain the complexity going forward, to develop a detailed hierarchy of stem cells, we can take a look at the HSCs after decades of research. Additional information is evolving on these stem cells, indicating that the mapping of lineages continues to be a subject in progress[15]. Thus, therapies will be developed in the realm of unresolved science.
Tumor initiating cells have been traditionally referred as dormant cancer cells, which are considered as those responsible for cancer resurgence[13,16,17]. There are arguments if CSCs differ from tumor initiating cells[18]. Since a stem cell should be able to repopulate a system and self-renew, it could be argued that a tumor initiating cell, which can repopulate a system, will not be a stem cell unless the cell can self-renew. Regardless, as the field of CSCs begin to develop into a hierarchy, the field of stem cell therapy will benefit because decisions could be made on which cancer cells will be affected by other stem cells, such as MSCs[19,20]. This point is important because MSCs, which are currently in clinical trials[21-23], can support and also protect cancer growth[19,20,24].
The major problem that is envisioned for stem cell therapy is linked to undiagnosed cancers. This population represents about 30% of middle aged subjects[25]. As would be expected, stem cell treatment would be likely indicated for a similar cohort of individuals who would be considered as middle-age. The delivery of stem cells to older and middle-aged individuals is particularly important since MSCs could support tumor growth as well as to protect the tumor cells from immune clearance[19,20]. Going forward, to ensure the safety of MSC treatments, it will be paramount to encourage scientific studies to understand how the delivery of stem cells might affect the undiagnosed cancers. Such studies will require indepth experimental analyses.
A difficult question to be answered is to predict whether stem cell therapy should be designed with strategies to retain the dormant cancer cells in their quiescent phase or to also be prepared to treat the cancer if the stem cell treatment induced tumor growth and metastasis. Answers to this question are not simple because MSCs have varied effects on tumors, including the ability to maintain a quiescent phenotype or to support tumor growth[19,20,24]. There is also an ethical issue if patients with a history of cancer should be disqualified for a treatment in which he or she could benefit.
MSCs are suggested and also in ongoing clinical trial to directly deliver drugs to target tumors[26]. The main disadvantage of using MSCs for this purpose is their ability to support cancer growth and progression[24,27]. While the use of MSCs for targeted therapy is an excellent method, the approach could affect the CSCs, through direct interaction and also through their immune suppressive effect[19,24]. It is expected that the drugs will target the rapidly dividing cancer cells. However, if the drugs cannot target the stem cell subset, the MSCs could protect these cells to encourage dormancy.
Perhaps MSCs could be protective by inducing and expanding immune suppressor regulatory T-cells and/or to deliver small RNA either through the formation of gap junctions or through exosomes[28,29]. There are reports to support the presence of small RNA within exosomes released from tumor cells[30,31]. In general, stem cells are not metabolically active cells and are therefore expected to be “rich” sources of RNA that could be delivered to the tumor cells. CSCs could benefit from the close location of MSCs to establish gap junctional intercellular communication, which can facilitate the passage of small microRNA that benefits a dormant phase of the cancer cell[29,32].
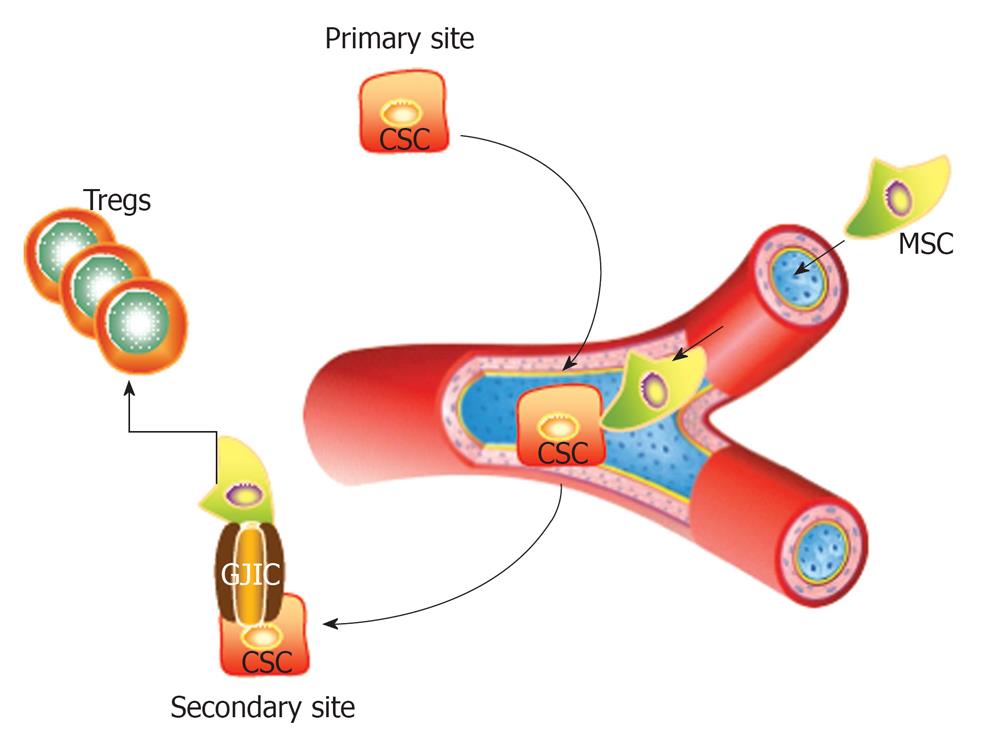
This editorial highlights the issue of CSCs as a serious consideration when MSCs and other similar cells are used in therapy. Shown in Figure 1 is a CSC from the primary site migrating through the circulation to a secondary site. If the CSC contact a MSC, this can lead to the formation of gap junctional intercellular communication for the exchange of small microRNA[29] and/or can prevent immune target by inducing the expansion of regulatory T-cells.
Dormancy of cancer has been described, although it is yet to be determined if the CSCs are responsible for a dormant phase. Regardless, robust research is required to understand how stem cells such as MSCs affect pre-existing CSCs and their progenies. There is intense research to develop methods to prevent the transition of dormant cancer cells into rapidly growing cells. However, these strategies are proposing to retain the dormant cells in the individuals. Perhaps this is a good strategy since 30% of individuals have undiagnosed occult cancer. On the other hand, this could be a problem if transplanted MSCs could support the transition of the dormant cancer cells into aggressively dividing cells. It is therefore a serious consideration to carefully review the issues on CSCs and MSCs for safe treatment.
Peer reviewer: Niels Olsen Saraiva Camara, MD, Full Professor of Immunology, Laboratory of Transplantation Immunobiology, Department of Immunology, Institute of Biomedical Sciences, University of São Paulo, Av Prof.Lineu Prestes 1730, ICB IV, Room 240, Brazil
S- Editor Li JY L- Editor A E- Editor Zheng XM
| 1. | Song C, Xiang J, Tang J, Hirst DG, Zhou J, Chan KM, Li G. Thymidine kinase gene modified bone marrow mesenchymal stem cells as vehicles for antitumor therapy. Hum Gene Ther. 2011;22:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Zhao D, Najbauer J, Annala AJ, Garcia E, Metz MZ, Gutova M, Polewski MD, Gilchrist M, Glackin CA, Kim SU. Human neural stem cell tropism to metastatic breast cancer. Stem Cells. 2012;30:314-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Ghaedi M, Soleimani M, Taghvaie NM, Sheikhfatollahi M, Azadmanesh K, Lotfi AS, Wu J. Mesenchymal stem cells as vehicles for targeted delivery of anti-angiogenic protein to solid tumors. J Gene Med. 2011;13:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Meyerrose T, Olson S, Pontow S, Kalomoiris S, Jung Y, Annett G, Bauer G, Nolta JA. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev. 2010;62:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Zhao D, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, Kim SU, Aboody KS. Neural stem cell tropism to glioma: critical role of tumor hypoxia. Mol Cancer Res. 2008;6:1819-1829. [PubMed] |
| 6. | Saito S, Nakayama T, Hashimoto N, Miyata Y, Egashira K, Nakao N, Nishiwaki S, Hasegawa M, Hasegawa Y, Naoe T. Mesenchymal stem cells stably transduced with a dominant-negative inhibitor of CCL2 greatly attenuate bleomycin-induced lung damage. Am J Pathol. 2011;179:1088-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62:1156-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | English K, Mahon BP. Allogeneic mesenchymal stem cells: agents of immune modulation. J Cell Biochem. 2011;112:1963-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Sherman LS, Munoz J, Patel SA, Dave MA, Paige I, Rameshwar P. Moving from the laboratory bench to patients' bedside: considerations for effective therapy with stem cells. Clin Transl Sci. 2011;4:380-386. [PubMed] |
| 10. | Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107:4817-4824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 11. | François M, Romieu-Mourez R, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114:2632-2638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Romieu-Mourez R, François M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol. 2007;179:1549-1558. [PubMed] |
| 13. | Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339-9344. [PubMed] |
| 14. | Krause M, Yaromina A, Eicheler W, Koch U, Baumann M. Cancer stem cells: targets and potential biomarkers for radiotherapy. Clin Cancer Res. 2011;17:7224-7229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 457] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 15. | Chi AW, Chavez A, Xu L, Weber BN, Shestova O, Schaffer A, Wertheim G, Pear WS, Izon D, Bhandoola A. Identification of Flt3+CD150- myeloid progenitors in adult mouse bone marrow that harbor T lymphoid developmental potential. Blood. 2011;118:2723-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Almog N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 2010;294:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Demicheli R, Terenziani M, Valagussa P, Moliterni A, Zambetti M, Bonadonna G. Local recurrences following mastectomy: support for the concept of tumor dormancy. J Natl Cancer Inst. 1994;86:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Badve S, Nakshatri H. Breast-cancer stem cells-beyond semantics. Lancet Oncol. 2012;13:e43-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010;184:5885-5894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 284] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 20. | Mishra PJ, Banerjee D. Activation and differentiation of mesenchymal stem cells. Methods Mol Biol. 2011;717:245-253. [PubMed] |
| 21. | Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, Nawata M, Tensho K, Kato H, Uematsu K. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 23. | Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1372] [Cited by in RCA: 1226] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 24. | Greco SJ, Patel SA, Bryan M, Pliner LF, Banerjee D, Rameshwar P. AMD3100-mediated production of interleukin-1 from mesenchymal stem cells is key to chemosensitivity of breast cancer cells. Am J Cancer Res. 2011;1:701-715. [PubMed] |
| 25. | Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 362] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 26. | Sun XL, Xu ZM, Ke YQ, Hu CC, Wang SY, Ling GQ, Yan ZJ, Liu YJ, Song ZH, Jiang XD. Molecular targeting of malignant glioma cells with an EphA2-specific immunotoxin delivered by human bone marrow-derived mesenchymal stem cells. Cancer Lett. 2011;312:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Grisendi G, Bussolari R, Veronesi E, Piccinno S, Burns JS, De Santis G, Loschi P, Pignatti M, Di Benedetto F, Ballarin R. Understanding tumor-stroma interplays for targeted therapies by armed mesenchymal stromal progenitors: the Mesenkillers. Am J Cancer Res. 2011;1:787-805. [PubMed] |
| 28. | Gregory LA, Ricart RA, Patel SA, Lim PK, Rameshwar P. microRNAs, Gap Junctional Intercellular Communication and Mesenchymal Stem Cells in Breast Cancer Metastasis. Curr Cancer Ther Rev. 2011;7:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS, Rameshwar P. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550-1560. [PubMed] |
| 30. | Silva J, Garcia V, Rodriguez M, Compte M, Cisneros E, Veguillas P, Garcia JM, Dominguez G, Campos-Martin Y, Cuevas J. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer. 2012;51:409-418. [PubMed] |
| 31. | Zhang HG, Zhuang X, Sun D, Liu Y, Xiang X, Grizzle WE. Exosomes and immune surveillance of neoplastic lesions: a review. Biotech Histochem. 2012;87:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Matuskova M, Hlubinova K, Pastorakova A, Hunakova L, Altanerova V, Altaner C, Kucerova L. HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett. 2010;290:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |