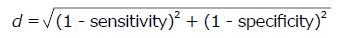Published online Nov 4, 2016. doi: 10.5492/wjccm.v5.i4.219
Peer-review started: March 9, 2016
First decision: May 19, 2016
Revised: July 1, 2016
Accepted: August 17, 2016
Article in press: August 18, 2016
Published online: November 4, 2016
Processing time: 241 Days and 10.7 Hours
To investigate the factors associated with the functional progress of hospitalized patients following an intensive care admission.
Retrospective study including data from a cohort of 198 hospitalized patients following an intensive care admission and not requiring mechanical ventilation in a single tertiary referral hospital. A generalized linear model was used to identify the main effects of clinical and demographic variables on the outcomes of functionality (KATZ Index of Independence in Activities of Daily Living) and muscle strength (MRC Scale). The covariates identified as independent predictors were analysed using the receiver operating characteristic curves. The analysis differentiated the periods in the intensive care unit (ICU), in the Ward (WARD) and the total time of hospital stay (TOT).
Considering the functional outcome (ΔKATZ), the variables that significantly contributed to the model (P < 0.05) were the KATZ and MRC on admission, age, sepsis (no), and total length of stay (TLS). Regarding the muscle strength outcome model (ΔMRC), the predictors were MRC on admission, Simplified Acute Physiology Score III, previous stroke, TLS, and sex (female). The variable age (AUC = 0.664) discriminated the ΔKATZICU. The variables age (AUC = 0.712), KATZ in ICU (AUC = 0.590) and on ward admission (AUC = 0.746), and MRC on ward admission (AUC = 0.721) were discriminative for ΔKATZWARD. For ΔKATZTOT the variables KATZ on ICU admission (AUC = 0.621) and TLS (AUC = 0.617) were discriminative. For ΔMRCICU the variables SAPSIII (AUC = 0.661) and MRC on ICU admission (AUC = 0.653) were discriminative. MRC on ICU (AUC = 0.681) and ward admission (AUC = 0.553) were discriminative for ΔMRCWARD. TLS (AUC = 0.649) and MRC on ward admission (AUC = 0.696) discriminative for the ΔMRCTOT.
Specific functional, clinical and demographical variables at ICU admission are associated with the functional prognosis during the hospitalization period.
Core tip: In spite of the advances in critical care, functional deficits are commonly observed during and after the hospitalization period. This retrospective study aimed to investigate the factors associated with the functional progress in a cohort of patients that underwent a mobilization protocol, from the intensive care unit (ICU) to the hospital discharge. As functional ability, muscle strength, low illness severity score at ICU and ward admission, absence of sepsis and stroke, longer total length of stay, male gender and younger age were predictors of favourable patients’ functional progress, these variables should be taken in consideration when planning rehabilitative strategies for hospitalized patients following an intensive care admission.
- Citation: Ferreira NA, Lopes AJ, Ferreira AS, Ntoumenopoulos G, Dias J, Guimaraes FS. Determination of functional prognosis in hospitalized patients following an intensive care admission. World J Crit Care Med 2016; 5(4): 219-227
- URL: https://www.wjgnet.com/2220-3141/full/v5/i4/219.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v5.i4.219
The advances in critical care have led to increased survival, however, physical and psychological deficits are commonly observed during and after the hospitalization period[1]. Intensive care unit acquired weakness (ICU-AW) is currently recognized as an important complication of critical illness. It is defined as symmetrical weakness in upper and lower limbs and is diagnosed with a medical research council (MRC) score sum less than 48[2]. Prolonged bed rest, systemic inflammation, hyperglycaemia, sedation, sepsis, malnutrition, patient immobilization, use of corticosteroids and neuromuscular blockers, among others, are considered risk factors for ICU-AW and, consequently, poorer functional outcome[3,4].
According to the World Health Organization International Classification of Functioning, Disability and Health[5,6], the key measures of physical function can be categorized as: (1) mobility: Including balance, lying, sitting, standing, shifting the body’s centre of gravity; (2) muscle function: Strength; (3) walking and moving: Including walking independently, walking with assistance, walking short and long distances; (4) self-care: Activities of daily living (ADL) such as washing, dressing, toileting, grooming and eating; and (5) self-reported quality of life (QOL). Although 26 different functional instruments have been described for use in critically ill patients, proper assessment tools addressing impairment, activity limitations and participation restrictions need to be developed to be utilized across different time points of recovery[7]. The KATZ Index of Independence in Activities of Daily Living (KATZ ADL)[8] was first developed to assess the functional status in non-hospitalized older adults, and has been applied across a wide range of patient populations[9] and is one of the few functional scales that have been used both in critical care[10-12] and in the ward settings[11,13-15]. Similarly, the MRC, which was initially developed to assess the volitional muscle strength in outpatients[16], has been broadly used in critical care patients with a very good interrater reliability[17], however there are limitations to the use of volitional measures of muscle function in critically ill patients[18].
The literature however is still scarce concerning the factors that may determine the patients’ functional recovery during the in-hospital period following a stay in intensive care. The ability to predict patient functional progress may contribute to the individualization of rehabilitative interventions. Additionally, the functional assessment throughout different time points of the in-hospital period, from the ICU admission/discharge to hospital discharge, using measures of muscle function and self-care may improve the understanding of the relationship between these two functional domains.
Thus, the primary objective of this study was to identify the factors that determine the functional progress of a cohort of patients that required an intensive care admission without the need for mechanical ventilation and underwent a mobilization protocol, from the ICU admission to hospital discharge. Secondarily, we aimed at evaluate the association between the KATZ ADL index and the MRC scale during the same hospitalization period.
Retrospective study including data from a cohort of 198 hospitalized patients in a single tertiary referral hospital. The medical records from patients admitted to the ICU at the Barra D’Or Hospital between September 2013 and December 2014 were screened according to the eligibility criteria.
The generalized linear model was used to identify the main effects of clinical and demographic variables on the outcomes of functionality and muscle strength during the hospitalization period. The study was approved by the Institutional Research Board and Ethics Committee.
Data was obtained from non-mechanically ventilated patients presenting with at least one of the following criteria: KATZ score ≥ 3, MRC ≤ 48, diagnosis of pulmonary or cardiovascular disease, and cooperative (Richmond Agitation-Sedation Scale between 0 and 2) who stayed at least 48 h at the ICU and underwent the mobilization protocol. Records of patients who died during the period of the study, with neuromuscular disease, returned to the ICU after discharge or with incomplete data were not included.
Eligibility criteria and procedures for the mobilization protocol followed the framework proposed by Hanekom et al[19] (2011). According to their clinical and functional condition, the patients progressed from passive and active mobilization and from being recumbent to ambulation as able. For patients unable to mobilize out of bed, lower limb strengthening exercises were included. Neuromuscular electrical stimulation and in-bed cycling exercises were not used. The interventions were undertaken daily on a twice a day basis.
The peripheral muscle strength was assessed by the MRC scale[16,17] which uses a 6-point scale from 0 (no contraction) to 5 (full contraction through range against resistance) of 12 muscle groups: Bilateral shoulder abduction, elbow flexion, wrist extension, hip flexion, knee extension and foot dorsiflexion. The representative score for each patient is the sum of the points given for each muscle group (maximum = 60).
The overall functional performance was assessed using the KATZ ADL index, which was culturally adapted for the Brazilian population[20]. This index assesses the subjects’ ability to perform the following activities of daily living independently: Bathing, dressing, toileting, transferring, continence, and feeding. The scoring system gives a numerical score from “0” (independent in the six functions) to “6” (dependent in the six functions).
The mobilization protocol, muscle strength and functional performance were performed by the physical therapy team, who were periodically trained to ensure a these procedures were applied in a standardised way. The patients underwent the mobilization protocol from the ICU admission until the hospital discharge. Functional and muscle strength assessments were undertaken on three key periods: ICU admission, ICU discharge and hospital discharge.
Differences (delta, ∆) between the output and input values of hospital sectors were established as categorical outcomes (∆SCOREperiod = OUTscore,period - INscore,period). Such differences were calculated for periods of stay only in the ICU (ICU), only in the ward (WARD) and for the total hospital stay (TOT). Thus, considering that in the KATZ scoring system higher values are representative of poor functionality, the following categorization was set as ΔKATZperiod = 1 if the sector’s output score is lower than the input or ΔKATZperiod = 0 if the sector’s output score is higher or equal than the input. Conversely, considering that the MRC score is directly proportional to the functionality, the categorization was set as ΔMRCperiod = 1 if the sector’s output score is greater than the input, ΔMRCperiod = 0 if the output score is equal or lower than the input.
Descriptive statistics included mean ± SD, median (minimum; maximum) or proportions for subjects aged < 65 years, ≥ 65 years, and the whole sample. Data between age groups were compared using independent-sample t tests for continuous variables, χ2 test for categorical variables, and Fisher’s exact test for dichotomous variables. Plots represent mean values alongside the 95%CI. Binary logistic, generalized linear model was used to identify the main effects of the factors (gender, “male” = 1; sepsis, “yes” = 1; COPD, “yes” = 1; dementia, “yes” = 1, previous stroke, “yes” = 1; and cause of ICU admission) and covariates (age; SAPSIII; length of stay; KATZ at admission; MRC at admission) on the outcomes of functionality and muscle strength (ΔKATZperiod and ΔMRCperiod, respectively). The covariates identified as independent predictors were analysed using the receiver operating characteristic (ROC) curves. The value of state variable was coded “0” or “1”, aiming to mirror the curve above the reference diagonal to facilitate the data interpretation. Cut-off points were determined by the minimal distance calculated as
Math 3
[21]. The association between MRC and KATZ scores was assessed through the Spearman’s correlation coefficient, and all the results were considered significant when P < 0.05. All statistical analyses were performed with SPSS software (IBM Inc., United States).
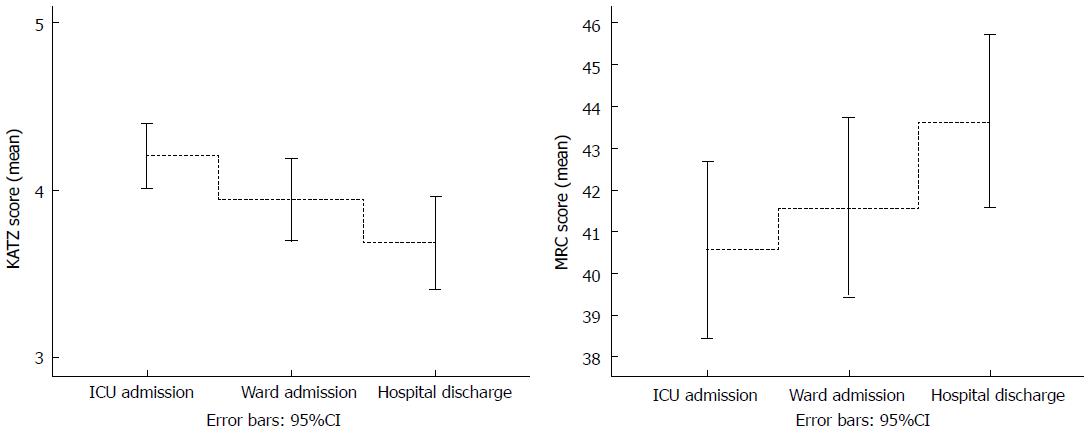
A total of 198 subjects (121 females, 61%) aged 24 to 101 years (76.4 ± 15.9 years) were considered eligible for the study. From these, 78 were hospitalized for lung disease, 105 for sepsis, 19 had COPD, 51 had dementia and 26 had a prior stroke. The average length of hospital stay was 12 ± 9 d, and the average SAPSIII score was 51.7 ± 9.5 (Table 1). In the analysis of deltas for KATZ, 24.7% of the patients improved and 63.1% remained stable in ΔKATZICU, 23.7% improved and 66.7% remained stable in ΔKATZWARD, and 36.4% improved and 51.5% remained stable in ΔKATZTOT. For MRC, 28.8% improved and 57.1% remained stable in ΔMRCICU, 35.4% improved and 52.5% remained stable in ΔMRCWARD and 47.0% showed improvement, and 35.4% remained stable in ΔMRCTOT. The proportion of elderly patients (≥ 65 years) in the group was 80.3%. One hundred and thirty-four (67.7%) patients had a MRC < 48 at ICU discharge (consistent with the definition of ICU-AW), with only minimal improvement at hospital discharge with 122 (61.6%) with the MRC < 48. The functional and muscle strength progress through the different time points of the study are shown in Figure 1.
| Variables | Levels | Age < 65 yr | Age≥65 yr | P value | All sample | |
| Mean ± SD | Mean ± SD | Mean ± SD | Median (Min; max) | |||
| n = 39 | n = 159 | n = 198 | ||||
| Age, yr | 50.5 ± 12.2 | 82.7 ± 8.6 | NT | 76.4 ± 15.9 | 80 (24; 101) | |
| KATZ at ICU admission, score | 3.9 ± 1.4 | 4.3 ± 1.4 | 0.207 | 4.2 ± 1.4 | 4 (0; 6) | |
| MRC at ICU admission, score | 46.6 ± 15.1 | 39.1 ± 14.9 | 0.007 | 40.6 ± 15.2 | 44 (0; 60) | |
| KATZ at ward admission, score | 2.7 ± 2.1 | 4.2 ± 1.6 | < 0.001 | 3.9 ± 1.8 | 4 (0; 6) | |
| MRC at ward admission, score | 48.1 ± 15.0 | 40.0 ± 15.1 | 0.004 | 41.6 ± 15.3 | 45.5 (0; 60) | |
| KATZ at hospital discharge, score | 2.5 ± 2.2 | 4.0 ± 1.8 | < 0.001 | 3.7 ± 2 | 4 (0; 6) | |
| MRC at hospital discharge, score | 50.6 ± 15.4 | 41.9 ± 14.0 | 0.002 | 43.6 ± 14.7 | 48 (0; 60) | |
| Hospital length of stay, d | 9.9 ± 7.0 | 12.1 ± 9.8 | 0.117 | 12 ± 9 | 9 (2; 52) | |
| SAPS3, % | 43.8 ± 8.8 | 53.7 ± 8.6 | < 0.001 | 51.7 ± 9.5 | 51 (27; 89) | |
| Gender, n (%) | Female | 17 (14) | 104 (86) | 0.017 | 121 (61) | - |
| Male | 22 (29) | 55 (71) | - | 77 (39) | - | |
| Sepsis, n (%) | No | 19 (20) | 74 (80) | 0.859 | 93 (47) | - |
| Yes | 20 (19) | 85 (81) | - | 105 (53) | - | |
| COPD, n (%) | No | 37 (21) | 142 (79) | 0.377 | 179 (90) | - |
| Yes | 2 (11) | 17 (89) | - | 19 (10) | - | |
| Dementia, n (%) | No | 39 (27) | 108 (73) | < 0.001 | 147 (74) | - |
| Yes | 0 (0) | 51 (100) | - | 51 (26) | - | |
| Previous stroke, n (%) | No | 38 (22) | 134 (78) | 0.032 | 172 (87) | - |
| Yes | 1 (4) | 25 (96) | - | 26 (13) | - | |
| Cause of ICU admission, n (%) | Cardiovascular | 4 (33) | 8 (67) | 0.646 | 12 (6) | - |
| Gastrointestinal | 3 (16) | 16 (84) | - | 19 (10) | - | |
| Neoplastic | 0 (0) | 1 (100) | - | 1 (1) | - | |
| Neurologic | 4 (25) | 12 (75) | - | 16 (8) | - | |
| Renal | 8 (23) | 27 (77) | - | 35 (18) | - | |
| Pulmonary | 16 (21) | 62 (79) | - | 78 (39) | - | |
| Others | 4 (11) | 33 (89) | - | 37 (19) | - | |
Considering the functional outcome during the ICU stay (ΔKATZICU), the variables that significantly contributed to the model were age (Wald χ2 = 10.060, P = 0.002), KATZ at ICU admission (Wald χ2 = 7.385, P = 0.007), MRC at ICU admission (Wald χ2 = 4.837, P = 0.028) and previous stroke (Wald χ2 = 4.671, P < 0.031). For the ward period (ΔKATZWARD) the significant predictors were age (Wald χ2 = 6.520, P = 0.011), KATZ at ward admission (Wald χ2 = 12.782, P < 0.001), MRC at ward admission (Wald χ2 = 4.418, P = 0.036), and sepsis (Wald χ2 = 4.528, P = 0.033). Similarly, age (Wald χ2 = 8.077, P = 0.004), total length of stay (Wald χ2 = 6.629, P = 0.010) and KATZ at ward admission (Wald χ2 = 10.099, P = 0.001) contributed for the functional outcome model considering the total length of stay (ΔKATZTOT) (Table 2).
| Functional outcome | ΔKATZ | ΔMRC | ||||||||
| Test for model effects | Parameter estimates | Test for model effects | Parameter estimates | |||||||
| Comparison | Variable | Waldχ2 | P (Sig.) | B | Lower 95%CI | Upper 95%CI | Waldχ2 | P (Sig.) | B | Lower 95%CI |
| ICU | Age, yr | 10.006 | 0.002 | 0.043 | 0.016 | 0.069 | 1.430 | 0.232 | 0.016 | -0.010 |
| SAPS3, % | 1.312 | 0.252 | -0.023 | -0.063 | 0.017 | 5.789 | 0.016 | -0.053 | -0.097 | |
| Length of stay in ICU, d | 1.295 | 0.255 | -0.040 | -0.110 | 0.029 | 3.145 | 0.076 | -0.068 | -0.144 | |
| KATZ at ICU admission, score | 7.385 | 0.007 | -0.562 | -0.967 | -0.157 | 0.399 | 0.528 | 0.119 | -0.251 | |
| MRC at ICU admission, score | 4.837 | 0.028 | -0.045 | -0.084 | -0.005 | 9.645 | 0.002 | 0.057 | 0.021 | |
| Sex (male = 1) | 0.069 | 0.792 | -0.108 | -0.913 | 0.697 | 0.733 | 0.392 | 0.341 | -0.440 | |
| Sepsis (no) | 0.105 | 0.745 | -0.133 | -0.934 | 0.669 | 0.088 | 0.766 | -0.122 | -0.924 | |
| COPD (no) | 0.184 | 0.668 | -0.302 | -1.683 | 1.078 | 0.479 | 0.489 | -0.488 | -1.870 | |
| Dementia (no) | 0.238 | 0.626 | -0.261 | -1.312 | 0.789 | 1.478 | 0.224 | -0.622 | -1.626 | |
| Previous stroke (no) | 4.671 | 0.031 | -1.782 | -3.398 | -0.166 | 8.815 | 0.003 | -2.667 | -4.428 | |
| Cause of ICU admission | 3.785 | 0.706 | NS | NS | NS | 7.956 | 0.241 | NS | NS | |
| Ward | Age, yr | 6.520 | 0.011 | 0.040 | 0.009 | 0.071 | 1.235 | 0.267 | 0.016 | -0.012 |
| SAPS3, % | 0.137 | 0.711 | 0.008 | -0.034 | 0.050 | 0.029 | 0.865 | -0.004 | -0.046 | |
| Length of stay in ward, d | 2.726 | 0.099 | -0.047 | -0.102 | 0.009 | 1.014 | 0.314 | -0.028 | -0.083 | |
| KATZ at ICU admission, score | 9.241 | 0.002 | -0.858 | -1.411 | -0.305 | 1.185 | 0.276 | 0.228 | -0.183 | |
| MRC at ICU admission, score | 1.339 | 0.247 | 0.034 | -0.024 | 0.092 | 20.013 | < 0.001 | 0.223 | 0.125 | |
| KATZ at ward admission, score | 12.782 | < 0.001 | 0.867 | 0.392 | 1.342 | 0.503 | 0.478 | 0.123 | -0.217 | |
| MRC at ward admission, score | 4.418 | 0.036 | -0.064 | -0.123 | -0.004 | 12.085 | 0.001 | -0.170 | -0.265 | |
| Sex (male = 1) | 0.548 | 0.459 | 0.306 | -0.504 | 1.115 | 0.971 | 0.324 | -0.361 | -1.079 | |
| Sepsis (no) | 4.528 | 0.033 | 0.940 | 0.074 | 1.805 | 0.740 | 0.390 | 0.332 | -0.424 | |
| COPD (no) | 0.035 | 0.852 | -0.122 | -1.404 | 1.160 | 1.978 | 0.160 | 0.881 | -0.347 | |
| Dementia (no) | 0.138 | 0.710 | -0.206 | -1.290 | 0.879 | 0.063 | 0.803 | 0.120 | -0.822 | |
| Previous stroke (no) | 0.362 | 0.548 | -0.377 | -1.606 | 0.852 | 0.067 | 0.796 | -0.144 | -1.231 | |
| Cause of ICU admission | 6.186 | 0.403 | NS | NS | NS | 4.222 | 0.647 | NS | NS | |
| Total | Age, yr | 8.077 | 0.004 | 1.465 | -3.56 | 6.490 | 0.243 | 0.622 | 0.007 | -0.019 |
| SAPS3, % | 0.816 | 0.366 | 0.044 | 0.014 | 0.075 | 0.832 | 0.362 | 0.019 | -0.021 | |
| Total length of stay, d | 6.629 | 0.010 | -0.021 | -0.066 | 0.024 | 4.799 | 0.028 | -0.039 | -0.075 | |
| KATZ at ICU admission, score | 10.269 | 0.001 | -0.056 | -0.098 | -0.013 | 0.024 | 0.876 | -0.031 | -0.424 | |
| MRC at ICU admission, score | 0.257 | 0.612 | 0.826 | 0.321 | 1.331 | 1.077 | 0.299 | -0.024 | -0.069 | |
| KATZ at ward admission, score | 10.099 | 0.001 | 0.014 | -0.040 | 0.069 | 0.006 | 0.937 | -0.014 | -0.364 | |
| MRC at ward admission, score | 2.743 | 0.098 | -0.752 | -1.215 | -0.288 | 4.406 | 0.036 | 0.049 | 0.003 | |
| Sex (male = 1) | 0.157 | 0.692 | -0.047 | -0.102 | 0.009 | 3.864 | 0.049 | -0.751 | -1.499 | |
| Sepsis (no) | 1.589 | 0.208 | 0.169 | -0.668 | 1.007 | 0.439 | 0.508 | 0.247 | -0.484 | |
| COPD (no) | 0.002 | 0.968 | 0.594 | -0.330 | 1.517 | 1.076 | 0.300 | 0.570 | -0.507 | |
| Dementia (no) | 0.028 | 0.866 | -0.026 | -1.318 | 1.265 | 0.629 | 0.428 | -0.363 | -1.261 | |
| Previous stroke (no) | 0.046 | 0.831 | -0.097 | -1.226 | 1.032 | 3.273 | 0.070 | 0.911 | -0.076 | |
| Cause of ICU admission | 12.048 | 0.061 | NS | NS | NS | 2.985 | 0.811 | NS | NS | |
Regarding the muscle strength outcome during the ICU stay (ΔMRCICU), the SAPSIII (Wald χ2 = 5.789, P = 0.016), MRC at ICU admission (Wald χ2 = 9.645, P = 0.002) and previous stroke (Wald χ2 = 8.815, P = 0.003) were significant predictors. For the ward period (ΔMRCWARD) the predictors were the MRC at ICU admission (Wald χ2 = 20.013, P < 0.001) and MRC at ward admission (Wald χ2 = 12.085, P = 0.001). At hospital discharge (ΔMRCTOT), the total length of stay (Wald χ2 = 4.799, P = 0.028), MRC at ward admission (Wald χ2 = 4.406, P = 0.036) and sex (Wald χ2 = 3.864, P = 0.049) contributed for the model (Table 2).
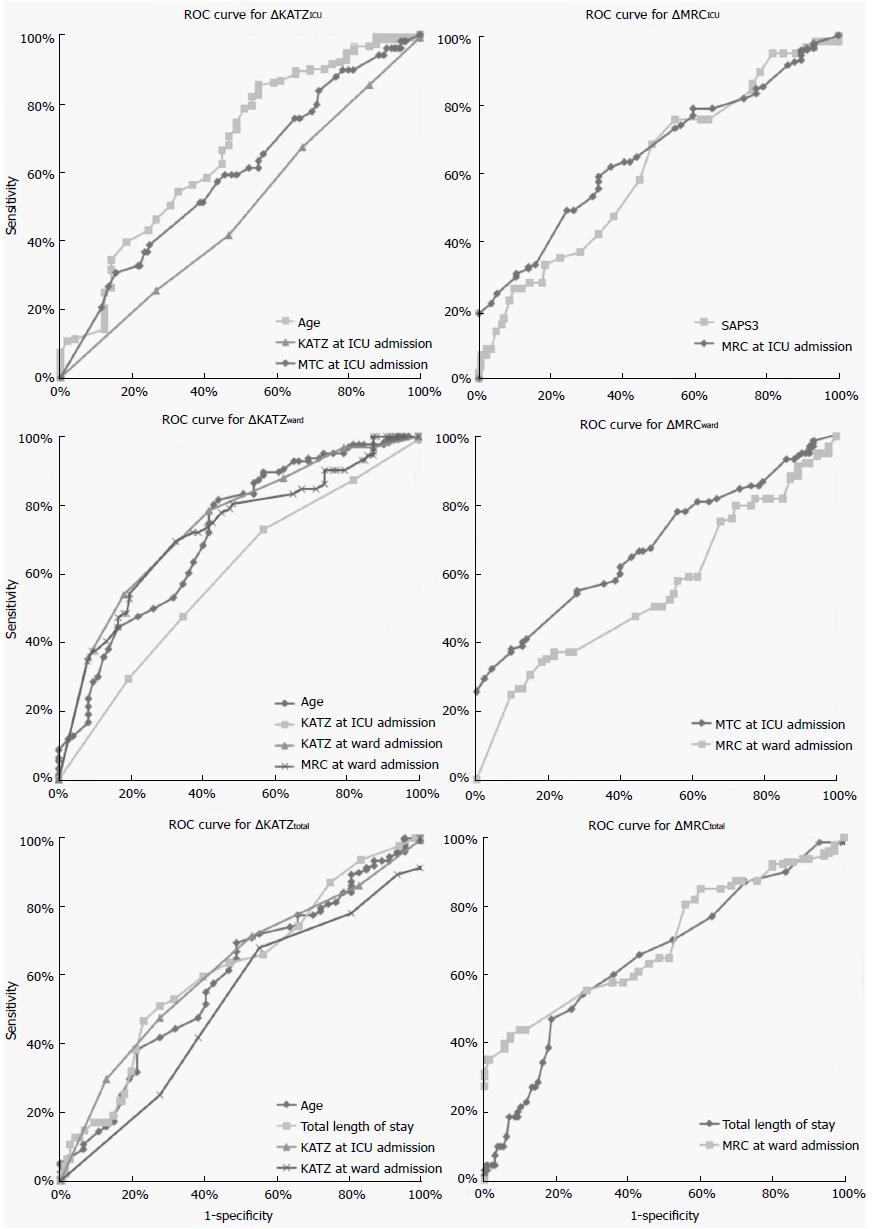
Among the significant predictors in the generalized linear model analysed using the ROC curve, age (AUC = 0.664, cut-off = 78.5 years) was the only variable that discriminated the ΔKATZICU. For the ΔMRCICU the variables SAPSIII (AUC = 0.661, cut-off = 50.0%) and MRC at ICU admission (AUC = 0.653, cut-off = 37 points) were discriminative. For ΔKATZWARD, the variables of age (AUC = 0.712, cut-off = 81.5 years), KATZ at ICU admission (AUC = 0.590, cut-off = 4.5 points), KATZ at ward admission (AUC = 0.746, cut-off = 2.5 points), and MRC at ward admission (AUC = 0.721, cut-off = 47 points) were discriminative. The variables that significantly discriminated the ΔMRCWARD were MRC at ICU admission (AUC = 0.681, cut-off = 44.5 points) and MRC at ward admission (AUC = 0.553, cut-off = 43.3 points). Finally, total length of stay (AUC = 0.617, cut-off = 11.5 d) and KATZ at ICU admission (AUC = 0.621, cut-off = 3.5 points) were the only variables that discriminated the ΔKATZTOT. Whereas total length of stay (AUC = 0.649, cut-off = 10.5 d) and MRC at ward admission (AUC = 0.696, cut-off = 37 points) were the variables that significantly discriminated the ΔMRCTOT (Figure 2 and Table 3).
| Functional outcomes | ΔKATZ | ΔMRC | |||||||||
| AUC | Cut-off | Sensitivity | Specificity | P (Sig.) | AUC | Cut-off | Sensitivity | Specificity | P (Sig.) | ||
| ICU | Age, yr | 0.664 | 78.5 | 41% | 42% | 0.001 | 1 | 1 | 1 | 1 | 1 |
| SAPS3, % | 1 | 1 | 1 | 1 | 1 | 0.661 | 50.5 | 68% | 52% | 0.014 | |
| KATZ at ICU admission, score | 0.515 | 4.5 | 47% | 58% | 0.753 | 1 | 1 | 1 | 1 | 1 | |
| MRC at ICU admission, score | 0.592 | 45.5 | 57% | 56% | 0.053 | 0.653 | 37 | 44% | 35% | 0.001 | |
| Ward | Age, yr | 0.712 | 81.5 | 32% | 47% | < 0.001 | 1 | 1 | 1 | 1 | 1 |
| KATZ at ICU admission, score | 0.590 | 4.5 | 35% | 52% | 0.035 | 1 | 1 | 1 | 1 | 1 | |
| MRC at ICU admission, score | 1 | 1 | 1 | 1 | 1 | 0.681 | 44.5 | 39% | 42% | < 0.001 | |
| KATZ at ward admission, score | 0.746 | 2.5 | 63% | 12% | < 0.001 | 1 | 1 | 1 | 1 | 1 | |
| MRC at ward admission, score | 0.721 | 47 | 69% | 67% | < 0.001 | 0.553 | 43.3 | 54% | 48% | 0.200 | |
| Total | Age, yr | 0.592 | 81.5 | 38% | 52% | 0.058 | 1 | 1 | 1 | 1 | 1 |
| Total length of stay, d | 0.617 | 11.5 | 51% | 72% | 0.016 | 0.649 | 10.5 | 54% | 73% | 0.001 | |
| KATZ at ICU admission, score | 0.621 | 3.5 | 53% | 28% | 0.012 | 1 | 1 | 1 | 1 | 1 | |
| KATZ at ward admission, score | 0.515 | 4.5 | 38% | 58% | 0.750 | 1 | 1 | 1 | 1 | 1 | |
| MRC at ward admission, score | 1 | 1 | 1 | 1 | 1 | 0.696 | 37 | 51% | 35% | < 0.001 | |
There were weak significant associations between ΔMRCICU and ΔKATZICU (ρ = 0.264; P < 0.001), ΔMRCICU and ΔKATZWARD (ρ = 0.151; P = 0.034) and ΔMRCTOT and ΔKATZTOT (ρ = 0.183; P = 0.010).
The results of this study showed that, in spite of being cooperative and undergoing a mobilization protocol, only a small percentage of the patients in ICU improved their muscle strength (28.8%) and functional status (24.7%) in this setting. The same was observed in the ward period, with improvements in 35.4% of patients for muscle strength and 23.7% of patients for KATZ ADL index. This cohort of patients had low functional and muscle strength scores at hospital discharge, i.e., KATZ = 3.7 and MRC = 43.6. Advanced age was identified as one of the determining factors of unfavourable functional progress in our study, and with the high prevalence of aged patients in our sample (80.3%, i.e., 167 patients older than 65 years of age) this must have contributed to the poor functional progress during hospitalization and at hospital discharge. As shown in Table 1, elderly patients had more severe clinical condition at admission, as well as lower MRC scores and higher prevalence of previous stroke. Besides aging, these factors may have also contributed to the poorer functional scores observed on the ward and at hospital discharge in comparison with the younger subgroup.
Nevertheless, when considering the sample as a whole, there was a clear trend in improving MRC and KATZ scores throughout the hospitalization period (Figure 1). These results are in accordance with the study of van der Schaaf et al[22] (2009) who followed a sample of 255 participants during the ICU stay. In this study, even with a younger sample (mean age = 58.8 years), they found that 69% of the subjects had persistent limitations for the activities daily living even one year after the hospital discharge. In our study length of stay was a significant predictor of improvement in muscle strength and functional status when considering the total time of hospitalization. This suggests that the in-hospital functional and muscle strength recovery may be time-dependent. Hence[12] the extent of improvements in functional outcomes during hospitalization should be incorporated in hospital discharge planning. However, the evidence is still inconclusive regarding the benefits of exercise-based interventions on functional exercise capacity and health-related quality of life for survivors of critical illness[23]. Further research into the rehabilitation during hospitalization and post hospital discharge are required[24].
A total of 134 (67.7%) and 122 (61.6%) of the patients presented with ICU-AW at ICU and hospital discharge, respectively. The high incidence of ICU-AW in this cohort of patients may be due to the high prevalence of sepsis (53%) and increased age in our sample[25,26].
Nonetheless, higher MRC scores at ICU admission were predictive for the improvement in muscle strength and functionality during the ICU stay. These results reinforce the concept that more attention by physical therapists should be given to patients with reduced muscle strength at ICU admission. In this way, the early application of rehabilitation may benefit selected patients. Specifically the application of electrical muscle stimulation[27] or in-bed cycling[28] in the patient group who are un-cooperative or with very poor muscle strength may be beneficial but requires more prospective evaluation.
Interestingly, the KATZ indexes at ICU admission were predictive only for the functional improvement. As a minimum degree of muscle strength is needed for performing the self-care activities, it is likely that individuals with higher KATZ scores had strength about 4-5 in the muscles assessed by the MRC scale. Thus, due to the ceiling effect, further improvements in muscle strength were not detected in patients with higher KATZ scores.
Surprisingly, dementia, considered an exclusion criterion in some mobilization trials 1[11] was not a predictor of no-improvement in muscle strength. Conversely, previous stroke, which results in variable degrees of neuromuscular disability, was predictive of non-improvements in KATZ and MRC scores during the ICU stay.
The functional progression of hospitalized patients is dependent on many factors. For example, nutritional status, use of certain drugs (corticosteroids, neuromuscular blockers, etc.), hyperglycaemia, and multiple organ failure[3,29,30], among others might have influenced the results of this research. Although it can be considered a limitation, we used a representative sample of critically ill patients, and our outcomes provided functional, demographic and clinical parameters to be used as general predictors of muscle strength and functional progress during the hospitalization period. We believe that the findings of this study add new relevant information for the early rehabilitation and mobilization protocols, as well as to serve as a starting point for further research on the individual factors to be considered for these types of intervention.
The functional assessment instrument used in this study was not originally developed for use in the intensive care unit. Similarly, other functional instruments designed for outpatients, such as the Barthel Index and the functional independence measure (FIM) have been used in intensive care[6]. As many ICU patients may present with a very low functional capacity, it is likely that these general functional instruments have some limitation concerning their clinimetric properties. For example in ICU there may be potential for a floor or ceiling effect; limited ability to detect meaningful change (responsiveness) and/or minimal clinically important difference[7]. To date there are no instruments designed to follow the functional progress of patients’ from ICU admission to hospital discharge and beyond. Therefore, such general scales seem to be useful for the long term prospective functional monitoring, for both clinical and research purposes.
As intubated and mechanically ventilated patients were not included in this study, it was not possible to analyse the influence of this factor on muscle strength and functional progress during hospitalization. Although muscle weakness is associated with prolonged weaning from mechanical ventilation, there is large variability in the reported prevalence of ICU-AW among mechanically ventilated patients[31]. Therefore, future studies should address if mechanical ventilation is an independent predictor of peripheral muscle strength and functional progress in critically ill patients. Another limitation of the study is that data from deceased patients were not included and analysed. As we used secondary records and patients with incomplete data were excluded, this information was not available in our database. Our study did not focus on mortality outcomes and it hence it would not have been possible to analyze functional progress of these subjects throughout all time points.
In conclusion, better functional patient condition and low severity of illness score at ICU admission, absence of sepsis and stroke, longer total length of stay and age lower than 78.5 years are predictors of favourable patient functional progress during hospitalization following an intensive care admission.
The advances in critical care have led to increased patient survival, however, severe deficits in physical and psychological status are commonly observed during and after the hospitalization period. The possibility of predicting the patients’ functional progress may assist to contribute to individualized rehabilitative interventions in this cohort of patients’. The authors investigated the determining factors of functional progress in a cohort of patients that required intensive care unit (ICU) admission without mechanical ventilation that underwent a mobilization protocol, from the ICU to hospital discharge.
It un-clear which factors influence the patients’ functional recovery during the in-hospital period after a stay in ICU. This study identifies the clinical and functional characteristics that influence the functional progress of hospitalized patients.
These results demonstrated a high prevalence of Intensive Care Unit Acquired Weakness and functional impairments at hospital discharge, similar to previous research. The authors have defined key clinical and functional parameters that predict the functional progress of hospitalized patients who underwent a standardized mobilization protocol through the ICU and the ward setting.
The rehabilitation of hospitalized patients who require an ICU admission without mechanical ventilation should take into account the predictors of poor functional outcomes identified in this study.
This is a well-written manuscript about functional rehabilitation and muscle strength following an ICU admission.
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Farand P, Hokama A, Juneja D S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Bemis-Dougherty AR, Smith JM. What follows survival of critical illness? Physical therapists’ management of patients with post-intensive care syndrome. Phys Ther. 2013;93:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Hough CL, Lieu BK, Caldwell ES. Manual muscle strength testing of critically ill patients: feasibility and interobserver agreement. Crit Care. 2011;15:R43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Koukourikos K, Tsaloglidou A, Kourkouta L. Muscle atrophy in intensive care unit patients. Acta Inform Med. 2014;22:406-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Ntoumenopoulos G. Rehabilitation during mechanical ventilation: Review of the recent literature. Intensive Crit Care Nurs. 2015;31:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. International Classification of Functioning, Disability and Health (ICF). [accessed. 2015;Jul 25] Available from: http://www.who.int/classifications/icf/en/. |
| 6. | Tipping CJ, Young PJ, Romero L, Saxena MK, Dulhunty J, Hodgson CL. A systematic review of measurements of physical function in critically ill adults. Crit Care Resusc. 2012;14:302-311. [PubMed] |
| 7. | Parry SM, Denehy L, Beach LJ, Berney S, Williamson HC, Granger CL. Functional outcomes in ICU - what should we be using? - an observational study. Crit Care. 2015;19:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | KATZ S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2637] [Cited by in RCA: 2682] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 9. | White DK, Wilson JC, Keysor JJ. Measures of adult general functional status: SF-36 Physical Functioning Subscale (PF-10), Health Assessment Questionnaire (HAQ), Modified Health Assessment Questionnaire (MHAQ), KATZ Index of Independence in activities of daily living, Functional Independence Measure (FIM), and Osteoarthritis-Function-Computer Adaptive Test (OA-Function-CAT). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S297-S307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Brorsson B, Asberg KH. KATZ index of independence in ADL. Reliability and validity in short-term care. Scand J Rehabil Med. 1984;16:125-132. [PubMed] |
| 11. | Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874-1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2077] [Cited by in RCA: 2123] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 12. | Winkelman C, Johnson KD, Hejal R, Gordon NH, Rowbottom J, Daly J, Peereboom K, Levine AD. Examining the positive effects of exercise in intubated adults in ICU: a prospective repeated measures clinical study. Intensive Crit Care Nurs. 2012;28:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Wallace M, Shelkey M. Monitoring functional status in hospitalized older adults. Am J Nurs. 2008;108:64-71; quiz 71-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Zaharias E, Cataldo J, Mackin L, Howie-Esquivel J. Simple measures of function and symptoms in hospitalized heart failure patients predict short-term cardiac event-free survival. Nurs Res Pract. 2014;2014:815984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Etxeberria-Lekuona D, Casas Fernández de Tejerina JM, Méndez López I, Oteiza Olaso J, Arteaga Mazuelas M, Jarne Betran V. Multiple hospitalizations at the Department of Internal Medicine of a tertiary hospital. Rev Clin Esp. 2015;215:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 654] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 17. | Vanpee G, Hermans G, Segers J, Gosselink R. Assessment of limb muscle strength in critically ill patients: a systematic review. Crit Care Med. 2014;42:701-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Connolly BA, Jones GD, Curtis AA, Murphy PB, Douiri A, Hopkinson NS, Polkey MI, Moxham J, Hart N. Clinical predictive value of manual muscle strength testing during critical illness: an observational cohort study. Crit Care. 2013;17:R229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Hanekom S, Gosselink R, Dean E, van Aswegen H, Roos R, Ambrosino N, Louw Q. The development of a clinical management algorithm for early physical activity and mobilization of critically ill patients: synthesis of evidence and expert opinion and its translation into practice. Clin Rehabil. 2011;25:771-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Lino VT, Pereira SR, Camacho LA, Ribeiro Filho ST, Buksman S. Cross-cultural adaptation of the Independence in Activities of Daily Living Index (KATZ Index). Cad Saude Publica. 2008;24:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 21. | Yarnold PR. UniODA vs. ROC Analysis: Computing the “Optimal” Cut-Point. Opitmal Data Analysis. 2014;3:117-120 Available from: http://optimalprediction.com/files/pdf/V3A29.pdf. |
| 22. | van der Schaaf M, Beelen A, Dongelmans DA, Vroom MB, Nollet F. Functional status after intensive care: a challenge for rehabilitation professionals to improve outcome. J Rehabil Med. 2009;41:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Connolly B, Salisbury L, O’Neill B, Geneen L, Douiri A, Grocott MP, Hart N, Walsh TS, Blackwood B. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database Syst Rev. 2015;CD008632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Denehy L, Elliott D. Strategies for post ICU rehabilitation. Curr Opin Crit Care. 2012;18:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Lipshutz AK, Gropper MA. Acquired neuromuscular weakness and early mobilization in the intensive care unit. Anesthesiology. 2013;118:202-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Batt J, dos Santos CC, Cameron JI, Herridge MS. Intensive care unit-acquired weakness: clinical phenotypes and molecular mechanisms. Am J Respir Crit Care Med. 2013;187:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | RETRACTED ARTICLE: Neuromuscular electrical stimulation in critically ill patients in the intensive care unit: a systematic review. Einstein (Sao Paulo). 2014;12:361-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Parry SM, Berney S, Warrillow S, El-Ansary D, Bryant AL, Hart N, Puthucheary Z, Koopman R, Denehy L. Functional electrical stimulation with cycling in the critically ill: a pilot case-matched control study. J Crit Care. 2014;29:695.e1-695.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | de Jonghe B, Lacherade JC, Sharshar T, Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37:S309-S315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 30. | Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591-1600. [PubMed] |
| 31. | Hashem MD, Nelliot A, Needham DM. Early Mobilization and Rehabilitation in the ICU: Moving Back to the Future. Respir Care. 2016;61:971-979. [PubMed] |