Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.106020
Revised: April 9, 2025
Accepted: May 18, 2025
Published online: September 20, 2025
Processing time: 180 Days and 2.4 Hours
Knee osteoarthritis (OA) imposes a substantial burden through pain, functional limitation, and progressive cartilage loss. Bone marrow aspirate concentrate (BMAC) has emerged as a promising regenerative therapy for OA due to its rich composition of mesenchymal stromal cells (MSCs) and bioactive factors. While intra-articular BMAC injections provide short-term symptomatic relief, recent literature suggests that targeting the subchondral bone—an area crucial to OA progression—may offer superior and longer-lasting clinical benefits.
To compares the outcomes of subchondral vs intra-articular BMAC injections in patients with primary knee OA.
In this unicentric, double-blinded, randomized controlled trial, 30 patients with radiologically confirmed primary knee OA (Kellgren-Lawrence grades II and III) will be equally randomized to receive either subchondral (Group A) or intra-articular (Group B) BMAC injections. BMAC will be harvested from the posterior iliac crest, processed using a standardized centrifugation protocol to yield a product with > 85% cell viability, and administered under image guidance. The primary outcome is the change in pain intensity at 12 months as measured by the Visual Analog Scale (VAS). Secondary outcomes include functional improvement assessed by Knee Injury and Osteoarthritis Outcome Score (KOOS), International Knee Documentation Committee (IKDC), and Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores, structural changes evaluated through advanced magnetic resonance imaging using (MRI) the whole-organ MRI Score, and safety as determined by the incidence of adverse events.
This study aims to evaluate pain reduction at 12 months post-injection, using the VAS as the primary outcome. Secondary outcomes include functional improvement (KOOS, WOMAC, IKDC), cartilage regeneration (T2 cartigram), adverse event incidence, patient satisfaction (standardized questionnaires, Likert scale), and quality of life (EQ-5D). Ethical considerations follow the Declaration of Helsinki and Good Clinical Practice, with institutional review board approval and participant informed consent ensured. Confidentiality and data security comply with regulations, and a data safety monitoring board oversees trial safety. Results will be shared via peer-reviewed journals, presentations at international orthopedic conferences, and detailed summaries for stakeholders and participants. The trial is registered under clinical trial registry of India/2024/04/065284. Findings emphasize patient-centered advancements in knee osteoarthritis management.
This trial aims to refine regenerative strategies for knee OA by comparing subchondral vs intra-articular BMAC injections, addressing long-term efficacy, safety, and treatment standardization to guide targeted interventions. This trial will provide critical insights into the comparative efficacy and safety of subchondral vs intra-articular BMAC injections in treating primary knee OA.
Core Tip: In this double-blinded, randomized controlled trial, 30 patients with primary knee osteoarthritis will receive either subchondral or intra-articular Bone marrow aspirate concentrate (BMAC) injections. The study aims to compare pain relief (measured by Visual Analog Scale) at 12 months. Secondary outcomes include functional improvement (Knee Injury and Osteoarthritis Outcome Score, International Knee Documentation Committee, and Western Ontario and McMaster Universities Arthritis Index scores), structural changes [magnetic resonance imaging (MRI) using the whole-organ MRI Score], and safety (adverse events). BMAC is harvested from the posterior iliac crest, processed, and administered under image guidance.
- Citation: Nallakumarasamy A, Shrivastava S, Rangarajan RV, Jeyaraman N, Ramasubramanian S, Muthu S, Jeyaraman M. Comparative outcome analyses of subchondral vs intra-articular bone marrow aspirate concentrate in primary osteoarthritis knee: A double-blinded randomized controlled trial protocol. World J Exp Med 2025; 15(3): 106020
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/106020.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.106020
Knee osteoarthritis (OA) is a degenerative joint disease characterized by progressive cartilage loss, subchondral bone remodeling, and persistent synovial inflammation[1–3]. This multifactorial pathology is a major cause of disability in older adults, affecting millions worldwide and imposing significant healthcare burdens[4–7]. Conventional treatments—ranging from pharmacological interventions to surgical procedures—often provide only transient symptomatic relief, without addressing the underlying structural deterioration[8–13]. Total knee arthroplasty, while effective for end-stage OA, is invasive and associated with potential complications such as persistent postoperative pain in up to 20% of cases[14,15]. These limitations have fueled interest in regenerative therapies that aim not only to alleviate symptoms but also to modulate the disease process.
Bone marrow aspirate concentrate (BMAC) has emerged as a promising orthobiologic treatment for knee OA[16,17]. BMAC is rich in mesenchymal stromal cells (MSCs), hematopoietic progenitors, and a spectrum of bioactive cytokines that collectively contribute to tissue repair, immunomodulation, and potentially cartilage regeneration[18]. BMAC’s therapeutic potential stems from MSC that secrete anti-inflammatory cytokines (e.g., interleukin-10, transforming growth factor-beta) and growth factors (e.g., vascular endothelial growth factor, platelet-derived growth factor), promoting paracrine effects that reduce synovial inflammation and stimulate chondrocyte proliferation.
Recent literature has demonstrated that BMAC injections can lead to significant improvements in pain, function, and even structural changes within the joint[19–21]. For example, multiple studies report enhancements in patient-reported outcome measures such as the Visual Analog Scale (VAS), Knee Injury and Osteoarthritis Outcome Score (KOOS), and International Knee Documentation Committee (IKDC) scores. In one study, KOOS subscales for pain, activities of daily living, and quality of life improved markedly within 12 weeks, while another investigation noted a reduction in VAS pain scores from 7.0 to 2.5 over 24 months[21,22].
A key advancement in the field is the recognition of the subchondral bone’s role in the pathophysiology of OA[23]. Subchondral bone marrow edema, a hallmark of OA progression, has been correlated with increased pain and joint deterioration[24]. Traditional intra-articular injections primarily target the synovial environment, but emerging evidence suggests that subchondral delivery of BMAC might offer superior benefits by directly addressing bone marrow edema and stimulating the repair by modulating osteoclast activity and enhancing osteochondral repair, as supported by preclinical evidence[25,26]. Magnetic resonance imaging (MRI) studies employing the Whole-Organ MRI Score (WORMS) have documented significant reductions in bone marrow edema following subchondral BMAC injections[27,28]. Moreover, there is an observed correlation between the extent of baseline bone marrow lesions and subsequent clinical improvement, highlighting the potential of this targeted approach.
Despite these promising findings, variability in BMAC preparation and delivery methods across studies has led to inconsistent outcomes. Some protocols involve centrifugation to concentrate cells, while others use non-centrifugation techniques; dosages also vary from 1 to 5 million cells per kilogram. Such heterogeneity underscores the need for well-controlled, comparative trials. This study is designed as a double-blinded randomized controlled trial to compare subchondral vs intra-articular BMAC injections in patients with primary knee OA (Kellgren-Lawrence grades II and III).
The study aims to assess the efficacy of intraarticular and subchondral BMAC injections with changes in patient-reported outcome measures such as VAS, WOMAC, and KOOS from baseline to different follow-up time points, to compare the efficacy of intraarticular and subchondral BMAC injections in knee OA from baseline to different follow-up time points, and to assess the healing of subchondral bone lesions (bone marrow lesions) and the integrity of cartilage during pre and post-procedural follow-up with intraarticular and subchondral BMAC in primary OA knees via MOCART at a 1-year time point using MRI.
This unicentric, prospective, double-blinded, randomized controlled trial will be conducted at the Department of Orthopedics, Mother Cell Regenerative Centre, Trichy. All requisite clearances and permissions have been obtained from the institutional ethical committee. The study is scheduled to run from September 2023 (post-DRC approval) to December 2025 (total duration: 30 months).
Based on preliminary data indicating clinically meaningful differences in pain (medium effect size, Cohen’d = 0.5 for VAS pain score) and functional outcomes (KOOS scores) following BMAC treatments—and after adjusting for an anticipated 15% dropout rate—a total of 30 patients (15 per group) will be enrolled. This sample size provides 80% power at a 95%CI to detect significant differences between the intervention arms.
A computer-generated, permuted block randomization (block size of four) will allocate patients in a 1:1 ratio to either Group A (subchondral BMAC injection) or Group B (intra-articular BMAC injection) (Figure 1). Base line characteristics will be compared statistically to confirm even distribution of samples. Blinding the injecting surgeon is challenging due to different techniques of injecting the BMAC. An independent study coordinator (a doctor not involved in the study analyses) will manage the randomization process. It includes preparing the patient with identically appearing syringes in the procedure room. The procedure involves the injecting surgeon performs either subchondral or intra-articular injections under local anaesthesia, who follows standardized procedural steps (focuses solely on technical execution) without knowing the clinical intent and patient’s profile. This is an inherent procedural variation not fully maskable, outcome assessors and the patients remain intact. Any pain difference is not expected to unblind patients, given intra-procedural local anaesthesia and standardized post-care [rest, ice, compression, and elevation (RICE) therapy]. Unblinding protocols are in place for the management of major adverse events and those patients will be excluded in the final assessment. This ensures that both the patient and outcome assessors remain blinded throughout the trial.
Inclusion criteria: Patients aged between 30 to 80 years. Radiographic confirmation of primary knee OA using Kellgren-Lawrence grades II and III. Persistent moderate-to-severe knee pain unresponsive to conservative treatment for at least 3 months. Ability to attend regular follow-up visits. Provision of informed written consent.
Exclusion criteria: Patients age outside the 30–80-year range. Advanced (grade IV) or very mild (grade I) OA or secondary OA. Prior intra-articular corticosteroid injections within 3 months. History of inflammatory, autoimmune, or polyarticular arthritis. Abnormal hematological parameters (e.g., hemoglobin < 10 gm/dL, platelet count < 105/μL). Active local or systemic infection. Coagulation disorders or contraindications to bone marrow aspiration.
Before intervention, all patients will undergo comprehensive workup. Weight-bearing radiographs and baseline MRI (including T2 mapping and proton density sequences) to assess cartilage integrity and subchondral bone status. Baseline laboratory investigations, including a complete hemogram, erythrocyte sedimentation rate and C-reactive protein renal function tests, and coagulation profile, are conducted. While the procedure can typically be performed under local anesthesia, some apprehensive or hypersensitive patients may require mild intravenous sedation.
Under aseptic conditions and local anesthesia (6 mL of 2% lignocaine), 60–80 mL of bone marrow will be aspirated from the anterior iliac crest using a Jamshidi needle. The aspirate will be collected in heparinized syringes to prevent coagulation. A standardized double centrifugation (density centrifugation) protocol will then be applied to concentrate mesenchymal stem cells (MSCs) and growth factors, ensuring a cell viability exceeding 85% (assessed via trypan blue exclusion). It include, the prepared tubes are subjected for first centrifugation at 2800 rpm for 12 minutes. BMAC portion is subjected for second centrifugation at 3200 rpm for 10 minutes in plain tubes. The final product will be adjusted to a volume of 5–6 mL for injection.
Pre-procedural protocol: Patients will be briefed regarding the procedure, potential risks, and the structured post-treatment rehabilitation program (including active quadriceps and range-of-motion exercises). Informed consent will be reconfirmed after the clinical and radiological evaluation.
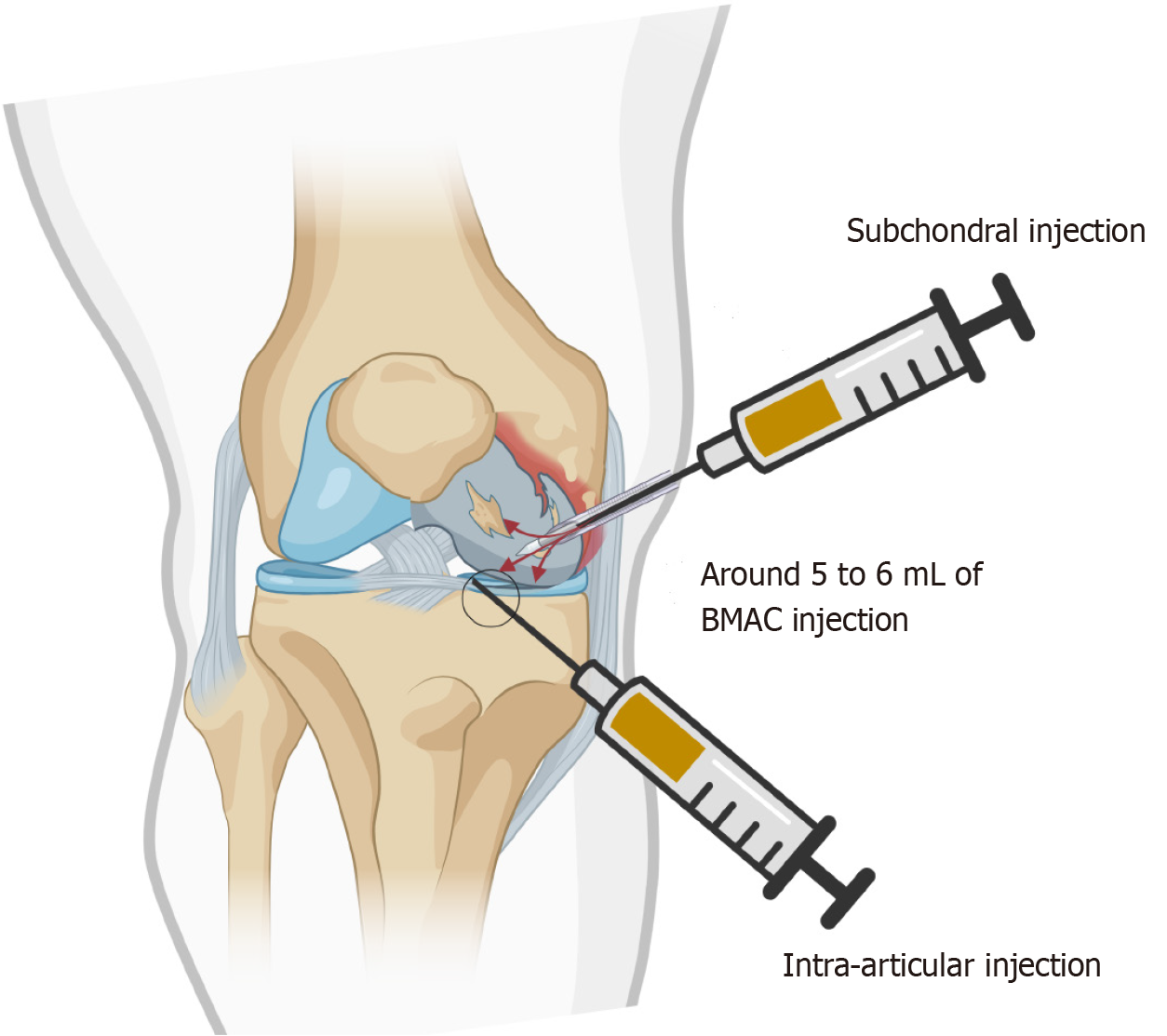
Group A (Subchondral injection; n = 15): With strict aseptic precautions, under local anaesthesia (5 mL of 2% lignocaine) and fluoroscopic guidance, a 20-gauge needle will be advanced into the subchondral bone (targeting regions of bone marrow edema as identified on MRI) of the affected compartment. A single dose of 5–6 mL BMAC (2 million BMAC cells per kilogram body weight) will be injected to modify the subchondral microenvironment to facilitate cartilage repair and long-term symptom relief.
Group B (Intra-articular injection; n = 15): Under similar sterile conditions and imaging guidance (ultrasound or fluoroscopy), a single dose of 5–6 mL BMAC (2 million BMAC cells per kilogram body weight) will be administered into the joint cavity using a standard anterolateral or superolateral approach. To maintain blinding, syringes will be masked with opaque sleeves.
Following injection, a crepe bandage will be applied, and patients will be observed for 10 minutes prior to initiating gentle knee mobilization exercises.
BMAC therapy is relatively safe but carries risks, particularly in patients with advanced OA or underlying medical conditions. Proper patient selection, sterile technique, and post-procedure monitoring can help minimize complications. The procedure-related complications, such as pain and swelling, are self-limiting.
Post-injection, patients will be advised to adhere to the following:
RICE therapy: RICE for the first 48–72 hours.
Weight bearing: Restricted for 48–72 hours, with gradual resumption based on clinical status.
Rehabilitation program: A structured program emphasizing range-of-motion exercises and quadriceps strengthening.
Follow-up evaluations: Scheduled at 1, 3, 6, and 12 months post-procedure, including repeat radiographs and MRI to assess cartilage and subchondral changes.
Data analysis will be performed using SPSS Version 26. Intention-to-treat and per-protocol analyses will be conducted.
Primary outcome: Change in pain intensity at 12 months, measured by the VAS.
Secondary outcomes: Functional improvements as assessed by KOOS, WOMAC, and IKDC scores. Structural changes in cartilage and subchondral bone (assessed by MRI, including T2 mapping and WORMS to evaluate bone marrow edema and cartilage morphology). Incidence and characterization of adverse events. Patient satisfaction and quality of life measured by the EQ-VAS.
Between-group comparisons: Paired t-tests (or the Wilcoxon signed-rank test for non-parametric data) will be used to compare baseline and follow-up outcomes.
Within-group comparisons: Repeated measures ANOVA with post-hoc Bonferroni corrections (or Friedman test with pairwise comparisons for non-normal data) will assess changes over time.
Categorical variables: Fisher’s exact test will be utilized to compare the incidence of adverse events. A P value < 0.05 will be considered statistically significant for all variables
This study will adhere to the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol has received ethical approval (IRB No. PR23AKS0 L5 dated 28.10.2023) and Clinical Trial Registry of India (CTRI) approval (CTRI/2024/04/065284 dated 05.04.2024) and informed consent will be obtained from all participants. Data confidentiality will be maintained, and any serious adverse events will be reported within 24 hours to the IRB.
Results will be published in high-impact, peer-reviewed journals and presented at national and international conferences. Detailed reports will be provided to participating institutions, and findings will be shared with healthcare stakeholders to inform future clinical practice.
The current study addresses a critical gap in the management of knee OA by directly comparing subchondral and intra-articular administration of BMAC.
One of the most compelling aspects of BMAC therapy is its potential to harness the regenerative capacity of MSC and other progenitor cells[29]. MSCs have been shown to secrete anti-inflammatory cytokines and growth factors that facilitate tissue repair and modulate the local inflammatory milieu[30,31]. This paracrine effect may help to restore joint homeostasis and promote cartilage regeneration—a key unmet need in OA management. Studies have reported significant improvements in clinical scores, such as the KOOS and IKDC, following BMAC injections. For instance, one study demonstrated that KOOS activities-of-daily-living scores improved from 61.1 to 89.3 within 12 weeks, while another reported VAS pain scores dropping from 7.0 at baseline to 2.5 at 24 months[22]. Such results underscore the therapeutic promise of BMAC in reducing pain and enhancing function in knee OA patients.
However, a central debate in the literature pertains to the optimal site for BMAC delivery[32,33]. Traditional intra-articular injections are thought to provide rapid symptomatic relief by modulating the synovial environment, yet their impact on subchondral bone pathology remains limited. Recent investigations have shifted focus toward the subchondral bone, given its role in the pathogenesis of OA[27]. The subchondral region is often characterized by bone marrow edema, which correlates with pain severity and disease progression. MRI assessments using the WORMS have provided objective evidence of a significant reduction in bone marrow edema following subchondral BMAC injections[23,27]. This reduction is clinically meaningful, as studies have shown that patients with higher baseline bone marrow edema tend to experience greater improvements in pain and function post-treatment.
Our study is designed to elucidate whether the subchondral route can offer superior long-term benefits compared to the traditional intra-articular approach. By ensuring a standardized BMAC preparation protocol with a cell viability exceeding 85% and a uniform injection volume, we aim to minimize variability and better isolate the effect of the injection site. The literature indicates that while both approaches yield improvements in pain and functional outcomes, subchondral injections may provide a more durable therapeutic effect. For example, one study noted that the improvement in the IKDC subjective score was maintained up to 12 months in patients receiving a combined approach that targeted both the joint cavity and subchondral bone[32]. Another study demonstrated sustained benefits in KL grade III–IV OA patients over 4 years with intra-articular BMAC[20].
The safety profile of BMAC is consistently reported as favorable, with minimal adverse events. Most studies, including those with follow-ups extending to five years, report no major complications related to BMAC administration. In our trial, close monitoring for adverse events will be integral, as previous research has noted minor complications such as localized hematomas or transient joint swelling. Nonetheless, the overall low incidence of adverse events supports the use of BMAC as a minimally invasive treatment modality. A randomized study comparing subchondral and intra-articular BMAC injections in knee OA, which utilized a concentrated BMAC dose delivering approximately 1.8–2.5 million nucleated cells per kilogram in the subchondral group, correlating with sustained clinical improvements (e.g., delayed need for arthroplasty) over 15 years. This supports the rationale for our selected dosage as being within a therapeutically effective range[33]. A systematic review on BMAC for cartilage repair, which highlights that doses ranging from 1 to 3 million cells per kilogram have been associated with improved histological and clinical outcomes in preclinical and clinical settings. This provides a broader evidence base for the dosage selected in our protocol[34]. An another study discusses the cellular composition of BMAC and suggests that a threshold of approximately 2 million MSC per kilogram may optimize regenerative outcomes due to sufficient paracrine signaling and tissue repair potential[35]. Future research must address these issues through larger, multicenter, randomized controlled trials that incorporate dose-response analyses and standardized protocols.
The mechanisms by which BMAC exerts its therapeutic effects are not fully understood. Although the paracrine secretion of anti-inflammatory cytokines and growth factors is a leading hypothesis, the precise molecular pathways remain to be elucidated. Understanding these mechanisms is critical for refining treatment protocols and identifying patient subgroups most likely to benefit from BMAC therapy. Our study includes advanced MRI techniques and comprehensive clinical scoring systems to capture both the biological and functional changes following treatment. The correlation between imaging findings—such as the reduction in bone marrow edema—and clinical outcomes will provide valuable insights into the structure–function relationship in OA treatment.
This trial will provide critical insights into the comparative efficacy and safety of subchondral vs intra-articular BMAC injections in treating primary knee OA. By addressing the current gaps in long-term outcome data and standardization of treatment protocols, the study seeks to refine regenerative strategies for knee OA and potentially shift clinical practice towards more targeted interventions that address both joint and subchondral pathology.
| 1. | Primorac D, Molnar V, Rod E, Jeleč Ž, Čukelj F, Matišić V, Vrdoljak T, Hudetz D, Hajsok H, Borić I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes (Basel). 2020;11:854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 2. | Coaccioli S, Sarzi-Puttini P, Zis P, Rinonapoli G, Varrassi G. Osteoarthritis: New Insight on Its Pathophysiology. J Clin Med. 2022;11:6013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 3. | Yao Q, Wu X, Tao C, Gong W, Chen M, Qu M, Zhong Y, He T, Chen S, Xiao G. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023;8:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 533] [Article Influence: 266.5] [Reference Citation Analysis (0)] |
| 4. | Leifer VP, Katz JN, Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage. 2022;30:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 203] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 5. | Wright EA, Katz JN, Cisternas MG, Kessler CL, Wagenseller A, Losina E. Impact of knee osteoarthritis on health care resource utilization in a US population-based national sample. Med Care. 2010;48:785-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e508-e522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 521] [Cited by in RCA: 576] [Article Influence: 288.0] [Reference Citation Analysis (0)] |
| 7. | Langworthy M, Dasa V, Spitzer AI. Knee osteoarthritis: disease burden, available treatments, and emerging options. Ther Adv Musculoskelet Dis. 2024;16:1759720X241273009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 8. | Fraenkel L, Bogardus ST Jr, Concato J, Wittink DR. Treatment options in knee osteoarthritis: the patient's perspective. Arch Intern Med. 2004;164:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Grässel S, Muschter D. Recent advances in the treatment of osteoarthritis. F1000Res. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 10. | Rönn K, Reischl N, Gautier E, Jacobi M. Current surgical treatment of knee osteoarthritis. Arthritis. 2011;2011:454873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Steinmeyer J, Bock F, Stöve J, Jerosch J, Flechtenmacher J. Pharmacological treatment of knee osteoarthritis: Special considerations of the new German guideline. Orthop Rev (Pavia). 2018;10:7782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Parker DA, Scholes C, Neri T. Non-operative treatment options for knee osteoarthritis: current concepts. Journal of ISAKOS. 2018;3:274-281. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Richard MJ, Driban JB, McAlindon TE. Pharmaceutical treatment of osteoarthritis. Osteoarthritis Cartilage. 2023;31:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 116] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 14. | Luo D, Fan Z, Yin W. Chronic post-surgical pain after total knee arthroplasty: a narrative review. Perioper Med (Lond). 2024;13:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Goree JH, Grant SA, Dickerson DM, Ilfeld BM, Eshraghi Y, Vaid S, Valimahomed AK, Shah JR, Smith GL, Finneran JJ, Shah NN, Guirguis MN, Eckmann MS, Antony AB, Ohlendorf BJ, Gupta M, Gilbert JE, Wongsarnpigoon A, Boggs JW. Randomized Placebo-Controlled Trial of 60-Day Percutaneous Peripheral Nerve Stimulation Treatment Indicates Relief of Persistent Postoperative Pain, and Improved Function After Knee Replacement. Neuromodulation. 2024;27:847-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Jeyaraman M, Karthik KS, Choudary D, Jeyaraman N, Nallakumarasamy A, Ramasubramian S. Autologous Bone Marrow Aspiration Concentrate (BMAC) Therapy for Primary Knee Osteoarthritis-An Observational and Dose Escalation Study. Indian J Orthop. 2024;58:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Baek JH, Lee SC, Lee DN, Ahn HS, Nam CH. Effectiveness and Complications of Bone Marrow Aspirate Concentrate in Patients with Knee Osteoarthritis of Kellgren-Lawrence Grades II-III. Medicina (Kaunas). 2024;60:977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Sugaya H, Yoshioka T, Kato T, Taniguchi Y, Kumagai H, Hyodo K, Ohneda O, Yamazaki M, Mishima H. Comparative Analysis of Cellular and Growth Factor Composition in Bone Marrow Aspirate Concentrate and Platelet-Rich Plasma. Bone Marrow Res. 2018;2018:1549826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Keeling LE, Belk JW, Kraeutler MJ, Kallner AC, Lindsay A, McCarty EC, Postma WF. Bone Marrow Aspirate Concentrate for the Treatment of Knee Osteoarthritis: A Systematic Review. Am J Sports Med. 2022;50:2315-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 20. | Pabinger C, Lothaller H, Kobinia GS. Intra-articular injection of bone marrow aspirate concentrate (mesenchymal stem cells) in KL grade III and IV knee osteoarthritis: 4 year results of 37 knees. Sci Rep. 2024;14:2665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 21. | El-Kadiry AE, Lumbao C, Salame N, Rafei M, Shammaa R. Bone marrow aspirate concentrate versus platelet-rich plasma for treating knee osteoarthritis: a one-year non-randomized retrospective comparative study. BMC Musculoskelet Disord. 2022;23:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Jeyaraman M, Jeyaraman N, Ramasubramanian S, Ranjan R, Jha SK, Gupta A. Bone Marrow Aspirate Concentrate for Treatment of Primary Knee Osteoarthritis: A Prospective, Single-Center, Non-randomized Study with 2-Year Follow-Up. Indian J Orthop. 2024;58:894-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, Zheng MH. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 563] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 24. | Hu Y, Chen X, Wang S, Jing Y, Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 280] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 25. | Kon E, Boffa A, Andriolo L, Di Martino A, Di Matteo B, Magarelli N, Trenti N, Zaffagnini S, Filardo G. Combined subchondral and intra-articular injections of bone marrow aspirate concentrate provide stable results up to 24 months. Knee Surg Sports Traumatol Arthrosc. 2023;31:2511-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 26. | Zhang K, Yu J, Li J, Fu W. The Combined Intraosseous Administration of Orthobiologics Outperformed Isolated Intra-articular Injections in Alleviating Pain and Cartilage Degeneration in a Rat Model of MIA-Induced Knee Osteoarthritis. Am J Sports Med. 2024;52:140-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Xu L, Hayashi D, Roemer FW, Felson DT, Guermazi A. Magnetic resonance imaging of subchondral bone marrow lesions in association with osteoarthritis. Semin Arthritis Rheum. 2012;42:105-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1158] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 29. | Kim GB, Seo MS, Park WT, Lee GW. Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis. Int J Mol Sci. 2020;21:3224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 30. | Nallakumarasamy A, Shrivastava S, Ravi V, Jeyaraman N, D AG, Ramasubramanian S, Jeyaraman M. Optimizing bone marrow harvesting sites for enhanced mesenchymal stem cell yield and efficacy in knee osteoarthritis treatment. World J Methodol. 2025;15:101458. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (12)] |
| 31. | Zhidu S, Ying T, Rui J, Chao Z. Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities. Stem Cell Res Ther. 2024;15:266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 32. | Kon E, Boffa A, Andriolo L, Di Martino A, Di Matteo B, Magarelli N, Marcacci M, Onorato F, Trenti N, Zaffagnini S, Filardo G. Subchondral and intra-articular injections of bone marrow concentrate are a safe and effective treatment for knee osteoarthritis: a prospective, multi-center pilot study. Knee Surg Sports Traumatol Arthrosc. 2021;29:4232-4240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Hernigou P, Bouthors C, Bastard C, Flouzat Lachaniette CH, Rouard H, Dubory A. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: what better postpone knee arthroplasty at fifteen years? A randomized study. Int Orthop. 2021;45:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 34. | Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated Bone Marrow Aspirate for the Treatment of Chondral Injuries and Osteoarthritis of the Knee: A Systematic Review of Outcomes. Orthop J Sports Med. 2016;4:2325967115625481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 35. | Cavallo C, Boffa A, Andriolo L, Silva S, Grigolo B, Zaffagnini S, Filardo G. Bone marrow concentrate injections for the treatment of osteoarthritis: evidence from preclinical findings to the clinical application. Int Orthop. 2021;45:525-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |