Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.107184
Revised: April 29, 2025
Accepted: June 19, 2025
Published online: September 20, 2025
Processing time: 148 Days and 1.2 Hours
Antimicrobial resistance (AMR) is an escalating global health threat, disproportionately impacting low-income and middle-income countries like India. The rise of multidrug-resistant organisms (MDROs) complicates treatment, increases healthcare costs, and contributes to higher morbidity and mortality. In India, factors such as antibiotic misuse, over-the-counter availability, and self-medication have exacerbated this crisis. Despite the urgency, regional data on MDRO prevalence remains limited.
To assess the burden and distribution of MDRO infections identified at a tertiary healthcare institute in North India.
This cross-sectional study analyzed inpatient data from All India Institute of Medical Sciences (AIIMS) Rishikesh using the E-hospital database from May 2021 to February 2024, except 2022. All inpatients with culture-confirmed MDRO infections were included. Patient charts and discharge summaries were reviewed. Data analysis was performed using Jamovi software, with descriptive statistics summarizing demographics. The χ² test was used to assess associations between MDROs and various factors. Ethical approval was obtained, and patient confidentiality was maintained.
This study included 820 participants having 1106 MDROs. The majority of patients were aged 35-64 years, with a male predominance (57.6%). Most cases were from Uttar Pradesh (49.1%) and Uttarakhand (47.6%), with Bijnor and Haridwar districts reporting the highest burden from their respective states. Klebsiella pneumoniae (K. pneumoniae) (30.6%), Acinetobacter baumannii (16.7%), and Escherichia coli (E. coli) (16.7%) were the most common MDROs. Urine was the most frequent sample type (51.0%), with K. pneumoniae as the leading pathogen. Infections varied significantly across departments (P < 0.001), with General Medicine and Surgery reporting the highest burden. E. coli and Klebsiella spp. were most prevalent in these departments respectively. MDRO prevalence fluctuated over time, with significant variations by quarter (P < 0.001). Following the second coronavirus disease 2019 wave, there was a rapid surge in MDROs, which stabilized after approximately three months. The overall mortality among patients harboring MDROs was 25.9%.
This study highlights the burden of MDROs among patients at AIIMS Rishikesh, with K. pneumoniae as the predominant pathogen. Strengthening antimicrobial stewardship and infection control measures is essential to combat rising AMR, with department-specific, and pathogen-specific stewardships.
Core Tip: The study assessed the burden of multidrug-resistant organisms (MDROs) in All India Institute of Medical Sciences Rishikesh from May 2021 to February 2024, except 2022. Klebsiella pneumoniae (30.6%) was the most common MDRO, with urine as the predominant sample type (51.0%). The highest burden was observed in the General Medicine and Surgery Departments, with significant quarterly variations (P < 0.001). Uttar Pradesh and Uttarakhand contributed the most cases, particularly from Bijnor and Haridwar districts. There was post-corona virus disease surge of MDROs and one-quarter died. Strengthening antimicrobial stewardship and infection control is crucial to curb antimicrobial resistance.
- Citation: Singh H, Patel AA, Pandy P, Omar BJ, Prasad A, Singh V, Sharma P, Bairwa M, Sihag RK, Tiwari A, Rajput D, Kulshrestha M, Saini S, Kumar A, Sarkar B, Duggal B, Agarwal A, Kaeley N, Dhingra G, Mahala P, Yesodharan V, Chauhan H, Kumari D, Choudhary S, Sharma AK, Yadav R, Panda PK. Burden of multi-drug-resistant organisms in a tertiary healthcare institute in North India: Implications for regional public health. World J Exp Med 2025; 15(3): 107184
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/107184.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.107184
Antimicrobial resistance (AMR) is an escalating global public health and developmental threat, with low-income and middle-income countries (LMICs) being the most affected[1]. Multidrug resistance (MDR) is defined as the ability of bacteria to resist or to become tolerant to several structurally and functionally distinct drugs simultaneously[2]. The increasing prevalence of multidrug-resistant organisms (MDROs) poses a significant challenge for clinicians and hospital staff, as it renders many antibiotics ineffective, making even common infections difficult to treat. This results in increased healthcare expenditure, prolonged hospital stays, higher morbidity and mortality, and an overall strain on healthcare systems. In 2019, an estimated 4.95 million fatalities worldwide were associated with bacterial AMR[3]. If adequate measures are not implemented promptly, annual AMR-related mortality could rise to 10 million by 2050[4].
The situation is further exacerbated in developing countries like India due to multiple factors, including poverty, high disease burden, inadequate healthcare infrastructure, and a lack of awareness among the general population regarding appropriate antibiotic use[5]. The widespread availability of antibiotics as over-the-counter medications is a major contributor to the emergence of MDROs[6]. Between 2000 and 2018, antibiotic consumption in India surged from 48% to 67%[7]. Additionally, self-medication practices, often driven by financial constraints, result in suboptimal antibiotic regimens, fostering AMR[8].
Despite growing concerns regarding AMR, data on the prevalence of MDROs in North India remains limited. Understanding the true burden of resistance is crucial for effective AMR mitigation strategies, which necessitates extensive surveillance and data collection[3]. The antibiogram of India differs significantly from that of Western Nations, making it imperative for clinicians to be well-versed in local resistance patterns to guide empirical antibiotic therapy appropriately[9]. Most existing studies focus on specific age groups or disease conditions, making it difficult to generalize findings at the regional level. This study aims to determine the prevalence of MDROs among all inpatients across various hospital departments at our institute. The collected data will also be stratified by state and district of patient origin to identify regional patterns of resistance.
A cross-sectional study was conducted using patient data from admitted patients at the All India Institute of Medical Sciences (AIIMS) (Rishikesh, India) extracted from the E-hospital database between May 2021 and February 2024, except 2022.
AIIMS Rishikesh is a government-run tertiary care referral hospital located in the northern region of India, which has been operational since 2013. It primarily received referrals from the states of Uttar Pradesh and Uttarakhand, although patients from other states were also occasionally admitted. However, due to existing limitations and inconsistencies in the primary-to-tertiary care referral pathways within the Indian healthcare system, the hospital's admission patterns did not accurately reflect a district-wise distribution of patients.
All hospital admissions at AIIMS Rishikesh with culture-positive MDRO infections were included. MDR was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories (as per the European Centre for Disease Prevention and Control and the Centers for Disease Control and Prevention). Culture and sensitivity was performed in an automated culture system (VITEK-2; bioMérieux SA, Marcy-l'Étoile, France). Admissions with incomplete or invalid data were excluded.
Patient charts and discharge summaries were retrieved from the E-hospital website, an online patient portal managed by the Government of India. Initially, all hospital admissions at AIIMS Rishikesh with multidrug-resistant infections between May 2021 and February 2024 were shortlisted. Data from 2022 and the first quarter of 2023 was excluded due to incomplete data. Pathogenic isolates were distinguished from non-pathogenic isolates using a stepwise model[10]. Relevant data were then extracted from patient discharge summaries.
A structured strengthening the reporting of observational studies in epidemiology flowchart was designed to illustrate the patient selection process, inclusion and exclusion criteria, and final dataset composition. This flowchart provides a clear visualization of the study methodology and patient categorization.
Inclusion criteria: (1) All admitted MDR-infected cases where the clinician treats as a pathogen; and (2) Patient medical records available from 2021 to 2024.
Exclusion criteria: (1) The MDR infections that were not reported to the investigators/for which an infectious disease reference was not sorted; and (2) Incomplete/incorrect data.
Jamovi software was used for data analyses[11]. Descriptive statistics summarized demographic data, while χ² tests assessed associations between MDRO prevalence and factors such as patient state of residence, hospital department, and temporal trends. P < 0.05 was considered statistically significant.
To ensure the confidentiality of study participants, no personal identifiers were disclosed. Ethical approval for this study was obtained from the Institutional Ethics Committee of AIIMS Rishikesh, and the Ethical Clearance number is 28/IEC/IM/NF/2023.
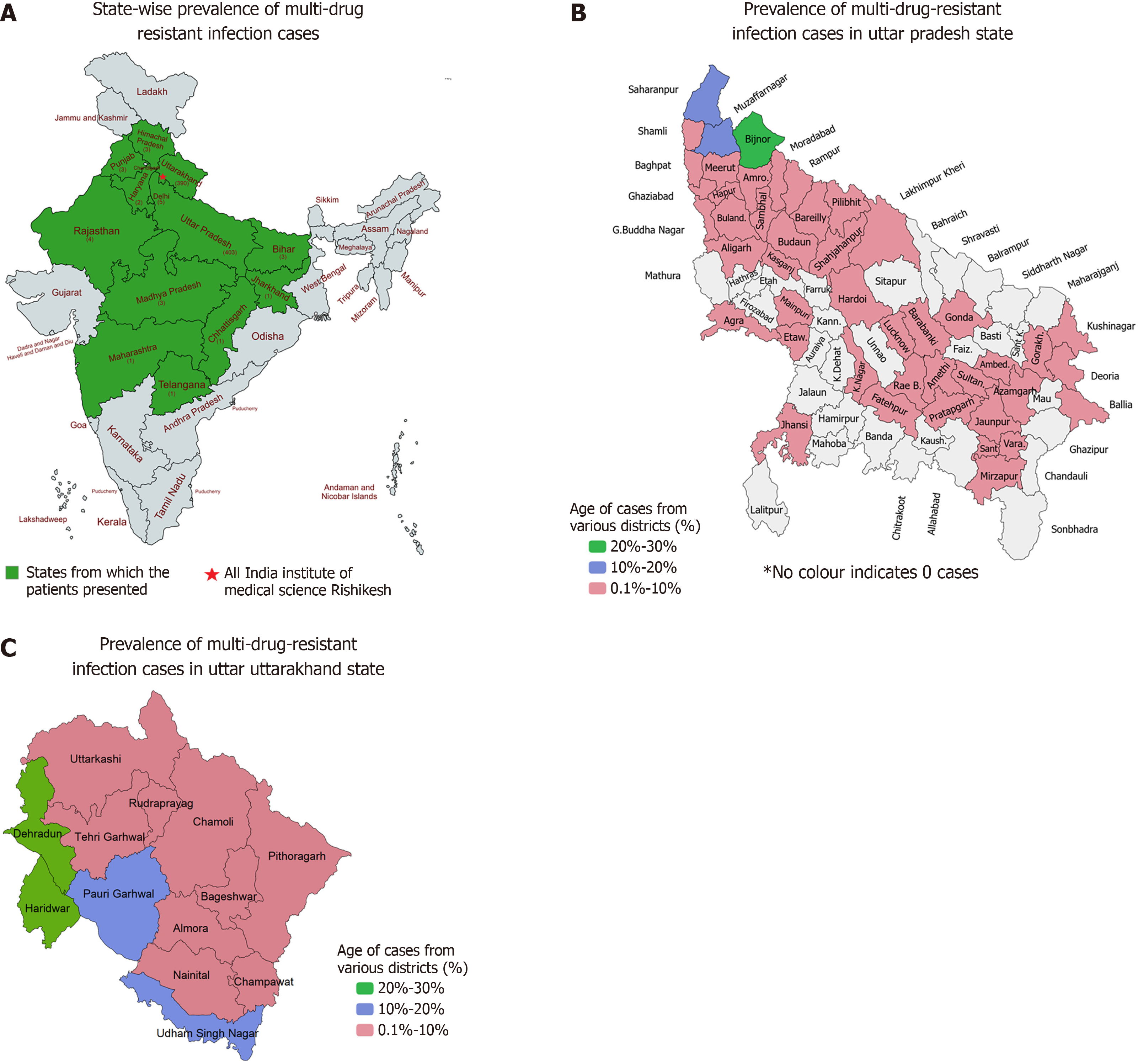
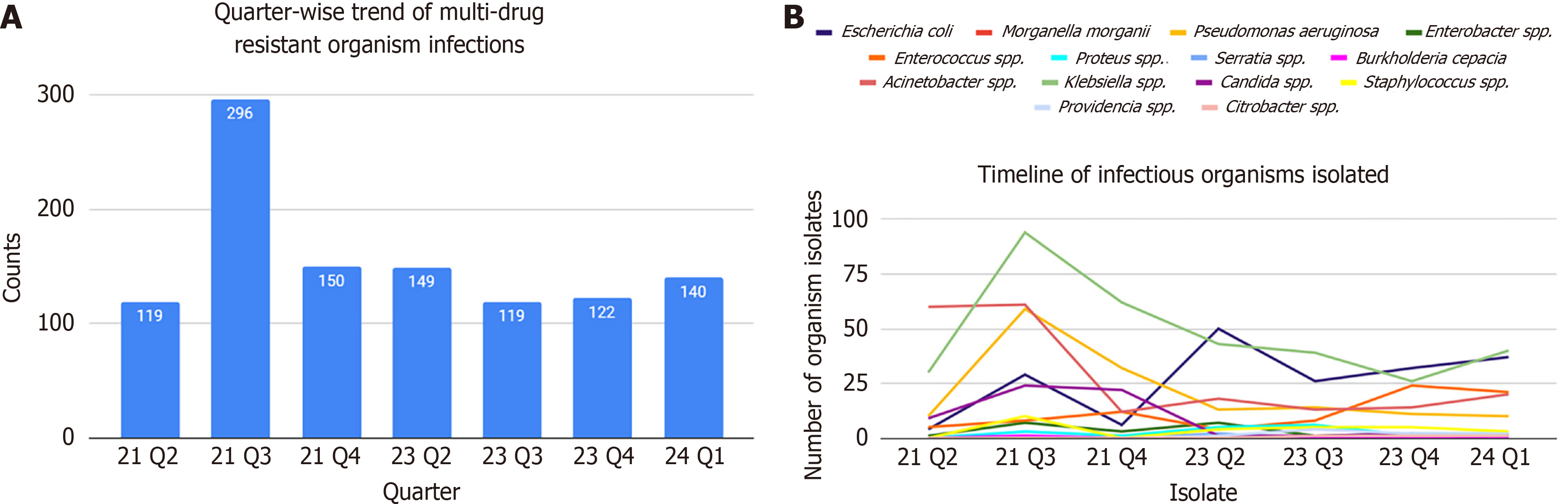
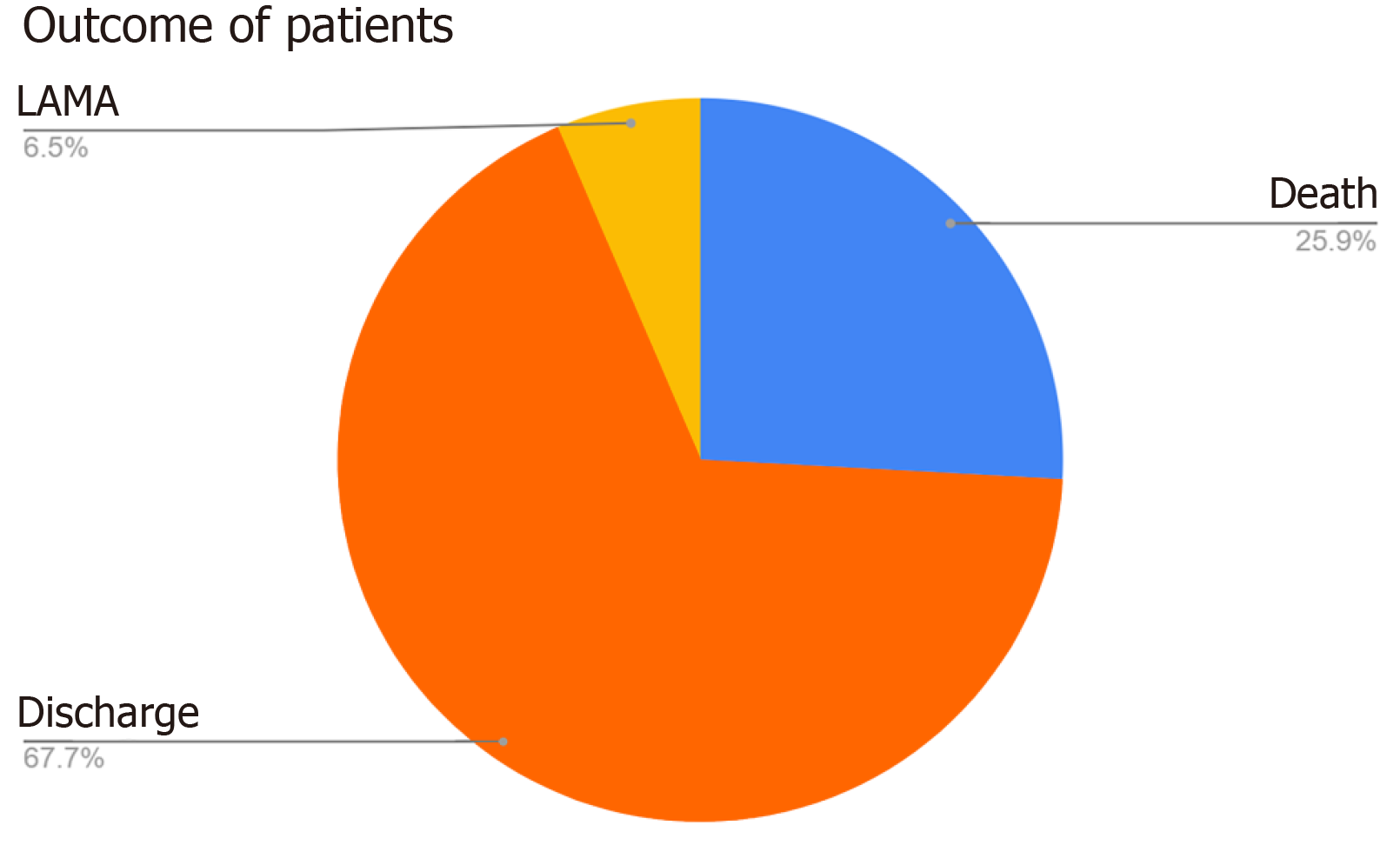
Total hospital admissions during the study period were 72678. Of the 932 patients initially shortlisted, 820 patients were included in the final analysis following exclusion of cases. The median age of patients was 44 years, with a male predominance (57.6%). Table 1 shows details of the age distributions of the participants. Geographical distribution indicated that 49.1% of cases originated from Uttar Pradesh and 47.6% from Uttarakhand, with Bijnor and Haridwar districts reporting the highest burden (Figure 1). A total of 1106 MDROs were isolated, which is during the period of our study (Figure 2). Klebsiella pneumoniae (K. pneumoniae) was the most frequently isolated MDRO (30.6%), followed by Acinetobacter baumannii (A. baumannii) (16.7%) and Escherichia coli (E. coli) (16.7%). Urine samples accounted for 51.0% of isolates, with Klebsiella spp. as the predominant organism (30.3%), followed by E. coli (22.9%). In blood, the most common isolates were Acinetobacter spp. (35.1%) and Klebsiella spp. (30.5%). Refer to Table 2 for details on isolates from various samples. No significant association was found between the MDROs and the state of presentation (P = 1). Considerable variations were observed in MDRO distribution across hospital departments (P < 0.001), with General Medicine and Surgery reporting the highest burden (Table 3). The most common MDROs isolated in the Department of General Medicine were E. coli (29.1%) and Klebsiella spp. (23.6%). Klebsiella spp. (37.9%) and E. coli (24.1%) also accounted for most of the Department of General Surgery infections. K. pneumoniae accounted for the most prevalent organism in the Klebsiella spp. The most frequent isolate in the Department of Neurosurgery was Klebsiella spp. (24.6%) followed by Acinetobacter spp. (18.9%) and E. coli (18.0%). A detailed prevalence of MDROs in various departments has been mentioned in Table 4. The outcome of the hospitalization of 820 patients was studied. Various outcomes included- discharge of the patient, death of the patient, and leave against medical advice (LAMA). Most hospitalizations resulted in discharge (67.7%) of the patients in a hemodynamically stable condition. About one-fourth of the patients admitted with MDRO infections died. Figure 3 shows the various outcomes of patients with MDRO infections.
| Age group (in years) | Number of participants | Percentage of total |
| < 14 | 29 | 3.5 |
| 14-34 | 257 | 31.3 |
| 35-64 | 424 | 51.7 |
| ≥ 65 | 110 | 13.4 |
| Samples | Number | Percentage |
| Urine | 564 | 51.0 |
| Blood | 151 | 13.7 |
| Pus | 142 | 12.8 |
| Endotracheal tube/tracheal tube/broncho-alveolar lavage | 98 | 8.9 |
| Tissue | 49 | 4.4 |
| Sputum/throat swab | 23 | 2.1 |
| Cerebrospinal fluid | 16 | 1.4 |
| Bile | 15 | 1.4 |
| Pleural fluid | 14 | 1.3 |
| Body fluids (not specified) | 12 | 1.1 |
| Peritoneal fluid | 11 | 1.0 |
| Ascitic fluid | 9 | 0.8 |
| Vaginal swab | 1 | 0.1 |
| Corneal scraping | 1 | 0.1 |
| Name of department | Number of multidrug resistance organisms | Percentage |
| General Medicine | 148 | 13.4 |
| General Surgery | 145 | 13.1 |
| Neurosurgery | 122 | 11.0 |
| Trauma Surgery | 102 | 9.2 |
| Medicine Intensive Care Unit | 90 | 8.1 |
| Emergency Medicine | 81 | 7.3 |
| Pulmonary Medicine | 62 | 5.6 |
| Urology | 43 | 3.9 |
| Medical Oncology/Hematology | 41 | 3.7 |
| Neurology | 41 | 3.7 |
| Obstetrics and Gynecology | 36 | 3.3 |
| Nephrology | 32 | 2.9 |
| Otorhinolaryngology | 28 | 2.5 |
| Corona Virus Disease | 21 | 1.9 |
| Orthopedics | 19 | 1.7 |
| Pediatrics | 17 | 1.5 |
| Gastroenterology | 16 | 1.4 |
| Surgical Gastroenterology | 10 | 0.9 |
| Surgical Oncology | 10 | 0.9 |
| Cardiology | 9 | 0.8 |
| Geriatric Medicine | 7 | 0.6 |
| Radiation Oncology | 7 | 0.6 |
| Endocrinology | 5 | 0.5 |
| Neonatology | 5 | 0.5 |
| Burns and Plastic Surgery | 4 | 0.4 |
| Physical Medicine and Rehabilitation | 3 | 0.3 |
| Dermatology | 1 | 0.1 |
| Pediatric Surgery | 1 | 0.1 |
| Department | Isolates | ||||||||||||||
| Acinetobacter spp. | Burkholderia cepacia | Candida spp. | Citrobacter spp. | Enterobacter spp. | Enterococcus spp. | Escherichia coli | Klebsiella spp. | Morganella morganii | Proteus spp. | Providencia spp. | Pseudomonas aeruginosa | Serratia spp. | Staphylococcus spp. | Total | |
| Burns and Plastic Surgery | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Cardiology | 1 | 0 | 0 | 0 | 0 | 0 | 5 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 9 |
| Coronavirus Disease | 10 | 0 | 2 | 0 | 0 | 0 | 0 | 7 | 0 | 1 | 0 | 1 | 0 | 0 | 21 |
| Dermatology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Emergency Medicine | 15 | 0 | 12 | 0 | 2 | 3 | 12 | 18 | 0 | 0 | 0 | 18 | 0 | 1 | 81 |
| Endocrinology | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Gastroenterology | 6 | 0 | 1 | 0 | 1 | 1 | 1 | 4 | 0 | 0 | 0 | 1 | 0 | 1 | 16 |
| General Medicine | 15 | 0 | 10 | 0 | 2 | 15 | 43 | 35 | 0 | 1 | 1 | 23 | 0 | 3 | 148 |
| General Surgery | 21 | 0 | 6 | 0 | 2 | 11 | 35 | 55 | 1 | 1 | 0 | 8 | 1 | 4 | 145 |
| Geriatric Medicine | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 7 |
| Medical Oncology/ Hematology | 3 | 0 | 2 | 0 | 3 | 1 | 10 | 14 | 0 | 1 | 0 | 5 | 1 | 1 | 41 |
| Medicine Intensive Care Unit | 23 | 0 | 2 | 0 | 0 | 10 | 5 | 24 | 0 | 0 | 5 | 14 | 1 | 6 | 90 |
| Neonatology | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 5 |
| Nephrology | 3 | 0 | 2 | 0 | 1 | 3 | 4 | 12 | 0 | 0 | 0 | 6 | 0 | 1 | 32 |
| Neurology | 4 | 0 | 2 | 0 | 0 | 7 | 9 | 14 | 0 | 1 | 1 | 3 | 0 | 0 | 41 |
| Neurosurgery | 23 | 0 | 7 | 0 | 1 | 7 | 22 | 30 | 1 | 5 | 0 | 20 | 2 | 4 | 122 |
| Obstetrics and Gynecology | 3 | 0 | 2 | 0 | 0 | 6 | 3 | 18 | 0 | 0 | 0 | 3 | 0 | 1 | 36 |
| Orthopedics | 6 | 0 | 2 | 0 | 1 | 0 | 0 | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 19 |
| Otorhinolaryngology | 9 | 0 | 1 | 0 | 0 | 1 | 0 | 9 | 0 | 0 | 0 | 6 | 0 | 2 | 28 |
| Pediatric Surgery | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pediatrics | 5 | 0 | 1 | 0 | 1 | 1 | 1 | 5 | 0 | 0 | 0 | 3 | 0 | 0 | 17 |
| Physical Medicine and Rehabilitation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 3 |
| Pulmonary Medicine | 25 | 0 | 1 | 0 | 1 | 7 | 6 | 16 | 0 | 0 | 1 | 3 | 0 | 2 | 62 |
| Radiation Oncology | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Surgical Gastroenterology | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| Surgical Oncology | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5 | 0 | 1 | 0 | 1 | 0 | 0 | 10 |
| Trauma Surgery | 14 | 0 | 0 | 2 | 4 | 4 | 18 | 37 | 0 | 4 | 1 | 15 | 1 | 2 | 102 |
| Urology | 5 | 0 | 5 | 0 | 1 | 3 | 6 | 11 | 0 | 1 | 0 | 11 | 0 | 0 | 43 |
| Total | 200 | 1 | 60 | 3 | 23 | 82 | 185 | 340 | 3 | 16 | 9 | 150 | 6 | 28 | 1106 |
AMR remains a growing global concern, particularly in LMICs like India, where the burden of infections is substantial. Our study provides critical insights into the prevalence and distribution of MDROs among inpatients at AIIMS Rishikesh. The findings highlight significant regional and departmental variations, underscoring the urgent need for targeted infection control and antimicrobial stewardship interventions.
MDRO infections were more common in males (57.6%), consistent with previous studies[12,13]. The age distribution ranged from neonates to elderly individuals (95 years), with the majority of affected patients (35–64 years) belonging to the middle-aged group. This differs from studies conducted in India and China, which identify the elderly as the most vulnerable to MDR infections[12,14]. These demographic patterns suggest that while older adults remain susceptible, the middle-aged population also experiences a high disease burden. It could be due to the demise of elderly patients with MDR infections before they reach the tertiary healthcare center.
Geographically, Uttar Pradesh (49.1%) and Uttarakhand (47.6%) contributed nearly equally to the MDRO burden, reflecting significant referral patterns to tertiary care institutions like ours. The highest number of cases originated from districts such as Bijnor and Saharanpur in Uttar Pradesh, and Haridwar and Dehradun in Uttarakhand. This could be attributed to easier accessibility to AIIMS Rishikesh from these areas, which could result in a greater patient burden from these areas in our institute. The observed regional clustering suggests that these districts would benefit from focused public health interventions to mitigate the spread of AMR.
Among the isolated pathogens, K. pneumoniae (30.6%) was the most prevalent MDRO, followed by A. baumannii (16.7%) and E. coli (16.7%). This differs from the findings of the National Antimicrobial Resistance Surveillance Network (NARS-Net) 2023, which reported E. coli as the most commonly isolated MDRO[13]. One key reason for this discrepancy is the methodological approach of our study. Unlike NARS-Net, which is laboratory-based and microbiologist-based, our study utilized a stepwise clinical model, incorporating bedside physician assessments to distinguish true pathogens from commensals, colonizers, or contaminants. This distinction is crucial, as laboratory-only data often do not account for clinical relevance, potentially leading to an overestimation of certain organisms, such as E. coli, which is frequently isolated as a commensal/colonizer in urine cultures.
The persistently high burden of MDRO infections in relatively underserved states such as Uttar Pradesh and Uttarakhand underscores the critical need for robust AMR containment strategies. Recent trends indicate an alarming rise in resistant K. pneumoniae strains. A North Indian study reported an increase in resistant Klebsiella infections from 7.5% in 2018 to 21.4% in 2022 among intensive care unit patients[15]. This rising trend presents significant treatment challenges, particularly in resource-limited healthcare settings where access to advanced antimicrobial therapies may be constrained.
Our temporal analysis showed that MDRO cases remained relatively stable from 2021 to February 2024, after excluding 2022 and the first quarter of 2023 due to incomplete data. However, a marked surge in MDRO infections was observed in Quarter 3 of 2021, likely linked to the aftermath of the second wave of coronavirus disease 2019 (April–May 2021), during which antibiotic overuse and misuse were rampant. This underscores the collateral impact of pandemic-driven antimicrobial prescribing practices on resistance patterns. The peak in quarter 3 of 2021 could also be because of initiation of Pseudomonas, Enterococcus, Acinetobacter, Klebsiella, Methicillin-resistant Staphylococcus aureus, E. coli eradication program started at the institution around that time, for which multiple cultures were sent to determine the sterility of isolates after these MDRO patients being isolated.
Departmental analysis revealed that MDRO infections were most prevalent in General Medicine and General Surgery. These departments manage high patient volumes, prolonged hospital stays, and frequent antibiotic usage—factors predisposing to MDRO colonization and infections. A study conducted in Spain noticed a higher burden of MDROs in medical wards as compared to surgical wards[16]. General Medicine wards predominantly cater to patients with chronic illnesses requiring recurrent hospitalizations, while surgical wards face higher risks of perioperative infections. The significant variation in MDRO distribution across departments (P < 0.001) highlights the necessity for department-specific infection control strategies, which may enhance the effectiveness of AMR mitigation efforts. Our study found that E. coli and Klebsiella spp. were the most common MDROs in the departments of General Medicine and General Surgery respectively, with K. pneumoniae being the predominant species within the Klebsiella group. The high prevalence of Klebsiella spp. in surgery and its allied departments emphasizes the risk of post-surgical infections and the challenge of managing AMR in surgical settings. A previous study conducted in India showed that most MDROs isolated from surgical site infections were E. coli, Klebsiella spp., Proteus spp., and Staphylococcus. aureus[17]. These findings emphasize the need for stringent infection control measures, targeted antibiotic stewardship programs, and improved surveillance to mitigate the spread of MDROs across different hospital departments.
MDRO infections pose serious threats to patient outcomes, being associated with increased morbidity, mortality, and prolonged hospital stays. They also escalate healthcare costs by complicating treatment protocols and increasing resource utilization. Our study showed a significant percentage (25.9%) of death among MDRO infections. A study conducted from 2021 to 2022 in Southern India showed 63.2% mortality in MDR patients[18]. Another 2019 study by Gandra et al[19] found that infections with MDR and extensively drug-resistant bacteria, particularly E. coli, K. pneumoniae, and A. baumannii, were associated with a 2–3-fold increase in patient mortality, with an overall in-hospital mortality rate of 13.1%. Furthermore, a 2018 study reported that 30.1% of healthy adults in a rural South Indian community were colonized with Enterobacteriaceae harboring MDR genes, including extended-spectrum beta-lactamase, AmpC, and New Delhi metallo-beta-lactamase, indicating a significant community reservoir of resistance[20]. Though the present study couldn’t answer these colonizer MDROs, definitely it is a concern. The 6.5% of LAMAs are concerning and may be due to gaps in patient education, ineffective communication, and socio-economic or domestic constraints[21]. LAMA could result in ineffective treatment and an eventual increase in morbidity and mortality[22].
To curb the rising rates of MDROs, the implementation of antimicrobial stewardship programs (ASP) is crucial. These programs should incorporate regular audits, feedback mechanisms, and educational initiatives tailored to local antibiograms[23]. Additionally, stringent infection prevention measures, such as hand hygiene, environmental disinfection, and contact precautions, are imperative to limit MDRO transmission[24]. At the present tertiary care hospital, there is an established ASP set-up; however, knowledge-practice gaps are rampant and may need time or a cultural mindset to come into real practice[25]. Community awareness campaigns can also help reduce unnecessary prescriptions by promoting rational antimicrobial use among patients.
This study had certain limitations. As it was a cross-sectional study conducted at a tertiary care government hospital, its findings may not be fully generalizable to primary and secondary healthcare settings. Additionally, gaps in microbiological testing due to limited culture facilities during certain periods may have influenced data representation. Moreover, our exclusive focus on inpatients, predominantly from lower socioeconomic backgrounds and with severe infections, might not reflect AMR patterns in the broader community. Simultaneously, this study has no implication of hospital-acquired MDROs. A 2024 study by Kumar et al[26] observed a high prevalence of antibiotic-resistant E. coli strains in clinical samples from South India compared to municipal samples, with notable resistance to commonly used antibiotics such as cephalosporins and fluoroquinolones. Future study will answer these hospital vs community MDROs and their inherent route of origin.
Our study describes the substantial burden of MDRO infections in a tertiary care hospital in North India, with Uttar Pradesh and Uttarakhand emerging as the major contributing states. The highest case burden was recorded in Bijnor, Saharanpur, Haridwar, and Dehradun, necessitating targeted public health interventions in these regions. K. pneumoniae was the predominant MDRO in the region, with urinary isolates being the most frequent sample type. The departments of General Medicine and General Surgery exhibited the highest infection burden, warranting stringent infection control measures, particularly enhanced contact precautions. E. coli and Klebsiella spp. were the most prevalent in these departments respectively. Immediate to second corona virus disease wave, there was a rapid surge of MDROs, settled after 3 months. There was 25% mortality of all MDROs harboring patients. Our findings highlight the critical need for region-specific and department-specific antimicrobial stewardship and infection prevention strategies to curb AMR in the region.
| 1. | World Health Organization. Antimicrobial resistance. 2025. [cited 13 June 2025]. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. |
| 2. | National Center for Biotechnology Information. Drug Resistance, Multiple, Bacterial-MeSH-NCBI. 2025. [cited 13 June 2025]. Available from: https://www.ncbi.nlm.nih.gov/mesh/68024901. |
| 3. | Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8908] [Cited by in RCA: 7328] [Article Influence: 2442.7] [Reference Citation Analysis (0)] |
| 4. | AMR Review. Home|AMR Review. 2025. [cited 13 June 2025]. Available from: https://amr-review.org/. |
| 5. | Dixit A, Kumar N, Kumar S, Trigun V. Antimicrobial Resistance: Progress in the Decade since Emergence of New Delhi Metallo-β-Lactamase in India. Indian J Community Med. 2019;44:4-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 6. | Kalluru S, Eggers S, Barker A, Shirley D, Sethi AK, Sengupta S, Yeptho K, Safdar N. Risk factors for infection with multidrug-resistant organisms in Haryana, India. Am J Infect Control. 2018;46:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Browne AJ, Chipeta MG, Haines-Woodhouse G, Kumaran EPA, Hamadani BHK, Zaraa S, Henry NJ, Deshpande A, Reiner RC Jr, Day NPJ, Lopez AD, Dunachie S, Moore CE, Stergachis A, Hay SI, Dolecek C. Global antibiotic consumption and usage in humans, 2000-18: a spatial modelling study. Lancet Planet Health. 2021;5:e893-e904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 415] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 8. | Suy S, Rego S, Bory S, Chhorn S, Phou S, Prien C, Heng S, Wu S, Legido-Quigley H, Hanefeld J, Saphonn V, Khan MS. Invisible medicine sellers and their use of antibiotics: a qualitative study in Cambodia. BMJ Glob Health. 2019;4:e001787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Vaithiyam VS, Rastogi N, Ranjan P, Mahishi N, Kapil A, Dwivedi SN, Soneja M, Wig N, Biswas A. Antimicrobial Resistance Patterns in Clinically Significant Isolates from Medical Wards of a Tertiary Care Hospital in North India. J Lab Physicians. 2020;12:196-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Panda PK, Yadav B, Sahu SN, Singh V. Pathogen vs Non-pathogen from a Culture-positive Report: How to Distinguish and Who Will? JASPI. 2024;2:10-14. [DOI] [Full Text] |
| 11. | Jamovi project. Jamovi-open statistical software for the desktop and cloud. 2025. [cited 13 June 2025]. Available from: https://www.jamovi.org/. |
| 12. | Wang M, Wei H, Zhao Y, Shang L, Di L, Lyu C, Liu J. Analysis of multidrug-resistant bacteria in 3223 patients with hospital-acquired infections (HAI) from a tertiary general hospital in China. Bosn J Basic Med Sci. 2019;19:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | National Centre for Disease Control. Annual Report National Antimicrobial Surveillance Network January-December 2023. 2025. [cited 13 June 2025]. Available from: https://ncdc.mohfw.gov.in/wp-content/uploads/2024/09/Final-Annual-Report-2023-06_08_2024.pdf. |
| 14. | Aich A, Naorem S, Devi KM, Bhattacharjee S, Bhaumik P, Subba S, Damrolien S. Prevalence, Etiology, Risk Factors and Antibiogram of Multi Drug Resistant Acinetobacter Isolates from ICUs of a Tertiary Care Hospital in North-East India. Indian J Crit Care Med. 2024;28: S446-S447. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Sharma A, Thakur A, Thakur N, Kumar V, Chauhan A, Bhardwaj N. Changing Trend in the Antibiotic Resistance Pattern of Klebsiella Pneumonia Isolated From Endotracheal Aspirate Samples of ICU Patients of a Tertiary Care Hospital in North India. Cureus. 2023;15:e36317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 16. | Peñalva G, Cantón R, Pérez-Rodríguez MT, González-López JJ, Rodríguez-Baño J, Barrio-Tofiño ED, Kirkegaard-Biosca C, Sánchez-Romero I, Gutiérrez-Villanueva A, Marrodán-Ciordia T, Guerra-Laso JM, Rosario-Quintana CD, Suárez-Hormiga L, Cámara J, Puig-Asensio M, Heredero E, Sepúlveda MA, Rodríguez-Díaz JC, Merino E, Cercenado E, Villa S, Siller M, Arnaiz F, Seral C, Lepe JA, Cisneros JM, Paño-Pardo JR; BMR. Burden of bacterial antimicrobial resistance among hospitalised patients in Spain: findings from three nationwide prospective studies. Lancet Reg Health Eur. 2025;51:101220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Shafaat Khan A, Sarwat T, Mohan S, Dutta R. Surgical Site Infection: Bacteriological and Clinicopathological Profile and Antibiogram in a Tertiary Care Hospital. J Med Sci Health. 2021;6:51-57. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Sanil M, Todur P, Nayak VR, Chaudhuri S. Incidence and Clinical Outcomes of Multidrug-resistant Respiratory Infection in the Intensive Care Units of a Tertiary Care Hospital: A Prospective, Observational Study. Indian J Chest Dis Allied Sci. 2024;66:89-93. [DOI] [Full Text] |
| 19. | Gandra S, Tseng KK, Arora A, Bhowmik B, Robinson ML, Panigrahi B, Laxminarayan R, Klein EY. The Mortality Burden of Multidrug-resistant Pathogens in India: A Retrospective, Observational Study. Clin Infect Dis. 2019;69:563-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 20. | Kumar CPG, Bhatnagar T, Sathya Narayanan G, Swathi SS, Sindhuja V, Siromany VA, VanderEnde D, Malpiedi P, Smith RM, Bollinger S, Babiker A, Styczynski A; Antibiotic Resistance in Communities and Hospitals India Team. High-level Colonization With Antibiotic-Resistant Enterobacterales Among Individuals in a Semi-Urban Setting in South India: An Antibiotic Resistance in Communities and Hospitals (ARCH) Study. Clin Infect Dis. 2023;77 (Suppl 1):S111-S117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Gaur A, Gilham E, Machin L, Warriner D. Discharge Against Medical Advice: The Causes, Consequences and Possible Corrective Measures. Br J Hosp Med (Lond). 2024;85:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Alagappan A, Chambers TJG, Brown E, Grecian SM, Lockman KA. How does discharge against medical advice affect risk of mortality and unplanned readmission? A retrospective cohort study set in a large UK medical admissions unit. BMJ Open. 2023;13:e068801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Jaggi N, Sissodia P, Sharma L. Control of multidrug resistant bacteria in a tertiary care hospital in India. Antimicrob Resist Infect Control. 2012;1:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Ji B, Ye W. Prevention and control of hospital-acquired infections with multidrug-resistant organism: A review. Medicine (Baltimore). 2024;103:e37018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Kaur S, Sethi P, Panda PK. Knowledge-Practice Gaps of Practicing Doctors on Antimicrobial Stewardship-A Single Center Experience. Am J Infect Dis. 2022;18:9-20. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Kumar G, Balakrishna K, Mukhopadhyay C, Kalwaje Eshwara V. Characterization and comparative analysis of antimicrobial resistance in Escherichia coli from hospital and municipal wastewater treatment plants. J Water Health. 2024;22: 2276-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |