Published online Dec 20, 2023. doi: 10.5493/wjem.v13.i5.123
Peer-review started: August 12, 2023
First decision: September 14, 2023
Revised: October 10, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: December 20, 2023
Processing time: 128 Days and 12.9 Hours
The overuse and misuse of antimicrobials contribute significantly to antimicrobial resistance (AMR), which is a global public health concern. India has particularly high rates of AMR, posing a threat to effective treatment. The World Health Or
To study the prescribing patterns of antimicrobials using WHO AWaRe classification through a cross-sectional study in All India Institute of Medical Sciences Rishikesh.
A descriptive, cross-sectional study was conducted from July 2022 to August 2022 at a tertiary care hospital. Prescriptions containing at least one antimicrobial were included in the study. Data on prescriptions, including patient demographics, de
The study involved a total of 123 patients, each of whom received at least one antimicrobial prescription. Most prescriptions were for inpatients, evenly dis
This study highlights the need for better antimicrobial prescribing practices and increased awareness of the WHO AWaRe classification and AMR among healthcare professionals. The findings indicate a high proportion of pre
Core Tip: With the rise in antimicrobial resistance (AMR) particularly in developing countries it is the need of the hour to adopt better prescribing practices. It is important to raise knowledge and awareness about AMR and improve adherence to guidelines. The research highlights areas of improvement in prescribing practices.
- Citation: Negi G, KB A, Panda PK. Ground level utility of Access, Watch, Reserve classification: Insights from a tertiary care center in North India. World J Exp Med 2023; 13(5): 123-133
- URL: https://www.wjgnet.com/2220-315X/full/v13/i5/123.htm
- DOI: https://dx.doi.org/10.5493/wjem.v13.i5.123
The discovery of antimicrobials is regarded as one of the most crucial advancements of the 20th century. It is imperative to prescribe antimicrobials judiciously, emphasizing their use in circumstances where they can offer significant advantages to patients with maximum efficiency, all the while mitigating the potential for exacerbating antimicrobial resistance (AMR)[1]. However, there is mounting evidence indicating that the overuse and misuse of antimicrobials have become leading factors in the complex causative web of AMR[2]. It poses a global public health problem, capable of spreading and causing significant human and economic burdens[3]. The situation in India is particularly concerning, as the country experiences some of the highest rates of AMR among bacteria commonly causing infections in both community and healthcare settings[4].
It is imperative to address the emergence of Multi-drug resistant organisms (MDR), which threaten the progress made in medical advancements over the past century. Conversely, in many parts of the world, there is not only overuse and misuse of antimicrobials but also inadequate access. Pneumonia remains a leading cause of childhood deaths globally, with over 2 million estimated deaths per year, primarily due to limited access to antimicrobials[5]. Thus, promoting access to these life-saving medicines for those in need should also be a priority[1].
Antimicrobial stewardship aims to bridge the gap between excessive and inadequate antimicrobial use[6]. However, assessing the impact of antimicrobial stewardship programs has proven challenging, as the commonly used metrics, such as defined daily doses (DDD) per 1000 inhabitants per day and days of therapy, provide limited information about the quality of antibiotic use[7]. The existing defined daily dose method used in adult antibiotic surveillance is unsuitable for neonates and children due to their widely variable bodyweights[8].
To address these issues, the World Health Organization (WHO) updated its Model List of Essential Medicines in 2017, introducing the Access, Watch, Reserve (AWaRe) classification system[9]. The AWaRe system categorizes antimicrobials based on their appropriateness, safety, and potential impact on AMR. The Access group includes antibiotics of choice for the 25 most common infections, with favourable safety profiles and a low likelihood of exacerbating AMR. These antimicrobials should be consistently available, affordable, and quality-assured. The Watch group comprises critically important antimicrobials that require strict monitoring and limited use due to their higher potential for negatively impacting AMR. The Reserve group includes last-resort antimicrobials effective against MDR bacteria, and their use should be minimized as they represent a valuable, non-renewable resource. The fourth category is of the discouraged antimicrobials which mostly includes antimicrobial combinations. Some fixed dose combinations of antibiotics, do not have any reasonable indications for the treatment of infectious diseases in humans and may negatively impact AMR and patient safety[1]. The AWaRe system is also represented as a traffic-light approach: Access = green, Watch = orange and Reserve = red. The overall goal was to reduce the use of Watch Group and Reserve Group, and to increase the use of Access antibiotics to > 60% by 2023[5].
Unlike previous measures of antibiotic consumption, the AWaRe classification allows for the quantification of anti
Given the ongoing struggle to identify appropriate measures for hospitals and their antimicrobial stewardship programs, this study aims to audit antimicrobial prescriptions and compare the utility of the AWaRe classification with other process measurements of antimicrobial utilization. Additionally, the study seeks to assess the knowledge and attitudes of prescribing physicians regarding AMR and the AWaRe classification. This study is particularly important in the context of North India, where the burden of resistance is increasing, and limited research has been conducted in this region. Furthermore, the study aims to examine the prescribing practices of antibiotics in a tertiary care center and compare them with the WHO Defined Daily Dosage to determine whether common antibiotics are being under or over-dosed.
Cross sectional study conducted on patient’s prescriptions of various departments in a tertiary care hospital from July 2022 to August 2022.
This study was carried out in a tertiary care centre in North India after approval by the Institutional Ethics Committee [All India Institute of Medical Sciences, Rishikesh, India (Reference number -AIIMS/IEC/22/252)].
Consenting treating physicians (faculty, junior and senior residents) and their prescriptions having at least one antimicrobial were studied. Universal sampling was used to select prescriptions for a duration of 2 mo.
Prescriptions containing at least one antimicrobial in both outpatient and inpatient settings and the physician prescribing it.
Treating physician not giving consent.
After obtaining an informed consent from the prescribing doctor, a questionnaire about their knowledge and practices on AWaRe classification was asked through online or offline modes. The questionnaire was self-structured after searching medical literature for comparable studies and adapting questions designed in other physicians’ surveys previously carried out[1,11]. The questionnaire consisted of two sets of questions: one designed to assess the knowledge of physicians and the other intended to gauge their attitudes towards AMR and the AWaRe classification. Pre-validation of questionnaire for its contents and relevance was done by experts (one clinician, one pharmacologist, one biostatistician). Data on prescriptions, including patient demographics, departments, types of antimicrobials prescribed, and duration of treatment, were collected.
The relevance of antimicrobial prescriptions was checked by measuring appropriateness (right drug, right dose and right duration) according to WHO guidelines, Infectious Disease Society of America guidelines and disease specific guidelines if needed. Simultaneously sub-group comparison on AWaRE classification was also performed.
Proportion of prescribed antimicrobials based on AWaRe classification was calculated. Then the knowledge and practices of prescribing doctor about the utility of AWaRe was determined. The appropriateness of AWaRe classification with days of therapy and defined daily doses of antimicrobial utilization was also estimated.
Data was collected on predefined proforma, google form, and excel sheet (Microsoft excel spreadsheet software, office 2016) and analysed by estimating the proportion of various variables including antimicrobial utilizations as per AWaRe classification. Univariate analysis was performed and the data was arranged as percentages of total. Mean daily dosing for a particular antimicrobial was determined and compared with WHO DDD using the Students T test. P value less than 0.05 was considered statistical significant.
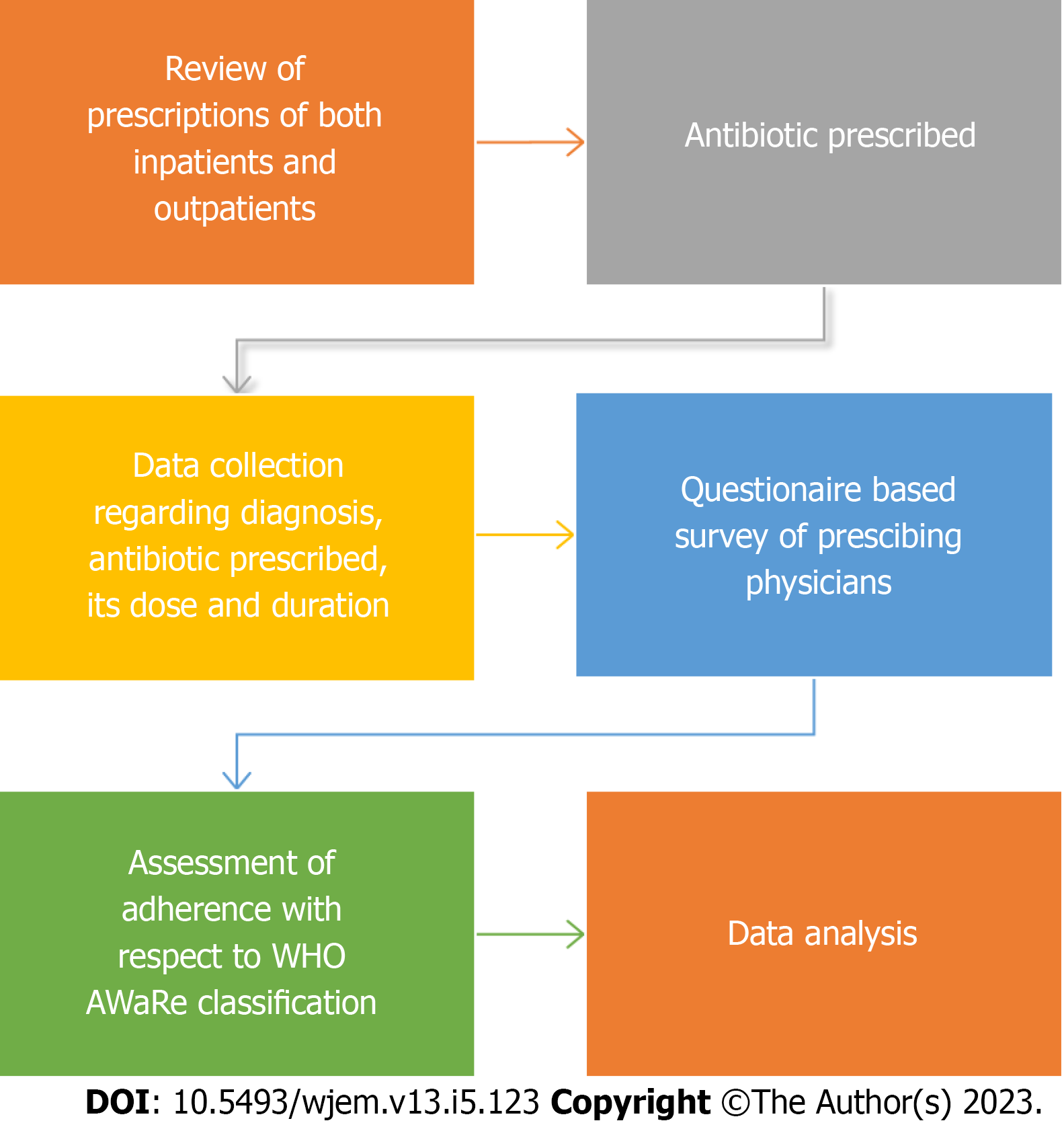
The research was carried out in accordance with the methodology presented in Figure 1. A total of n = 123 patients were enrolled in this study, with each of them receiving antibiotic prescriptions. The majority of these prescriptions were issued to inpatients (75.4%), and both the Medicine and Surgical departments were equally represented, accounting for 49.6% and 50.4%, respectively. Among the healthcare providers responsible for prescribing antibiotics, 72% were Junior Residents, 18.7% were Senior Residents, and 9.3% were Consultants. These findings have been summarized in Table 1.
| Category | Counts | % of total |
| Total number of patients | 123 | |
| Total prescriptions | 123 | |
| No. of different antibiotics used | 27 | |
| Most common antibiotic | Metronidazole | 19 |
| Designation | ||
| Senior resident | 42 | 18.7 |
| Junior resident | 162 | 72.0 |
| Consultant | 21 | 9.3 |
| OPD/IPD | ||
| OPD | 55 | 24.6 |
| IPD | 169 | 75.4 |
| Department | ||
| Medicine | 112 | 49.6 |
| Surgery | 114 | 50.4 |
| WHO AWaRe classification | ||
| Access | 140 | 57.61 |
| Watch | 93 | 38.27 |
| Reserve | 10 | 4.11 |
| Antibiotics used in a patient | Duration of antibiotics | |
| Mean | 1.84 | 6.63 |
| Median | 2 | 6.00 |
| Standard deviation | 0.833 | 3.83 |
| Minimum | 1 | 1.00 |
| Maximum | 5 | 19.0 |
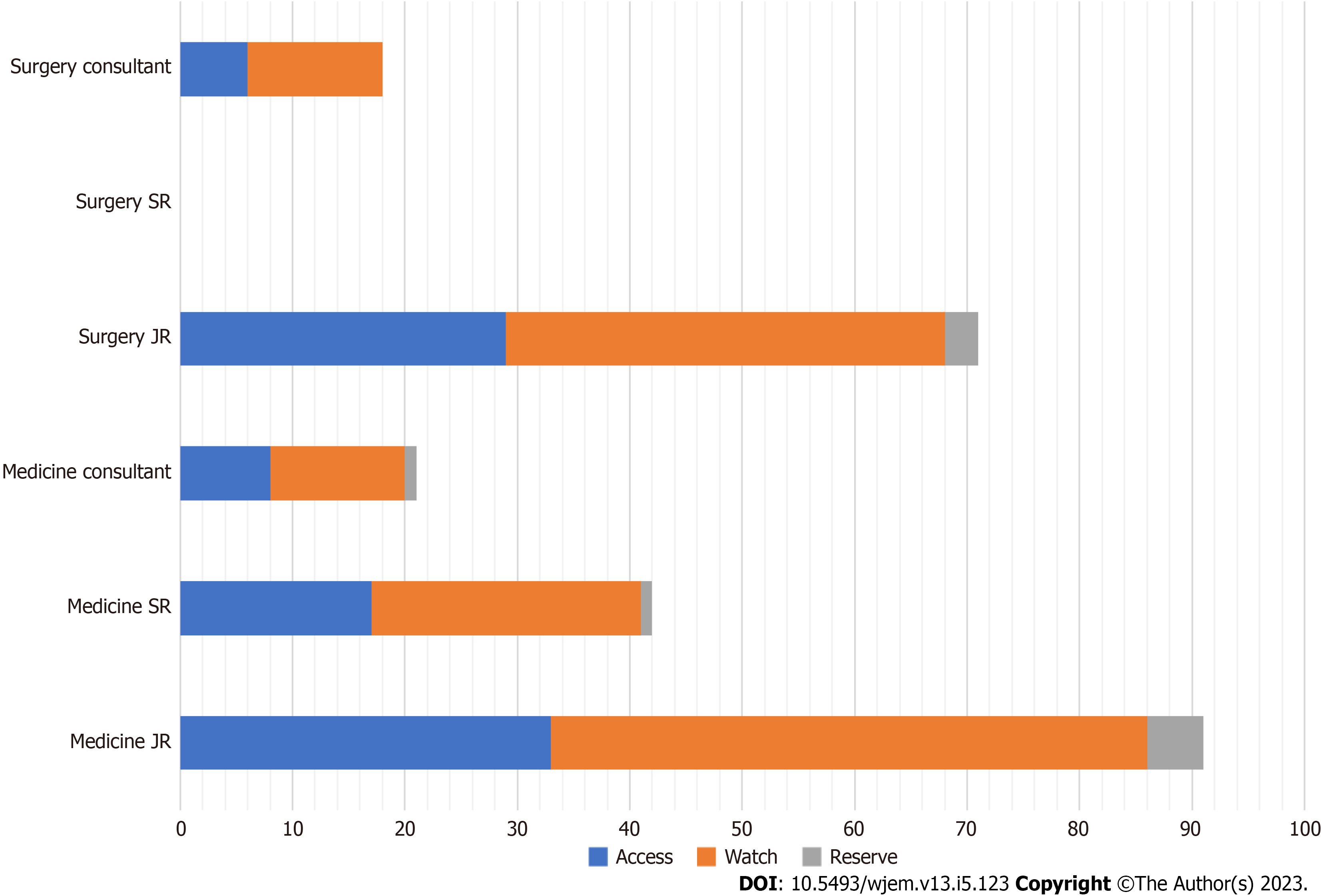
The prescriptions included 27 different antibiotics, with metronidazole being the most prescribed (19%) followed by ceftriaxone (17%). The mean number of antibiotics used per patient was 1.84 ± 0.83. The mean duration of antibiotics prescribed was 6.63 ± 3.83 days. The maximum number of antibiotics prescribed per patient was five. According to the AWaRe classification, 57.61% of antibiotics fell under the Access, 38.27% in Watch, and 4.11% in Reserve categories, suggesting appropriate antibiotic selection according to these criteria. The distribution of antibiotics prescribed according to the WHO AWaRe categories is presented in Figure 2. The difference in prescribing frequencies amongst departments can be noted. Most of the antibiotics prescribed in the Access category were from the Medicine department (75.4%), followed by Surgery (24.6%). For Watch antibiotics, Medicine had a higher proportion (63.4%) compared to Surgery (36.6%). In terms of seniority, Junior Residents prescribed the highest number of antibiotics for both Access and Watch categories in Medicine and Surgery departments. Senior residents and Consultants prescribed a lower number of antibiotics in all categories and departments. Only a few antibiotics were prescribed in the Reserve category, with most prescriptions being from the Medicine department.
The study also evaluated the Knowledge and Awareness of Healthcare professionals towards the WHO AWaRe classification through a questionnaire survey. A total of 93 participants responded to the survey. Among them, most parti
| Category | Counts | % of total |
| Position | ||
| Junior resident | 65 | 69.9 |
| Senior resident | 24 | 25.8 |
| Faculty | 4 | 4.3 |
| Do you know about WHO AWaRe classification | ||
| No details | 22 | 23.7 |
| Yes | 31 | 33.3 |
| Never heard | 21 | 22.6 |
| Little Idea | 19 | 20.4 |
| How did you hear about AWaRe? | ||
| The internet | 29 | 31.2 |
| The WHO website | 10 | 10.8 |
| The antimicrobial policy of our institution | 14 | 15.1 |
| Other sources | 19 | 20.4 |
| No idea about it | 21 | 22.6 |
The survey results on the knowledge and awareness of AMR among healthcare professionals are also presented in Tables 3 and 4. Out of the 93 participants, 68 (73.1%) agreed that the emergence of AMR is inevitable, while only 13 (14.0%) disagreed that AWaRe usage will result in the inability to treat serious infections. Additionally, 58 (62.4%) agreed that it will lead to lengthier hospital stays, 43 (46.2%) agreed that the success of chemotherapy and major surgery will be hampered, and the majority also agreed that its use will lead to increased cost of treatment and increased mortality rates. Regarding the utilization of AWaRe in the hospital summarized in Tables 4 and 5, 35.5% of the participants agreed that it should be used, while only 2.2% disagreed. Additionally, 34.4% agreed that AWaRe reduces adverse effects of inappropriate prescription. However, 37.6% of the participants considered that AWaRe threatens a clinician's autonomy and 30.1% thought that its use can delay treatment.
| Total score | Junior resident (n = 21) | Senior resident (n = 9) | Consultant (n = 3) | Medicine (n = 24) | Surgery (n = 9) | |
| n | 33 | - | - | - | - | - |
| Mean | 3.91 | 3.81 | 4.22 | 3.67 | 3.79 | 4.22 |
| Standard deviation | 2.17 | 2.25 | 2.33 | 1.53 | 2.08 | 2.49 |
| Minimum | 1 | 1 | 1 | 2 | 1 | 1 |
| Maximum | 8 | 8 | 8 | 5 | 8 | 8 |
| Statement | False | True | No idea | n |
| Emergence of Antimicrobial Resistance is inevitable | 24 | 68 | 1 | 93 |
| It will result in inability to treat serious infections | 13 | 80 | 0 | 93 |
| Lengthier hospital stays will be a result | 35 | 58 | 0 | 93 |
| Success of chemotherapy and major surgery will be hampered | 50 | 43 | 0 | 93 |
| Cost of treatment will be increased | 52 | 41 | 0 | 93 |
| MMR and IMR will increase | 62 | 31 | 0 | 93 |
| Question | Strongly disagree | Disagree | Neutral | Agree | Strongly agree |
| Should AWaRe be used in the hospital? | 18 (19.4%) | 2 (2.2%) | 21 (22.6%) | 33 (35.5%) | 19 (20.4%) |
| AWaRe reduces adverse effects of inappropriate prescription | 12 (12.9%) | 5 (5.4%) | 25 (26.9%) | 32 (34.4%) | 19 (20.4%) |
| AWaRe threatens a clinician's autonomy | 9 (9.7%) | 35 (37.6%) | 35 (37.6%) | 11 (11.8%) | 3 (3.2%) |
| It can delay treatment | 16 (17.2%) | 37 (39.8%) | 28 (30.1%) | 8 (8.6%) | 4 (4.3%) |
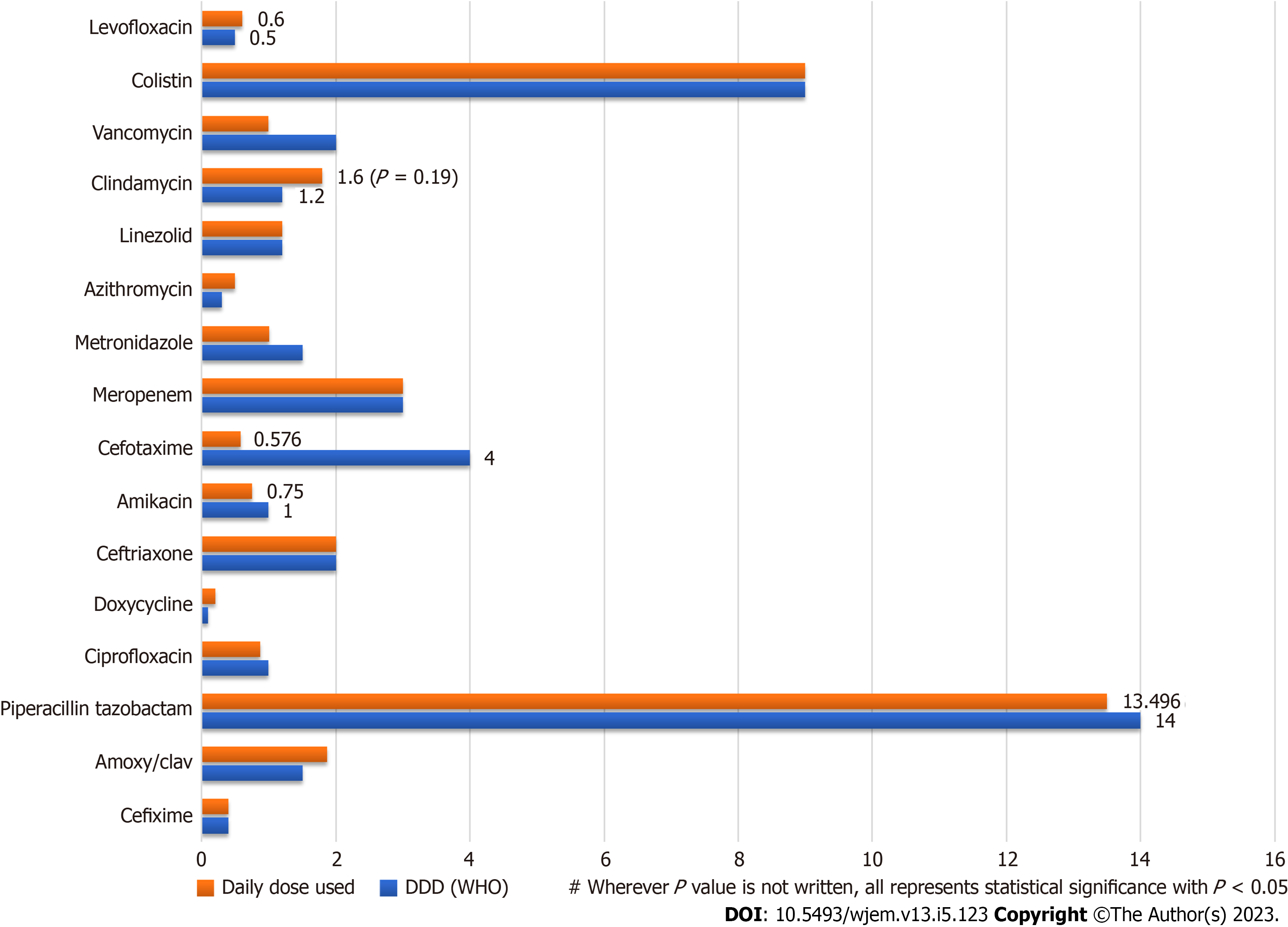
Additionally, the DDD of each drug was also evaluated. The usage of various antimicrobial drugs in a hospital setting, along with their daily doses and DDD according to the WHO's Anatomical Therapeutic Chemical classification system was calculated. Some of the important findings include high usage rates of ceftriaxone and metronidazole, and relatively low usage rates of drugs like colistin and clindamycin. Additionally, some drugs had wider ranges than others. Comparison of WHO defined DDD with Daily Drug dose (Mean) in the studied prescriptions is represented in the Clustered Bar chart in Figure 3.
Finally, the Mean Daily Drug Dose for prescribed drugs was compared with WHO defined DDD for each drug using a Student’s T test. The mean daily drug dose of amoxy/clav was significantly higher than the WHO DDD (1.8 vs 1.50, P = 0.014), while the mean daily drug dose of metronidazole and doxycycline were significantly lower than the WHO DDD (P < 0.001 and P = 0.008, respectively). The mean daily drug dose of piperacillin/tazobactam, amikacin, clindamycin, and levofloxacin did not show significant differences compared to the WHO DDD (P > 0.05).
The findings of this study highlight several important issues related to antimicrobial prescribing practices and awareness among healthcare professionals. This study, conducted in a tertiary care institute in North India included prescriptions having at least one antibiotic in both inpatient (75.4%) and outpatient (24.6%) settings collected over a duration of 2 months by universal sampling. A total of 123 patient prescriptions were included which included 243 individual antibiotics. The mean number of antibiotics used per patient was 1.84 ± 0.83 which coincides with a study conducted in Brazil[9] with a mean of 2.4 antibiotics per patient. This finding is in line with the WHO prescribing indicators which state that each prescription should contain an average of 1.6–1.8 antibiotics[7]. The use of multiple antibiotics can increase the risk of adverse effects, drug interactions, and development of AMR. The mean duration of antibiotics prescribed was 6.63 ± 3.83 d. A study conducted in a tertiary care hospital in India reported a mean duration of 5.7 ± 3.1 d[12], while another in Ethiopia reported a mean duration of 10.2 d[13].These variations could be attributed to differences in patient demo
In the present study, prescriptions included 27 different antibiotics of which Metronidazole was the most prescribed antibiotic, which could be due to its activity against anaerobic bacteria, which are commonly implicated in infections of the gastrointestinal tract, genitourinary tract, and skin. It is prescribed mostly in combination with amoxicillin in obstetrics and gynaecological care (mainly in post-delivery prophylaxis), and occasionally in caesarean section. Similar results were found study at Ghana Police Hospital[14]. This was followed by Ceftriaxone, a third-generation cepha
The WHO AWaRe classification system was used to analyse the antibiotics prescribed in this study, and the results showed that most antibiotics fell under the Access (57.61%) category. These antibiotics have a narrow spectrum of activity, lower cost, a good safety profile and generally low resistance potential and should be widely available and affordable. While this is a positive finding, it is also important to note that a significant proportion of antibiotics prescribed were from the Watch category (38.27%), which includes antibiotics that are at risk of becoming ineffective due to overuse and misuse. The small percentage of antibiotics prescribed in the Reserve category (4.11%) is reassuring, as these antibiotics are reserved for use as a last resort and should only be used in highly specific circumstances. The overall prescribing of antibiotics in our study is lower than the WHO recommendations, which state that more than 60% of all prescribed antibiotics must be from the Access group by 2023. Figures in our study are consistent with another Indian study which had Access (53.31%), Watch (40.09%) from, and Reserve (3.40%) category[16], and study done in Zambia with (n = 384) which had Access (55.5%), Watch (43.1%) and Reserve (1.4%)[7]. Our findings differ from another study in India[17] in which Watch group antibiotics accounted for 53.19 % of the total antibiotics and a Bangladesh study in which 64.0% of the patients were treated with antibiotics from the Watch group, 35.6% were treated with antibiotics from the Access group, and only 0.1% were treated with antibiotics from the Reserve group. The higher proportion of Access category antibiotics prescribed in our study could be attributed to the fact that these antibiotics are commonly used for the treatment of common infections encountered in the hospital, such as urinary tract infections, respiratory tract infections, and skin and soft tissue infections. The low proportion of Reserve category antibiotics prescribed in our study is consistent with the recommended sparing use of these antibiotics by WHO and other guidelines, which recommend their use only as a last resort in the treatment of severe infections[18]. In terms of departments and designations, our study found that the Medicine department prescribed the majority of antibiotics in both the Access and Watch categories, followed by the Surgery department. This finding is consistent with previous studies conducted in India and other low- and middle-income countries, where the Medicine department was found to be the highest prescriber of antibiotics[19].
To assess knowledge and attitude of prescribing doctor about the utility of AWaRe a Questionnaire based survey was carried out. Although (73.1%) agreed that the emergence of AMR is inevitable the lack of awareness about the pro
Despite the WHO AWaRe classification being included in the antibiotic policy of our institution only 33.3% of the responders knew about it which shines a light on the gap between the measures being taken and their implementation. The institute antibiotic policy authorizes only the Senior Residents and Consultants to prescribe antibiotics from Watch and Reserve groups but in this study Junior Residents prescribed the highest number of antibiotics for both Access and Watch categories which coincides with previous studies conducted in India and other countries. In a study conducted in a tertiary care hospital in India, junior residents prescribed the majority (68.7%) of antibiotics[19]. The higher proportion of antibiotics prescribed by Junior Residents in our study could be attributed to their relatively higher workload and less clinical experience compared to Senior Residents and Consultants. Overall, the findings of this study suggest that there is a moderate level of awareness among healthcare professionals about AMR, there is a need for further education and awareness programs to ensure the appropriate use of antibiotics in the hospital setting. It must be noted that most of the responders of the survey were also Junior Residents and hence education programmes can hence play a vital role in improving the results by working at grassroot levels.
Another analysis of the study depicts that there is a significant difference between the WHO DDD and the prescribed daily dose in the analysed prescriptions. Several studies conducted in the West have compared the mean daily drug dose of prescribed drugs with the WHO defined DDD. In a study conducted in a hospital in Italy found that the mean daily dose of antibiotics was generally lower than the WHO DDD for most antibiotics[22]. These findings are similar to the present study, which found higher mean daily doses of amoxicillin/clavulanic acid and piperacillin/tazobactam, and a lower mean daily dose of levofloxacin. The WHO DDD is a standardized measure of drug consumption used to compare the drug consumption between different countries and regions. It represents the assumed average maintenance dose per day for a drug used for its main indication in adults. On the other hand, the prescribed daily dose refers to the actual dose that a physician prescribes to a patient.
The difference between the WHO DDD and the prescribed daily dose can have several implications. Firstly, it can lead to overuse or underuse of medications, which can affect the therapeutic outcomes of patients. For example, if the prescribed daily dose is lower than the WHO DDD, the patient may not receive the optimal therapeutic effect of the medication. Conversely, if the prescribed daily dose is higher than the WHO DDD, the patient may be at risk of adverse effects or toxicity. Secondly, the difference between the WHO DDD and the prescribed daily dose can affect the comparability of drug consumption data between different regions and countries. If different regions or countries use different prescribed daily doses, it can be difficult to compare their drug consumption patterns. Therefore, adherence to the WHO DDD is important to ensure that drug consumption data are standardized and comparable across different regions and countries. In conclusion, the difference between the WHO DDD and the prescribed daily dose is an important issue that needs to be addressed in the prescribing practices of physicians. Adherence to the WHO DDD can help to ensure optimal therapeutic outcomes for patients and comparability of drug consumption data between different regions and countries.
Overall, the findings of this study emphasize the importance of improving antimicrobial prescribing practices and increasing awareness among healthcare professionals regarding the WHO AWaRe classification system and the threat of AMR. Effective antimicrobial stewardship programs that promote appropriate antibiotic use can help reduce the risk of AMR and improve patient outcomes. Future research should focus on implementing such programs in hospital settings and evaluating their effectiveness in reducing inappropriate prescribing practices.
This research indicates an appropriate proportion of prescriptions falling under the Access category (57.61%), suggesting appropriate antibiotic selection, a significant proportion also belongs to the Watch category (38.27%), emphasizing the need for greater caution to prevent the escalation of AMR. There is a moderate level of awareness among healthcare professionals about AMR and the steps being taken to tackle it, highlighting the gap in implementation of policies and need for more steps to be taken in spreading the knowledge about the subject. However, there is a significant difference between the WHO DDD and the prescribed daily dose in the analysed prescriptions suggesting overuse and underuse of antibiotics.
India has particularly high rates of antimicrobial resistance (AMR), posing a threat to effective treatment. The World Health Organization (WHO) Access, Watch, Reserve classification system was introduced to address this issue and guide appropriate antibiotic prescribing. However, there is a lack of studies examining the prescribing patterns of antimicrobials using the AWaRe classification, especially in North India.
This study aimed to assess the prescribing patterns of antimicrobials using the WHO AWaRe classification in a tertiary care centre in North India.
(1) To audit the prescribing patterns of antimicrobials among clinicians using WHO’s AWaRe classification in a tertiary care centre; (2) To assess knowledge and practices of prescribing doctor about the utility of AWaRe by Questionnaire based assessment; and (3) To compare the appropriateness of AWaRe classification with days of therapy and defined daily doses of antimicrobial utilization.
A descriptive, cross-sectional study was conducted from July 2022 to August 2022 at a tertiary care hospital. Prescriptions containing at least one antimicrobial were included in the study. A questionnaire-based survey was also conducted to assess the knowledge and practices of prescribing doctors regarding the utility of AWaRe classification.
The study involved a total of 123 patients, each of whom received at least one antimicrobial prescription. Most prescri
This study highlights the need for better antimicrobial prescribing practices and increased awareness of the WHO AWaRe classification and AMR among healthcare professionals.
The findings indicate a high proportion of prescriptions falling under the Access category, suggesting appropriate antibiotic selection. There is room for improvement and educational interventions and antimicrobial stewardship programs should be implemented to enhance knowledge and adherence to guidelines, ultimately contributing to the containment of AMR.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gajdács M, Hungary S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH
| 2. | Amaha ND, Weldemariam DG, Berhe YH. Antibiotic consumption study in two hospitals in Asmara from 2014 to 2018 using WHO's defined daily dose (DDD) methodology. PLoS One. 2020;15:e0233275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 3. | World Health Assembly 49. The world health report 1996: fighting disease, fostering development [Internet]. World Health Organization; 1996. [DOI] [Full Text] |
| 6. | Godman B, Egwuenu A, Haque M, Malande OO, Schellack N, Kumar S, Saleem Z, Sneddon J, Hoxha I, Islam S, Mwita J, do Nascimento RCRM, Dias Godói IP, Niba LL, Amu AA, Acolatse J, Incoom R, Sefah IA, Opanga S, Kurdi A, Chikowe I, Khuluza F, Kibuule D, Ogunleye OO, Olalekan A, Markovic-Pekovic V, Meyer JC, Alfadl A, Phuong TNT, Kalungia AC, Campbell S, Pisana A, Wale J, Seaton RA. Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 7. | Mudenda S, Nsofu E, Chisha P, Daka V, Chabalenge B, Mufwambi W, Kainga H, Kanaan MHG, Mfune RL, Mwaba F, Zulu M, Tembo R, Mwasinga W, Chishimba K, Mwikuma G, Monde N, Samutela M, Chiyangi HK, Mohamed S, Matafwali SK. Prescribing Patterns of Antibiotics According to the WHO AWaRe Classification during the COVID-19 Pandemic at a Teaching Hospital in Lusaka, Zambia: Implications for Strengthening of Antimicrobial Stewardship Programmes. Pharmacoepidemiology. 2023;2:42-53. [DOI] [Full Text] |
| 8. | Amponsah OKO, Buabeng KO, Owusu-Ofori A, Ayisi-Boateng NK, Hämeen-Anttila K, Enlund H. Point prevalence survey of antibiotic consumption across three hospitals in Ghana. JAC Antimicrob Resist. 2021;3:dlab008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Fonseca LG, de Oliveira Conterno L. Audit of antibiotic use in a Brazilian University Hospital. Braz J Infect Dis. 2004;8:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 10. | Gajdács M, Ábrók M, Lázár A, Burián K. Urinary Tract Infections in Elderly Patients: A 10-Year Study on Their Epidemiology and Antibiotic Resistance Based on the WHO Access, Watch, Reserve (AWaRe) Classification. Antibiotics (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | AWaRe [Internet]. [cited May 23, 2023]. Home. Available from: https://adoptaware.org/. |
| 12. | Ahmad A, Khan MU, Malik S, Mohanta GP, Parimalakrishnan S, Patel I, Dhingra S. Prescription patterns and appropriateness of antibiotics in the management of cough/cold and diarrhea in a rural tertiary care teaching hospital. J Pharm Bioallied Sci. 2016;8:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Demoz GT, Kasahun GG, Hagazy K, Woldu G, Wahdey S, Tadesse DB, Niriayo YL. Prescribing Pattern of Antibiotics Using WHO Prescribing Indicators Among Inpatients in Ethiopia: A Need for Antibiotic Stewardship Program. Infect Drug Resist. 2020;13:2783-2794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Darkwah TO, Afriyie DK, Sneddon J, Cockburn A, Opare-Addo MNA, Tagoe B, Amponsah SK. Assessment of prescribing patterns of antibiotics using National Treatment Guidelines and World Health Organization prescribing indicators at the Ghana Police Hospital: a pilot study. Pan Afr Med J. 2021;39:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Das N, Madhavan J, Selvi A, Das D. An overview of cephalosporin antibiotics as emerging contaminants: a serious environmental concern. 3 Biotech. 2019;9:231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Bansal A, Sharma R, Prakash R. Adoption of the World Health Organization access, watch reserve index to evaluate and monitor the use of antibiotics at a tertiary care hospital in India. Perspect Clin Res. 2022;13:90-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Mugada V, Mahato V, Andhavaram D, Vajhala SM. Evaluation of Prescribing Patterns of Antibiotics Using Selected Indicators for Antimicrobial Use in Hospitals and the Access, Watch, Reserve (AWaRe) Classification by the World Health Organization. Turk J Pharm Sci. 2021;18:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Access, watch, reserve: How a key policy tool can accelerate the fight against antimicrobial resistance [Internet]. World Health Organization; [cited Oct 12, 2023]. Available from: https://www.who.int/southeastasia/news/opinion-editorials/detail/access-watch-reserve-how-a-key-policy-tool-can-accelerate-the-fight-against-antimicrobial-resistance. |
| 19. | Patel A, Naagar M, Singh R. A study on prescribing pattern of antibiotics in medicine ward of tertiary care teaching hospital. Int J Basic Clin Pharmacol. 2020;9:887-890. [DOI] [Full Text] |
| 20. | Owens MR, Brito-Silva F, Kirkland T, Moore CE, Davis KE, Patterson MA, Miketinas DC, Tucker WJ. Prevalence and Social Determinants of Food Insecurity among College Students during the COVID-19 Pandemic. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 21. | Kraus S, Schiavone F, Pluzhnikova A, Invernizzi AC. Digital transformation in healthcare: Analyzing the current state-of-research. J Bus Res. 2021;123:557-567. [DOI] [Full Text] |
| 22. | Giacomelli A, Ridolfo AL, Oreni L, Vimercati S, Albrecht M, Cattaneo D, Rimoldi SG, Rizzardini G, Galli M, Antinori S. Consumption of antibiotics at an Italian university hospital during the early months of the COVID-19 pandemic: Were all antibiotic prescriptions appropriate? Pharmacol Res. 2021;164:105403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |