Published online Feb 4, 2015. doi: 10.5492/wjccm.v4.i1.77
Peer-review started: October 21, 2014
First decision: November 27, 2014
Revised: December 16, 2014
Accepted: January 9, 2015
Article in press: January 12, 2015
Published online: February 4, 2015
Processing time: 116 Days and 14.3 Hours
AIM: To investigate the diagnostic yield, therapeutic efficacy, and rate of adverse events related to flexible fiberoptic bronchoscopy (FFB) in critically ill children.
METHODS: We searched PubMed, SCOPUS, OVID, and EMBASE databases through July 2014 for English language publications studying FFB performed in the intensive care unit in children < 18 years old. We identified 666 studies, of which 89 full-text studies were screened for further review. Two reviewers independently determined that 27 of these studies met inclusion criteria and extracted data. We examined the diagnostic yield of FFB among upper and lower airway evaluations, as well as the utility of bronchoalveolar lavage (BAL).
RESULTS: We found that FFB led to a change in medical management in 28.9% (range 21.9%-69.2%) of critically ill children. The diagnostic yield of FFB was 82% (range 45.2%-100%). Infectious organisms were identified in 25.7% (17.6%-75%) of BALs performed, resulting in a change of antimicrobial management in 19.1% (range: 12.2%-75%). FFB successfully re-expanded atelectasis or removed mucus plugs in 60.3% (range: 23.8%-100%) of patients with atelectasis. Adverse events were reported in 12.9% (range: 0.5%-71.4%) of patients. The most common adverse effects of FFB were transient hypotension, hypoxia and/or bradycardia that resolved with minimal intervention, such as oxygen supplementation or removal of the bronchoscope. Serious adverse events were uncommon; 2.1% of adverse events required intervention such as bag-mask ventilation or intubation and atropine for hypoxia and bradycardia, normal saline boluses for hypotension, or lavage and suctioning for hemorrhage.
CONCLUSION: FFB is safe and effective for diagnostic and therapeutic use in critically ill pediatric patients.
Core tip: Flexible fiberoptic bronchoscopy (FFB) is effective and safe for diagnostic and therapeutic use among critically ill pediatric patients. FFB led to change in management in 28.9% of patients, with a diagnostic yield of 82%. Bronchoalveolar lavage obtained during FFB may assist with identifying infectious organisms (25.7%) and optimizing antimicrobial therapy (19.1%). FFB had therapeutic benefit with removal of mucus plugs or resolution of atelectasis in 60.3%. The majority of reported adverse events were transient and included hypotension, hypoxia and/or bradycardia requiring minimal intervention.
- Citation: Field-Ridley A, Sethi V, Murthi S, Nandalike K, Li STT. Utility of flexible fiberoptic bronchoscopy for critically ill pediatric patients: A systematic review. World J Crit Care Med 2015; 4(1): 77-88
- URL: https://www.wjgnet.com/2220-3141/full/v4/i1/77.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v4.i1.77
Flexible fiberoptic bronchoscopy (FFB) is recognized as an essential tool to diagnose and treat pediatric pulmonary disorders. Even though the first published report on the utility of FFB in children was in 1978, rigid bronchoscopy by surgeons remained standard of practice for many years due to instrument size limitations[1,2]. With the advent of smaller-sized bronchoscopes, FFB use has increased in pediatric and neonatal patients[3-6].
In 1987, the first published FFB guideline for adults provided recommendations for the use of bronchoscopy for diagnosis and management of a broad spectrum of inflammatory, infectious, and malignant diseases[7]. Updated guidelines published by the British Thoracic Society further defined the indications, patient selection criteria, and potential adverse events in adult bronchoscopy[8]. However, the guidelines for adult FFB cannot necessarily be extrapolated to children given the smaller airways, differences in pulmonary diagnoses, and sedation needs for FFB in children. Guidelines about the use of FFB in pediatric patients are over a decade old[9,10]. Despite increased use of FFB by pediatric pulmonologists, intensivists and anesthesiologists, there are no current guidelines regarding the safety and utility of FFB in the pediatric critically ill population.
Our objective was to systematically review the published literature on the utility and safety of FFB in pediatric and neonatal intensive care settings. Our specific questions were: (1) what is the diagnostic yield of FFB; (2) what is the therapeutic efficacy of FFB; and (3) what is the rate of adverse events secondary to FFB?
This systematic review was conducted according to PRISMA guidelines[11]. The protocol for our study was registered online at PROSPERO (CRD42014010801)[12]. The National Library of Medicine through PubMed was searched for “bronchoscopy” (MeSH and all fields) and “intensive care units” (MeSH and all fields) and English and “journal article” AND infant (MeSH) or child (MeSH) or adolescent (MeSH). In addition, we searched the following databases for the terms “bronchoscopy” and “intensive care unit” and (infant or child or adolescent) and “journal article” and English language: SCOPUS, OVID, and EMBASE. Our search strategy included studies published in English from database inception to July 20, 2014. References of identified articles were searched for additional relevant articles.
Articles eligible for inclusion were English-language manuscripts reporting either diagnostic, therapeutic or adverse events related to FFB performed on children (< 18 years old) in intensive care units (ICUs). Cohort, case control, or randomized controlled trials that reported either diagnostic, therapeutic, or adverse events related to FFB were included. Articles focusing on bronchoscopy in patients with foreign body aspiration were excluded, as rigid bronchoscopy is indicated for removal of foreign bodies[9]. For the purposes of this systematic review, we defined a positive diagnostic FFB as one identifying anatomic or functional airway abnormality, foreign body/obstruction, mucus plugging/atelectasis, hemorrhage, and/or airway inflammation.
One author (SM) screened article titles for initial inclusion. Two authors (SM and SL) independently screened abstracts in duplicate for inclusion. All authors (SM, SL, AF, VS and KN) piloted the standardized electronic data extraction form on two articles. Two authors independently assessed each article for study eligibility and extracted data. Data extracted included study design, participant demographics, and bronchoscopy outcomes (including diagnostic results, change in therapy, bronchoalveolar lavage (BAL) results, ICU length of stay, hospital length of stay, length of mechanical ventilation, rate of successful extubation, and adverse events). Risk of bias was not assessed. Discrepancies were resolved after joint article review and discussion. Results were presented as a narrative synthesis. Pooled estimates of diagnostic yield, therapeutic efficacy, and adverse events were estimated as weighted averages with weights proportional to study denominators from the relevant subpopulations, making the assumption that study-specific proportions are homogeneous. No formal tests for homogeneity were conducted in light of the wide variation in denominator counts, including very small studies[13].
The statistical methods of this study were reviewed by Daniel J. Tancredi, PhD, from the University of California Davis.
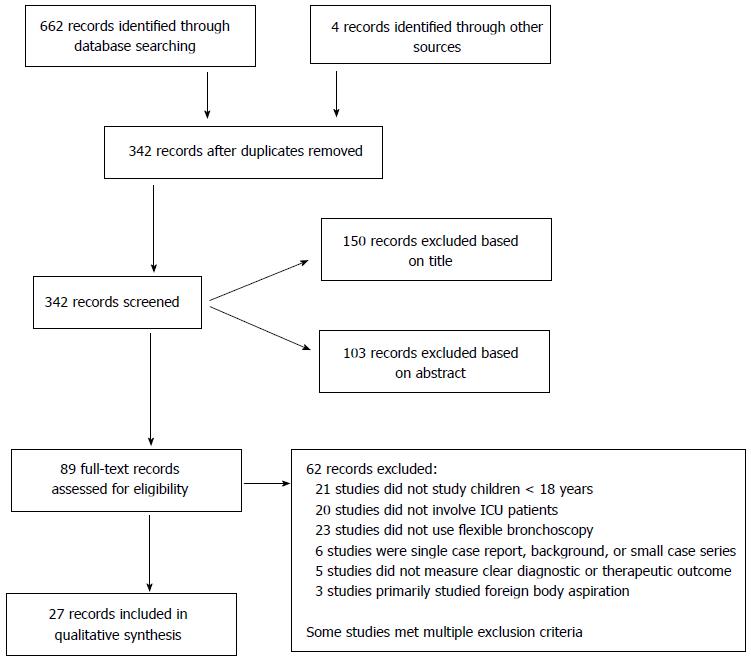
We identified 666 studies, of which 89 full-text studies were screened for further review. Two reviewers independently determined that 27 of these studies met inclusion criteria (Figure 1).
Two-thirds of the included studies were retrospective cohort, the remainder consisted of case control or prospective cohort studies (Table 1). Sixteen studies (59%) investigated patients admitted to a pediatric intensive care unit (PICU), eight studies (30%) investigated neonatal intensive care Unit (NICU) patients, while three (11%) included both PICU and NICU patients. Almost all FFB were performed at the bedside, with the exception of routine evaluation for esophageal atresia, where the procedure took place in the operating room[14]. The patient populations undergoing FFB included patients evaluated for a spectrum of anatomic airway or intrinsic pulmonary abnormalities, including patients with congenital heart disease (CHD) (7/27; 26% studies) and patients on extracorporeal life support (ECLS) (4/27; 15% studies)[4,15-23]. FFB was performed multiple times on patients in 55% of the studies.
| Ref. | Population | Indications | Diagnostic yield | Diagnostic BAL findings | Therapeutic outcomes |
| Abu-Kishk et al[25], 2012 | 9 PICU: hemoptysis (age 2 mo-17 yr) | Hemoptysis | 77.8% (7/9) | ||
| Atzori et al[14], 2006 | 62 NICU: esophageal atresia (mean age 37.5 WGA) | Airway evaluation | 24.2% (15/62): Change in surgical management 9.7% (6/62): Change in anatomic class 11.3% (7/62): Tracheomalacia | ||
| Bar-Zohar et al[24], 2004 | 100 PICU: medical, non-airway surgery, and airway surgery groups (age 2 d-17 yr) | Airway evaluation; BAL; extubation failure | 73% (65/89): Upper airway 56% (14/25): Lower airway 63.6% (28/44): Extubation failure 38.6% (44/114): Change in medical management 20% (11/31): Airway surgical re-exploration | 46.7% (14/30) identified organism 50% (15/30) change in antimicrobials 40% (12/30) clinical improvement after change in antimicrobials 36.4% (4/11) concordance between BAL and blind tracheal aspirate | 84.6% (11/13) extubated after lavage 74.3% (26/35) re-expanded collapsed lobe |
| Chapotte et al[18], 1998 | 72 PICU: CHD (age 1 d-14 yr) | Perioperative evaluation; respiratory symptoms; radiologic respiratory signs | 70.8% (51/72) 48.6% (35/72) identified extra-luminal compression | 33.3% (2/6) identified organisms in patients with mucosal inflammation | |
| Davidson et al[17], 2008 | 129 PICU: ECLS, CHD (age 2.9 mo-3 yr) | Airway evaluation; atelectasis; BAL; ETT position; respiratory distress | 68.4% (78/114): Overall 46.3% (37/80): ECLS 60.3% (41/68): CHD identified extra-luminal compression | 45.3% (53/117): Overall identified organism 53.8% (28/52): ECLS subgroup identified organism | 82.1% (32/39) successful procedures: removed blood and mucous plugs, or instilled surfactant, placed endovascular stents |
| de Blic et al[4], 1991 | 33 NICU: CHD, lung disease and/or congenital malformations (age 2 d-9 mo) | Anatomic evaluation; atelectasis/emphysema; respiratory distress | 62.2% (23/37): Overall 52.8% (19/36): Change in management 13.9% (5/36): Change in surgical management 50% (5/10): CHD | ||
| Efrati et al[16], 2009 | 319 PICU: CHD, oncology (age 1-22 yr) | Anatomic evaluation; BAL; trauma | 79.3% (253/319): Overall 90.2% (46/51): CHD 83.3% (50/60): Oncology 21.9% (70/319): Change in management 3.4% (11/319): Change in surgical management | 17.6% (56/319): Identified organism 12.2% (39/319): Change in antimicrobials 88% (22/25): Abnormal cytology consistent with infection | |
| Fan et al[26], 1988 | 87 PICU: (age 1 wk-18 yr) | Anatomic evaluation; decannulation; difficult intubation; respiratory symptoms; tracheostomy | 94.8% (91/96) | 87.5%(7/8) 100% (5/5): Difficult airways intubated 66.7% (2/3): Re-expanded collapsed lobe | |
| Hintz et al[22], 2002 | 8 NICU: CDH on ECLS | Atelectasis | 87.5% (7/8): Improved lung expansion after lavage | ||
| Kamat et al[19], 2011 | 79 PICU: ECLS (10 d-21 yr) | Atelectasis; BAL; anatomic evaluation; surfactant instillation | 21.3% (33/155): Identified organism | 76.1% (118/155): Atelectasis 15.4% (10/65): Improved CXR 2.6% (4/155): Surfactant | |
| Kohelet et al[27], 2011 | 19 NICU: (age 1 d-8 mo) | Anatomic evaluation; atelectasis; BAL; difficulty weaning MV; respiratory symptoms | 60% (15/25): Overall 100% (6/6): Wean from MV 52% (13/25): Abnormal anatomy | 60% (6/10): Identified organism 50% (5/10): Change in antimicrobials | 75% (6/8): Re-expanded collapsed lobe |
| Kolatat et al[28], 2002 | 45 NICU: (mean age 33 WGA) | Respiratory distress post-extubation | 93.3% (42/45) | ||
| Kotby et al[29], 2008 | 35 PICU: suspected pulmonary fungal infections (age 1-15 yr) | BAL | 40% (14/35): Identified organism 77.1% (27/35): Diagnosed probable pulmonary fungal infection (+ BAL culture or + BAL fungal antigen) | ||
| Maggi et al[36], 2012 | 44 PICU: status asthmaticus requiring MV (age 6 mo-18 yr) | Atelectasis; lavage; respiratory distress; | 100% (29/29): Improved A-a gradient, shunt fraction, decreased FiO2, improved compliance. 37.9% (11/29): Extubated within 6 h 69% (20/29): Extubated within 12 h Reduced PICU LOS (3.06 d vs 3.4 d in control (P < 0.05)) Reduced length of time on MV [10 h vs 20.5 h (P < 0.0005)] | ||
| Manna et al[30], 2006 | 134 PICU: CHD (age 4 mo-6 yr) | Anatomic evaluation; atelectasis; BAL; extubation failure; hemorrhage | 76.4% (113/148): Overall 84.4% (27/32): Upper airway 80% (56/70): Lower airway 18.6% (13/70): CHD identified extraluminal compression 90.5% (19/21): Extubation failure 44% (11/25): Pulmonary disease | 35.3% (6/17): Identified organism | 92.3% (24/26): Re-expanded collapsed lobe |
| Myer et al[30], 1988 | 10 NICU: (age 1 d-16 mo) | Atelectasis; hemorrhage; hypercarbia; hypoxia; hyperinflation; respiratory distress | 50% (5/10): Overall 20% (2/10): Granuloma | 60% (3/5): Re-expanded collapsed lobe 40% (2/5): Granuloma required rigid bronchoscopy | |
| Nakano et al[5], 2004 | 16 NICU: esophageal atresia, Trisomy 21, CDH, hydrocephalus, Goldenhaar, and Kasabach-Merritt (age 3 d-8.5 mo) | Anatomic evaluation; extubation failure; hemorrhage; respiratory distress | 66.7% (14/21) | 23.8% (5/21): Removed obstruction (mucus plug, clot/local tissue) or altered suction practice | |
| Nayak et al[21], 2012 | 30 PICU: CHD requiring mechanical ventilation prior to extubation (age 1 d-6 mo) | Anatomic evaluation; extubation failure | 50% (15/30): Overall significant tracheobronchial narrowing 50% (4/8): Extubation failure | 73.3% (22/30): Extubated | |
| Nussbaum et al[31], 2002 | 2836 PICU: (age 1 d-15 yr) | Anatomic evaluation; atelectasis; BAL; hemorrhage; ETT position; intubation; tracheostomy evaluation; plastic bronchitis; respiratory distress | 84.8% (2405/2836): Overall 95.2% (766/805): Upper airway 82.6% (1862/2254): Lower airway 47.9% (1358/2836): Inflammatory changes | 24.1% (411/1705): Identified organism 41.7% (5/12): Transbronchial biopsy positive dyskinetic cilia syndrome 72.4% (21/29): Acute chest SCD plastic bronchitis | |
| Peng et al[32], 2011 | 358 PICU and NICU: (age 1 d-17.5 yr) | Anatomic evaluation; BAL; intubation; respiratory distress | 87.2% (312/358): Overall 47.8% (171/358): Airway malacia 39.4% (141/358): Inflammatory changes | 56.1% (201/358): Interventional FFB 71.4% (518/725): of all FFB were interventional | |
| Pietsch et al[37], 1985 | 19 NICU: necrotizing tracheobronchitis (mean age 6.53 d) | Therapeutic removal of obstruction | 66.7% (10/15): Survived after removal of debris | ||
| Prentice et al[23], 2011 | 7 PICU: ECLS (age 8 d-27 yr) | Persistent atelectasis | 100% (7/7) 57.1% (4/7): Bronchus compression/narrowing 71.4% (5/7): Mucus plugs | 75% (3/4): Identified organism 75% (3/4): Change in antimicrobials | 28.7% (2/7): Removed mucus plugs, ECLS subsequently weaned |
| Sachdev et al[35], 2010 | 30 PICU: clinical suspicion of VAP (age 1 mo-12 yr) | BAL | 65% (26/40): Identified organism | ||
| Soong et al[43], 2011 | 8 PICU and NICU: obstructive fibrinous tracheal pseudomembrane (age 2 mo-13 yr) | Therapeutic ablation | 100% (8/8): Ablation of obstructive membrane | ||
| Soong et al[33], 1995 | 207 NICU and PICU: (age 1 d-10 yr) | Respiratory symptoms; intractable pneumonia | 81.1% (172/212) | 35.4% (75/212): Resolution of atelectasis, improved secretions | |
| Tang et al[3], 2009 | 47 PICU: (age 1 d-13 yr) | Anatomic evaluation, BAL; therapeutic (FB, clot removal, hemoptysis, intubation) | 80.9% (38/47) | 36.8% (7/19): Identified organism 57.9% (11/19): Change in antimicrobials | 87.0% (20/23): Re-expanded collapsed lobe. 44.8% (13/29): Extubated < 24 h after mucus plug, blood clot, FB removed |
| Ward et al[15], 1987 | 25 PICU: CHD (n = 7), (age 1 d-11 yr) | Anatomic evaluation; atelectasis; confirm ETT/tracheostomy position; hyperinflation; respiratory distress | 64% (16/25): Overall 62.5% (5/8): Tracheostomy - change in management 80% (4/5): Hemoptysis - change in management 85.7% (6/7): CHD |
Six studies reported a change in clinical management secondary to FFB in 28.9% (range 21.9%-69.2%; 157/540)[4,14-16,24]. Changes in clinical management included unanticipated surgical intervention, modification of surgical intervention, and alteration of endotracheal suctioning techniques. The change in clinical management due to FFB findings was similar for non-surgical patients (22.3%; range 18.5%-69.2%; 82/368) and lower for airway surgery patients (8.9%; range 3.4%-24.2%; 42/472). Atzori et al[14] reported that FFB was instrumental in delineating the type of tracheoesophageal fistula and altered surgical planning in 24.2% (15/62) of children with esophageal atresia[14]. De Blic et al[4] reported that in children with CHD, FFB findings of external compression of the airways by cardiovascular anomalies prompted earlier cardiac surgery in 50% (5/10)[4].
Twenty-one studies reported an overall diagnostic yield of 82% using FFB (range 45.2%-100%; 3791/4622)[3-5,14-18,20-33]. FFB was more likely to be positive in patients with suspected upper airway abnormalities (92.7%; range 73%-95.2%; 858/926) than in patients with suspected lower airway abnormalities (74.3%; range 11.3%-90.2%; 2274/3061). Upper airway findings included airway stenosis, compression or malacia, edema, foreign body, pseudomembrane, and vocal cord dysfunction[20,24,31]. Lower airway findings included airway stenosis, compression, malacia, mucus plugs, thrombus, and malpositioned endotracheal tube[3,14,15,17,18,20,21,23,24,31,32].
The diagnostic yield of FFB varied amongst different patient populations. The populations with the highest diagnostic yield of FFB were patients with extubation failure, patients with CHD, patients with hemoptysis, and patients undergoing ECLS. In patients with extubation failure, FFB identified a cause, such as mucus plugs, laryngotracheomalacia, laryngeal trauma/edema or compression, in 69.9% (range 50%-90.5%; 51/73)[3,16-21,24,27,31,32]. In children with CHD, the diagnostic yield of FFB was 57.5% (range 18.5%-90.2%; 177/308). External airway compression was the most commonly reported finding[15-18,20,21,34]. In patients with hemoptysis, FFB identified a cause in 56% of patients (range 20%-100%; 14/25)[5,15,25,30]. In patients receiving ECLS, 31% (range 21.5%-46.2%; 108/349) had a positive finding on FFB, including airway compression, abnormal bronchial anatomy, malpositioned or occluded endotracheal tube, or mucus plugging[17,19,22,23].
BAL was a common indication for FFB and findings were reported in 12 studies[3,16-20,23,24,27,29,31,35]. An infectious organism was identified in 25.7% (range 17.6%-75%; 631/2455) of all BALs performed. The highest yield of BAL was in immunocompromised patients, where 79.1% of BALs were found to be positive (range 42.9%-83.3%; 53/67)[16,20]. In ECLS patients, BAL identified an organism in 30.3% of procedures (range 21.3%-75%; 64/211)[17,19,23]. Five studies reported that BAL led to a change in antimicrobial therapy in 19.1% (range 12.2%-75%; 73/382) of patients[3,16,23,24,27]. Bar-Zohar et al[24] reported that in the 50% (15/30) of patients whose antibiotics were changed as a result of BAL findings, only 33% (10/30) of them improved clinically[24]. Concordance between BAL isolates and blind tracheal swab isolates was 47% (range 36%-67%; 8/17)[24,27]. In critically ill children, the use of BAL for non-infectious causes of pulmonary infiltrates was uncommon. Two isolated studies reported findings associated with aspiration in 67% (2/3) of cases and evidence of hemoptysis in 63% (5/8)[20,24].
Therapeutic outcomes were reported in 17 of 27 studies. Overall, the therapeutic yield for FFB was 60.3% (range 23.8%-100%; 595/987). Interventions performed with FFB included lavage, removal of partial obstructions, and assistance with difficult intubations or failed extubations. An improvement in atelectasis after FFB was reported in 44.9% (range 15.4%-92%; 173/385) of procedures[3,19,20,22,24,26,27,30,33]. In one study of 44 intubated children with status asthmaticus, FFB, compared to no FFB, was associated with decreased length of time on mechanical ventilation (20.5 h vs 10 h) and decreased PICU length of stay (3.4 d vs 3.1 d), but no change in total hospital length of stay[36]. In the three studies that examined the utility of FFB in assessing the etiology of extubation failure, FFB assisted in successful extubation in 69.9% of procedures (range 50%-90.5%; 51/73)[20,21,24] by removing mucus plugs or thrombus to assist with weaning from the ventilator. FFB was also used to identify patients with a normal exam who were ready for extubation[3,5,15,17,24]. Kohelet et al[27] found that in neonatal patients, therapeutic FFB in the NICU improved atelectasis in 75% (6/8) and decreased mechanical ventilation time.
Four studies reported the therapeutic yield of FFB in 174 patients receiving ECLS[17,19,22,23]. In these patients, repeat FFB to re-expand collapsed lobes was successful in 42.9% (range 15.4%-87.5%; 51/119). Furthermore, repeat therapeutic lavage was associated with decreased ventilator support, increased lung expansion and tidal volumes. Improved lung recruitment was associated with reduced ECLS support and, ultimately, separation from ECLS[17,19,22,23].
Sixteen studies that included 5060 bronchoscopies reported adverse events (Table 2). Overall, adverse events were reported in 12.9% (range 0.5%-71.4%; 654/5060) of FFBs performed. Serious adverse events requiring intervention were uncommon (2.1%; 108/5060). The most common adverse events were hypoxia, bradycardia, hypotension, and bleeding. Mild to moderate hypoxia (with oxygen saturations greater than 80%) was reported in 2.3% (range 0%-70.3%; 114/5060) of FFBs and usually resolved with removal of the FFB from the airway and/or supplemental oxygen[3,4,16,20,24,26,29,31,32]. In 6.1% (7/114) of patients with hypoxia, bag-mask ventilation was required for recovery. Bradycardia with hypoxia was reported in 0.4% (range 0%-4%; 21/5060) of FFBs performed[16,24,26,27,31-33]. A single study reported that 3.4% (11/319) of patients required atropine to treat bradycardia in addition to supplemental oxygen for hypoxia[16]. Hypotension occurred in 1.2% (range 0%-19.4%; 58/5060) of procedures performed, and a fluid bolus was given in 0.9% (46/5060) of all procedures[20,24,29]. Bleeding occurred in 4% (range 0%-37.5%; 198/5060) of overall procedures performed, and in most cases, resolved spontaneously or with suction[3,16,22,23,29,31,33]. In the 0.4% (range 0.4%-5.9%; 21/5060) of procedures that required intervention for hemostasis, saline or epinephrine lavage was sufficient to stop bleeding[16,19,23,31]. In patients receiving ECLS, who are at higher risk for bleeding secondary to systemic anticoagulation, 15.9% (range 0%-37.5%; 60/260) had bleeding after FFB[19,23]. Other reported complications included local trauma, such as pneumothorax or perforation (0.2%; 8/5060), stridor (0.3%; 14/5060), bronchospasm (0.5%; 24/5060), and fever (4.1%; 217/5060). Data on specific anesthetic risks were rarely reported. Three patients (0.1%; 3/2984), who received fentanyl in preparation for FFB, had rigid chest, and two of the three required intubation[20,23]. Two deaths were reported in high-risk neonates due to perforation of the mainstem bronchus. Both infants were subsequently found to have full thickness necrotizing tracheobronchitis[37].
| Ref. | Hypoxia | Bradycardia/Hypoxia | Hypotension | Hemorrhage | Other |
| Bar-Zohar et al[24], 2004 | 0% (0/155) | 0% (0/155) | 19.3% (30/155) 12.9% (20/155) NS bolus | 0% (0/155) | 1.3% (2/155) intubated for mucus plug |
| Davidson et al[17], 2008 | 0% (0/200) | 0.5% (1/200) patient “instability” | |||
| de Blic et al[4], 1991 | 70.3% (26/37) transient moderate hypoxia (SaO2 > 80) | 0% (0/37) | |||
| Efrati et al[16], 2009 | 6.6% (21/319), resolved - O2 0.3% (1/319) - BMV 0.3% (1/319) required intubation | 3.4% (11/319), resolved - O2 and atropine | 1.6% (5/319), resolved-saline lavage | 1.6% (5/319) stridor resolved -steroids or epinephrine 0.9% (3/319) fever | |
| Fan et al[26], 1988 | 2.3% (2/87), resolved - removal of scope or O2 | 0% (0/87) | |||
| Hintz et al[22], 2002 | 37.5% (3/8) | ||||
| Kamat et al[19], 2011 | 34.2% (53/155) mild to moderate blood tinged secretions 2% (3/155) placed on HFOV for increased bloody secretions | ||||
| Kohelet et al[27], 2011 | Transient (number not reported) | 0% (0/25) | 0% (0/25) | 4% (1/25) pneumothorax | |
| Kotby et al[29], 2008 | 42.9% (15/35), transient | 5.7% (2/35), transient | 22.9% (8/35) | Decreased PaO2 | |
| Manna et al[20], 2006 | 10.8% (16/148) transient; 16.7% (3/18) of ARDS patients | 17.6% (26/148), NS bolus | 0.6% (1/148) rigid chest after fentanyl | ||
| Nussbaum et al[31], 2002 | 0.7% (21/2836), of those 76.2% (16/21) resolved - removal of scope or O2; 23.8% (5/21) emergency intubation; 2/5 apneic prior to FFB | Transient (number not reported) | 0% (0/2836) | 4% (113/2836) mild nasopharyngeal bleeding 0.4% (12/2836) bleeding after biopsy, resolved - epinephrine lavage | Transient stridor (number not reported) 0.6% (17/2836) laryngo/bronchospasm, resolved - albuterol and O2, BMV 9.5% (2/21) rigid chest after fentanyl |
| Peng et al[32], 2011 | Transient (number not reported) | Transient (number not reported) | 0.8% (6/725) laryngospasm, resolved - lidocaine spray and NIPPV 0.3% (2/725) pneumothorax 29.5% (214/725) fever | ||
| Pietsch et al[37], 1985 | 13.3% (2/15) death secondary to mainstem bronchus perforation 6.7% (1/15) pneumothorax - chest tube | ||||
| Prentice et al[23], 2011 | 5.9% (1/17), resolved - epinephrine lavage | ||||
| Soong et al[33], 1995 | 4% (10/247) transient, resolved - removal of scope or O2 1.2% (3/247) required BMV | Self-limited nasal bleeding (number not reported) | 2% (5/247) stridor | ||
| Tang et al[3], 2009 | 20.8% (11/53), mild | 3.8% (2/53), mild | 1.9% (1/53) SVT 1.9% (1/53) pneumothorax 1.9% (1/53) bronchospasm |
FFB contributes to changes in clinical management, can assist in the diagnosis of upper and lower anatomic lesions of the respiratory tract, and is integral in identifying causes of respiratory distress and prolonged mechanical ventilation. Furthermore, FFB can be used for therapeutic interventions such as removal of obstructions and re-expansion of collapsed lung. Despite a consensus statement adopted by the American Thoracic Society in 1991, and guidelines by the European Respiratory Journal in 2003, there are no specific guidelines for FFB in critically ill pediatric and neonatal patients[9,10]. We have determined that there are populations for whom FFB is a high yield procedure and should be strongly considered (Table 3).
| Recommend | Consider |
| Upper airway symptoms (e.g., stridor) | CHD with persistent atelectasis |
| BAL in immunocompromised + respiratory distress | ECLS with persistent atelectasis |
| BAL in immunocompetent + respiratory distress AND + new/persistent fever AND infiltrate on chest X-ray on existing therapy | Prolonged mechanical ventilation |
| Esophageal atresia Asthma intubated + persistent atelectasis |
Change in clinical management is an important measure of the utility of FFB. We found that, in more than a third of cases, FFB was integral in changing patient care. This is similar to a study of adult ICU patients, in which 33% (29/87) of FFB led to a change in patient management[38,39]. We found that FFB significantly contributed to surgical planning in those without known respiratory anomalies, earlier surgical intervention in children with CHD, and change in the type of surgical intervention in children with esophageal atresia. FFB was also important in altering medical management, such as adjusting endotracheal suction techniques after identifying airway granulomas.
The overall diagnostic yield for FFB was 82%. While some studies included inflammation as a significant finding, even when these studies were excluded, the diagnostic yield was 75.2% (range 45.2%-100%; 1074/1428)[3,31,32]. This is higher than the 44% (44/87) diagnostic yield reported in critically ill adults[38]. The higher incidence of positive FFB in pediatric ICU patients may be secondary to the reluctance to perform early FFB in children, leading to severe and persistent symptoms prior to FFB. Specific populations in whom there was high diagnostic yield with FFB included children with CHD, children who failed extubation, and children with concern for upper airway abnormalities. Therefore, we propose that FFB should be strongly considered in the early evaluation of patients with CHD, children who failed extubation, and children with suspected upper airway abnormalities (Table 3).
BAL was used to identify causative organisms and tailor antibiotic management. The emergence of antibiotic resistant organisms requires that clinicians have the ability to tailor therapy. Thus, BAL may play a critical role in antibiotic stewardship. The 50% concordance of BAL with blind tracheal aspirates supports the use of BAL rather than blind tracheal aspirates in patients who are not improving on current antibiotic management. We found the highest yield of BAL culture was in patients who are immunocompromised (79%) or had a new fever with infiltrate on chest X-ray. Similar findings have been reported in critically ill adults, where BAL identified an organism in 24% (150/616) of procedures, with the highest yield (36%; 47/129) among immunocompromised patients[39]. Our findings support the use of FFB to obtain BAL in the immunocompromised host with respiratory insufficiency or in patients with pneumonia not responding to current antibiotic therapy (Table 3). Additional consideration should be given to FFB in the ECLS population[17,19,23]. In patients receiving ECLS, common clinical signs of infection may be obscured since body temperature is controlled via the ECLS circuit and systemic inflammatory response syndrome can be induced by ECLS. A high index of suspicion for infection is warranted in patients receiving ECLS who develop new infiltrates or have difficulty weaning from ECLS support. Therefore, FFB should be considered promptly in these patients.
FFB is an important therapeutic option for patients with respiratory compromise. Overall, greater than 50% of patients who underwent FFB for a therapeutic intervention achieved some benefit. This is similar to adult studies where 44% (range 22%-89%; 64/147) of patients received therapeutic benefit from bronchoscopy[38,40,41]. In pediatric studies, several specific populations appeared to derive the most benefit from therapeutic FFB. In a single study of patients with respiratory failure from asthma who underwent FFB, mucus plug removal was associated with improved oxygenation and lung expansion on chest X-ray, reduced ventilator support, and shorter PICU length of stay[36]. In patients receiving ECLS, FFB was associated with reduced need for ECLS support, particularly when bronchoscopy was performed multiple times. FFB may have the ability to decrease morbidity and mortality associated with prolonged ECLS support since FFB may improve respiratory mechanics and thus need for ECLS[42]. FFB has only recently become a treatment modality in the NICU with the advent of ultrathin bronchoscopes. An important consideration in this population is the impact of mechanical ventilation on premature and developing lungs. By treating atelectasis and decreasing time on mechanical ventilation, FFB in the NICU may ameliorate subglottic stenosis and chronic lung disease seen with prolonged ventilation. We suggest that therapeutic bronchoscopy be considered in intubated patients with asthma and atelectasis, patients receiving ECLS, and NICU patients with difficulty weaning from mechanical ventilation (Table 3). The recommendation to perform FFB in neonates to evaluate difficulty weaning from mechanical ventilation is in accord with the European Respiratory Journal guidelines, which support the use of FFB in neonates to evaluate for subglottic stenosis and other airway abnormalities. However, recommendations for FFB for asthmatic and ECLS dependent populations are not specified in either the European Respiratory Journal or the American Thoracic Society guidelines[9,10].
We found that 2.1% of pediatric patients who undergo FFB had adverse events that required a medical intervention, which is similar to the 2% (range 1.6%-4%; 17/814) reported in the adult populations[38,39]. Interventions were minor, including halting the procedure to allow spontaneous recovery from hypoxia, providing supplemental oxygen, and administering fluid boluses for hypotension. The patient populations with the highest proportion of complications were those receiving ECLS and immunocompromised patients. Patients receiving ECLS were systemically anticoagulated, and had more frequent bleeding complications requiring intervention with suctioning, saline lavage or local epinephrine. Whether the higher proportion of complications in immunocompromised patients is secondary to higher disease burden or directly related to the procedure itself is unclear. Nonetheless, adverse events requiring interventions including bag-mask ventilation and intubation were higher in this group. Due to insufficient data, we were not able to derive any meaningful interpretation regarding adverse events from sedatives used during FFB. In the studies that reported complications related to sedation, the most serious was rigid chest from fentanyl given pre-procedure in three patients who were not intubated. In our review, studies reported a mix of intubated and non-intubated patients who underwent FFB. While there was not a reported difference in adverse events in those with a secured airway as compared to those with a natural airway, the considerations to undertake the procedure may be different. For example, sedation choices may vary, and consideration of bronchoscope size relative to airway becomes important when the approach is through the nares. Finally, there may be increased risk of adverse events in patients who undergo multiple FFBs, although this finding was not born out in our review.
Our study has several limitations. We did not assess study quality in this review. Our inclusion criteria were broad to maximize our assessment of the available literature on the use of flexible bronchoscopy in critically ill children. Thus, the only studies excluded were case reports. We used standard methodology to identify papers to include in our review; however, it is possible that we may have missed publications. We limited our review to papers in English, and may have seen different results in non-English language publications. Included studies did not always distinguish between patients admitted to the ICU for procedural sedation and those that were critically ill. Thus, it is possible that not all patients included in this review were critically ill. Children with foreign body aspiration were excluded from our study because foreign body aspiration should be removed by rigid bronchoscopy.
Many of the included studies did not report quantitative outcomes after FFB, making it difficult to draw conclusions about specific risks or benefits of the procedure (e.g., a study may have mentioned improvement in ventilator settings, but did not quantify this in a meaningful way). Some studies also reported normal examinations as part of their diagnostic yield. Furthermore, one of the concerns regarding the use of FFB in pediatric populations is the anesthetic risk in these patients. According to the pediatric guidelines by the American Thoracic Society, adverse reactions to medications account for at least half of complications associated with FFB[9]. In many of the included studies, it was difficult to differentiate anesthetic complications from procedural complications. Future studies should examine complications due to sedatives among patients who undergo FFB.
We have identified patient populations in whom FFB should be strongly considered. Given the overall high diagnostic and therapeutic yield, there is a rationale to perform FFB more frequently in critically ill children. Our data suggest that experienced bronchoscopists be readily available to evaluate and treat critically ill neonates and children. This begs the question: how will this demand be met? Currently, the majority of pediatric bronchoscopists are pulmonologists or otolaryngologists. Our data supports the need for pediatric intensivists to be trained in this procedure. Indeed, Kohelet et al[27] proposed that neonatologists be trained in bedside FFB, given the high incidence of respiratory pathology in the NICU[27]. Finally, more outcomes-based research regarding FFB and its impact on morbidity and mortality is needed in the NICU and PICU. Well-designed prospective, randomized multi-center trials to investigate clinical outcomes including mortality, length of mechanical ventilation, and length of ICU and hospital stay are needed. Furthermore, unlike in adults, the use of interventional FFB for procedures such as endobronchial stents, airway laser procedures, and endobronchial or transbronchial lung biopsies has received limited investigation in the pediatric population[32]. Further studies of the safety and efficacy of interventional FFB could have significant impact in reducing open surgical procedures in children.
Our study identified indications, as well as diagnostic and therapeutic utility for FFB in critically ill children. In this review, FFB was associated with very few complications. This study provides the foundation for guidelines for FFB in critically ill children. Randomized studies are needed to investigate the impact of FFB on clinical outcomes.
Flexible fiberoptic bronchoscopy (FFB) is used with increasing frequency in neonatal and pediatric populations. However, there are no recent guidelines regarding its use in these populations.
The indications for use of FFB in critically ill children are not well delineated. Understanding the diagnostic yield, therapeutic efficacy, and rate of adverse events related to FFB in critically ill children will help determine the indications for use of FFB in critically ill children.
FFB led to a change in medical management in 28.9% of critically ill children, with a diagnostic yield of 82%. Bronchoalveolar lavage obtained during FFB may assist with identifying infectious organisms (25.7%) and optimizing antimicrobial therapy (19.1%). FFB had therapeutic benefit with removal of mucus plugs or resolution of atelectasis in 60.3%. The majority of reported adverse events were transient and included hypotension, hypoxia and/or bradycardia requiring minimal intervention.
FFB is effective and safe for diagnostic and therapeutic use among critically ill pediatric patients. In particular, FFB is recommended in patients with upper airway symptoms (e.g., stridor), in immunocompromised patients with respiratory distress, and in immunocompetent patients with respiratory distress in addition to fever and/or persistent infiltrates on chest X-ray.
FFB is a procedure that allows visualization of the upper and lower airways using a flexible bronchoscope. FFB can also be used to remove fluid or mucous plugs from the airways. Bronchoalveolar lavage is a procedure where fluid is squirted through the bronchoscope into the lungs and then recollected in order to diagnose lung disease.
A well written paper with good research of English literature.
P- Reviewer: Gow KW, Sinha R, Watanabe T S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Wood RE, Fink RJ. Applications of flexible fiberoptic bronchoscopes in infants and children. Chest. 1978;73:737-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Barbato A, Magarotto M, Crivellaro M, Novello A, Cracco A, de Blic J, Scheinmann P, Warner JO, Zach M. Use of the paediatric bronchoscope, flexible and rigid, in 51 European centres. Eur Respir J. 1997;10:1761-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Tang LF, Chen ZM. Fiberoptic bronchoscopy in neonatal and pediatric intensive care units: a 5-year experience. Med Princ Pract. 2009;18:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | de Blic J, Delacourt C, Scheinmann P. Ultrathin flexible bronchoscopy in neonatal intensive care units. Arch Dis Child. 1991;66:1383-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Nakano T, Shikada M, Nomura M, Kuwayama-Komaki F, Suganuma E, Ishikawa-Kato M, Sakai T, Hirakawa H, Ueno S, Yokoyama S. Feasibility of fiberoptic bronchoscopy for small infants including newborns. Tokai J Exp Clin Med. 2004;29:1-5. [PubMed] |
| 6. | Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest. 2003;123:1693-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 378] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Guidelines for fiberoptic bronchoscopy in adults. American Thoracic Society. Medical Section of the American Lung Association. Am Rev Respir Dis. 1987;136:1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Du Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S, Mandal S, Martin J, Mills J, Navani N. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax. 2013;68 Suppl 1:i1-i44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 560] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 9. | Green CG, Eisenberg J, Leong A, Nathanson I, Schnapf BM, Wood RE. Flexible endoscopy of the pediatric airway. Am Rev Respir Dis. 1992;145:233-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Midulla F, de Blic J, Barbato A, Bush A, Eber E, Kotecha S, Haxby E, Moretti C, Pohunek P, Ratjen F. Flexible endoscopy of paediatric airways. Eur Respir J. 2003;22:698-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9207] [Cited by in RCA: 8037] [Article Influence: 535.8] [Reference Citation Analysis (2)] |
| 12. | Moher D; PROSPERO. International prospective register of systematic reviews [Accessed 2014 September]. Available from: http: //www.crd.york.ac.uk/PROSPERO/. |
| 13. | Trikalinos TA, Trow P, Schmid CH. Simulation-Based Comparison of Methods for Meta-Analysis of Proportions and Rates [Internet]. Available from: http: //www.ncbi.nlm.nih.gov/pubmed/?term=24404633. |
| 14. | Atzori P, Iacobelli BD, Bottero S, Spirydakis J, Laviani R, Trucchi A, Braguglia A, Bagolan P. Preoperative tracheobronchoscopy in newborns with esophageal atresia: does it matter? J Pediatr Surg. 2006;41:1054-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Ward RF, Arnold JE, Healy GB. Flexible minibronchoscopy in children. Ann Otol Rhinol Laryngol. 1987;96:645-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Efrati O, Sadeh-Gornik U, Modan-Moses D, Barak A, Szeinberg A, Vardi A, Paret G, Toren A, Vilozni D, Yahav Y. Flexible bronchoscopy and bronchoalveolar lavage in pediatric patients with lung disease. Pediatr Crit Care Med. 2009;10:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Davidson MG, Coutts J, Bell G. Flexible bronchoscopy in pediatric intensive care. Pediatr Pulmonol. 2008;43:1188-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Chapotte C, Monrigal JP, Pezard P, Jeudy C, Subayi JB, De Brux JL, Cottineau C, Granry JC. Airway compression in children due to congenital heart disease: value of flexible fiberoptic bronchoscopic assessment. J Cardiothorac Vasc Anesth. 1998;12:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Kamat PP, Popler J, Davis J, Leong T, Piland SC, Simon D, Harsch A, Teague WG, Fortenberry JD. Use of flexible bronchoscopy in pediatric patients receiving extracorporeal membrane oxygenation (ECMO) support. Pediatr Pulmonol. 2011;46:1108-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Manna SS, Durward A, Moganasundram S, Tibby SM, Murdoch IA. Retrospective evaluation of a paediatric intensivist-led flexible bronchoscopy service. Intensive Care Med. 2006;32:2026-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Nayak PP, Sheth J, Cox PN, Davidson L, Forte V, Manlhiot C, McCrindle BW, Schwartz SM, Solomon M, Van Arsdell GS. Predictive value of bronchoscopy after infant cardiac surgery: a prospective study. Intensive Care Med. 2012;38:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Hintz SR, Sheehan AM, Halamek LP, Rhine WD, Van Meurs KP, Benitz WE, Frankel LR. Use of bronchoscopy for congenital diaphragmatic hernia patients on extracorporeal membrane oxygenation. Clin Intens Care. 2002;13:103-108. [DOI] [Full Text] |
| 23. | Prentice E, Mastropietro CW. Flexible bronchoscopy for children on extracorporeal membrane oxygenation for cardiac failure. Pediatr Crit Care Med. 2011;12:422-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Bar-Zohar D, Sivan Y. The yield of flexible fiberoptic bronchoscopy in pediatric intensive care patients. Chest. 2004;126:1353-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Abu-Kishk I, Klin B, Eshel G. Hemoptysis in children: a single institutional experience. Pediatr Emerg Care. 2012;28:1206-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Fan LL, Sparks LM, Fix FJ. Flexible fiberoptic endoscopy for airway problems in a pediatric intensive care unit. Chest. 1988;93:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Kohelet D, Arbel E, Shinwell ES. Flexible fiberoptic bronchoscopy--a bedside technique for neonatologists. J Matern Fetal Neonatal Med. 2011;24:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Kolatat T, Aunganon K, Yosthiem P. Airway complications in neonates who received mechanical ventilation. J Med Assoc Thai. 2002;85 Suppl 2:S455-S462. [PubMed] |
| 29. | Kotby AA, Shaheen MA, Basim HH, El Masry AA, Mansour MG, Abdel Fattah MA. Diagnostic bronchoalveolar lavage (BAL) for pulmonary fungal infections in critically ill children. J Bronchology. 2008;15:4-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Myer CM, Thompson RF. Flexible fiberoptic bronchoscopy in the neonatal intensive care unit. Int J Pediatr Otorhinolaryngol. 1988;15:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Nussbaum E. Pediatric fiberoptic bronchoscopy: Clinical experience with 2,836 bronchoscopies. Pediatr Crit Care Med. 2002;3:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Peng YY, Soong WJ, Lee YS, Tsao PC, Yang CF, Jeng MJ. Flexible bronchoscopy as a valuable diagnostic and therapeutic tool in pediatric intensive care patients: a report on 5 years of experience. Pediatr Pulmonol. 2011;46:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Soong WJ, Jeng MJ, Hwang B. The application of a modified mini-flexible-fiberoptic endoscopy in pediatric practice. Zhonghua Yixue Zazhi (Taipei). 1995;56:338-344. [PubMed] |
| 34. | de Blic J, Marchac V, Scheinmann P. Complications of flexible bronchoscopy in children: prospective study of 1,328 procedures. Eur Respir J. 2002;20:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Sachdev A, Chugh K, Sethi M, Gupta D, Wattal C, Menon G. Diagnosis of ventilator-associated pneumonia in children in resource-limited setting: a comparative study of bronchoscopic and nonbronchoscopic methods. Pediatr Crit Care Med. 2010;11:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Maggi JC, Nussbaum E, Babbitt C, Maggi FE, Randhawa I. Pediatric fiberoptic bronchoscopy as adjunctive therapy in acute asthma with respiratory failure. Pediatr Pulmonol. 2012;47:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Pietsch JB, Nagaraj HS, Groff DB, Yacoub UA, Roberts JL. Necrotizing tracheobronchitis: a new indication for emergency bronchoscopy in the neonate. J Pediatr Surg. 1985;20:391-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Olopade CO, Prakash UB. Bronchoscopy in the critical-care unit. Mayo Clin Proc. 1989;64:1255-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Joos L, Patuto N, Chhajed PN, Tamm M. Diagnostic yield of flexible bronchoscopy in current clinical practice. Swiss Med Wkly. 2006;136:155-159. [PubMed] |
| 40. | Haenel JB, Moore FA, Moore EE, Read RA. Efficacy of selective intrabronchial air insufflation in acute lobar collapse. Am J Surg. 1992;164:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Turner JS, Willcox PA, Hayhurst MD, Potgieter PD. Fiberoptic bronchoscopy in the intensive care unit--a prospective study of 147 procedures in 107 patients. Crit Care Med. 1994;22:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Paden ML, Rycus PT, Thiagarajan RR. Update and outcomes in extracorporeal life support. Semin Perinatol. 2014;38:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 43. | Soong WJ, Jeng MJ, Lee YS, Tsao PC, Yang CF, Soong YH. Pediatric obstructive fibrinous tracheal pseudomembrane--characteristics and management with flexible bronchoscopy. Int J Pediatr Otorhinolaryngol. 2011;75:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |