Copyright
©2014 Baishideng Publishing Group Inc.
World J Exp Med. May 20, 2014; 4(2): 16-26
Published online May 20, 2014. doi: 10.5493/wjem.v4.i2.16
Published online May 20, 2014. doi: 10.5493/wjem.v4.i2.16
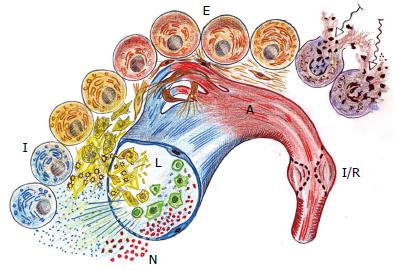
Figure 1 Schematic representation of the different stages of wound repair.
During the post-traumatic local inflammatory response three successive and overlapped phases: in the arterial side of the microcirculation (red), a nervous (N) or immediate phase with ischemia-reperfusion (I/R) occurs; in the post-capillary venule (blue), an immune (I) or intermediate phase with a leukocytic (L) phenotype is expressed; and, finally an endocrine (E) or late with an angiogenic (A) phenotype is developed, which implies the capillaries neoformation.
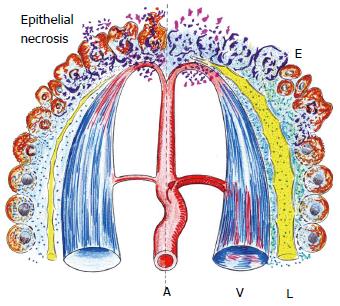
Figure 2 First or immediate phase of the acute inflammatory response.
On the left side, a schematic representation in which the tissue suffers the injury and therefore necrosis of the epithelial cells are produced. In turn, on the right side, the beginning of the tissue inflammatory response in response to necrosis is shown. This initial phase presents ischemia-reoxygenation and interstitial edema (E) with interstitial infiltration of mediators of the stress response as well as substrates including glucose, amino acids and lipids. In addition, the lymphatic circulation (L) is activated. A: Arterial microcirculation; V: Post-capillary venous circulation.
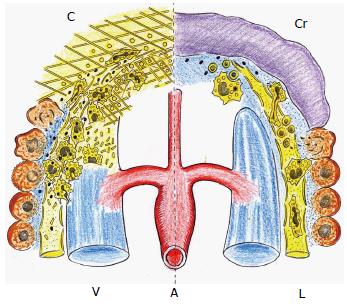
Figure 3 Immune or intermediate phase of the post-traumatic acute inflammatory response.
Interstitial infiltration by platelets and leukocytes, all of them entrapped in the provisional extracellular matrix (left). Underlying the wound crust (Cr) that is formed later, the leukocytes change their phenotype to promote the resolution of the inflammatory response and wound repair by re-epithelization and scar formation (right). C: Coagulation with fibrin-platelet clot. A: Arterial microcirculation; V: Post-capillary venous circulation; L: Lymphatic circulation.
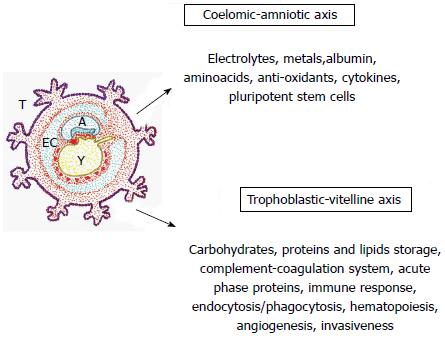
Figure 4 Hypothesized functions by ontogenic recapitulation in the traumatized tissue.
These functions could be similar to the extra-embryonic coelomic-amniotic and trophoblastic-vitelline functions during early embryonic development. The extra-embryonic coelom or exocoelomic cavity surrounds the blastocyst, which is composed of the amnion and the primary yolk sac. EC: Exocoelomic cavity; A: Amnion; T: Trophoblast; Y: Yolk sac or vitellum.
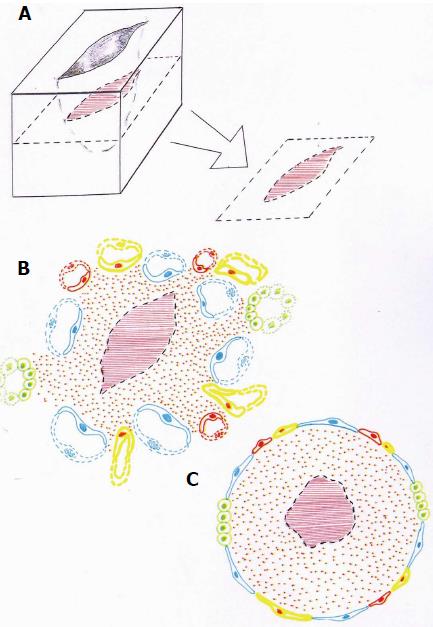
Figure 5 Figurative representation of a skin wound.
The wound (A) is surrounded by different types of inflammatory venous, arterial and lymphatic endothelia (B). This heterogeneous inflammatory endothelium could be represented like a sheath of the inflamed interstitium that surrounds in turn the wound or broken tissue (C).
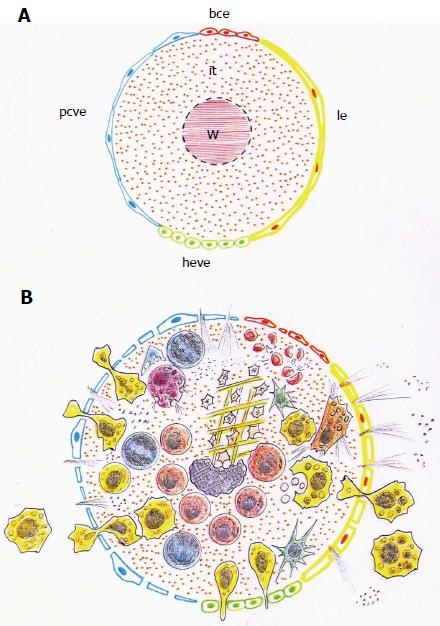
Figure 6 Schematic representations of the heterogeneous endothelium that surrounds the wounded tissue.
A: The endothelium that cover the wound (W) and the damaged interstitium (it) are made up by the post-capillary venous endothelium (pcve), the high endothelial venular endothelium (heve), the lymphatic endothelium (le) and the blood capillary endothelium (bce); B: The inflammatory response is produced into the injured interstitium. The inflammatory mediators, molecules and cells, invade this interstitial space crossing through a sheath of heterogeneous endothelia.
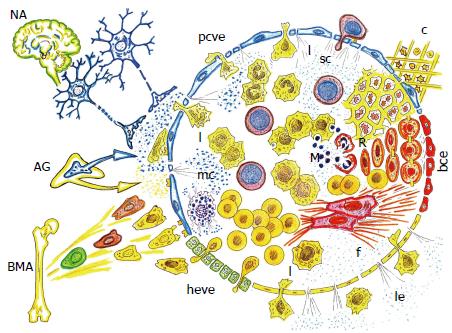
Figure 7 Neurogenic and bone marrow-related axes coupled in the inflamed endothelial egg, after wound.
The upregulated extra-embryonic functions, i.e., coelomic-amniotic or neurogenic, and trophoblastic-vitelline or bone marrow-related, are focused in the endothelial inflammatory egg, favoring the induction of a gastrulation-like phenotype, which evolves towards re-epithelization and fibrosis (scar) in post-natal life. NA: Neurogenic axis; AG: Adrenal gland; BMA: Bone marrow-related Axis; c: Coagulation; sc: Stem cell; mc: Mast cell. R: Regeneration; f: Fibrosis; l: Leukocytes; M: Microbiome. ve: Post-capillary venous endothelium; heve: High endothelial venular endothelium; le: Lymphatic endothelium; bce: Blood capillary endotelium; pcve: Psot capillary venous endothelium.
- Citation: Aller MA, Arias JI, Arraez-Aybar LA, Gilsanz C, Arias J. Wound healing reaction: A switch from gestation to senescence. World J Exp Med 2014; 4(2): 16-26
- URL: https://www.wjgnet.com/2220-315X/full/v4/i2/16.htm
- DOI: https://dx.doi.org/10.5493/wjem.v4.i2.16