Published online Aug 4, 2016. doi: 10.5492/wjccm.v5.i3.187
Peer-review started: February 25, 2016
First decision: March 24, 2016
Revised: April 8, 2016
Accepted: April 21, 2016
Article in press: April 22, 2016
Published online: August 4, 2016
Processing time: 161 Days and 17.8 Hours
AIM: To investigate posttraumatic cytokine alterations and their value for predicting complications and mortality in polytraumatized patients.
METHODS: Studies on the use of specific cytokines to predict the development of complications and mortality were identified in MEDLINE, EMBASE, Web of Science and the Cochrane Library. Of included studies, relevant data were extracted and study quality was scored.
RESULTS: Forty-two studies published between 1988 and 2015 were identified, including 28 cohort studies and 14 “nested” case-control studies. Most studies investigated the cytokines interleukin (IL)-6, IL-8, IL-10 and tumor necrosis factor (TNF-α). IL-6 seems related to muliorgan dysfunction syndrome, multiorgan failure (MOF) and mortality; IL-8 appears altered in acute respiratory distress syndrome, MOF and mortality; IL-10 alterations seem to precede sepsis and MOF; and TNF-α seems related to MOF.
CONCLUSION: Cytokine secretion patterns appear to be different for patients developing complications when compared to patients with uneventful posttraumatic course. More research is needed to strengthen the evidence for clinical relevance of these cytokines.
Core tip: Early identification of patients at risk for developing complications is one of the most challenging problems in the therapy of multiple injuries. Close monitoring of cytokine secretion patterns could give physicians an impression of the individual risk for development of complications. Further, physicians are directed to the appropriate prophylactic treatment, as well as optimal timing of surgical interventions, thereby reducing “second hits” with subsequent risks of development of sepsis and multiorgan failure. This article provides an overview of the results from literature concerning posttraumatic immune alterations leading to various complications and death.
- Citation: Dekker ABE, Krijnen P, Schipper IB. Predictive value of cytokines for developing complications after polytrauma. World J Crit Care Med 2016; 5(3): 187-200
- URL: https://www.wjgnet.com/2220-3141/full/v5/i3/187.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v5.i3.187
The term polytrauma is used to describe a combination of serious injuries in at least two different anatomical regions. Polytraumatized patients that survive the initial impact of trauma, are confronted with an enormous host defence reaction, which is associated with morbidity and mortality. Trauma initiates a local pro-inflammatory response, encompassing the activation of effector cells, complement cascade, coagulation system, cytokines, acute phase proteins and neuroendocrine mediators[1,2]. This sequence of events is part of the physiologic response to trauma, as it serves to initiate the healing process, prevents the host from additional injury and acts as a barrier against infection[3]. Yet extensive trauma can arouse a comprehensive systemic inflammatory state known as the systemic inflammatory response syndrome (SIRS). An overactivated pro-inflammatory reaction leads to progressive sequestration of leukocytes in vital organs, predisposing patients to the development of organ failure. In an attempt to mediate these deleterious effects, immunesuppressive mediators are released. This counter regulatory response syndrome (CARS) becomes active almost immediately after the onset of SIRS[4]. Despite dampening inflammation, CARS itself may have unfavorable effects as well, as it can induce an increased susceptibility to infections and sepsis[2]. The posttraumatic immunologic alterations of combined SIRS and CARS have been termed CHAOS (cardiovascular shock, homeostasis, apoptosis, organ dysfunctions and immune suppression)[5]. With an overwhelming initial traumatic insult, an overstimulated SIRS response initiates the chaos that results in early multiorgan failure (MOF), present within 72 h after injury[2,6]. A less severe initial insult may prime immune cells while eliciting a moderate inflammatory reaction. In this setting, a second insult (“hit”) may strengthen the inflammatory reaction towards immune suppression, predisposing the patient to sepsis[7,8].
Cytokines play a pivotal role in both the pro-inflammatory and the anti-inflammatory reaction to trauma[9,10]. The pro-inflammatory cytokine interleukin-6 (IL-6) is secreted by a wide range of cells including neutrophils, T- and B-lymphocytes and endothelial cells[8,11]. Release of IL-6 is enhanced after stimulation by micro-organisms and cytokines (TNF-α, IL-1β), and liberated after tissue damage and infection. The biologic activity of IL-6 includes increased T- and B-cell activation and proliferation, differentiation of cytotoxic T cells and enhanced activity of natural killer (NK) cells[12]. In addition, IL-6 mediates the induction of the acute phase response and reduces apoptosis in neutrophil granulocytes[4,11]. Combined actions lead to an effective SIRS response early after trauma. The pro-inflammatory cytokine IL-8 is an endogenous chemoattractant. Monocytes, macrophages, neutrophils and endothelial cells secrete IL-8, and its release is enhanced after stimulation with IL-1, TNF-α, C5a and LPS[9,13]. After activation, IL-8 induces expression of adhesion molecules on neutrophils and endothelial cells, which enables the migration of neutrophils to the site of production[4,9]. The anti-inflammatory cytokine IL-10 is primarily synthesized by CD4+ TH2 lymphocytes and, to a lesser extent, by B lymphocytes, monocytes and macrophages[8]. Activated IL-10 decreases the cytokine production of TH1 cells, reduces antigen presentation of macrophages and subsequent proliferation of T-lymphocytes, and suppresses monocyte function[4,14,15]. These actions make IL-10 one of the most important mediators in the anti-inflammatory immune response. The pro-inflammatory cytokine TNF-α is one of the first cytokines to be released after trauma. The cytokine is produced by monocytes, macrophages, lymphocytes and T lymphocytes. After secretion, TNF-α increases endothelial cell permeability and adhesion properties, and activates macrophages, NK cells and lymphocytes. TNF-α also induces the secretion of various cytokines [IL-6, -8, -10, interferon (IFN-γ)] and immunoglobulin production[7,12]. Release of excessive TNF-α ultimately leads to accumulation of leukocytes in the injured tissues. Many of these cytokines attributed to the potential development of complications in polytrauma patient. Their exact causal role has not been detected yet.
Early identification of patients at risk for developing complications is one of the most challenging problems in the therapy of multiple injuries. Close monitoring of cytokine secretion patterns could give physicians an impression of the individual risk for development of complications. Further, physicians are directed to the appropriate prophylactic treatment, as well as optimal timing of surgical interventions, thereby reducing “second hits” with subsequent risks of development of sepsis and MOF. Previous studies have acknowledged the correlation between markers of inflammation and clinical condition after polytrauma. The aim of the current review was: (1) to summarize the available knowledge on specific cytokines that are involved in the posttraumatic immune alterations; and (2) to assess the value of cytokines for predicting the development of acute respiratory distress syndrome (ARDS), sepsis, multiorgan dysfunction syndrome (MODS), MOF and mortality.
The systematic review was performed in concordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement[16]. Due to heterogeneity across the studies in terms of patient population, study design and analytical techniques used, and the small amount of studies for each biomarker-complication combination, a meta-analysis was not feasible.
Studies addressing the relation between complications after multiple trauma and cytokine concentrations, were identified in the following databases: MEDLINE (1988 - 18 January 2014), Embase (1988 - 18 January 2014), Web of Science (1988 - 18 January 2014) and the Cochrane Library (to Issue 1, 2014). The search strategy was developed by an information specialist, and carried out using various combinations of the key words “multiple trauma”, “cytokines” and the complications “systemic inflammatory response sydrome (SIRS)”, “ARDS”, “sepsis”, “MODS”, “MOF” and “mortality”. In addition, forward citation searches of selected studies and literature reviews were carried out. The initial search was not limited by language, publication date and type of publication. In February 2016, an additional literature search of the mentioned databases was carried out. One relevant new article was found.
Primary outcomes were the development of one or more of the following complications: (1) ARDS, determined in concordance with the American-European Consensus Conference 1994 definitions[17]; (2) sepsis, diagnosed when SIRS (defined according to the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference 1992[18]) occurred in combination with a septic focus or positive blood culture; (3) MODS; and (4) MOF, in the included studies diagnosed based on different scoring systems[19-24]. The secondary outcome was mortality during a predetermined follow-up period of individual studies.
Studies were scanned for eligibility based on title and abstract. Subsequently, eligibility of selected studies was assessed by retrieving the full text of the article. Inclusion criteria were prospective or retrospective cohort, case-control and cross-sectional studies including at least 10 adult multiple trauma patients (ISS ≥ 16). Excluded were articles in other language than English or German, animal studies and ex vivo studies, studies involving pediatric populations, case reports, review articles and letters/editorials. Studies not elaborating on the primary or secondary outcomes investigated in this review were also excluded. In addition, studies measuring cytokine concentrations in samples other than serum (e.g., wound exsudate, broncho-alveolar lavage fluid) were not eligible for inclusion, as local alterations in concentration may not reflect the systemic changes in the immune reaction.
The following data were extracted from included studies: Title, study design, date of publication, size of study population, patient demographics, incidence of complications and mortality, follow-up period, type of cytokines studied, mean cytokine concentrations measured at specific moments during follow-up, and cut-off points with sensitivity and specificity. Data were extracted from figures when raw data were not available. In the case of duplicate publications, the most relevant or informative article was chosen.
The quality of included studies was critically evaluated with the strengthening the reporting of observational studies in epidemiology (STROBE) statement[25].
In this review of the literature no biostatistical methods were used. For this reason, no biomedical statistician was involved for statistical review.
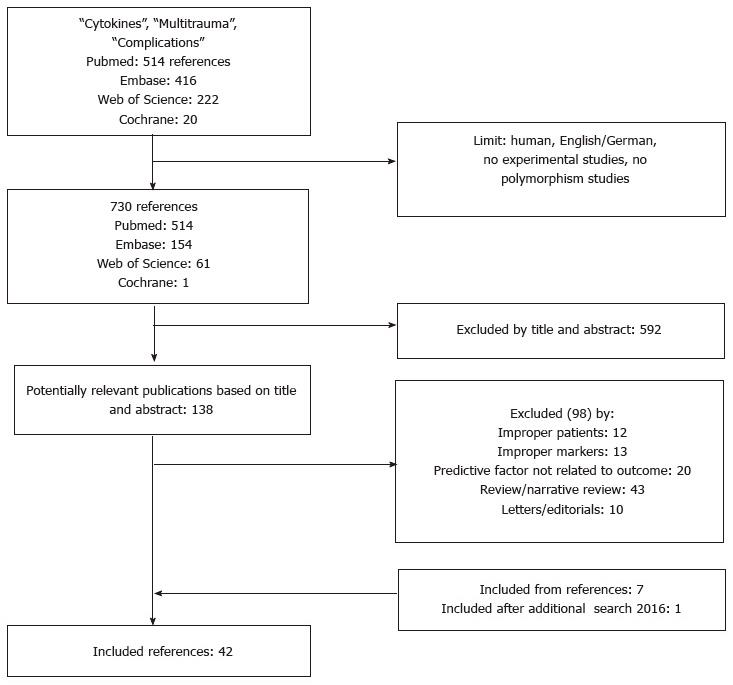
After exclusion of duplicate studies, the literature search yielded 730 potentially relevant articles. One hundred and thirty-eight articles passed the first screening and were retrieved for closer examination. Of the retrieved articles, 40 were eligible for study inclusion. The full text of six potentially relevant studies could not be obtained, which were therefore excluded from the analysis. Seven citations were found assessing reference lists of the included studies. One relevant article was encountered in the additional search carried out in 2016. The study selection procedure is outlined in Figure 1.
The 42 included articles consisted of 28 cohort studies[3,13,26-51] and 14 “nested” case-control studies[11,14,52-63]. Two studies were retrospective[14,52]; the other 40 studies were prospective in study design. Studies were published between 1988 and 2015, and together included 5756 patients. The development of ARDS in relation to cytokine levels was investigated in seven studies; sixteen studies determined cytokine concentrations in sepsis; MODS development was assessed in ten studies; and eleven studies reported cytokine alterations in MOF. Twenty studies investigated the relation between cytokine concentrations and mortality. Only seven studies reported a cytokine cut-off value for the development of complications, five of which stated sensitivity (and specificity) for the cut-off value. Ten studies reported some kind of prediction value for the investigated cytokines (i.e., odds ratio, area under the curve, sensitivity and specificity, 95%CI and positive/negative predictive value). All included studies are listed in Table 1. The overall study quality according to the STROBE statement was good, with a median total score of 18 points (range 12-24), suggesting a low risk of bias.
| No. | Ref. | Year | Design | No pts. (control) | Cytokines | ARDS (%) | Sepsis (%) | MODS (%) | MOF (%) | Mortality (%) |
| 1 | Billeter et al[35] | 2009 | P-coh | 1032 | IL-6 | 10% | ||||
| 2 | Bogner et al[36] | 2009 | P-coh | 58 | IL-6, -8, -10 | 74% | 19% | |||
| 3 | Cook et al[58] | 2013 | P-cc | 83 (18) | G-CSF | 7% | 7% | |||
| 4 | Cuschieri et al[34] | 2010 | P-coh | 152 | IL-6 | 37% | 5% | |||
| 5 | Donnelly et al[37] | 1994 | P-coh | 15 | IL-6, -8, -1β; TNF- | 49% | 33% | |||
| 6 | Dresing et al[26] | 2004 | P-coh | 30 | IL-6; TNF- | 13% | 19% | |||
| 7 | Egger et al[38] | 2004 | P-coh | 26 | IL-6, -8 | 35% | ||||
| 8 | Flores et al[39] | 2001 | P-coh | 43 | IL-6 | 49% | 16% | |||
| 9 | Frangen et al[59] | 2008 | P-cc | 71 (25) | IL-17, -6 | 22% | ||||
| 10 | Frank et al[11] | 2002 | P-cc | 77 (15) | IL-6, -8 | 9% | ||||
| 11 | Frink et al[3] | 2009 | P-coh | 143 | IL-1β, -6, -8, -10; TNF- | 29% | 17% | 15% | ||
| 12 | Gebhard et al[40] | 2000 | P-coh | 94 | IL-6 | 19% | ||||
| 13 | Giamarellos-Bourboulis et al[55] | 2008 | P-cc | 69 (10) | IL-6, -8; TNF-, IFN-γ | 62% | 35% | |||
| 14 | Gouel-Chéron et al[53] | 2012 | P-cc | 100 (18) | IL-6, -10 | 37% | 5% | |||
| 15 | Haasper et al[28] | 2010 | P-coh | 94 | IL-6 | 16% | 22% | 13% | ||
| 16 | Hayakawa et al[31] | 2011 | P-coh | 45 | TNF- | 53% | 25% | |||
| 17 | Heizmann et al[52] | 2008 | R-cc | 195 (10) | IL-2, -4, -10, -11, -12, -18; IFN-γ | 19% | ||||
| 18 | Jastrow et al[32] | 2009 | P-coh | 48 | IL-6, -8, -10, -1β, -2, -4, -12; TNF- | 23% | 17% | |||
| 19 | Keel et al[41] | 2009 | P-coh | 83 | IL-6 | 40% | 12% | |||
| 20 | Lausevic et al[33] | 2008 | P-coh | 65 | IL-6, -10 | 62% | 55% | 51% | ||
| 21 | Lausevic et al[29] | 2010 | P-coh | 65 | IL-6, -10 | 63% | 51% | |||
| 22 | Law et al[42] | 1994 | P-coh | 13 | IL-6, -8; TNF- | 46% | 23% | |||
| 23 | Lendemans et al[13] | 2004 | P-coh | 16 | IL-6, -10; TNF- | 56% | ||||
| 24 | Liener et al[43] | 2002 | P-coh | 94 | IL-8 | 0% | 0% | 0% | 19% | |
| 25 | Livingston et al[44] | 1988 | P-coh | 20 | IFN-γ | 30% | 15% | |||
| 26 | Maier et al[27] | 2007 | P-coh | 251 | IL-6, -8, -10 | 34% | 12% | |||
| 27 | Meade et al[45] | 1994 | P-coh | 25 | IL-6, -8; TNF- | 36% | ||||
| 28 | Menges et al[50] | 1999 | P-coh | 68 | IL-10, -1; TNF- | 25% | 25% | 1% | ||
| 29 | Mommsen et al[30] | 2009 | P-coh | 55 | IL-18 | 42% | 13% | 13% | ||
| 30 | Neidhardt et al[54] | 1997 | P-cc | 417 (137) | IL-10 | 5% | 11% | 22% | 22% | |
| 31 | Oberholzer et al[46] | 2000 | P-coh | 1276 | IL-6, IL-10 | 14% | 40% | 7% | ||
| 32 | Partrick et al[56] | 1996 | P-cc | 27 (6) | IL-6, -8 | 33% | 7% | |||
| 33 | Paunel-Görgülü et al[47] | 2011 | P-coh | 47 (17) | IL-6 | 38% | 11% | |||
| 34 | Raymondos et al[48] | 2012 | P-coh | 24 | IL-6, -8, -1β, TNF- | 29% | 4% | |||
| 35 | Roetman et al[60] | 2008 | P-cc | 229 (110) | IL-18, -4; IFN-γ | 16% | ||||
| 36 | Schinkel et al[61] | 2005 | P-cc | 216 (110) | IL-11 | 4% | 16% | |||
| 37 | Sherry et al[14] | 1996 | R-cc | 66 (10) | IL-10 | 8% | 39% | 2% | ||
| 38 | Sousa et al[51] | 2015 | P-coh | 99 | IL-6, -10; TNF- | 19% | 34% | 28% | ||
| 38 | Spielmann et al[57] | 2001 | P-cc | 47 (15) | TNF- | 11% | 30% | 51% | 23% | |
| 39 | Svoboda et al[62] | 1994 | P-cc | 42 (12) | IL-1β, -2, -6; TNF- | 33% | 26% | |||
| 40 | Wick et al[49] | 2000 | P-coh | 37 | IL-12 | 11% | 16% | |||
| 41 | Yagmur et al[63] | 2005 | P-cc | 99 (10) | IL-1, -2, -6, -8; TNF- | 17% |
IL-6: (1) ARDS; two studies[37,45] could not relate ARDS to IL-6 concentration alterations, whereas two other studies[48,51] found a positive correlation (Table 2); (2) Sepsis; five studies[35,41,46,47,53] found an increased IL-6 production to be predictive for the development of sepsis, whereas five other studies[28,29,38,39,55] did not (Table 3); (3) MODS; all five prospective cohort studies[3,28,34,46,51] concluded that IL-6 is markedly increased in the early development of MODS (Table 4); and (4) MOF; of the nine prospective studies, six[13,27,32,33,36,56] studies found a positive correlation between increased serum concentrations and development of MOF. Three[11,42,62] investigators demonstrated an elevated IL-6 in MOF patients, which was not predictive according to these studies (Table 5). Also, IL-6 tends to be higher in non-survivors (Table 6).
| Ref. | Year | Design | No pts. | ARDS n (%) | Predicts ARDS | Results |
| IL-6 | ||||||
| Donnelly et al[37] | 1994 | P-coh | 15 | 7 (49%) | N | [IL-6] is not significantly different in ARDS |
| Meade et al[45] | 1994 | P-coh | 25 | 9 (36%) | N | [IL-6] is higher in patients with ARDS after onset of symptoms; does not predict development of ARDS |
| Raymondos et al[48] | 2012 | P-coh | 24 | 7 (29%) | Y | [IL-6] is significantly higher in patients at high risk for ARDS |
| Sousa et al[51] | 2015 | P-coh | 99 | 19 (19%) | Y | [IL-6] is significantly higher at 72 h post injury |
| IL-8 | ||||||
| Donnelly et al[37] | 1994 | P-coh | 15 | 7 (49%) | Y | [IL-8] is significantly higher in patients with ARDS, starting at 16 h post injury |
| Meade et al[45] | 1994 | P-coh | 25 | 9 (36%) | N | [IL-8] is higher in patients with ARDS after onset of symptoms; does not predict development of ARDS |
| Raymondos et al[48] | 2012 | P-coh | 24 | 7 (29%) | Y | [IL-8] is significantly higher in patients at high risk for ARDS |
| IL-10 | ||||||
| Neidhardt et al[54] | 1997 | P-cc | 417 | 19 (5%) | N | [IL-10] is not related to the development of ARDS |
| Sherry et al[14] | 1996 | R-cc | 66 | 5 (8%) | N | [IL-10] is not related to the development of ARDS |
| Sousa et al[51] | 2015 | P-coh | 99 | 19 (19%) | Y | [IL-10] is significantly higher in patients with ARDS upon admission, at 24 + 48 + 72 h post injury |
| Spielmann et al[57] | 2001 | P-cc | 47 | 5 (11%) | N | [IL-10] is not related to the development of ARDS |
| TNF-α | ||||||
| Donnelly et al[37] | 1994 | P-coh | 15 | 7 (49%) | N | [TNF-α] below detection limit |
| Meade et al[45] | 1994 | P-coh | 25 | 9 (36%) | N | [TNF-α] below detection limit |
| Sousa et al[51] | 2015 | P-coh | 99 | 19 (19%) | N | [TNF-α] is not related to the development of ARDS |
| IL-1β | ||||||
| Donnelly et al[37] | 1994 | P-coh | 15 | 7 (49%) | N | [IL-1β] below detection limit |
| Meade et al[45] | 1994 | P-coh | 25 | 9 (36%) | N | [IL-1β] below detection limit |
| Ref. | Year | Design | No pts. | Sepsis n (%) | Diagnostic tests | Predicts sepsis | Results |
| IL-6 | |||||||
| Billeter et al[35] | 2009 | P-coh | 1032 | Y | [IL-6] is significantly higher in sepsis between days 3-7 | ||
| Egger et al[38] | 2004 | P-coh | 26 | 9 (35%) | N | [IL-6] is significantly higher in sepsis before clinical manifestations; does not predict sepsis | |
| Flores et al[39] | 2001 | P-coh | 43 | 21 (49%) | N | [IL-6] is not significantly altered in sepsis | |
| Giamarellos-Bourboulis et al[55] | 2008 | P-cc | 69 | 43 (62%) | ROC AUC 0.500 (95%CI: 0.304-0.696, P > 0.05) | N | [IL-6] is not related to the development of sepsis |
| Gouel-Chéron et al[53] | 2012 | P-cc | 100 | 37 (37%) | > 67.1 pg/mL: Sensitivity 85%; specificity 73% | Y | [IL-6] > 67.1 pg/mL is predictive for sepsis on days 1 + 2 (OR = 10.9) |
| Haasper et al[28] | 2010 | P-coh | 94 | 15 (16%) | N | [IL-6] is not significantly different in sepsis | |
| Keel et al[41] | 2009 | P-coh | 83 | 33 (40%) | Y | [IL-6] is significantly higher in sepsis on days 5 + 14 | |
| Lausevic et al[33] | 2010 | P-coh | 65 | 41 (63%) | N | [IL-6] is not predictive for sepsis | |
| Oberholzer et al[46] | 2000 | P-coh | 1276 | 179 (14%) | Y | [IL-6] is significantly higher in septic patients | |
| Paunel-Görgülü et al[47] | 2011 | P-coh | 47 | 18 (38%) | AUC ROC 0.79 (day 5 post injury) | Y | [IL-6] is significantly elevated on days 5 + 9 in sepsis |
| IL-8 | |||||||
| Egger et al[38] | 2004 | P-coh | 26 | 9 (35%) | N | [IL-8] is not significantly altered in sepsis | |
| Giamarellos-Bourboulis et al[55] | 2008 | P-cc | 69 | 43 (62%) | AUC ROC 0.453 (95%CI: 0.254-0.652, P > 0.05) | N | [IL-8] is not predictive for sepsis |
| IL-10 | |||||||
| Gouel-Chéron et al[53] | 2012 | P-cc | 100 | 37 (37%) | N | [IL-10] is not related to the development of sepsis | |
| Lausevic et al[33] | 2010 | P-coh | 65 | 41 (63%) | Y | [IL-10] is significantly lower in sepsis on days 1 + 2 | |
| Menges et al[50] | 1999 | P-coh | 68 | 17 (25%) | Y | [IL-10] is significantly higher in sepsis and MOF after 6 d | |
| Neidhardt et al[54] | 1997 | P-cc | 417 | 45 (11%) | Y | [IL-10] is significantly higher in sepsis on days 1 + 3 + 5 + 7 + 10 + 14 + 21 | |
| Sherry et al[14] | 1996 | R-cc | 66 | 26 (39%) | Y | [IL-10] is significantly higher in sepsis | |
| TNF-α | |||||||
| Giamarellos-Bourboulis et al[55] | 2008 | P-cc | 69 | 43 (62%) | AUC ROC 0.466 (95%CI: 0.274-0.657, P > 0.05) | N | [TNF-α] is not related to the development of sepsis |
| Menges et al[50] | 1999 | P-coh | 68 | 17 (25%) | Y | [TNF-α] is significantly higher in sepsis and MOF after 8 d | |
| IFN-γ | |||||||
| Giamarellos-Bourboulis et al[55] | 2008 | P-cc | 69 | 43 (62%) | N | [IFN-γ] below detection limit | |
| Livingston et al[44] | 1988 | P-coh | 20 | 6 (30%) | Y | [IFN-γ] is markedly lower in sepsis after 14 d | |
| G-CSF | |||||||
| Cook et al[58] | 2013 | P-cc | 83 | 6 (7%) | Y | [G-CSF] > 500 pg/mL is significantly associated with sepsis | |
| IL-18 | |||||||
| Mommsen et al[30] | 2009 | P-coh | 55 | 23 (42%) | Y | [IL-18] is significantly higher in sepsis on days 3-6 post injury | |
| IL-1 | |||||||
| Menges et al[50] | 1999 | P-coh | 68 | 17 (25%) | Y | [IL-1] is significantly higher in sepsis and MOF on days 3 + 5 + 6 + 9 - 13 | |
| Study | Year | Design | No pts. | MODSn (%) | Diagnostic tests | Predicts MODS | Results |
| IL-6 | |||||||
| Cuschieri et al[34] | 2010 | P-coh | 152 | 29 (37%) | > 350 pg/mL: Sensitivity 79%, specificity 76%; OR = 3.87 (95%CI: 1.13-11.19) | Y | [IL-6] > 350 pg/mL is highly associated with MODS |
| Frink et al[3] | 2009 | P-coh | 143 | 24 (17%) | r = 0.35; > 761.7 pg/μL: Sensitivity 16.7%, specificity 98.3% | Y | [IL-6] > 76.6 pg/μL is associated with MODS with accuracy of 84.7% |
| Haasper et al[28] | 2010 | P-coh | 94 | 21 (22%) | Y | [IL-6] is significantly higher in MODS on days 1 + 7 | |
| Oberholzer et al[46] | 2000 | P-coh | 1276 | 516 (40%) | Y | [IL-6] is significantly higher in (severe) MODS | |
| Sousa et al[51] | 2015 | P-coh | 99 | 34 (34%) | > 294 pg/mL: AUC ROC 0.769 (95%CI: 0.414-0.736) | Y | [IL-6] > 294 pg/mL is associated with MODS at 48 + 72 h post injury |
| IL-8 | |||||||
| Frink et al[3] | 2009 | P-coh | 143 | 24 (17%) | r = 0.53; sensitivity 0% | N | [IL-8] is significantly higher in MODS; does not predict development of MODS |
| IL-10 | |||||||
| Frink et al[3] | 2009 | P-coh | 143 | 24 (17%) | r = 0.31; sensitivity 0% | N | [IL-10] is significantly higher in MODS; does not predict development of MODS |
| Neidhardt et al[54] | 1997 | P-cc | 417 | 92 (22%) | Y | [IL-10] is significantly higher in MODS on days 1 + 3 + 5 + 7 + 10 + 14 + 21 post injury | |
| Spielmann et al[57] | 2001 | P-cc | 47 | 24 (51%) | N | [IL-10] is not related to the development of MODS | |
| Sousa et al[51] | 2015 | P-coh | 99 | 34 (34%) | > 4.93 pg/mL: AUC ROC 0.700 (95%CI: 0.506-0.841) | Y | [IL-10] > 4.93 pg/mL is associated with MODS at 24 + 72 h post injury |
| TNF-α | |||||||
| Frink et al[3] | 2009 | P-coh | 143 | 24 (17%) | r = 0.32; sensitivity 0% | N | [TNF-α] is significantly higher in MODS; does not predict development of MODS |
| Hayakawa et al[31] | 2010 | P-coh | 45 | 24 (53%) | Y | [TNF-α] is significantly higher in MODS on days 3 + 5 | |
| Sousa et al[51] | 2015 | P-coh | 99 | 34 (34%) | Y | [TNF-α] is associated with MODS at 48 h post injury | |
| Spielmann et al[57] | 2001 | P-cc | 47 | 24 (51%) | N | [TNF-α) is not associated with MODS | |
| IL-1β | |||||||
| Frink et al[3] | 2009 | P-coh | 143 | 24 (17%) | r = 0.00; sensitivity 0% | N | [IL-1β] is not related to development of MODS |
| IL-12 | |||||||
| Wick et al[49] | 2000 | P-coh | 37 | 4 (11%) | Y | [IL-12] is significantly lower in patients with MODS | |
| IL-18 | |||||||
| Mommsen et al[30] | 2009 | P-coh | 55 | 7 (13%) | Y | [IL-18] is significantly higher in MODS on days 2 + 3 + 6 + 7 + 9 + 10 + 13 + 14 | |
| MIF | |||||||
| Hayakawa et al[31] | 2010 | P-coh | 45 | 24 (53%) | Y | [MIF] is significantly higher in MODS | |
| Ref. | Year | Design | No pts. | MOFn (%) | Diagnostic tests | PredictsMOF | Results |
| IL-6 | |||||||
| Bogner et al[36] | 2009 | P-coh | 58 | 43 (74%) | Y | [IL-6] is significantly higher in MOF at 0 - 24 + 72 h | |
| Frank et al[11] | 2002 | P-cc | 77 | r = 0.25 on day 2 | N | [IL-6] is significantly higher in MOF; no reliable predictor due to low r | |
| Jastrow et al[32] | 2009 | P-coh | 48 | 11 (23%) | AUC ROC 0.816; (IL-6) > 0.861 pg/mL: sensitivity 57%, PPV 100% | Y | [IL-6] > 0.861 pg/mL is highly predictive for MOF |
| Lausevic et al[33] | 2008 | P-coh | 65 | 36 (55%) | Y | [IL-6] is significantly higher in MOF on all days of hospitalization | |
| Lendemans et al[13] | 2004 | P-coh | 16 | 9 (56%) | Y | [IL-6] is significantly higher in MOF after two weeks | |
| Law et al[42] | 1994 | P-coh | 13 | 6 (46%) | N | [IL-6] is elevated in MOF, does not predict MOF | |
| Maier et al[27] | 2007 | P-coh | 251 | 85 (34%) | AUC ROC 0.70 for late-onset MOF | Y | [IL-6] is predictive for (late) MOF |
| Partrick et al[56] | 1996 | P-cc | 27 | 9 (33%) | Y | [IL-6] is significantly higher in MOF at 12 + 36 h | |
| Svoboda et al[62] | 1994 | P-cc | 42 | 14 (33%) | N | [IL-6] is higher in MOF at day 1, does not predict MOF | |
| IL-8 | |||||||
| Bogner et al[36] | 2009 | P-coh | 58 | 43 (74%) | Y | [IL-8] is significantly higher in MOF from 0-72 h | |
| Frank et al[11] | 2002 | P-cc | 77 | r = 0.32 on day 2 | N | [IL-8] is significantly higher in MOF; not reliable due to low r | |
| Jastrow et al[32] | 2009 | P-coh | 48 | 11 (23%) | Y | [IL-8] is significantly higher in MOF from 0-24 h | |
| Law et al[42] | 1994 | P-coh | 13 | 6 (46%) | N | [IL-8] is elevated in MOF, does not predict MOF | |
| Maier et al[27] | 2007 | P-coh | 251 | 85 (34%) | AUC ROC 0.69 for late-onset MOF | Y | [IL-8] is predictive for (late) MOF |
| Partrick et al[56] | 1996 | P-cc | 27 | 9 (33%) | Y | [IL-8] is significantly higher in MOF at 12 + 36 + 84 h | |
| IL-10 | |||||||
| Bogner et al[36] | 2009 | P-coh | 58 | 43 (74%) | Y | [IL-10] is significantly higher in MOF in early post-injury phase (< 12 h) | |
| Jastrow et al[32] | 2009 | P-coh | 48 | 11 (23%) | AUC ROC 0.776; (IL-10) > 38.6 pg/mL: Sensitivity 71%, PPV 77% | Y | [IL-10] > 38.6 pg/mL is predictive for MOF |
| Lausevic et al[33] | 2008 | P-coh | 65 | 36 (55%) | Y | [IL-10] is significantly higher in MOF in very early post injury phase | |
| Lendemans et al[13] | 2004 | P-coh | 16 | 9 (56%) | Y | [IL-10] is significantly higher in MOF on days 3 + 4 | |
| Maier et al[27] | 2007 | P-coh | 251 | 85 (34%) | AUC ROC 0.60 for late-onset MOF | N | [IL-10) is not predictive for MOF |
| Menges et al[50] | 1999 | P-coh | 68 | 17 (25%) | Y | [IL-10] is significantly higher in sepsis and MOF after 6 d | |
| TNF-α | |||||||
| Jastrow et al[32] | 2009 | P-coh | 48 | 11 (23%) | Y | [TNF-α] is significantly higher in MOF from 2 – 6 + 10 – 24 h | |
| Lendemans et al[13] | 2004 | P-coh | 16 | 9 (56%) | Y | [TNF-α] is significantly higher in MOF on days 7 + 8 + 10 + 11 | |
| Menges et al[50] | 1999 | P-coh | 68 | 17 (25%) | Y | [TNF-α] is significantly higher in sepsis and MOF after 8 d | |
| Svoboda et al[62] | 1993 | P-cc | 42 | 14 (33%) | Y | [TNF-α] is higher in MOF, but only after onset of symptoms | |
| IL-1(β) | |||||||
| Menges et al[50] | 1999 | P-coh | 68 | 17 (25%) | Y | [IL-1] is significantly higher in sepsis and MOF on days 3 + 5 + 6 + 9 - 13 | |
| Svoboda et al[62] | 1994 | P-xx | 42 | 14 (33%) | N | [IL-1β] is not related to MOF | |
| IL-2 | |||||||
| Svoboda et al[62] | 1994 | P-cc | 42 | 14 (33%) | N | [IL-2] is not related to MOF | |
| IP-10 | |||||||
| Jastrow et al[32] | 2009 | P-coh | 48 | 11 (23%) | > 889.9 pg/mL has a sensitivity of 71% and PPV of 100% | Y | [IP-10] is highly predictive for MOF (AUC ROC 0.939) |
| Eotaxin | |||||||
| Jastrow et al[32] | 2009 | P-coh | 48 | 11 (23%) | > 193.8 pg/mL has a sensitivity of 71% and PPV of 62% | Y | [Eotaxin] is highly predictive for MOF (AUC ROC 0.810) |
| MIP-1β | |||||||
| Jastrow et al[32] | 2009 | P-coh | 48 | 11 (23%) | > 248.6 pg/mL has a sensitivity of 71% and PPV of 77% | Y | [MIP-1β] is highly predictive for MOF (AUC ROC 0.871) |
| IL-11 | |||||||
| Schinkel et al[61] | 2005 | P-cc | 216 | 9 (4%) | N | [IL-11[ is not significantly different in MOF | |
| Ref. | Design | No pts. | Mortalityn (%) | Follow-up | Diagnostic tests | Predicts mortality | Results |
| IL-6 | |||||||
| Bogner et al[36] | P-coh | 58 | 11 (19%) | 90 d | Y | [IL-6] is significantly higher in non-survivors at 0 + 6 h | |
| Cuschieri et al[34] | P-coh | 152 | 4 (5%) | In-hospital | N | [IL-6] is not significantly higher in non-survivors | |
| Dresing et al[26] | P-coh | 30 | 6 (19%) | 29 d | Y | [IL-6] is significantly higher in non-survivors on days 3 + 5 | |
| Frink et al[3] | P-coh | 143 | 21 (15%) | In-hospital | > 2176.0 pg/mL: Sensitivity 28.6%, specificity 100% on day 1 | Y | [IL-6] is highly predictive for non-survival (AUC ROC 0.858) |
| Frangen et al[59] | P-cc | 71 | 16 (22%) | In-hospital | Y | [IL-6] is significantly higher in non-survivors | |
| Gebhard et al[40] | P-coh | 94 | 18 (19%) | In-hospital | Y | [IL-6] is significantly higher in non-survivors at 4 + 6 + 12 h post injury | |
| Maier et al[27] | P-coh | 251 | 29 (12%) | In-hospital | AUC ROC 0.60 | N | [IL-6] is not predictive for non-survival |
| Sousa et al[51] | P-coh | 99 | 28 (28%) | 72 h | > 276 pg/mL: AUC ROC2 0,775 (95%CI: 0.591-0.960) | Y | [IL-6] > 276 pg/mL is significantly correlated with non-survival |
| Svoboda et al[62] | P-cc | 42 | 11 (26%) | In-hospital | > 400 pg/mL has a sensitivity of 100% | Y | [IL-6] > 400 pg/mL is significantly correlated with non-survival |
| Yagmur et al[63] | P-cc | 99 | 17 (17%) | 60 d | Y | [IL-6] is significantly elevated in non-survivors | |
| IL-8 | |||||||
| Bogner et al[36] | P-coh | 58 | 11 (19%) | 90 d | Y | [IL-8] is significantly higher in non-survivors at 6 + 24 h | |
| Liener et al[43] | P-coh | 94 | 18 (19%) | 15 d | Y | [IL-8] is significantly higher in non-survivors from 30 min-24 h | |
| Maier et al[27] | P-coh | 251 | 29 (12%) | In-hospital | AUC ROC 0.45 | N | [IL-8] is not predictive for non-survival |
| Yagmur et al[63] | P-cc | 99 | 17 (17%) | 60 d | Y | [IL-8] is significantly elevated in non-survivors | |
| IL-10 | |||||||
| Bogner et al[36] | P-coh | 58 | 11 (19%) | 90 d | Y | [IL-10] is significantly higher in non-survivors at 72 h post injury | |
| Gouel-Chéron et al[53] | P-cc | 100 | 5 (5%) | 14 d | Y | [IL-10] is significantly higher in non-survivors when detectable on days 1 + 2 | |
| Heizmann et al[52] | R-cc | 195 | 37 (19%) | 42 d | N | [IL-10] tends towards lower levels in non-survivors; not significant | |
| Maier et al[27] | P-coh | 251 | 29 (12%) | In-hospital | AUC ROC 0.51 | N | [IL-10] is not predictive for non-survival |
| Neidhardt et al[54] | P-cc | 417 | 92 (22%) | 21 d | Y | [IL-10] is significantly increased in non-survivors on days 1 + 3 | |
| Sherry et al[14] | R-cc | 66 | 1 (2%) | 50 d | N | [IL-10] is not related to non-survival | |
| Sousa et al[51] | P-coh | 99 | 28 (28%) | 72 h | > 8.24 pg/mL: AUC ROC 0.871 (95%CI: 0.715-1.000) | Y | [IL-10] > 8.24 pg/mL is associated with non-survival at 48 + 72 h post injury |
| TNF-α | |||||||
| Dresing et al[26] | P-coh | 30 | 6 (19%) | 29 d | N | [TNF-α] is not significantly elevated in non-survivors | |
| Sousa et al[51] | P-coh | 99 | 28 (28%) | 72 h | N | [TNF-α] is not significantly elevated in non-survivors | |
| Spielmann et al[57] | P-cc | 47 | 11 (23%) | 6 d | N | [TNF-α] is not significantly elevated in non-survivors | |
| Svoboda et al[62] | P-cc | 42 | 11 (26%) | In-hospital | Y | [TNF-α] is significantly elevated in non-survivors | |
| Yagmur et al[63] | P-cc | 99 | 17 (17%) | 60 d | N | [TNF-α] is not significantly elevated in non-survivors | |
| IL-18 | |||||||
| Heizmann et al[52] | R-cc | 195 | 37 (19%) | 42 d | N | [IL-18] tends towards lower levels in non-survivors; not significant | |
| Mommsen et al[30] | P-coh | 55 | 7 (13%) | 14 d | Y | [IL-18] is significantly increased in non-survivors on days 2-7 | |
| Roetman et al[60] | P-cc | 229 | 36 (16%) | 30 d | N | [IL-18] median value is significantly lower in non-survivors | |
| IL-2 | |||||||
| Heizmann et al[52] | R-cc | 195 | 37 (19%) | 42 d | N | [IL-2] tends towards lower levels in non-survivors; not significant | |
| Svoboda et al[62] | P-cc | 42 | 11 (26%) | In-hospital | N | [IL-2] is not related to non-survival | |
| Yagmur et al[63] | P-cc | 99 | 17 (17%) | 60 d | Y | [IL-2] is significantly increased in non-survivors | |
| IL-1 | |||||||
| Svoboda et al[62] | P-cc | 42 | 11 (26%) | In-hospital | N | [IL-1] is not related to non-survival | |
| Yagmur et al[63] | P-cc | 99 | 17 (17%) | 60 d | N | [IL-1] is not related to non-survival | |
| IL-12 | |||||||
| Heizmann et al[52] | R-cc | 195 | 37 (19%) | 42 d | N | [IL-12] tends towards lower levels in non-survivors; not significant | |
| Wick et al[49] | P-coh | 37 | 6 (16%) | In-hospital | Y | [IL-12] is significantly lower in non-survivors | |
| IL-11 | |||||||
| Schinkel et al[61] | P-cc | 216 | 34 (16%) | In-hospital | N | [IL-11] is lower in non-survivors, only reaching significance after week 4 | |
| Heizmann et al[52] | R-cc | 195 | 37 (19%) | 42 d | N | [IL-11] tends towards lower levels in non-survivors; not significant | |
| IL-17 | |||||||
| Frangen et al[59] | P-cc | 71 | 16 (22%) | In-hospital | N | [IL-17] is not related to non-survival | |
| IL-4 | |||||||
| Heizmann et al[52] | R-cc | 195 | 37 (19%) | 42 d | N | [IL-4] tends towards lower levels in non-survivors; not significant | |
| Roetman et al[60] | P-cc | 229 | 36 (16%) | 30 d | N | [IL-4] is not related to mortality | |
| IFN-γ | |||||||
| Heizmann et al[52] | R-cc | 195 | 37 (19%) | 42 d | N | [IFN-γ] tends towards lower levels in non-survivors; not significant | |
| Roetman et al[60] | P-cc | 229 | 36 (16%) | 30 d | N | [IFN-γ] inconsistently detectable | |
IL-8: (1) Two prospective cohort studies[37,48] reported a positive correlation between increased serum IL-8 concentrations and development of ARDS, whereas one[45] found no predictive value; (2) Two studies[38,55] reported that IL-8 was not significantly different between patients developing sepsis and those with an uneventful posttraumatic course; (3) One cohort study[3] found a higher IL-8 serum concentration in patients with MODS, which could however not predict the development of multiorgan dysfunction; and (4) Of the six included studies, four prospective studies[27,32,36,56] concluded that IL-8 is significantly higher in MOF. Two prospective studies[11,42] also found a significantly increased serum concentration, but concluded that this could not be translated into a predictive value for adverse outcome. Further, IL-8 concentrations seemed elevated in non-survivors.
IL-10: (1) Three studies, two prospective[54,57] and one retrospective[14], could not relate the serum IL-10 concentrations to the development of ARDS. One study[51] found IL-10 to be significantly higher in patients with ARDS; (2) Of the five reviewed studies, three prospective[29,50,54] and one retrospective study[14] found the IL-10 concentration to be predictive for the development of sepsis, whereas one prospective study[53] did not; (3) Two studies[51,54] reported IL-10 to be significantly elevated in patients with MODS, and two studies[3,57] could not find an association between the cytokine and development of MODS; and (4) According to five studies[13,32,33,36,50] the serum IL-10 concentration was significantly higher in MOF patients. One study showed no significant elevation[27].
TNF-α: (1) Three studies found no relation between TNF-α and development of ARDS[37,45,51]; (2) One study[55] concluded that concentrations were not related to development of sepsis, while one study[50] found significantly increased concentrations in septic patients; (3) Of the four studies reporting on TNF-α concentrations after trauma, two studies[31,51] found TNF-α to be related to the development of MODS, and two studies[3,57] could not relate serum concentrations to MODS; and (4) Four studies[13,32,50,62] showed that patients with MOF had significantly higher TNF-α concentrations compared to patients with uneventful course, although Svoboda et al[62] found no predictive value for the cytokine.
Polytraumatized patients are at risk for the development of various complications, leading to considerable morbidity and mortality. Early identification of “high risk” patients could improve outcome after accidental injury, because physicians are directed to the appropriate treatment. Further, close monitoring of the immune response could direct physicians to the appropriate timing of surgical interventions, thereby reducing “second hits” with subsequent development of sepsis and organ failure. The aim of the present review was to summarize the knowledge on cytokines predicting the development of ARDS, sepsis, MODS, MOF and mortality. According to the investigated studies, some cytokines seem to predict specific complications: Patients with ARDS seem to have higher IL-8 concentrations; IL-10 secretion seems increased in septic patients; and MODS/MOF development is preceded by an enhanced IL-6, IL-8, IL-10, and TNF-α release. With respect to the other cytokines studied (IFN-γ, G-CSF, IL-1β, -2, -4, -11, -12, -17, -18, MIF, MIP-1β, eotaxin, IP-10), study results are either inconsistent, or the small amount of current evidence makes an objective conclusion for the present study impossible.
Release of IL-6 is enhanced after stimulation by micro-organisms and cytokines (TNF-α, IL-1β)[7,8]. It is liberated after tissue damage and infection. The relatively late release and long half-life of IL-6 renders the cytokine a convenient parameter for clinical monitoring of the immune response of individual patients. The conflicting results of the reviewed studies lead to the conclusion that IL-6 cannot be used as a marker for ARDS and sepsis; elevated IL-6 concentrations do appear to precede the development of MODS, MOF and mortality. In future, physicians might therefore use IL-6 as a predictor of MODS, MOF and mortality in polytraumatized patients.
IL-8 induces expression of adhesion molecules, thereby enabling migration of neutrophils to the site of production[4,9]. Production of IL-8 takes place early in the inflammatory response and can persist for days or weeks[13]. According to the reviewed studies, IL-8 is higher in patients developing ARDS, MOF and in non-survivors. Of note, when IL-8 is used to investigate the development of ARDS, measuring local concentrations in bronchoalveolar lavage fluid generally leads to earlier identification of patients at risk[64-67]. The causal relation between the chemotaxis IL-8 exerts on PMN’s, and subsequent autodestructive changes in remote organs leading to ARDS and MOF[64], likely explains the consistent results of included studies. In line with these results, IL-8 might be used to identify patients prone to develop ARDS and MOF. Such a predictive value could not be demonstrated for the development of sepsis and MODS.
IL-10 decreases cytokine production of TH1 cells and reduces antigen presentation of macrophages and subsequent proliferation of T lymphocytes[14]. Release of high amounts of IL-10 occurs rapidly, generally within 60 min after trauma[54]. According to our study, an enhanced IL-10 secretion is related to the development of sepsis and MOF. Clearly, a vigorous anti-inflammatory IL-10 release makes the host susceptible to infections with subsequent sepsis and (sepsis-related) MOF. Therefore, IL-10 concentrations might direct physicians to the patients prone to develop sepsis and MOF. Concentrations of IL-10 could not be related to the development of ARDS, MODS and mortality.
TNF-α
The pro-inflammatory cytokine TNF-α is one of the first cytokines to be released after trauma[4]. Peak concentrations of TNF-α can be observed within one to two hours after trauma. Previous studies have demonstrated a positive correlation between elevated TNF-α and poor outcome[68-70]. However, as reported in this review, the elevation of TNF-α could only be related to the development of MOF. This might be explained by the very short half-time of the cytokine (14-18 min), suggesting that peak concentrations early in the posttraumatic course have already returned to baseline by the time a septic event and subsequent organ failure is recognized[2,9,13].
According to Cook et al[58], elevation of G-CSF significantly related to the development of hospital-acquired pneumonia. Wick et al[49] demonstrated that all patients with continuous decreased IL-12 levels died from septic MOF; comparable findings were demonstrated by Hensler et al[71]. Increased IL-12 production could, however, have unfavorable effects as well[72,73]. According to previous studies, IL-18 release is significantly correlated with sepsis, and its activation might be enhanced after infiltration of micro-organisms[74,75]. This effect could also be demonstrated by Mommsen et al[30]. Jastrow et al[32] determined a predictive value for several cytokines, among which IP-10, MIP-1β and eotaxin appear to be most accurate. More research has to be done before the value of these cytokines can be reviewed.
The principal limitation in this study was the heterogeneity across studies in terms of patient population, study design and statistical techniques used. Hence, meta-analysis of presented data could not be performed. Further, variations between patients in an individual study can result from differences in injury severity or injury pattern, diverse individual immunologic responses (gene polymorphisms), and general confounders such as age, sex, pre-existing diseases, number and amount of administrated therapeutic agents and secondary surgery. These aspects were not clearly outlined in most of the included studies. All these factors may alter the individual inflammatory response, and contribute to a low correlation between investigated cytokine and certain complication. Further, only a small amount of studies for each biomarker-complication combination was selected, due to the very specific research question. This made it difficult to draw clear conclusions from presented results. Also, some studies reported predictive values for the ratio of different cytokines. According to these studies, complications could be predicted more accurately when combining several cytokines in one prediction model. However, we could not include these findings in our results because of the small amount of studies investigating these specific ratios. Additionally, systemic concentrations of cytokines not necessarily reflect concentrations in end-organs. It might therefore be well possible that local concentrations of cytokines can more accurately predict the development of complications. Despite these concerns, the results presented in this review can be useful in the clinical appraisal of critically ill patients. For future studies on cytokines and polytrauma patients, we recommend the development of specific polytrauma protocols. Implementation of such protocols provides the possibility for meta-analysis in the future, as previously mentioned confounding factors would then be handled similarly. Important confounding factors that most studies did not elaborate on, include amount of resuscitation fluids administered, length of mechanical ventilation, need for nutritional support and secondary surgery. Monitoring cytokine secretion patterns without considering these factors, would give an unrealistic representation of posttraumatic immune alterations. Therefore, more research is needed to better understand the specific role of these factors in the individual immune response to trauma.
In conclusion, this article provides an overview of the results from literature concerning posttraumatic immune alterations leading to various complications and death. According to the current review, cytokine secretion patterns are different for patients developing complications, compared to patients with an uneventful posttraumatic course. Some of these cytokines, such as IL-6, IL-8 and IL-10, seem to be of value in the prediction of secondary deleterious effects after trauma. Close monitoring of these cytokines could direct physicians to the appropriate therapy of “high risk” patients, thereby reducing morbidity and mortality after polytrauma.
Severe trauma represents the most frequent cause of death in people below the age of 45. Early identification of patients at risk for developing complications is one of the most challenging problems in the treatment of multiple injuries. Close monitoring of cytokine secretion patterns may provide physicians with an impression of the patients’ risk for developing complications. Further, cytokine secretion patterns may pose an indication for the appropriate prophylactic treatment, as well as optimal timing of surgical interventions, thereby reducing the risk of sepsis and multiorgan failure. The aim of the current review was: (1) to summarize the available knowledge on specific cytokines that are involved in the posttraumatic immune alterations; and (2) to assess the value of cytokines for predicting the development of acute respiratory distress syndrome, sepsis, muli-organ dysfunction syndrome, multi-organ failure and mortality.
Polytraumatized patients that survive the initial impact of trauma, are confronted with an enormous host defence reaction, which is associated with morbidity and mortality. Over the past 20-25 years, cytokines have gained attention in the understanding of the posttraumatic pathophysiological immune alterations. Cytokines play a pivotal role in the pro- and anti-inflammatory reaction to trauma, and are essential in the subsequent defence and repair mechanisms. As cytokines serve as messenger molecules in cell-to-cell communication, they are likely to play an important role in the development of posttraumatic complications such as sepsis and multi organ failure.
Previous studies have acknowledged the correlation between cytokine concentrations and patients’ clinical condition after polytrauma. Yet, specific predictors for the development of posttraumatic complications have not been identified. The available literature concerning the relation between cytokine concentrations and development of posttraumatic complications was systematically reviewed by the authors, and the data were extracted using a standardized collection tool.
This review suggests that interleukin (IL)-6, IL-8 and IL-10 are of value in the prediction of secondary deleterious effects after trauma. Close monitoring of these cytokines could direct physicians to the appropriate therapy of “high risk” patients, thereby reducing morbidity and mortality after polytrauma.
SIRS: Systemic inflammatory response syndrome, defined according to the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference 1992; ARDS: Acute respiratory distress syndrome, determined in concordance with the American-European Consensus Conference 1994 definitions; Sepsis: Diagnosed when SIRS occurs in combination with a septic focus or positive blood culture; MODS and MOF: Multi-organ dysfunction syndrome/multi-organ failure, diagnosed based on different scoring systems.
This is an excellent literature analysis on an important issue. The paper was very well-structured and written.
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cotogni P, Wang Y S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Flohé S, Flohé SB, Schade FU, Waydhas C. Immune response of severely injured patients--influence of surgical intervention and therapeutic impact. Langenbecks Arch Surg. 2007;392:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009;40:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Frink M, van Griensven M, Kobbe P, Brin T, Zeckey C, Vaske B, Krettek C, Hildebrand F. IL-6 predicts organ dysfunction and mortality in patients with multiple injuries. Scand J Trauma Resusc Emerg Med. 2009;17:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Hietbrink F, Koenderman L, Rijkers G, Leenen L. Trauma: the role of the innate immune system. World J Emerg Surg. 2006;1:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 572] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 6. | Tschoeke SK, Ertel W. Immunoparalysis after multiple trauma. Injury. 2007;38:1346-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Ciriello V, Gudipati S, Stavrou PZ, Kanakaris NK, Bellamy MC, Giannoudis PV. Biomarkers predicting sepsis in polytrauma patients: Current evidence. Injury. 2013;44:1680-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Giannoudis PV, Hildebrand F, Pape HC. Inflammatory serum markers in patients with multiple trauma. Can they predict outcome? J Bone Joint Surg Br. 2004;86:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Hildebrand F, Pape HC, Krettek C. The importance of cytokines in the posttraumatic inflammatory reaction. Unfallchirurg. 2005;108:793-794, 796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Sears BW, Stover MD, Callaci J. Pathoanatomy and clinical correlates of the immunoinflammatory response following orthopaedic trauma. J Am Acad Orthop Surg. 2009;17:255-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Frank J, Maier M, Koenig J, Rose S, Bouma M, Buurman WA, Marzi I. Circulating inflammatory and metabolic parameters to predict organ failure after multiple trauma. Eur J Trauma. 2002;28:333-339. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | DeLong WG, Born CT. Cytokines in patients with polytrauma. Clin Orthop Relat Res. 2004;57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Lendemans S, Kreuzfelder E, Waydhas C, Nast-Kolb D, Flohé S. Clinical course and prognostic significance of immunological and functional parameters after severe trauma. Unfallchirurg. 2004;107:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Sherry RM, Cue JI, Goddard JK, Parramore JB, DiPiro JT. Interleukin-10 is associated with the development of sepsis in trauma patients. J Trauma. 1996;40:613-666; discussion 616-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Keel M, Schregenberger N, Steckholzer U, Ungethüm U, Kenney J, Trentz O, Ertel W. Endotoxin tolerance after severe injury and its regulatory mechanisms. J Trauma. 1996;41:430-47; discussion 430-437; 437-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17510] [Article Influence: 1094.4] [Reference Citation Analysis (1)] |
| 17. | Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 481] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6211] [Cited by in RCA: 6521] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 19. | Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1949] [Cited by in RCA: 1744] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 20. | Goris RJ, te Boekhorst TP, Nuytinck JK, Gimbrère JS. Multiple-organ failure. Generalized autodestructive inflammation? Arch Surg. 1985;120:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 629] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 21. | Sauaia A, Moore FA, Moore EE, Norris JM, Lezotte DC, Hamman RF. Multiple organ failure can be predicted as early as 12 hours after injury. J Trauma. 1998;45:291-301; discussion 301-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 144] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Moore FA, Moore EE, Poggetti R, McAnena OJ, Peterson VM, Abernathy CM, Parsons PE. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J Trauma. 1991;31:629-636; discussion 636-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 315] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Lefering R, Goris RJ, van Nieuwenhoven EJ, Neugebauer E. Revision of the multiple organ failure score. Langenbecks Arch Surg. 2002;387:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Grotz M, von Griensven M, Stalp M, Kaufmann U, Hildebrand F, Pape HC. Scoring multiple organ failure after severe trauma. Comparison of the Goris, Marshall and Moore scores. Chirurg. 2001;72:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5754] [Cited by in RCA: 9899] [Article Influence: 582.3] [Reference Citation Analysis (0)] |
| 26. | Dresing K, Armstrong VW, Leip CL, Streit F, Burchardi H, Stürmer KM, Oellerich M. Real-time assessment of hepatic function is related to clinical outcome in critically ill patients after polytrauma. Clin Biochem. 2007;40:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Maier B, Lefering R, Lehnert M, Laurer HL, Steudel WI, Neugebauer EA, Marzi I. Early versus late onset of multiple organ failure is associated with differing patterns of plasma cytokine biomarker expression and outcome after severe trauma. Shock. 2007;28:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Haasper C, Kalmbach M, Dikos GD, Meller R, Müller C, Krettek C, Hildebrand F, Frink M. Prognostic value of procalcitonin (PCT) and/or interleukin-6 (IL-6) plasma levels after multiple trauma for the development of multi organ dysfunction syndrome (MODS) or sepsis. Technol Health Care. 2010;18:89-100. [PubMed] |
| 29. | Lausević Z, Vuković G, Stojimirović B, Trbojević-Stanković J, Resanović V, Lausevic M. Kinetics of C-reactive protein, interleukin-6 and -10, and phospholipase A2-II in severely traumatized septic patients. Vojnosanit Pregl. 2010;67:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Mommsen P, Frink M, Pape HC, van Griensven M, Probst C, Gaulke R, Krettek C, Hildebrand F. Elevated systemic IL-18 and neopterin levels are associated with posttraumatic complications among patients with multiple injuries: a prospective cohort study. Injury. 2009;40:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Hayakawa M, Katabami K, Wada T, Minami Y, Sugano M, Shimojima H, Kubota N, Uegaki S, Sawamura A, Gando S. Imbalance between macrophage migration inhibitory factor and cortisol induces multiple organ dysfunction in patients with blunt trauma. Inflammation. 2011;34:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Jastrow KM, Gonzalez EA, McGuire MF, Suliburk JW, Kozar RA, Iyengar S, Motschall DA, McKinley BA, Moore FA, Mercer DW. Early cytokine production risk stratifies trauma patients for multiple organ failure. J Am Coll Surg. 2009;209:320-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Lausevic Z, Lausevic M, Trbojevic-Stankovic J, Krstic S, Stojimirovic B. Predicting multiple organ failure in patients with severe trauma. Can J Surg. 2008;51:97-102. [PubMed] |
| 34. | Cuschieri J, Bulger E, Schaeffer V, Sakr S, Nathens AB, Hennessy L, Minei J, Moore EE, O’Keefe G, Sperry J. Early elevation in random plasma IL-6 after severe injury is associated with development of organ failure. Shock. 2010;34:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Billeter A, Turina M, Seifert B, Mica L, Stocker R, Keel M. Early serum procalcitonin, interleukin-6, and 24-hour lactate clearance: useful indicators of septic infections in severely traumatized patients. World J Surg. 2009;33:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Bogner V, Keil L, Kanz KG, Kirchhoff C, Leidel BA, Mutschler W, Biberthaler P. Very early posttraumatic serum alterations are significantly associated to initial massive RBC substitution, injury severity, multiple organ failure and adverse clinical outcome in multiple injured patients. Eur J Med Res. 2009;14:284-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Donnelly TJ, Meade P, Jagels M, Cryer HG, Law MM, Hugli TE, Shoemaker WC, Abraham E. Cytokine, complement, and endotoxin profiles associated with the development of the adult respiratory distress syndrome after severe injury. Crit Care Med. 1994;22:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 108] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Egger G, Aigner R, Glasner A, Hofer HP, Mitterhammer H, Zelzer S. Blood polymorphonuclear leukocyte migration as a predictive marker for infections in severe trauma: comparison with various inflammation parameters. Intensive Care Med. 2004;30:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Flores JM, Jiménez PI, Rincón MD, Márquez JA, Navarro H, Arteta D, Murillo F. Early risk factors for sepsis in patients with severe blunt trauma. Injury. 2001;32:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Gebhard F, Pfetsch H, Steinbach G, Strecker W, Kinzl L, Brückner UB. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 242] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 41. | Keel M, Härter L, Reding T, Sun LK, Hersberger M, Seifert B, Bimmler D, Graf R. Pancreatic stone protein is highly increased during posttraumatic sepsis and activates neutrophil granulocytes. Crit Care Med. 2009;37:1642-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Law MM, Cryer HG, Abraham E. Elevated levels of soluble ICAM-1 correlate with the development of multiple organ failure in severely injured trauma patients. J Trauma. 1994;37:100-109; discussion 109-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Liener UC, Brückner UB, Knöferl MW, Steinbach G, Kinzl L, Gebhard F. Chemokine activation within 24 hours after blunt accident trauma. Shock. 2002;17:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Livingston DH, Appel SH, Wellhausen SR, Sonnenfeld G, Polk HC. Depressed interferon gamma production and monocyte HLA-DR expression after severe injury. Arch Surg. 1988;123:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 134] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Meade P, Shoemaker WC, Donnelly TJ, Abraham E, Jagels MA, Cryer HG, Hugli TE, Bishop MH, Wo CC. Temporal patterns of hemodynamics, oxygen transport, cytokine activity, and complement activity in the development of adult respiratory distress syndrome after severe injury. J Trauma. 1994;36:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48:932-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 170] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 47. | Paunel-Görgülü A, Flohé S, Scholz M, Windolf J, Lögters T. Increased serum soluble Fas after major trauma is associated with delayed neutrophil apoptosis and development of sepsis. Crit Care. 2011;15:R20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Raymondos K, Martin MU, Schmudlach T, Baus S, Weilbach C, Welte T, Krettek C, Frink M, Hildebrand F. Early alveolar and systemic mediator release in patients at different risks for ARDS after multiple trauma. Injury. 2012;43:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Wick M, Kollig E, Walz M, Muhr G, Köller M. [Does liberation of interleukin-12 correlate with the clinical course of polytraumatized patients?]. Chirurg. 2000;71:1126-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Menges T, Engel J, Welters I, Wagner RM, Little S, Ruwoldt R, Wollbrueck M, Hempelmann G. Changes in blood lymphocyte populations after multiple trauma: association with posttraumatic complications. Crit Care Med. 1999;27:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | Sousa A, Raposo F, Fonseca S, Valente L, Duarte F, Gonçalves M, Tuna D, Paiva JA. Measurement of cytokines and adhesion molecules in the first 72 hours after severe trauma: association with severity and outcome. Dis Markers. 2015;2015:747036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Heizmann O, Koeller M, Muhr G, Oertli D, Schinkel C. Th1- and Th2-type cytokines in plasma after major trauma. J Trauma. 2008;65:1374-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Gouel-Chéron A, Allaouchiche B, Guignant C, Davin F, Floccard B, Monneret G. Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: a powerful association to predict the development of sepsis after major trauma. PLoS One. 2012;7:e33095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 54. | Neidhardt R, Keel M, Steckholzer U, Safret A, Ungethuem U, Trentz O, Ertel W. Relationship of interleukin-10 plasma levels to severity of injury and clinical outcome in injured patients. J Trauma. 1997;42:863-870; discussion 870-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 154] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Giamarellos-Bourboulis EJ, Mouktaroudi M, Tsaganos T, Koutoukas P, Spyridaki E, Pelekanou A, Kotzampassi K. Evidence for the participation of soluble triggering receptor expressed on myeloid cells-1 in the systemic inflammatory response syndrome after multiple trauma. J Trauma. 2008;65:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Partrick DA, Moore FA, Moore EE, Biffl WL, Sauaia A, Barnett CC. Jack A. Barney Resident Research Award winner. The inflammatory profile of interleukin-6, interleukin-8, and soluble intercellular adhesion molecule-1 in postinjury multiple organ failure. Am J Surg. 1996;172:425-429; discussed 429-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Spielmann S, Kerner T, Ahlers O, Keh D, Gerlach M, Gerlach H. Early detection of increased tumour necrosis factor alpha (TNFalpha) and soluble TNF receptor protein plasma levels after trauma reveals associations with the clinical course. Acta Anaesthesiol Scand. 2001;45:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Cook KM, Sifri ZC, Baranski GM, Mohr AM, Livingston DH. The role of plasma granulocyte colony stimulating factor and bone marrow dysfunction after severe trauma. J Am Coll Surg. 2013;216:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Frangen TM, Bogdanski D, Schinkel C, Roetman B, Kälicke T, Muhr G, Köller M. Systemic IL-17 after severe injuries. Shock. 2008;29:462-467. [PubMed] |
| 60. | Roetman B, Schinkel C, Wick M, Frangen T, Muhr G, Köller M. Elevated systemic interleukin-18 in multiple injured patients is not related to clinical outcome. J Interferon Cytokine Res. 2008;28:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Schinkel C, Wick M, Muhr G, Köller M. Analysis of systemic interleukin-11 after major trauma. Shock. 2005;23:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Svoboda P, Kantorová I, Ochmann J. Dynamics of interleukin 1, 2, and 6 and tumor necrosis factor alpha in multiple trauma patients. J Trauma. 1994;36:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Yagmur Y, Ozturk H, Unaldi M, Gedik E. Relation between severity of injury and the early activation of interleukins in multiple-injured patients. Eur Surg Res. 2005;37:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Muehlstedt SG, Lyte M, Rodriguez JL. Increased IL-10 production and HLA-DR suppression in the lungs of injured patients precede the development of nosocomial pneumonia. Shock. 2002;17:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, Grant IS, Pollok AJ, Haslett C. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet. 1993;341:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 470] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 66. | Aggarwal A, Baker CS, Evans TW, Haslam PL. G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur Respir J. 2000;15:895-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 67. | Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992;145:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 367] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 68. | Strieter RM, Kunkel SL, Bone RC. Role of tumor necrosis factor-alpha in disease states and inflammation. Crit Care Med. 1993;21:S447-S463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 286] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 69. | Ozturk H, Yagmur Y, Ozturk H. The prognostic importance of serum IL-1beta, IL-6, IL-8 and TNF-alpha levels compared to trauma scoring systems for early mortality in children with blunt trauma. Pediatr Surg Int. 2008;24:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Zedler S, Faist E. The impact of endogenous triggers on trauma-associated inflammation. Curr Opin Crit Care. 2006;12:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 71. | Hensler T, Heidecke CD, Hecker H, Heeg K, Bartels H, Zantl N, Wagner H, Siewert JR, Holzmann B. Increased susceptibility to postoperative sepsis in patients with impaired monocyte IL-12 production. J Immunol. 1998;161:2655-2659. [PubMed] |
| 72. | Car BD, Eng VM, Schnyder B, LeHir M, Shakhov AN, Woerly G, Huang S, Aguet M, Anderson TD, Ryffel B. Role of interferon-gamma in interleukin 12-induced pathology in mice. Am J Pathol. 1995;147:1693-1707. [PubMed] |
| 73. | Ryffel B. Interleukin-12: role of interferon-gamma in IL-12 adverse effects. Clin Immunol Immunopathol. 1997;83:18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Emmanuilidis K, Weighardt H, Matevossian E, Heidecke CD, Ulm K, Bartels H, Siewert JR, Holzmann B. Differential regulation of systemic IL-18 and IL-12 release during postoperative sepsis: high serum IL-18 as an early predictive indicator of lethal outcome. Shock. 2002;18:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Oberholzer A, Steckholzer U, Kurimoto M, Trentz O, Ertel W. Interleukin-18 plasma levels are increased in patients with sepsis compared to severely injured patients. Shock. 2001;16:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |