Published online Nov 19, 2019. doi: 10.5492/wjccm.v8.i7.106
Peer-review started: June 26, 2019
First decision: August 2, 2019
Revised: September 17, 2019
Accepted: October 27, 2019
Article in press: October 27, 2019
Published online: November 19, 2019
Processing time: 153 Days and 17.2 Hours
Prolonged cardiac arrest (CA) produces extensive neuronal death and microglial proliferation and activation resulting in neuro-cognitive disabilities. Among other potential mechanisms, microglia have been implicated as triggers of neuronal death after hypoxic-ischemic insults. Minocycline is neuroprotective in some brain ischemia models, either by blunting the microglial response or by a direct effect on neurons.
To improve survival, attenuate neurologic deficits, neuroinflammation, and histological damage after ventricular fibrillation (VF) CA in rats.
Adult male isoflurane-anesthetized rats were subjected to 6 min VF CA followed by 2 min resuscitation including chest compression, epinephrine, bicarbonate, and defibrillation. After return of spontaneous circulation (ROSC), rats were randomized to two groups: (1) Minocycline 90 mg/kg intraperitoneally (i.p.) at 15 min ROSC, followed by 22.5 mg/kg i.p. every 12 h for 72 h; and (2) Controls, receiving the same volume of vehicle (phosphate-buffered saline). The rats were kept normothermic during the postoperative course. Neurologic injury was assessed daily using Overall Performance Category (OPC; 1 = normal, 5 = dead) and Neurologic Deficit Score (NDS; 0% = normal, 100% = dead). Rats were sacrificed at 72 h. Neuronal degeneration (Fluoro-Jade C staining) and microglia proliferation (anti-Iba-1 staining) were quantified in four selectively vulnerable brain regions (hippocampus, striatum, cerebellum, cortex) by three independent reviewers masked to the group assignment.
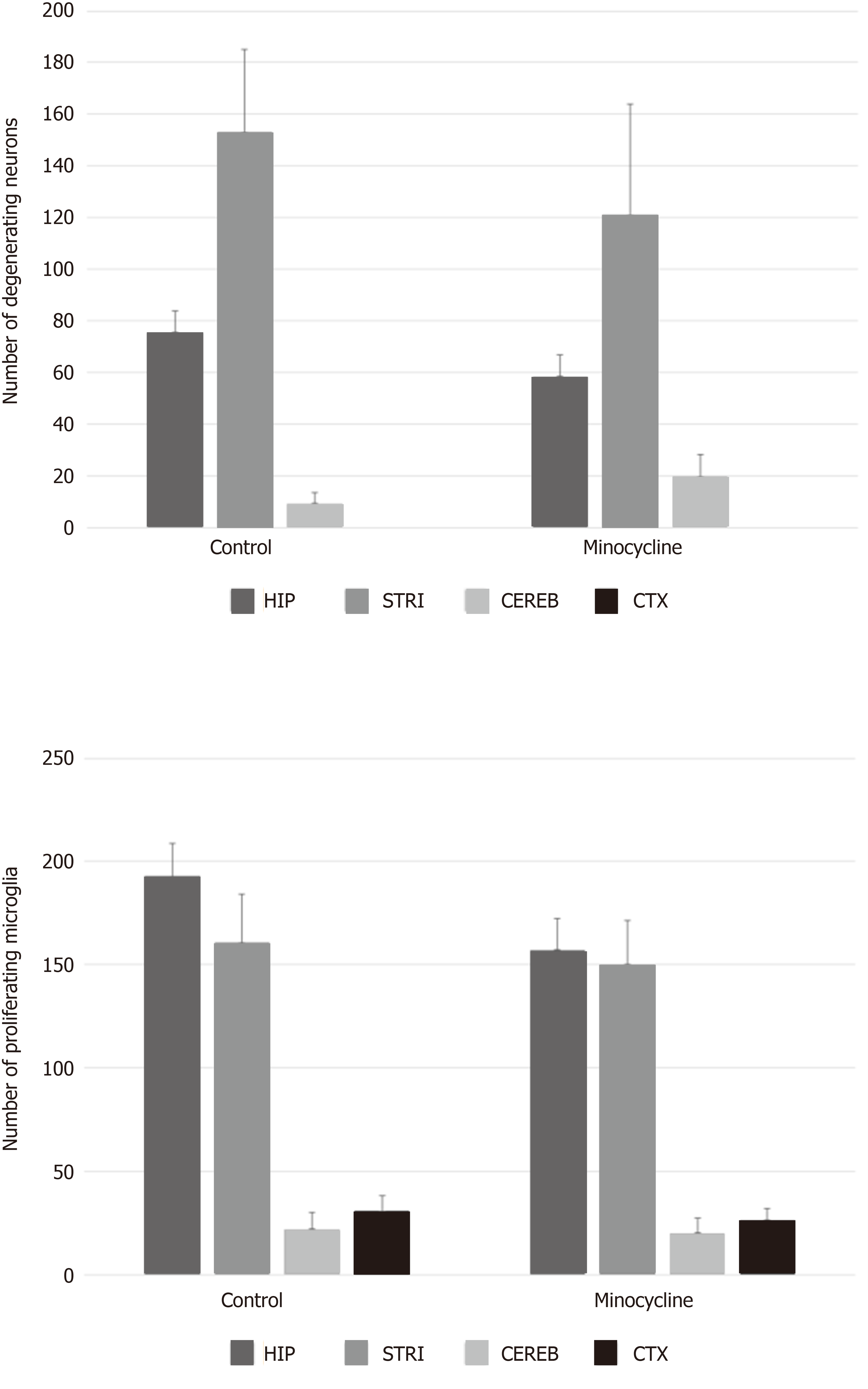
In the minocycline group, 8 out of 14 rats survived to 72 h compared to 8 out of 19 rats in the control group (P = 0.46). The degree of neurologic deficit at 72 h [median, (interquartile range)] was not different between survivors in minocycline vs controls: OPC 1.5 (1-2.75) vs 2 (1.25-3), P = 0.442; NDS 12 (2-20) vs 17 (7-51), P = 0.328) or between all studied rats. The number of degenerating neurons (minocycline vs controls, mean ± SEM: Hippocampus 58 ± 8 vs 76 ± 8; striatum 121 ± 43 vs 153 ± 32; cerebellum 20 ± 7 vs 22 ± 8; cortex 0 ± 0 vs 0 ± 0) or proliferating microglia (hippocampus 157 ± 15 vs 193 cortex 0 ± 0 vs 0 ± 0; 16; striatum 150 ± 22 vs 161 ± 23; cerebellum 20 ± 7 vs 22 ± 8; cortex 26 ± 6 vs 31 ± 7) was not different between groups in any region (all P > 0.05). Numerically, there were approximately 20% less degenerating neurons and proliferating microglia in the hippocampus and striatum in the minocycline group, with a consistent pattern of histological damage across the individual regions of interest.
Minocycline did not improve survival and failed to confer substantial benefits on neurologic function, neuronal loss or microglial proliferation across multiple brain regions in our model of rat VF CA.
Core tip: Prolonged cardiac arrest (CA) produces extensive neuronal death and neuroinflammation resulting in neuro-cognitive disabilities via ischemia-reperfusion injury. Minocycline was shown neuroprotective in some brain ischemia models, in part by blunting the microglial response or by a direct effect on neurons. In our established experimental CA model in adult rats, minocycline did not improve survival and failed to confer substantial benefits on survival, neurobehavioral outcome, neuronal loss or microglial proliferation across multiple brain regions.
- Citation: Janata A, Magnet IA, Schreiber KL, Wilson CD, Stezoski JP, Janesko-Feldman K, Kochanek PM, Drabek T. Minocycline fails to improve neurologic and histologic outcome after ventricular fibrillation cardiac arrest in rats. World J Crit Care Med 2019; 8(7): 106-119
- URL: https://www.wjgnet.com/2220-3141/full/v8/i7/106.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v8.i7.106
Currently, outcomes after cardiac arrest (CA) are poor, with an approximately 10% survival rate, and significant seuro-cognitive disabilities in survivors. No pharmacological adjuncts have as yet been shown to improve outcomes after CA in a clinical setting. Exploration of novel strategies and compounds for neuroprotection thus has scientific merit.
Janata et al[1], Drabek et al[2], Uray et al[3], and others[4-6] have reported that experimental CA produces extensive neuronal death and microglial proliferation and activation. Among other potential mechanisms, microglial activation have been implicated as significantly contributing to neuronal death and cerebral edema after insults to the central nervous system (CNS). Minocycline is suggestive to be neuroprotective in multiple brain ischemia models including CA[4-9], in part by blunting the microglial response[6], or by a direct effect on neurons[10].
Minocycline is neuroprotective in chronic inflammation models and stroke, most likely via attenuation of microglial activation. Minocycline was effective in improving functional outcome and neuronal death in a pediatric asphyxial CA model, concurrent with decrease in microglial proliferation and CNS cytokine expression at 72 h[7].
We have previously reported that minocycline at sufficient doses had only modest effect in our prolonged deep hypothermic CA model[2]. We concluded that the expected salutary effects of minocycline might have been masked by the concomitant beneficial effects of hypothermia, leaving little space for the detection of benefits of minocycline. Thus, in the current study, we chose to test minocylcine’s effects in our newly established model of normothermic ventricular fibrillation (VF) CA. We tested the hypothesis that minocycline would improve survival, functional and histological outcome after VF CA in rats. Primary outcomes were survival and functional outcome; secondary outcomes were histological damage (neuronal death and microglial activation) in multiple selectively vulnerable brain regions.
The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh (Protocol #13021161). We used our previously established model of VF CA[1].
In brief, adult male Sprague-Dawley rats (350-400 g) were obtained from a licensed vendor (Hilltop Lab Animals, Scottdale, PA, United States) and housed under 12 h/12 h light/dark in a holding facility for at least two days prior to the experiment. Water was provided ad libitum until the experiment. Standard chow was removed 12 h prior to experiment. On the day of the experiment, rats were anesthetized with 4% isoflurane (Baxter, Deerfield, IL, United States) in FiO2 1.0 in a plexiglass jar, intubated with a 14-gauge cannula (Becton Dickinson, Sandy, UT), and mechanically ventilated (Harvard Ventilator 683, Harvard Rodent Apparatus, South Natick, MA, United States) with tidal volume 8 mL/kg, PEEP 3 cm H2O and respiratory rate 30-40/min to maintain normocapnia. Anesthesia was maintained with 2% isoflurane (FiO2 of 0.5). Arterial (PE50) and venous (PE90) femoral lines were inserted via cut-downs for blood pressure monitoring and drug administration. Electrocardiogram (ECG), mean arterial pressure (MAP) and central venous pressure were continuously monitored and recorded (Polygraph, Grass Instruments, Quincy, MA, United States). Rectal temperature (Trec) was controlled at 37.0 ± 0.5 °C with a temperature controlled operating table, overhead heating lamp and a fan. After surgery, FiO2 was reduced to 0.3 and isoflurane was weaned over 5 min. VF CA was induced by a 2 min impulse of 12 V/50 Hz alternating current and ensured by ECG readings and reduction in MAP.
After 6 min CA, manual chest compressions were started at a rate approximately 275/min along with mechanical ventilation with FiO2 1.0. Epinephrine (Abbott, Abbott Park, IL, United States) 20 mg/kg was given with start of compressions; additional epinephrine 10 mg/kg was given at 1 min resuscitation time (RT). Sodium bicarbonate (Abbott, Abbott Park, IL, United States) 1 mEq/kg was given at start of resuscitation. At 2 min RT, defibrillation was attempted with biphasic 10 J impulse (Zoll M series defibrillator; Zoll, Chelmsford, MA, United States). If unsuccessful, subsequent shocks were delivered every 30 s, with maximum 5 attempts over 4 min resuscitation effort.
A Mini-mitter probe (Mini-mitter Co. Inc., Bend, OR) was advanced into the peritoneal cavity via small laparotomy to allow postoperative temperature control using overhead heating lamp and fan and monitoring via advanced telemetry system. Rats were weaned to spontaneous ventilation at 30 min RT. The rats were extubated and lines were removed at 60 min RT.
Controlled normothermia (36.5-37.5 °C) was maintained for 12 h, followed by an additional 12 h monitoring period. Rats that did not resume eating/drinking were given D5W/0.9NS (Baxter, Deerfield, IL, United States) sq twice daily. Morphine 0.15 mg was given twice daily subcutaneously for pain/distress. Neurologic status was assessed daily using Overall Performance Category (OPC, 1 = normal, 5 = dead)[11] and Neurologic Deficit Score (NDS, 0-10% = normal, 100% max deficit)[12].
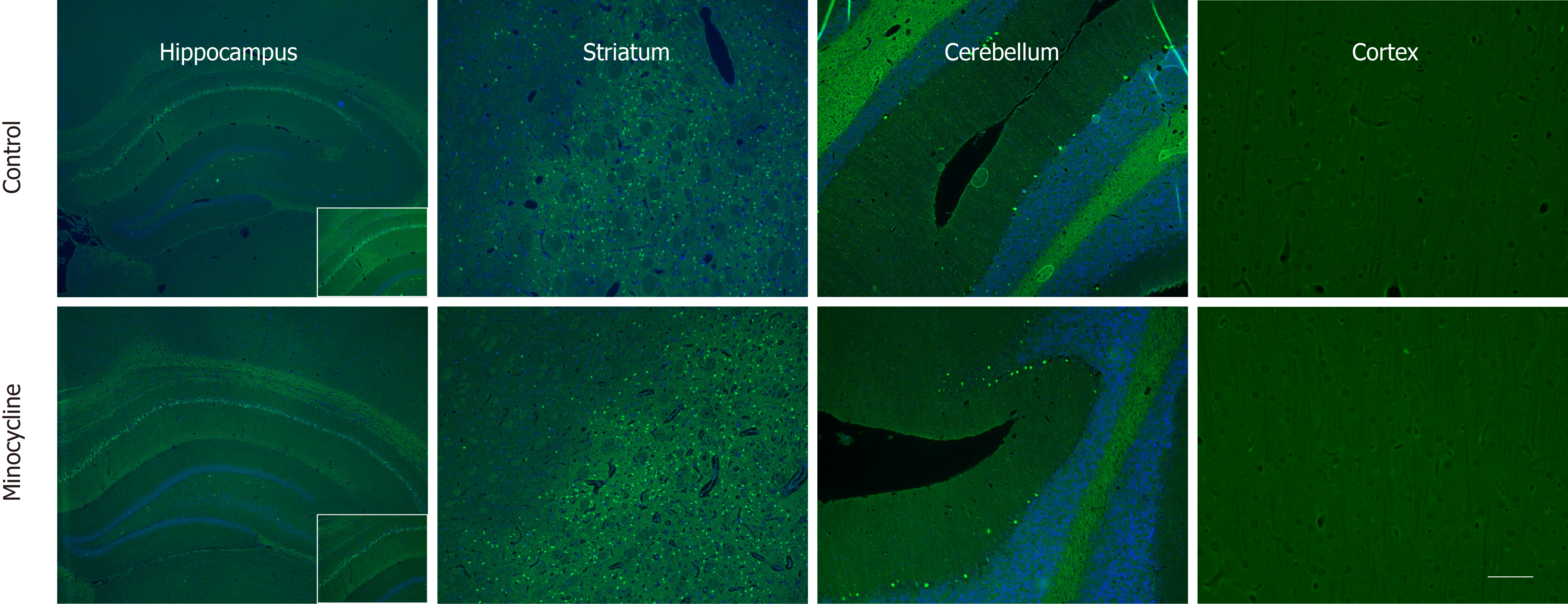
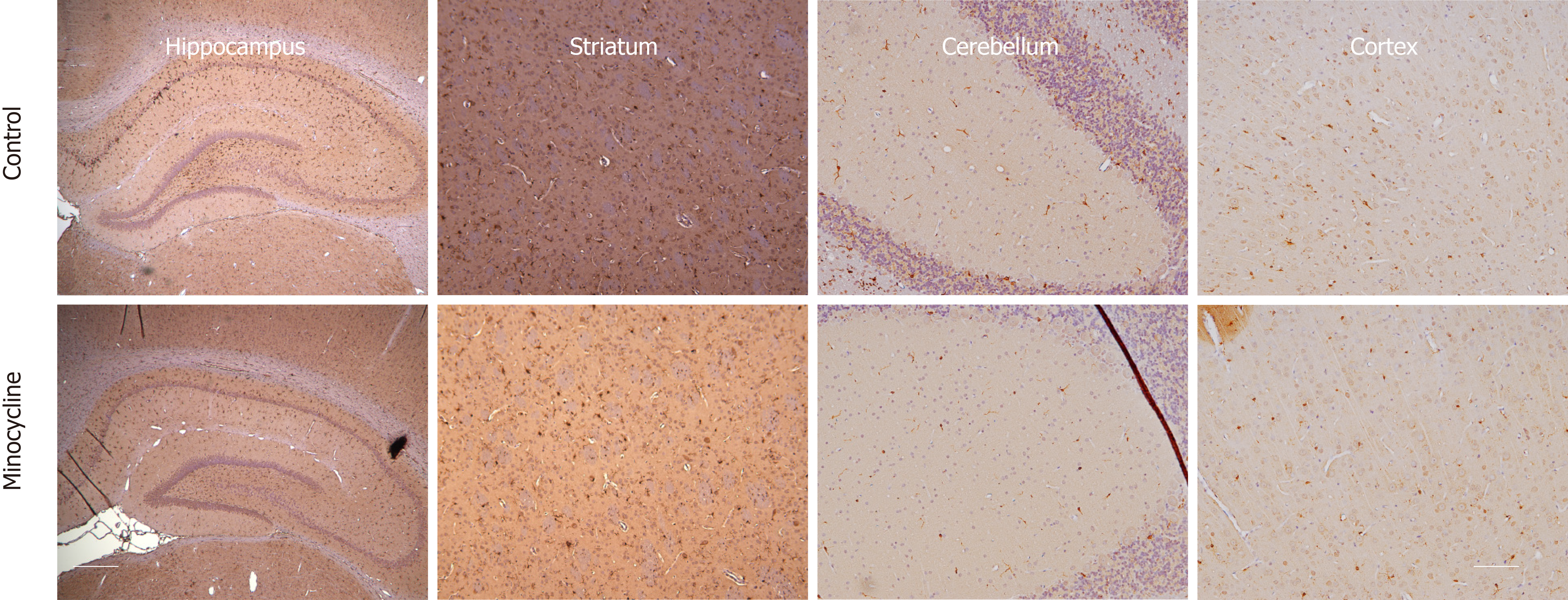
At 72 h, rats were deeply anesthetized with isoflurane and perfused transcardially with normal saline followed by 10% formalin. Fixed tissues were paraffin-embedded and standard coronal sections were performed at levels of aforementioned regions. Histological damage score was assessed in the striatum, hippocampus, cerebellum and cortex. Fluoro-Jade C was used to assess neuronal degeneration. Anti-Iba-1 staining was used to label activated microglia.
A randomization schedule was created prior to study commencement with balancing for each sequential groups of 4 rats, with two rats in each block assigned to receive minocycline and two rats assigned to receive vehicle treatment, in order to balance the number of rats allocated to each condition for each shipping container, thus reducing the possibility of bias and confounding. Rats that either did not achieve return of spontaneous circulation (ROSC), or died prior to the scheduled time-point of sacrifice were replaced at the end of the study following the same randomization protocol. The ongoing block was finished as originally designed. Minocycline (Sigma-Aldrich, Cat. No. M9511, St. Louis, MO) 90 mg/kg i.p. was administered at 15 min RT, followed by 22.5 mg/kg i.p. twice daily (6 am-6 pm) for 72 h. This regimen was based on prior studies which have demonstrated benefits[13]. In controls, vehicle (phosphate-buffered saline) was administered at the same time points and at the same volume.
The tissue samples were processed for embedding in paraffin. The resulting paraffin blocks were sequentially sectioned at 5 micrometer slices. All sections were stained with Fluoro-Jade C (Millipore, CA, United States) as a marker neuronal degeneration[14] and with anti-Iba-1 staining visualizing microglia. Iba-1 is a calcium-binding protein expressed specifically in activated microglia[15]. For the Iba-1 staining, sections were washed in tris-buffered saline and Tween 20 (TBST) (Biocare Medical, CA, United States), incubated in 0.3% H2O2 in TBST for 30 min to inhibit endogenous peroxidase activity, washed in TBST, and blocked in TBST containing 3% normal goat serum for 30 min. The sections were incubated with a rabbit anti-Iba1 polyclonal antibody (1:250, Wako, Richmond, VA, United States) overnight at 4 °C. The sections were then washed in TBST and incubated with a FITC-conjugated goat anti-rabbit IgG secondary antibody (Invitrogen, Carlsbad, CA, United States) for 1 h at room temperature. Sections were then washed and cover-slipped with Vectashield Mounting media containing 4',6-diamidino-2-phenylindole counterstain.
In addition, colorimetric visualization of Iba-1 immunostaining using diaminobenzamide (DAB) (Vector, CA, United States) was used as a secondary confirmatory method to visualize microglia. In short, sections were incubated with a primary antibody using a 1:250 dilution of anti-rabbit Iba-1 overnight at 4 °C. Sections were washed with TBST, incubated at RT for 1 h with a biotinylated secondary anti-rabbit IgG (Sigma-Aldrich Cat. No. 21537, St. Louis, MO, United States), followed by 1 h of avidin-biotin complex binding using an ABC kit (Vector, CA, United States). Sections were washed and incubated for 10 min with DAB followed by hematoxylin counterstaining. Tissue was dehydrated, cleared and cover-slipped for microscopic analysis. For control staining, normal rabbit IgG was used as the primary antibody.
A photograph of the representative section of the aforementioned regions of interest was taken under 10 × magnification (Nikon Eclipse 90i). The regions of interest were defined as follows: Hippocampus and cortex, bregma -3.2 mm; striatum, bregma +0.48 mm; and cerebellum, bregma -10.04 mm. Fluoro-Jade C positive neurons and Iba-1 positive activated microglia (characterized by ameboid cell body and retracted processes without thin ramifications)[16] were then quantitated morphometrically by three independent evaluators (KLS, CDW, KJ-F) masked to the treatment assignment using the National Institutes of Health Image-J software. No automated features of the software were used. Image-J was used solely to track the cell counts and provide a controlled feedback between the independent evaluators.
The analysis was performed using IBM SPSS Statistics 24.0 software (International Business Machines Corporation, Armonk, United States). All data were expressed as the mean ± SD except stated otherwise. We have targeted n = 8 survivors in each group to allow for evaluation of neuronal death. This was based on power sample size calculation for a continuous parameter (neuronal count), using two independent sample analysis with alpha = 0.05, power = 0.8, to detect 20% reduction in neuronal death. Survival and favorable (OPC 1-2) vs unfavorable (OPC 3-5) outcome was evaluated with Fisher’s exact test. Survival time was evaluated using a Kaplan-Meier survival analysis. Differences in OPC categories were evaluated with chi square test. NDS was evaluated with Mann-Whitney U-test. Differences in HDS and physiological and biochemical values between groups were evaluated with an independent samples t-test. Differences in physiological and biochemical parameters vs. respective baselines were evaluated by a paired samples t-test. P < 0.05 was considered statistically significant.
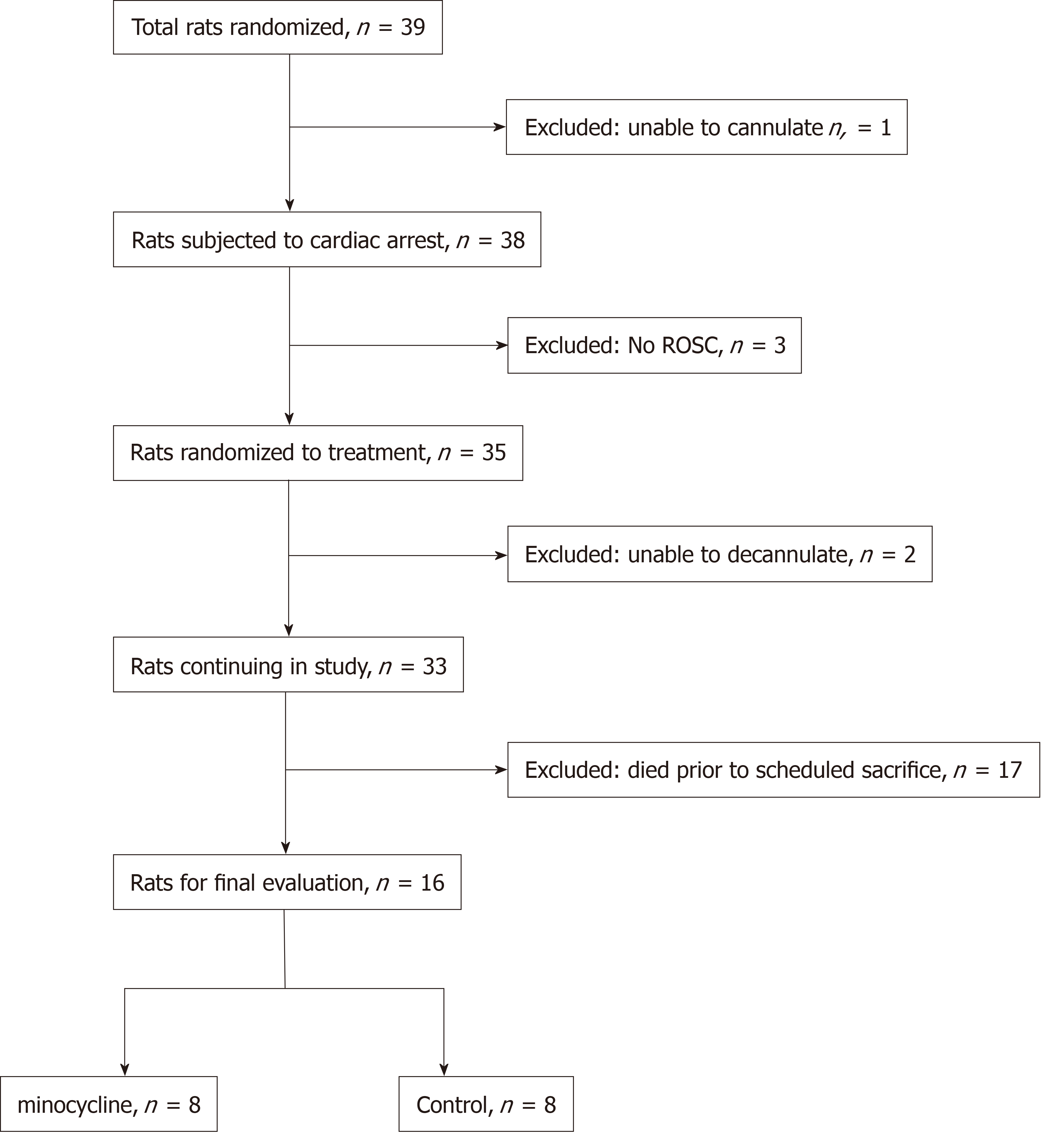
A total of 39 rats was used (Figure 1). Prolonged CA resulted in significant early biochemical derangements heralded by marked metabolic acidosis and significantly increased lactate levels that clearly indicated a severe insult. Post-resuscitation treatment with minocycline did not change survival rate or survival time, neurologic outcome or histological damage at 72 h that included marked neuronal degeneration and microglial activation in multiple selectively vulnerable brain regions.
Three rats did not achieve ROSC. Three additional rats were excluded for technical complications (bleeding during cannulation or decannulation). ROSC was achieved after 155 ± 51 s in the control group vs 141 ± 16 s in the minocycline group, respectively (P = 0.492). The number of defibrillation shocks (median, interquartile range) did not differ between groups [control: 1.9 (0, 4) vs minocycline: 1.7 (1, 2); P = 0.779].
Biochemical and physiological profiles were similar between groups at baseline and in the early post-resuscitation period, characterized by transient marked increased in lactate and metabolic acidosis. These changes were mostly normalized by RT 60 min (Table 1).
| BL | RT5 | RT15 | RT30 | RT60 | RT72H | ||
| HR (bpm) | Control | 355 ± 21 | 335 ± 47 | 354 ± 20 | 378 ± 45 | 366 ± 26 | N/A |
| Minocycline | 348 ± 20 | 335 ± 38 | 355 ± 21 | 369 ± 22 | 374 ± 28 | N/A | |
| MAP (mmHg) | Control | 91 ± 5 | 98 ± 31 | 100 ± 20 | 89 ± 13 | 94 ± 19 | N/A |
| Minocycline | 90 ± 9 | 98 ± 13 | 86 ± 11 | 94 ± 13 | 102 ± 9 | N/A | |
| pHa | Control | 7.39 ± 0.02 | 7.12 ± 0.06c | 7.24 ± 0.08c | 7.37 ± 0.05 | 7.41 ± 0.03 | 7.38 ± 0.15 |
| Minocycline | 7.46 ± 0.13 | 7.14 ± 0.04c | 7.27 ± 0.05c | 7.39 ± 0.03 | 7.42 ± 0.02 | 7.46 ± 0.15 | |
| paO2 (mmHg) | Control | 136 ± 15 | 381 ± 56c | 373 ± 41c | 144 ± 36 | 136 ± 37 | 306 ± 187 |
| Minocycline | 133 ± 14 | 378 ± 35c | 384 ± 43c | 145 ± 48 | 145 ± 38 | 427 ± 56c | |
| paCO2 (mmHg) | Control | 39 ± 3 | 51 ± 2c | 45 ± 2c | 40 ± 4 | 45 ± 5c | 41 ± 17 |
| Minocycline | 40 ± 4 | 51 ± 5c | 47 ± 4c | 40 ± 2 | 41 ± 2 | 35 ± 15 | |
| BE (mEq/L) | Control | -1.3 ± 1.5a | -12.6 ± 1.3c | -8.1 ± 4.2c | -1.8 ± 3.3 | 2.7 ± 1.9c | -1.8 ± 5 |
| Minocycline | 0.5 ± 1.6 | -12.1 ± 1.3c | -5.9 ± 2.1c | -0.2 ± 1.5 | 1.9 ± 1.6 | 0.0±3.5 | |
| Lactate (mmol/L) | Control | 1.7 ± 0.6 | 13.0 ± 2.6c | 9.7 ± 2.8c | 5.4 ± 2.0c | 2.0 ± 1.2 | 3.9 ± 1.1c |
| Minocycline | 1.2 ± 0.4 | 12.5 ± 1.6c | 8.7 ± 1.4c | 4.6 ± 1.0c | 1.9 ± 0.7c | 3.0 ± 0.7c | |
| Hct (%) | Control | 39 ± 1a | 38 ± 2 | 39 ± 2 | 39 ± 2 | 37 ± 3a | 43 ± 5c |
| Minocycline | 40 ± 1 | 38 ± 3 | 39 ± 2 | 40 ± 3 | 40 ± 1 | 41 ± 6 | |
| Glucose (g/dL) | Control | 218 ± 28 | 325 ± 31c | 277 ± 44c | 213 ± 41 | 134 ± 24c | 243 ± 48 |
| Minocycline | 223 ± 16 | 337 ± 19c | 293 ± 18c | 205 ± 41 | 162 ± 51c | 183 ± 15ac |
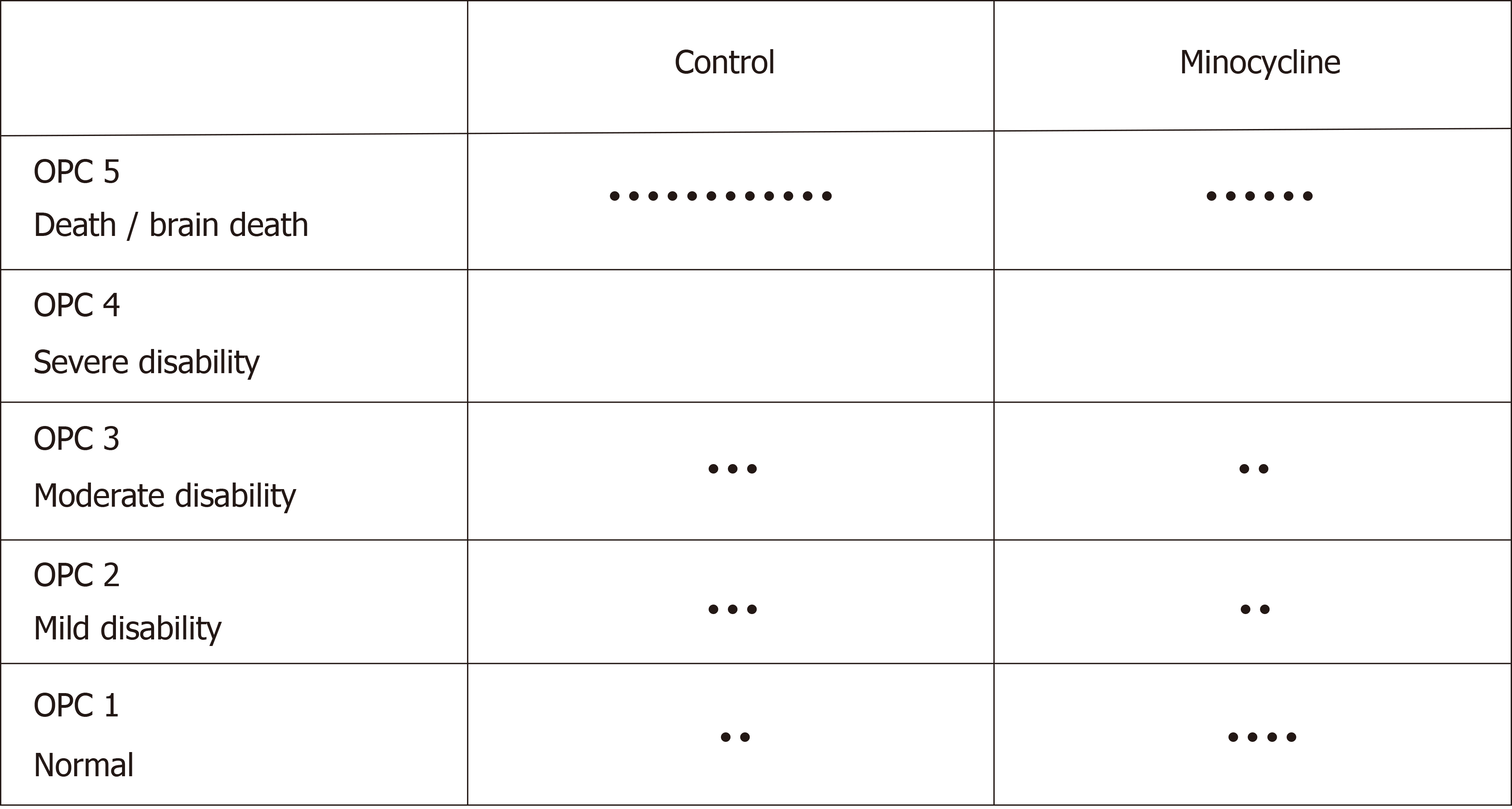
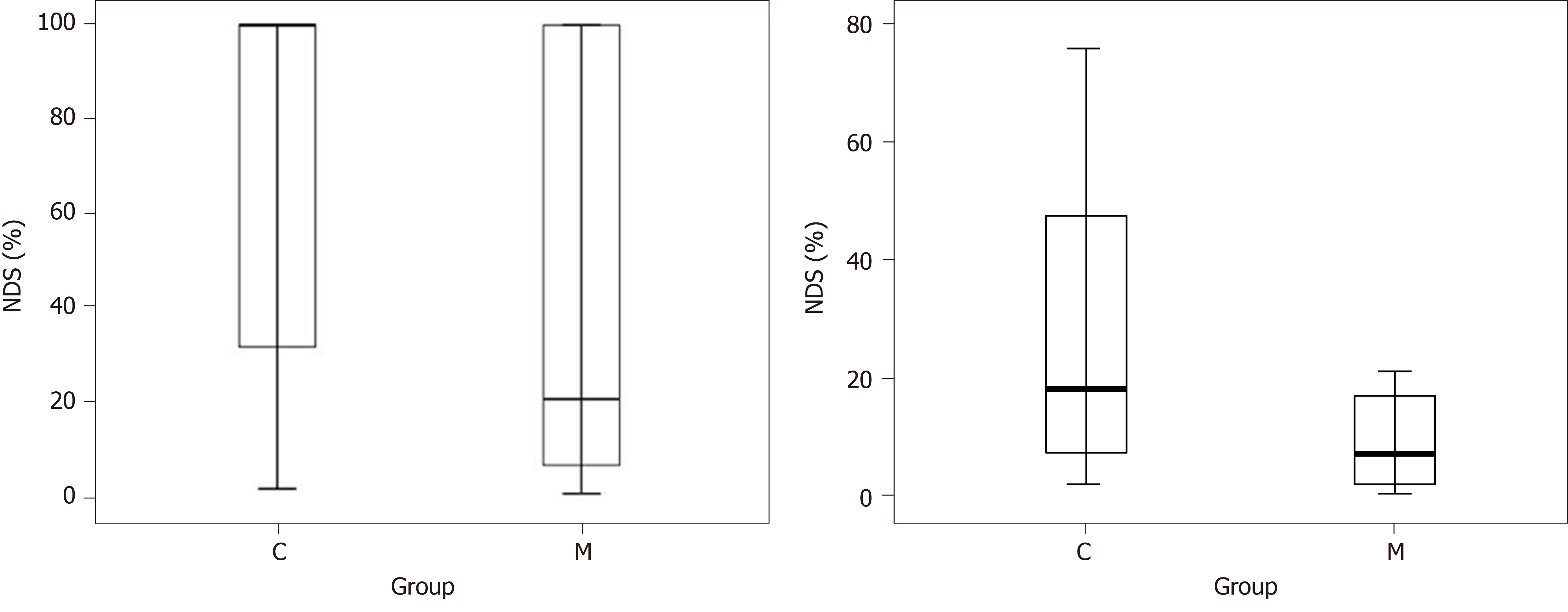
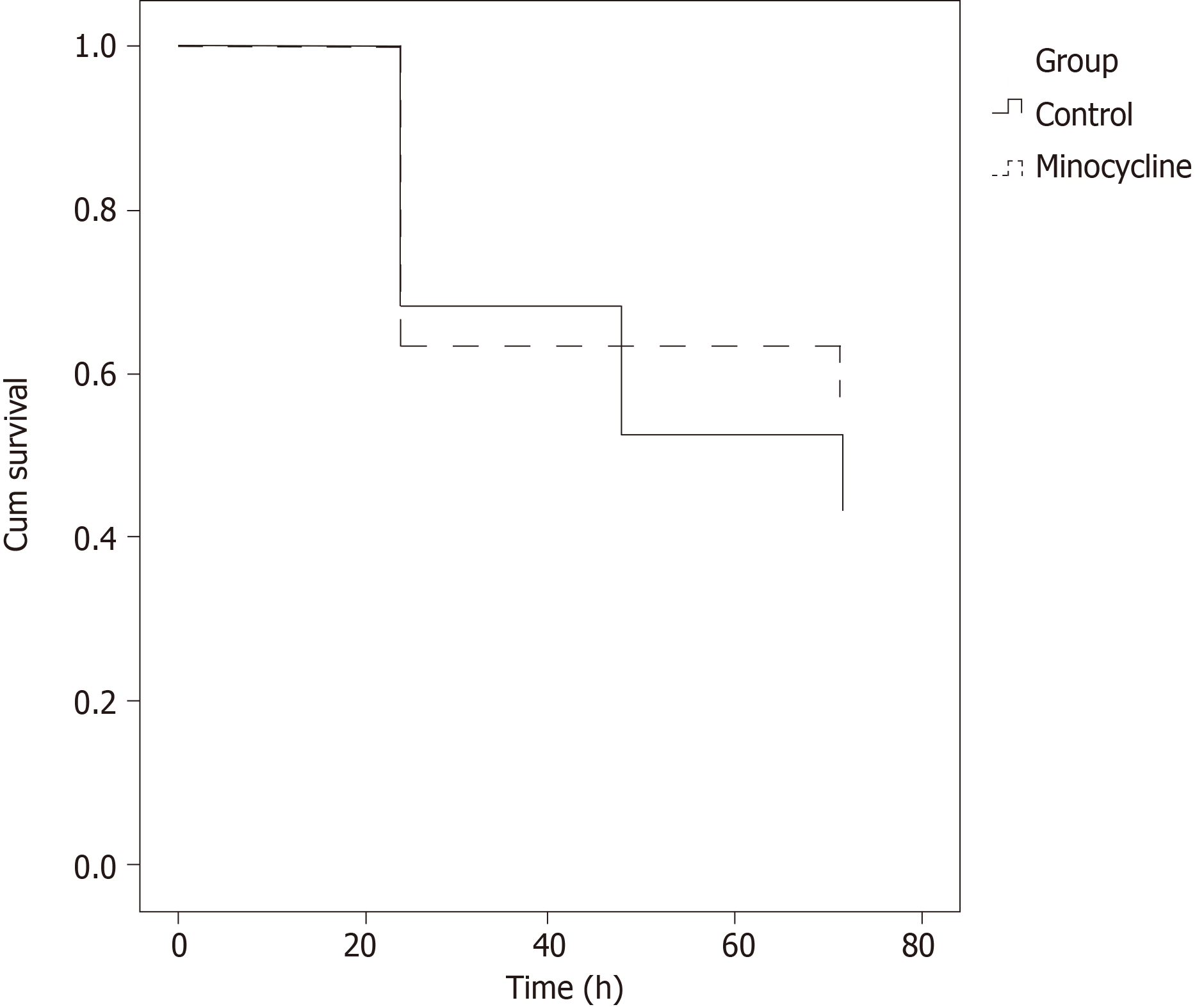
There were no differences in overall survival (8/19 in control group vs 8/14 in the minocycline group; Figure 2). The final NDS at 72 h was not different in all animals entering the study or in survivors only (Figure 3). Similarly, survival time was not different between groups (Figure 4). The weight of the rats did not different at baseline or on individual survival days. However, the overall weight decrease (baseline - D3) was greater in the control group (63 ± 19 g) vs minocycline group (42 ± 14 g; P = 0.04).
Histological damage assessed in survivors showed similar degree of neuronal loss and microglia proliferation in hippocampus, striatum, cerebellum and cortex in both control and minocycline groups at 72 h. Despite a relatively lower number of degenerating neurons and proliferating microglia (approximately 20% decrease) in the minocycline group, histologic damage score was not different between groups (Figure 5). Hippocampal neuronal degeneration was limited to the CA1 region, which is known to be selectively vulnerable hypoxia-ischemia, and to neurons in dentate gyrus. In striatum, medium spiny neurons, comprising about 80% of striatal population, showed extensive neurodegeneration. Large Purkinje neurons in cerebellum were also affected. No evidence of neuronal degeneration was apparent in cortex in either group (Figure 6). Regions with extensive neurodegeneration were also hallmarked by extensive microglia activation and proliferation with thickened, shortened processes. Interestingly, signs of mild microglial activation and proliferation were present in the cortex even in the absence of neuronal death at this stage (Figure 7).
Our model of VF CA is characterized by extensive neuronal cell death and microglial activation across multiple brain regions. We have characterized in detail the temporal pattern of evolving neuronal death in this model previously[1], identifying several selectively vulnerable regions as potential therapeutical targets. In this study, minocycline did not improve survival and failed to confer substantial benefits on neurologic injury, neuronal loss or microglial proliferation in multiple brain regions in our rat model of VF CA. The relative lack of effect of minocycline in this normothermic VF CA model is consistent with our prior results from deep hypothermic CA[2], as well as with the previous work by others documenting limited effect of minocycline in asphyxial CA in adult rats[5]. In contrast, other groups have demonstrated sustained beneficial effects of minocycline on hippocampal cell death and neuro-behavioral cognitive tasks both after a single dose pretreatment of minocycline[17], or after once-daily treatment for 7 d[18]. It is possible that differences between our models and treatment regimens might have contributed to these conflicting results. Also, minocycline has previously showed benefits in immature rats subjected to asphyxial CA[7], suggesting significant age-dependent differences in neuroinflammation after CA.
Traditionally, microglia have been viewed as the resident immune cell of the CNS, which serve a role of immune surveillance. While the early brain injury in ischemia-reperfusion is caused by release of excitatory mediators resulting from energy failure, secondary damage could also be triggered by microglia, which transform into phagocytes, purportedly aggravating the injury. From a temporal standpoint, microglial activation starts immediately after ischemia and thus importantly precedes morphologically detectable neuronal damage.
Microglial activation has been suggested to be a major cause of delayed neuronal death, most likely through releasing neurotoxic substances, including reactive oxygen radicals, nitric oxide, and pro-inflammatory cytokines[19]. Microglial activation could contribute to neuronal death or microglial-mediated synaptic injury and/or neuronal dysfunction – which could mediate cognitive deficits even in the absence of overt neuronal death. After hypoxic-ischemic injury, inactive microglia and macrophages in the neurovasculature change expression patterns, producing active substances, affecting survival vs. apoptosis[20].
Minocycline is a widely used antibiotic with anti-inflammatory and anti-apoptotic properties, and has been tested in several models of neurologic injury, including global[21-23] and focal brain ischemia[24-27], traumatic brain injury[28,29], spinal cord injury[30,31] and intracerebral hemorrhage[32]. Most recently, minocycline has showed promise in a clinical trial in acute stroke patients[33]. Minocycline has been shown to penetrate the blood-brain barrier well[34], reduce tissue injury and also improve functional recovery[9,21,35]. On a molecular level, minocycline inhibits inflammatory cell migration and degranulation and formation of free oxygen radicals[36-38], leads to decreased expression of inducible nitric-oxide synthetase[39-42] and augments expression of cyclooxygenase-2[43]. It also suppresses both caspase-dependent[28,44-47] and caspase-independent apoptotic pathways[37,47] which are relevantly expressed in our deep hypothermic CA model and similar models described elsewhere[48-50]. The primary effect of minocycline is probably inhibition of activation of microglia[21,22,24,30,51]. A few studies suggest that minocycline may exert its effects independent of microglia inhibition[9,52]. Both motor and neurocognitive behavior have improved after treatment with minocycline, even when the initiation of treatment was delayed for several hours after the insult[8,9,25,28].
Several studies have previously disputed a neuroprotective role of minocycline[53,54] or showed only transient protection[5,52,55]. Surprisingly, minocycline ablated hypoxic-ischemic injury in neonatal rat models[21,56-58] but was detrimental in a neonatal mouse model[59]. A combination of drugs including minocycline, but not minocycline alone, targeting multiple mechanisms operating after hypoxic-ischemic injury seemed to be more effective than either drug alone[60].
We have demonstrated in prior studies that reperfusion after prolonged ischemia results in extensive region-specific neuroinflammatory response. Interleukin (IL)-1a, IL-1b and tumor necrosis factor (TNF)-α were the most prominent cytokines detected early after reperfusion[61,62]. One of the purported actions of minocycline includes blocking TNF-α release from activated microglia. In our hypothermic CA model, minocycline was able to attenuate the increase in TNF-α most prominently in the striatum, although this was not associated with improved early outcome (24 h)[10]. One important caveat may be that, in our model of VF CA, neurons rather than microglia appeared to be a major source of early TNF-a release[62]. This finding has been anecdotally reported earlier[63]. Direct protective effects of minocycline on neurons subjected to hypoxic-ischemic injury might be associated with the mitigation of neuronal excitability, glutamate release, Ca(2+) overloading, and neu-roinflammation[64,65].
In this study, we eliminated hypothermia as a confounding factor, and extended the window of observation from 24 h to 72 h, in order to allow a more sensitive detection of potential benefits of minocycline. Our treatment protocol was based on prior studies[55]. Intraperitoneal administration of minocycline results in a bioavailability of 10%-80% with variable serum concentrations. Peak concentrations are achieved after 2.5 h, with half-life of 3 h. With this regard, we chose a more frequent dosing (b.i.d. rather than once daily) to ascertain adequate trough levels of the drug. It should be noted that minocycline penetrates blood-brain barrier well.
We chose a model with considerable mortality to allow for the tested drug to demonstrate its beneficial or detrimental effects. Most studies exploring neuronal death after brain ischemia have focused on neuronal death in the hippocampus, making this selectively vulnerable region a proxy for therapeutic efficacy. Hippocampal structure and neuronal circuits have been well defined but represent only one of the many selectively vulnerable regions with a unique cell population. However, prior reports have suggested that microglial activation is dominant in the striatum and neocortex rather than in the hippocampus[66]. This prompted us to broaden our histological damage assessment to other relevant selectively vulnerable brain regions with different neuronal populations, using various populations predominantly. For example, large pyramidal neurons in the CA1 sector of the hippocampus receive mostly glutamatergic input; Purkinje cells in the cerebellum are solely GABAergic; medium spiny neurons in the striatum are glutamatergic. Large pyramidal cells in cortical layer V use glutamate as the primary neurotransmitter; a smaller population of inhibitory interneurons with local projections and chandelier cells that make synaptic connections only to the axons protruding from other neurons, are also found in layer V, and are GABAergic. We acknowledge that neuronal death is a continuum and that individual brain regions may have different cell death trajectories, and thus our selection of regions of interest thus represents multiple types of neuronal cells across several brain regions. However, we did not observe a breakthrough effect of minocycline in any of these selectively vulnerable brain regions. In conclusion, in our experimental model of VF CA, minocycline did not confer benefits on neurologic outcomes or histological damage at 72 h.
Outcomes from cardiac arrest (CA) are suboptimal and survivors are often left with significant neuro-cognitive disabilities. No pharmacological adjuncts have been shown to improve outcomes after CA in a clinical setting. Exploration of novel therapeutical adjuncts for neuroprotection in clinically relevant animal models is thus warranted.
Minocycline has been shown to be neuroprotective in several models of ischemia-reperfusion, attenuating microglial activation as a dominant effect. Minocycline seemed a promising candidate to be tested in an experimental CA model that is characterized by extensive neuronal degeneration and microglial activation.
We tested the hypothesis that early treatment with minocycline at a sufficient dose would improve survival rate, survival time, neurologic outcome and histological damage in adult male rats subjected to prolonged CA.
Rats were subjected to CA and randomized to either minocycline treatment or control group, treated with vehicle, for 72 h. Minocycline treatment regimen was selected based on prior studies that demonstrated benefits.
Minocycline did not improve survival rate, survival time, neurologic outcome or histological damage (neuronal degeneration or microglial proliferation) in multiple selectively vulnerable brain regions.
Minocycline did not provide a breakthrough beneficial effect on neurologic injury or histological damage resulting from prolonged experimental CA.
Alternative pharmacological strategies should be explored to augment the outcome from CA.
The abstract of this work was awarded “Best of Category” Abstract for the Critical Care, Trauma and Resuscitation and “Best of Meeting” Abstract Finalist at the International Anesthesia Research Society, Montreal, Canada.
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tiruvoipati R S-Editor: Yan JP L-Editor: A E-Editor: Liu MY
| 1. | Janata A, Drabek T, Magnet IA, Stezoski JP, Janesko-Feldman K, Popp E, Garman RH, Tisherman SA, Kochanek PM. Extracorporeal versus conventional cardiopulmonary resuscitation after ventricular fibrillation cardiac arrest in rats: a feasibility trial. Crit Care Med. 2013;41:e211-e222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Drabek T, Tisherman SA, Beuke L, Stezoski J, Janesko-Feldman K, Lahoud-Rahme M, Kochanek PM. Deep hypothermia attenuates microglial proliferation independent of neuronal death after prolonged cardiac arrest in rats. Anesth Analg. 2009;109:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Uray T, Lamade A, Elmer J, Drabek T, Stezoski JP, Missé A, Janesko-Feldman K, Garman RH, Chen N, Kochanek PM, Dezfulian C, Callaway CW, Doshi AA, Frisch A, Guyette FX, Reynolds JC, Rittenberger JC; University of Pittsburgh Post-Cardiac Arrest Service. Phenotyping Cardiac Arrest: Bench and Bedside Characterization of Brain and Heart Injury Based on Etiology. Crit Care Med. 2018;46:e508-e515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Neigh GN, Karelina K, Glasper ER, Bowers SL, Zhang N, Popovich PG, DeVries AC. Anxiety after cardiac arrest/cardiopulmonary resuscitation: exacerbated by stress and prevented by minocycline. Stroke. 2009;40:3601-3607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Keilhoff G, Schweizer H, John R, Langnaese K, Ebmeyer U. Minocycline neuroprotection in a rat model of asphyxial cardiac arrest is limited. Resuscitation. 2011;82:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Wang W, Lu R, Feng DY, Liang LR, Liu B, Zhang H. Inhibition of microglial activation contributes to propofol-induced protection against post-cardiac arrest brain injury in rats. J Neurochem. 2015;134:892-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Tang M, Alexander H, Clark RS, Kochanek PM, Kagan VE, Bayir H. Minocycline reduces neuronal death and attenuates microglial response after pediatric asphyxial cardiac arrest. J Cereb Blood Flow Metab. 2010;30:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Wang QY, Sun P, Zhang Q, Yao SL. Minocycline attenuates microglial response and reduces neuronal death after cardiac arrest and cardiopulmonary resuscitation in mice. J Huazhong Univ Sci Technolog Med Sci. 2015;35:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Hewlett KA, Corbett D. Delayed minocycline treatment reduces long-term functional deficits and histological injury in a rodent model of focal ischemia. Neuroscience. 2006;141:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Drabek T, Janata A, Wilson CD, Stezoski J, Janesko-Feldman K, Tisherman SA, Foley LM, Verrier JD, Kochanek PM. Minocycline attenuates brain tissue levels of TNF-α produced by neurons after prolonged hypothermic cardiac arrest in rats. Resuscitation. 2014;85:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Carrillo P, Takasu A, Safar P, Tisherman S, Stezoski SW, Stolz G, Dixon CE, Radovsky A. Prolonged severe hemorrhagic shock and resuscitation in rats does not cause subtle brain damage. J Trauma. 1998;45:239-248; discussion 248-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Neumar RW, Bircher NG, Sim KM, Xiao F, Zadach KS, Radovsky A, Katz L, Ebmeyer E, Safar P. Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation. 1995;29:249-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Cho KO, Kim SK, Cho YJ, Sung KW, Kim SY. Regional differences in the neuroprotective effect of minocycline in a mouse model of global forebrain ischemia. Life Sci. 2007;80:2030-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 1066] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 15. | Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1007] [Cited by in RCA: 1193] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 16. | Koshinaga M, Suma T, Fukushima M, Tsuboi I, Aizawa S, Katayama Y. Rapid microglial activation induced by traumatic brain injury is independent of blood brain barrier disruption. Histol Histopathol. 2007;22:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Naderi Y, Sabetkasaei M, Parvardeh S, Moini Zanjani T. Neuroprotective effects of pretreatment with minocycline on memory impairment following cerebral ischemia in rats. Behav Pharmacol. 2017;28:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Naderi Y, Sabetkasaei M, Parvardeh S, Zanjani TM. Neuroprotective effect of minocycline on cognitive impairments induced by transient cerebral ischemia/reperfusion through its anti-inflammatory and anti-oxidant properties in male rat. Brain Res Bull. 2017;131:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Gehrmann J, Banati RB, Wiessner C, Hossmann KA, Kreutzberg GW. Reactive microglia in cerebral ischaemia: an early mediator of tissue damage? Neuropathol Appl Neurobiol. 1995;21:277-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 146] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Barakat R, Redzic Z. The Role of Activated Microglia and Resident Macrophages in the Neurovascular Unit during Cerebral Ischemia: Is the Jury Still Out? Med Princ Pract. 2016;25 Suppl 1:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Fan LW, Lin S, Pang Y, Rhodes PG, Cai Z. Minocycline attenuates hypoxia-ischemia-induced neurological dysfunction and brain injury in the juvenile rat. Eur J Neurosci. 2006;24:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769-15774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 794] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 23. | Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 2002;52:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Yrjänheikki J, Tikka T, Keinänen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496-13500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 820] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 25. | Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 26. | Wang CX, Yang T, Shuaib A. Effects of minocycline alone and in combination with mild hypothermia in embolic stroke. Brain Res. 2003;963:327-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Wang CX, Yang T, Noor R, Shuaib A. Delayed minocycline but not delayed mild hypothermia protects against embolic stroke. BMC Neurol. 2002;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393-9; discussion 1399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, Morganti-Kossmann MC. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol. 2007;204:220-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24:2182-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 381] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 31. | Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006;97:1314-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, Yong VW, Peeling J. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53:731-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 293] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 33. | Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 322] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 34. | Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet. 1988;15:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 244] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 298] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 36. | Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 376] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 37. | Morimoto N, Shimazawa M, Yamashima T, Nagai H, Hara H. Minocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damage. Brain Res. 2005;1044:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Lin S, Wei X, Xu Y, Yan C, Dodel R, Zhang Y, Liu J, Klaunig JE, Farlow M, Du Y. Minocycline blocks 6-hydroxydopamine-induced neurotoxicity and free radical production in rat cerebellar granule neurons. Life Sci. 2003;72:1635-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci USA. 2001;98:14669-14674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 602] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 40. | Lin S, Zhang Y, Dodel R, Farlow MR, Paul SM, Du Y. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition p38 MAP kinase in rat cerebellar granule neurons. Neurosci Lett. 2001;315:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Tomás-Camardiel M, Rite I, Herrera AJ, de Pablos RM, Cano J, Machado A, Venero JL. Minocycline reduces the lipopolysaccharide-induced inflammatory reaction, peroxynitrite-mediated nitration of proteins, disruption of the blood-brain barrier, and damage in the nigral dopaminergic system. Neurobiol Dis. 2004;16:190-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 42. | Ryu JK, McLarnon JG. Minocycline or iNOS inhibition block 3-nitrotyrosine increases and blood-brain barrier leakiness in amyloid beta-peptide-injected rat hippocampus. Exp Neurol. 2006;198:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Attur MG, Patel RN, Patel PD, Abramson SB, Amin AR. Tetracycline up-regulates COX-2 expression and prostaglandin E2 production independent of its effect on nitric oxide. J Immunol. 1999;162:3160-3167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 506] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 45. | Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME, Friedlander RM. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A. 2004;101:3071-3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 258] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 46. | Kelly KJ, Sutton TA, Weathered N, Ray N, Caldwell EJ, Plotkin Z, Dagher PC. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am J Physiol Renal Physiol. 2004;287:F760-F766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci U S A. 2003;100:10483-10487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 325] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 48. | Sato Y, Laskowitz DT, Bennett ER, Newman MF, Warner DS, Grocott HP. Differential cerebral gene expression during cardiopulmonary bypass in the rat: evidence for apoptosis? Anesth Analg. 2002;94:1389-1394, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Hindman BJ, Moore SA, Cutkomp J, Smith T, Ross-Barta SE, Dexter F, Brian JE. Brain expression of inducible cyclooxygenase 2 messenger RNA in rats undergoing cardiopulmonary bypass. Anesthesiology. 2001;95:1380-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Zhang TJ, Hang J, Wen DX, Hang YN, Sieber FE. Hippocampus bcl-2 and bax expression and neuronal apoptosis after moderate hypothermic cardiopulmonary bypass in rats. Anesth Analg. 2006;102:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Fan LW, Pang Y, Lin S, Rhodes PG, Cai Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience. 2005;133:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Fox C, Dingman A, Derugin N, Wendland MF, Manabat C, Ji S, Ferriero DM, Vexler ZS. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2005;25:1138-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 53. | Sriram K, Miller DB, O'Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem. 2006;96:706-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 54. | Cornet S, Spinnewyn B, Delaflotte S, Charnet C, Roubert V, Favre C, Hider H, Chabrier PE, Auguet M. Lack of evidence of direct mitochondrial involvement in the neuroprotective effect of minocycline. Eur J Pharmacol. 2004;505:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26:515-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 56. | Carty ML, Wixey JA, Colditz PB, Buller KM. Post-insult minocycline treatment attenuates hypoxia-ischemia-induced neuroinflammation and white matter injury in the neonatal rat: a comparison of two different dose regimens. Int J Dev Neurosci. 2008;26:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Buller KM, Carty ML, Reinebrant HE, Wixey JA. Minocycline: a neuroprotective agent for hypoxic-ischemic brain injury in the neonate? J Neurosci Res. 2009;87:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Cikla U, Chanana V, Kintner DB, Covert L, Dewall T, Waldman A, Rowley P, Cengiz P, Ferrazzano P. Suppression of microglia activation after hypoxia-ischemia results in age-dependent improvements in neurologic injury. J Neuroimmunol. 2016;291:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Tsuji M, Wilson MA, Lange MS, Johnston MV. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Exp Neurol. 2004;189:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 60. | Yu IC, Kuo PC, Yen JH, Paraiso HC, Curfman ET, Hong-Goka BC, Sweazey RD, Chang FL. A Combination of Three Repurposed Drugs Administered at Reperfusion as a Promising Therapy for Postischemic Brain Injury. Transl Stroke Res. 2017;8:560-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Drabek T, Wilson CD, Janata A, Stezoski JP, Janesko-Feldman K, Garman RH, Tisherman SA, Kochanek PM. Unique brain region-dependent cytokine signatures after prolonged hypothermic cardiac arrest in rats. Ther Hypothermia Temp Manag. 2015;5:26-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Janata A, Magnet IA, Uray T, Stezoski JP, Janesko-Feldman K, Tisherman SA, Kochanek PM, Drabek T. Regional TNFα mapping in the brain reveals the striatum as a neuroinflammatory target after ventricular fibrillation cardiac arrest in rats. Resuscitation. 2014;85:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 582] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 64. | González JC, Egea J, Del Carmen Godino M, Fernandez-Gomez FJ, Sánchez-Prieto J, Gandía L, García AG, Jordán J, Hernández-Guijo JM. Neuroprotectant minocycline depresses glutamatergic neurotransmission and Ca(2+) signalling in hippocampal neurons. Eur J Neurosci. 2007;26:2481-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Huang WC, Qiao Y, Xu L, Kacimi R, Sun X, Giffard RG, Yenari MA. Direct protection of cultured neurons from ischemia-like injury by minocycline. Anat Cell Biol. 2010;43:325-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Liu J, Bartels M, Lu A, Sharp FR. Microglia/macrophages proliferate in striatum and neocortex but not in hippocampus after brief global ischemia that produces ischemic tolerance in gerbil brain. J Cereb Blood Flow Metab. 2001;21:361-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |