Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.108318
Revised: April 24, 2025
Accepted: June 13, 2025
Published online: September 20, 2025
Processing time: 123 Days and 23.6 Hours
Hyperglycemia secondary to diabetes can lead to organ failure unless properly controlled, and it is associated with numerous health issues, particularly meta
To investigate the relationship between preoperative blood glucose levels and cancer prognostic factors in patients diagnosed with breast cancer.
In this study patients diagnosed with breast cancer were classified into two groups: Those with blood glucose levels < 125 mg/dL and those with levels > 125 mg/dL. Estrogen and progesterone receptor positivity, Ki-67 percentage, Cerb2 positivity, number of positive lymph nodes, and presence of lymphovascular invasion were examined histopathologically.
The study included 246 patients with breast cancer: 196 were in the normal glucose group and 50 were in the elevated glucose group. In the normal glucose group, 68% of the tumors were left-sided and 32% were right-sided, while all tumors in the elevated glucose group were left-sided. This difference was statistically significant (P < 0.001). Tumor size, Ki-67, and the number of positive lymph nodes were not associated with blood glucose in patients with breast cancer (P > 0.05 for all). However, blood glucose was correlated with age in this population. Correlation analyses revealed that blood glucose was positively correlated with age (r = 0.23, P < 0.001). Additionally, Ki-67 was correlated with tumor size (r = 0.14, P = 0.03) and age (r = -0.18, P = 0.005).
Our study highlighted the complex interplay between metabolic factors, such as fasting glucose levels, and breast cancer characteristics. Elevated glucose levels were associated with older age. The significant association between tumor laterality and glucose levels underscores the need for further investigation into the collective influence of metabolic and anatomical factors on breast cancer development and progression.
Core Tip: Hyperglycemia secondary to diabetes can lead to organ failure if not properly controlled and is associated with numerous health complications, particularly metabolic disorders. This study investigated the relationship between preoperative blood glucose levels and cancer prognostic factors in patients diagnosed with breast cancer. The results of the study indicated that tumors in patients with high blood glucose were left sided. The significant association between tumor laterality and glucose levels underscores the need for further investigation into the influence of metabolic and anatomical factors on breast cancer development and progression.
- Citation: Ozer SP, Keyif F, Bolat F, Ozer B, Aktas G. Relationship of diabetes mellitus with prognostic factors in breast cancer. World J Exp Med 2025; 15(3): 108318
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/108318.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.108318
Hyperglycemia secondary to diabetes can lead to organ failure if not properly controlled and is associated with numerous health complications, particularly metabolic disorders[1]. Recent studies have shown both positive and negative associations between diabetes and cancer although the exact nature of this relationship is still being investigated. One of the key mechanisms by which diabetes may contribute to cancer development is through increased insulin resistance. Elevated insulin resistance can promote cell proliferation, oxidative damage, and the release of growth factors, all of which may lead to malignant cellular transformation. The association between diabetes and various cancers has been demonstrated in multiple studies, with increased risk particularly noted for breast, pancreatic, liver, colorectal, endometrial, and urinary system cancers[2,3].
Breast cancer is one of the most common malignancies affecting females worldwide and remains a major health concern due to its high rates of morbidity and mortality. The progression of the disease and the response to treatment de
This study investigated the relationship between preoperative blood glucose levels and cancer prognostic factors in patients diagnosed with breast cancer.
In this study patients diagnosed with breast cancer who presented to Bolu Abant İzzet Baysal University Faculty of Medicine Hospital between January 2016 and December 2024 were retrospectively analyzed. Ethical approval was obtained from the Bolu Abant İzzet Baysal University Clinical Research Ethics Committee.
The subjects diagnosed with breast cancer were included. Patients diagnosed with ductal carcinoma in situ or lobular carcinoma in situ were excluded from the study.
Medical records were reviewed using the hospital automation system, and study groups were created based on demographic data and laboratory parameters recorded at the time of admission. Data collected included age, tumor location, tumor diameter, stage, number of metastatic lymph nodes, estrogen receptor (ER), progesterone receptor (PR), Ki-67 index, Cerb2 status, tumor grade, presence of lymphovascular invasion, and blood glucose levels.
Histopathological examination was conducted by hematoxylin and eosin staining. Tumor type (e.g., invasive ductal carcinoma, lobular carcinoma), tumor grade (based on Nottingham grading system), lymphovascular invasion, and margins of excision were evaluated. Immunohistochemistry was used to determine hormone receptor status (ER and PR), HER2/neu expression, and Ki-67 (proliferation index).
Preoperative fasting blood glucose levels (measured after at least 8 h of fasting) were used for classification. Patients were divided into two groups: Those with blood glucose levels < 125 mg/dL and those with levels > 125 mg/dL. ER and PR positivity, Ki-67 percentage, Cerb2 positivity, number of positive lymph nodes, and presence of lymphovascular invasion were evaluated histopathologically. Tumor laterality was categorized as right or left breast.
Statistical analyses were performed using IBM SPSS (Statistical Package for the Social Sciences). Descriptive statistics were used to summarize the data. The Kolmogorov-Smirnov and Shapiro-Wilk tests were employed to assess normality. For normally distributed variables the t-test was used. For non-normally distributed variables the Mann-Whitney U test was employed. A P value < 0.05 was considered statistically significant. For variables with statistically significant diffe
We retrospectively analyzed the data of 261 patients. However, 15 were excluded due to incomplete data. Figure 1 shows the flow diagram. The present study included the remaining 246 patients diagnosed with breast cancer: 196 in the normal glucose group and 50 in the elevated glucose group. The mean age in the normal glucose group was 55.6 ± 13.2 years, while it was 64.0 ± 11.1 years in the elevated glucose group. The age difference between the groups was statistically significant (P < 0.001).
No statistically significant differences were found between the groups in terms of Ki-67 index, tumor size, or the number of positive lymph nodes (all P > 0.05). Table 1 presents the detailed characteristics of the study groups.
| Normal blood glucose group | Elevated blood glucose group | P value | |
| Age (years, mean ± SD) | 55.6 ± 13.2 | 64 ± 11.1 | < 0.001 |
| Ki-67 [unit, median (min-max)] | 12 (1-90) | 10 (1-70) | 0.475 |
| Tumor size [cm, median (min-max)] | 21 (3-170) | 23 (6-100) | 0.637 |
| Number of positive lymph nodes [n, median (min-max)] | 1 (0-51) | 0 (0-36) | 0.783 |
| Blood glucose [mg/dL, median (min-max)] | 97 (69-125) | 154 (127-364) | < 0.001 |
In the normal glucose group, 68% of tumors were left-sided and 32% were right-sided, whereas in the elevated glucose group, all tumors were left-sided. This difference in tumor laterality was statistically significant (P < 0.001). However, the presence of ER, PR, Cerb2 expression, tumor grade, tumor stage, and lymphovascular invasion was similar between the groups (all P > 0.05).
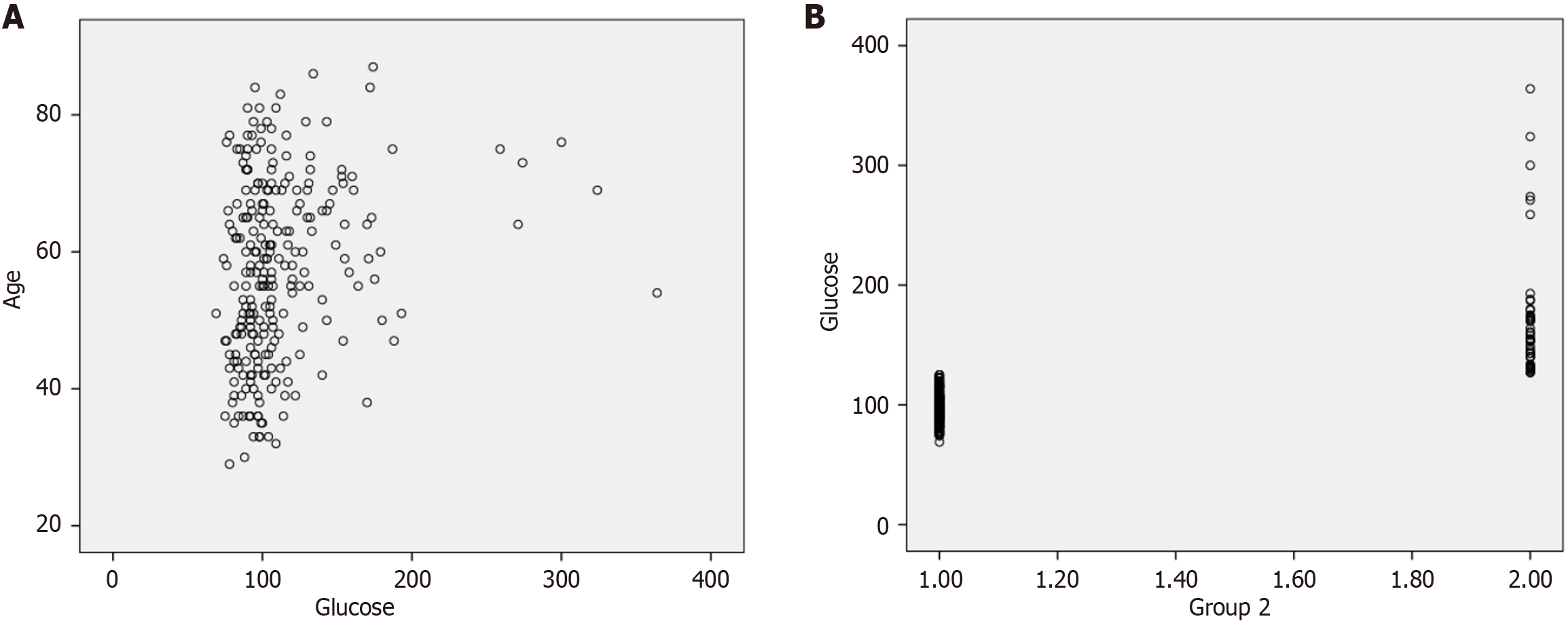
Correlation analysis showed a positive correlation between blood glucose and age (r = 0.23, P < 0.001), as illustrated in Figure 2A. Additionally, Ki-67 was positively correlated with tumor size (r = 0.14, P = 0.03) and negatively correlated with age (r = -0.18, P = 0.005). A significant correlation was also found between tumor size and the number of positive lymph nodes (r = 0.32, P < 0.001). Figure 2B shows the association between the study group and blood glucose.
The present study provided insights into the relationship between fasting glucose levels and various breast cancer characteristics. To contextualize our findings relevant literature from PubMed was reviewed. Our data indicated that elevated fasting glucose levels were associated with older age in patients with breast cancer, a finding consistent with previous research. Aging is often accompanied by metabolic alterations such as increased insulin resistance and a higher prevalence of hyperglycemia, which may contribute to elevated glucose levels in older individuals. A study comparing fasting glucose levels in patients with T2DM and healthy controls found that patients with T2DM were older and had significantly higher glucose levels than controls[8]. Similar findings have been reported in another study in the literature[9]. Patients with diabetes as well as prediabetes showed similar characteristics. Furthermore, individuals with prediabetes also tended to be older and exhibited elevated plasma glucose levels, as shown in the study by Balci et al[10]. These findings align with our results, reinforcing the link between aging, glucose dysregulation, and metabolic risk.
Despite the significant age and glucose level differences observed, no substantial variations were found in tumor characteristics such as Ki-67 expression, tumor size, or the number of positive lymph nodes between the normal and elevated glucose groups. This is particularly interesting given that hyperglycemia has been implicated in cancer progression. Elevated glucose levels may promote tumor development and progression through various mechanisms. Cancer cells often exhibit increased glucose uptake and metabolism, a phenomenon known as the Warburg effect, whereby glycolysis is favored even in the presence of oxygen. This metabolic reprogramming supports rapid cell proliferation and growth. High plasma glucose levels provide the necessary substrates for biosynthesis and energy, further fueling this process[11].
Additionally, glucose transporter proteins, particularly GLUT1, are frequently overexpressed in cancer cells, facilitating increased glucose influx. Overexpression of GLUT1 has been associated with tumor aggressiveness and poor prognosis[11]. High glucose levels may also activate oncogenic signaling pathways, such as the PI3K/AKT/mTOR pathway, which is sensitive to glucose availability and plays a crucial role in cell survival and proliferation. Hyper
Moreover, hyperglycemia has been linked to reduced survival in cancer patients undergoing treatment. For instance, high fasting glucose levels were associated with poorer outcomes in patients with locally advanced non-small cell lung cancer receiving chemoradiotherapy[12]. These observations underscore the importance of maintaining glucose control in patients with cancer. The lack of a significant association between fasting glucose and Ki-67 in our study may be due to the relatively small sample size, which could limit the statistical power to detect subtle differences.
Hyperglycemia may influence tumor biology through additional mechanisms, such as enhancing malignant cell proliferation. Elevated glucose levels can promote proliferation in breast cancer cells by increasing leptin and insulin-like growth factor-1 receptor signaling and activating pathways such as AKT/mTOR[13]. Another potential consequence is chemoresistance. High glucose conditions may contribute to resistance against chemotherapy by altering glycolipid metabolism. Hyperglycemia and hyperlipidemia together have been shown to negatively impact treatment outcomes in breast cancer patients[14].
The absence of significant differences in tumor characteristics in our study could be attributed to several factors. First, the small sample size may limit the ability to detect subtle yet clinically relevant differences. Second, the impact of hyperglycemia on tumor progression may depend on its duration and severity, which were not assessed in our study. Finally, variations in treatment regimens and adherence could have confounded the relationship between glucose levels and tumor features.
Interestingly, our results revealed a significant association between tumor laterality and glucose levels, particularly in relation to left vs right breast involvement. The clinical significance of breast cancer laterality has been explored in several studies. Some have reported differences in cancer-specific mortality depending on tumor location. For instance, tumors located in the upper-inner and lower-inner quadrants have been associated with a higher hazard ratio for mortality compared with those in the upper-outer quadrant[15].
Genetic factors may also influence tumor laterality. A study investigating the genetic basis of breast cancer laterality observed a slight predominance of left-sided breast cancer although the clinical implications remain unclear[16]. The observed relationship between tumor laterality and glucose levels in our analysis may suggest an underlying interaction between anatomical, physiological, or genetic factors and metabolic dysregulation.
It is well established that breast cancer exhibits a slight but consistent predilection for the left breast across various populations[16]. While some hypotheses suggest that differences in breast size may account for this asymmetry since the left breast is typically slightly larger, research has not consistently supported a direct link between breast size and cancer risk[17]. Other theories explore the role of genetic and developmental factors, including left-right asymmetry established during embryogenesis. Additionally, there has been speculation about the influence of brain hemispheric dominance on breast tissue development although evidence remains inconclusive[16].
Regarding the association between glucose metabolism and breast cancer laterality, the current literature does not provide substantial evidence linking hyperglycemia directly to tumor sidedness. While elevated glucose levels have been associated with cancer risk and progression through systemic mechanisms, there is no clear indication that these effects are lateralized. For example, while high glucose may promote tumor growth by impairing angiotensinogen expression, there is no known mechanism by which this would favor one breast over the other[18]. Thus, although left-sided breast cancer predominance is a recognized phenomenon, its association with blood glucose levels remains speculative. Further investigation is necessary to determine whether metabolic factors, such as glucose dysregulation, play a contributory role.
Breast cancer exhibits a notable tendency to occur more frequently in the left breast than in the right. This left-sided predominance has been consistently observed across various populations and studies[19]. Large-scale analyses, including data from the United States SEER program, have demonstrated that breast cancer is approximately 5% more likely to develop in the left breast compared with the right. This trend persists across different races, ages, and tumor stages[20]. Emerging research suggests that left-sided breast cancers may be associated with a more aggressive tumor biology. For instance, studies have found that left-sided tumors often exhibit higher expression of proliferation-related gene sets, such as E2F targets and MYC targets, indicating a more aggressive phenotype.
Additionally, left-sided tumors have been linked to lower rates of pathological complete response to neoadjuvant chemotherapy compared with right-sided tumors[19,21]. The distribution of molecular subtypes also appears to vary with tumor laterality. Left-sided breast cancers are more frequently associated with luminal B (HER2-positive) subtypes, while right-sided tumors show a higher prevalence of luminal A subtypes[22]. Several hypotheses have been proposed to explain the left-sided predominance in breast cancer incidence, including anatomical asymmetries, differences in breast size, and variations in hormonal exposure. However, no definitive explanation has been established, and further research is needed to elucidate the underlying causes. While the laterality of breast cancer can influence certain biological characteristics and treatment responses, it is not currently considered a standalone prognostic factor. Nonetheless, awareness of these differences may inform clinical decision-making and personalized treatment approaches[17].
This study had several limitations. Its retrospective design limits the ability to infer causality. The relatively small sample size may restrict the generalizability of our findings. Moreover, potential confounding factors such as the duration and severity of hyperglycemia as well as treatment-related variables were not controlled.
This study highlighted the complex interplay between metabolic factors, such as fasting glucose levels, and breast cancer characteristics. While elevated glucose levels were associated with older age, no significant differences in tumor proliferation markers or size were observed. However, the significant association between tumor laterality and glucose levels suggests a potential link between metabolic and anatomical factors in breast cancer development. Further research with larger, prospective cohorts is warranted to clarify these relationships and inform personalized treatment strategies aimed at improving prognostic assessments and clinical outcomes in breast cancer care.
| 1. | Kang C, LeRoith D, Gallagher EJ. Diabetes, Obesity, and Breast Cancer. Endocrinology. 2018;159:3801-3812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 2. | Guo M, Liu T, Li P, Wang T, Zeng C, Yang M, Li G, Han J, Wu W, Zhang R. Association Between Metabolic Syndrome and Breast Cancer Risk: An Updated Meta-Analysis of Follow-Up Studies. Front Oncol. 2019;9:1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Hardell KNL, Schonfeld SJ, Ramin C, Vo JB, Morton LM. Association between diabetes and subsequent malignancy risk among older breast cancer survivors. JNCI Cancer Spectr. 2024;8:pkae036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Zhao XB, Ren GS. Diabetes mellitus and prognosis in women with breast cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2016;95:e5602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 630] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 6. | Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM, Thomson CA, Caan BJ, Tinker LF, Urrutia RP, Knudtson J, Anderson GL. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women's Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015;1:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 438] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 7. | American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1413] [Article Influence: 471.0] [Reference Citation Analysis (1)] |
| 8. | Aktas G. Association between the Prognostic Nutritional Index and Chronic Microvascular Complications in Patients with Type 2 Diabetes Mellitus. J Clin Med. 2023;12:5952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 9. | Aktas G, Duman TT, Atak Tel B. Controlling Nutritional Status (CONUT) score is a novel marker of type 2 diabetes mellitus and diabetic microvascular complications. Postgrad Med. 2024;136:496-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Balci B, Tekce BK, Aktas G. Evaluation of serum oxidative stress levels and antioxidant capacity in prediabetes. Advances in Redox Research. 2024;12:100106. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Shin E, Koo JS. Glucose Metabolism and Glucose Transporters in Breast Cancer. Front Cell Dev Biol. 2021;9:728759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 12. | Bergamino M, Rullan AJ, Saigí M, Peiró I, Montanya E, Palmero R, Ruffinelli JC, Navarro A, Arnaiz MD, Brao I, Aso S, Padrones S, Cardenal F, Nadal E. Fasting plasma glucose is an independent predictor of survival in patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. BMC Cancer. 2019;19:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Li W, Zhang X, Sang H, Zhou Y, Shang C, Wang Y, Zhu H. Effects of hyperglycemia on the progression of tumor diseases. J Exp Clin Cancer Res. 2019;38:327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 14. | Qiu J, Zheng Q, Meng X. Hyperglycemia and Chemoresistance in Breast Cancer: From Cellular Mechanisms to Treatment Response. Front Oncol. 2021;11:628359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Bao J, Yu KD, Jiang YZ, Shao ZM, Di GH. The effect of laterality and primary tumor site on cancer-specific mortality in breast cancer: a SEER population-based study. PLoS One. 2014;9:e94815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Amer MH. Genetic factors and breast cancer laterality. Cancer Manag Res. 2014;6:191-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Cheng SA, Liang LZ, Liang QL, Huang ZY, Peng XX, Hong XC, Luo XB, Yuan GL, Zhang HJ, Jiang L. Breast cancer laterality and molecular subtype likely share a common risk factor. Cancer Manag Res. 2018;10:6549-6554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Sun S, Sun Y, Rong X, Bai L. High glucose promotes breast cancer proliferation and metastasis by impairing angiotensinogen expression. Biosci Rep. 2019;39:BSR20190436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Perkins CI, Hotes J, Kohler BA, Howe HL. Association between breast cancer laterality and tumor location, United States, 1994-1998. Cancer Causes Control. 2004;15:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Weiss HA, Devesa SS, Brinton LA. Laterality of breast cancer in the United States. Cancer Causes Control. 1996;7:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Abdou Y, Gupta M, Asaoka M, Attwood K, Mateusz O, Gandhi S, Takabe K. Left sided breast cancer is associated with aggressive biology and worse outcomes than right sided breast cancer. Sci Rep. 2022;12:13377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 22. | Barbara RC, Piotr R, Kornel B, Elżbieta Z, Danuta R, Eduardo N. Divergent Impact of Breast Cancer Laterality on Clinicopathological, Angiogenic, and Hemostatic Profiles: A Potential Role of Tumor Localization in Future Outcomes. J Clin Med. 2020;9:1708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |