INTRODUCTION
Central hypovolemia and systemic hypotension can compromise myocardial perfusion, thereby depleting cellular energy reserves and generating cytotoxic reactive oxygen species (ROS). Interventions to stabilize blood pressure after hemorrhage include fluid resuscitation to expand intravascular volume and application of tourniquets to slow bleeding from wounded extremities[1]. Tourniquets impose ischemia on the distal tissue; reintroduction of oxygenated blood upon tourniquet removal triggers massive production of ROS which provoke systemic inflammation[2,3].
In myocardium, ROS can destabilize electrophysiological function by decreasing mitochondrial membrane potential[4], disrupting ATP production[5,6] and increasing K+ flux through sarcolemmal ATP-sensitive potassium (KATP) channels[4,5]. Creatine kinase (CK), which catalyzes high energy phosphate shuttling from the mitochondria to the cytosol, can be reversibly inactivated by ROS[6-8], potentially producing a pro-arrhythmic state[7]. Dzeja et al[9] proposed that CK inactivation by ROS causes ADP accumulation that opens KATP channels, thereby shortening cardiac action potentials and provoking arrhythmias. CK was shown to be physically associated with the SUR2A subunit of the sarcolemmal KATP channel[10], raising the possibility that changes in CK activity may directly modulate KATP channel current. Thus, CK inactivation by ROS may contribute to cardiac electrical instability.
Ringer’s lactate ranks among the mainstay fluids for resuscitation of trauma victims[11]. A non-antioxidant, lactate does not protect myocardium from oxidative stress[12-14]. In contrast, pyruvate is a potent physiological antioxidant[7,13,15,16]. Accordingly, fluid resuscitation with pyruvate-enriched Ringer’s solution could dampen ROS formation in the myocardium, protecting CK and ameliorating cardiac electrical instability during hypovolemia and tourniquet application and release.
This study tested the hypothesis that substituting pyruvate resuscitation for lactate can suppress myocardial ROS formation, preserve CK activity and ATP metabolism and, thus, maintain cardiac electrical stability in goats subjected to hemorrhage and hindlimb ischemia-reperfusion. We also examined whether these beneficial effects would persist at least 3.5 h after completing pyruvate-enriched resuscitation. This study revealed that pyruvate-enhanced Ringer’s resuscitation effectively and persistently suppressed ROS formation in the myocardium, prevented loss of CK activity, augmented energy reserves, and stabilized cardiac electrical rhythm more effectively than lactated Ringer’s.
MATERIALS AND METHODS
Animal experimentation was approved by the Institutional Animal Care and Use Committee of the University of North Texas Health Science Center and conducted in accordance with the Guide to the Care and Use of Laboratory Animals (NIH publication 85-23, revised 1996) and the Position of the American Heart Association on Research Animal Use. Forty-five male Boer goats, 25-30 kg, were randomly assigned to time control (TC), lactate Ringer’s (LR) resuscitation, or pyruvate Ringer’s (PR) resuscitation.
Surgical preparation
Goats were anesthetized by iv injection of ketamine (5 mg/kg) and midazolam (0.2 mg/kg), intubated, and mechanically ventilated with 1%-2% isoflurane supplemented with 100% O2. The right carotid artery, jugular vein, and femoral vein were isolated and cannulated with saline-filled polyurethane catheters. A pressure transducer (Argon Medical Devices; Athens, TX) was connected to the arterial catheter to monitor blood pressure and heart rate. Blood O2 saturation was continuously monitored by pulse oximetry. Anticoagulant heparin (650 U/kg) was injected iv to prevent thrombosis within the catheters. Needle electrodes were applied to the forelimbs and left hindlimb. Standard lead 2 electrocardiogram was captured with a National Instruments analog-to-digital acquisition board sampling at 500 Hz and stored on a Dell laptop running Windaq (Dataq, Akron, OH) acquisition software.
Hemorrhage, hindlimb ischemia, and fluid resuscitation
Mean arterial pressure was lowered to 48 ± 1 mmHg by controlled blood withdrawal (20 mL/min) from the jugular vein (total withdrawal 220 ± 13 mL, i.e., 8.5 ± 0.6 mL/kg). The target mean arterial pressure was chosen to impose moderately severe hypotension while avoiding irreversible hemodynamic collapse. Next, the right femoral artery was occluded with an atraumatic vasoclamp and a veterinary tourniquet was applied to the right hindlimb proximal to the femoral cutdown to impose hindlimb ischemia. The tourniquet was placed and tightened until the locking mechanism could no longer be advanced. 30 min later, resuscitation was administered for 90 min by jugular venous infusion (10 mL/min) of freshly prepared Ringer’s solution containing the sodium salts of pyruvate or the natural lactate stereoisomer, L-lactate (Sigma, St. Louis MO)[17] at concentrations (110-150 mmol/L) adjusted to deliver 0.05 mmol pyruvate or lactate/kg per minute. At 60 min of resuscitation, the vasoclamp and tourniquet were released to reperfuse the hindlimb. Time control (TC) goats were surgically prepared and instrumented, but were not subjected to hemorrhage, resuscitation, or hindlimb ischemia. Left ventricular myocardium was biopsied at 90 min fluid resuscitation (n = 6 per group), or 3.5 h after resuscitation, i.e., 4 h after hindlimb reperfusion (n = 9 LR, 10 PR). In TC experiments, myocardium was harvested at times corresponding to 90 min resuscitation (n = 6) and 3.5 h post-resuscitation (n = 8).
QTc interval variability
Variability of the QT interval provides a measure of pro-arrhythmic electrical instability[18,19]. The QT interval was the time from the initial deflection from the isoelectric PR segment to the point at which voltage returned to the isoelectric TP segment. No U waves were detected in the electrocardiograms. The Bazett equation[20] was used to adjust QT intervals for variations in heart rate, yielding corrected QT (QTc) intervals. A minimum of 20 consecutive cardiac cycles per timepoint were analyzed; the standard deviation of the QTc values represented QTc interval variability[21].
Myocardial metabolites and enzymes
Left ventricular myocardium was snap-frozen in situ with liquid N2-precooled Wollenberger tongs, quickly excised, immersed in liquid N2 and stored at -80 °C. These biopsies were pulverized under liquid N2, and then phosphocreatine (PCr), creatine (Cr), inorganic phosphate (Pi), ATP, pyruvate, lactate and citrate were extracted in 0.3 mol/L HClO4[22,23] and measured by spectrophotometry[24-30]. Intracellular free ADP concentration ([ADP]) was estimated from the CK equilibrium[31], where [ADP] = {[ATP] [Cr]}/{[PCr] KCK/[H+]}. The equilibrium constant for CK was taken to equal 5.18 × 10-10 mol/L at a cytosolic free Mg2+ concentration of 0.6 mmol/L which was estimated from indicator metabolite analyses in guinea-pig myocardium[32]. Intracellular [H+] was assumed to equal 63 × 10-10 mol/L, based on measurements in in situ canine left ventricular myocardium[33]. Phosphocreatine phosphorylation potential, [PCr]/{[Cr] [Pi]}, provided a measure of the free energy state of ATP in the myocardium[22,23]. The intracellular space estimates used to calculate the phosphorylation potential were calculated as 1 – [(dry mass/wet mass) + extracellular volume], where extracellular volume was taken as 0.2 mL/g wet mass based on measurements in in situ canine myocardium[22].
Proteins in frozen myocardium were extracted in phosphate buffer[34]. Protein concentrations in the extracts were measured colorimetrically[35] with a Coomassie Plus Bradford kit (Pierce, Rockford, IL). CK and lactate dehydrogenase (LDH) activities were spectrophotometrically assayed[36,37] and expressed as IU/mg protein.
Isolation and analysis of creatine kinase MB isoform
Left ventricular protein extracts, 6 μg per sample, were electrophoretically separated in a gel composed of 30 mL 1.5% agarose in 30 mM barbital buffer (pH 8.6) at 70 V for 4 h at 4 °C. Next, gels were incubated at 37 °C in a reaction buffer containing 100 mmol/L Tris-HCl (pH 7.4), 5 mmol/L MgCl2, 5 mmol/L glucose, 1 mmol/L ADP, 15 mmol/L PCr, 3 mmol/L AMP, 10 mmol/L N-acetylcysteine, 0.3 mmol/L NADP+, 10 μg/mL glucose-6-phosphate dehydrogenase, and 10 μg/mL hexokinase for 20 min. Lastly, gels were imaged in ultraviolet light in an Alpha Innotech Fluorchem Imaging system. Band densitometry was performed with AlphaEase Fluorchem. Activities of the CKMB isoform in the LR and PR long protocols were identified from the position of standard CKMB and normalized to the long protocol mean TC value.
Myocardial 8-isoprostane
A product of phospholipid oxidation by ROS, 8-isoprostane provided a stable marker of oxidative stress[38]. 8-isoprostane was extracted from frozen myocardium and assayed at 412 nm in a 96 well plate reader (BioTek KCJunior, Winooski, VT) using an ELISA kit (Cayman Chemical, Ann Arbor, MI). 8-isoprostane contents in the LR, PR and long protocol TC groups were normalized to the short protocol TC values.
Statistical analysis
Presented values are mean ± SD. QTc variability was analyzed using a two-factor (treatment, time) ANOVA with repeated measures. All other variables were analyzed by single-factor (treatment) ANOVA. When ANOVA detected statistically significant effects, Student-Newman Keuls post hoc tests were applied to identify the specific between-group differences. Statistical analyses were performed using SigmaStat v10. Values of P < 0.05 were considered statistically significant.
RESULTS
Effects of pyruvate- vs lactate-enriched fluid resuscitation on systemic arterial pressure
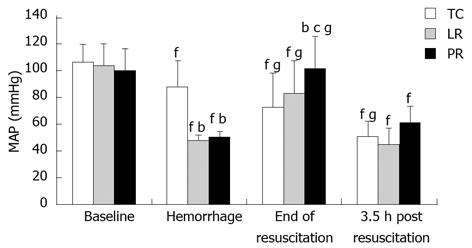
Controlled exsanguination of the goats lowered mean arterial pressure from 100 to 48-50 mmHg (Figure 1). Intravenous LR resuscitation, initiated 30 min after exsanguination and imposition of hindlimb ischemia, restored mean arterial pressure to that of time control goats. PR resuscitation was more effective than LR; at 90 min resuscitation, mean arterial pressure in the PR-resuscitated goats was appreciably higher than that of LR (P < 0.05) and TC (P < 0.05) goats. Mean arterial pressure fell after resuscitation. At 3.5 h post-resuscitation, the difference in mean arterial pressure in PR vs LR goats persisted but was no longer statistically significant (P = 0.084).
Figure 1 Systemic arterial pressure during hemorrhage, resuscitation and recovery.
Values are mean ± SD from 8 time control (TC), 9 lactate Ringer’s (LR), and 10 pyruvate Ringer’s (PR) experiments. bP < 0.01 vs TC; cP < 0.05 vs LR; fP < 0.01 vs baseline of same group; gP < 0.05 vs pre-resuscitation hemorrhage of same group.
Cardiac electrical instability
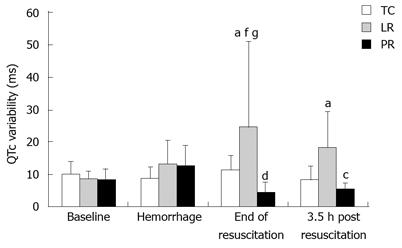
Increased variability of the heart rate adjusted ventricular depolarization-repolarization period, i.e. QTc interval, indicates cardiac electrical instability[39]. QTc variability sharply increased during LR resuscitation (Figure 2) to twice the TC value (P < 0.05). In contrast, PR lowered QTc variability to 18% of the respective LR value (P < 0.01). QTc variability was still elevated 3.5 h after LR resuscitation vs TC (P < 0.05) but remained suppressed post-PR vs post-LR (P < 0.05). Hence, PR stabilizes cardiac electrical function both during and for 3.5 h after its administration.
Figure 2 QTc variability during fluid resuscitation and recovery.
Values are mean ± SD from 8 time control (TC), 9 lactate Ringer’s (LR), and 10 pyruvate Ringer’s (PR) experiments. aP < 0.05 vs TC; cP < 0.05, dP < 0.01 vs LR; fP < 0.01 vs baseline of same group; gP < 0.05 vs pre-resuscitation hemorrhage of same group.
Myocardial pyruvate, lactate and citrate
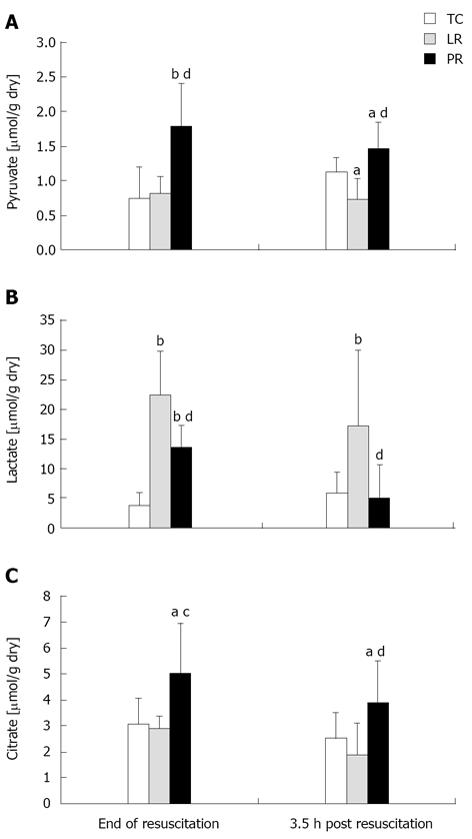
In the cytosol, LDH maintains an equilibrium between pyruvate and lactate[13]; in the mitochondria, pyruvate carboxylation generates oxaloacetate and, thus, Krebs cycle intermediates including citrate[15,16]. To assess pyruvate metabolism, pyruvate, lactate, and citrate were measured in left ventricular myocardium at 90 min resuscitation and 3.5 h post-resuscitation, i.e., 30 min and 4 h hindlimb reperfusion. As expected, pyruvate content (Figure 3A) was higher during PR vs both LR resuscitation (P < 0.01) and the corresponding TC value (P < 0.01). Notably, pyruvate content remained elevated for 3.5 h after PR resuscitation. Lactate contents (Figure 3B) were increased by LR and, to a lesser extent, PR (P < 0.01 vs TC); the latter result indicated conversion of pyruvate to lactate in PR-resuscitated myocardium. Lactate content remained elevated 3.5 h after LR resuscitation (P < 0.01 vs TC) but subsided post-PR (P < 0.01 vs LR). Myocardial citrate content (Figure 3C) paralleled pyruvate content. Thus, PR resuscitation increased citrate content by 62% and 77% vs TC and LR, respectively (P < 0.05). Citrate content fell by 22% during the 3.5 h after PR resuscitation, but remained well above the respective post-LR (P < 0.01) and TC (P < 0.05) contents.
Figure 3 Myocardial pyruvate (A), lactate (B) and citrate (C) contents.
Values (mean ± SD) are from left ventricular myocardium biopsied at 90 resuscitation (n = 6 per group) or at 3.5 h post resuscitation [8 time control (TC), 9 lactate Ringer’s (LR), and 10 pyruvate Ringer’s (PR)]. aP < 0.05, bP < 0.01 vs TC; cP < 0.05, dP < 0.01 vs LR.
8-Isoprostane
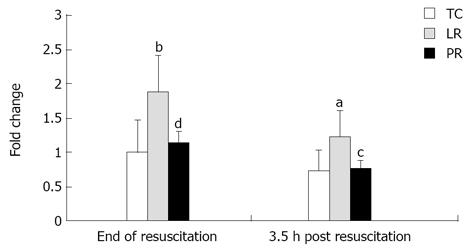
Oxidative stress is potentially arrhythmogenic[4,40]. The lipid peroxide 8-isoprostane is a marker of oxidative stress and antioxidant deficiency[7,38]. The combination of hemorrhage, LR resuscitation and hindlimb ischemia-reperfusion imposed oxidative stress on the myocardium: 8-isoprostane content, measured 30 min after hindlimb reperfusion, increased 88% (P < 0.01) vs TC (Figure 4). In contrast, PR resuscitation suppressed oxidative stress: 8-isoprostane content in the PR group was 39% below the LR value (P < 0.01), and did not differ from TC (Figure 4). This pattern persisted until 4 h of hindlimb reperfusion: 8-isoprostane content in the post-LR myocardium was 65% above the TC value (P < 0.05), but in the post-PR myocardium, 8-isoprostane content was 37% below post-LR content (P < 0.05) and similar to that of TC. Thus, the combination of hemorrhage, LR resuscitation, and hindlimb ischemia-reperfusion imposed oxidative stress in left ventricular myocardium which was prevented by PR resuscitation.
Figure 4 Myocardial 8-isoprostane content.
Values (mean ± SD) are normalized to the ‘end of resuscitation’ time control (TC) values [8 time control (TC), 9 lactate Ringer’s (LR), and 10 pyruvate Ringer’s (PR)]. aP < 0.05, bP < 0.01 vs TC; cP < 0.05, dP < 0.01 vs lactate Ringer’s (LR).
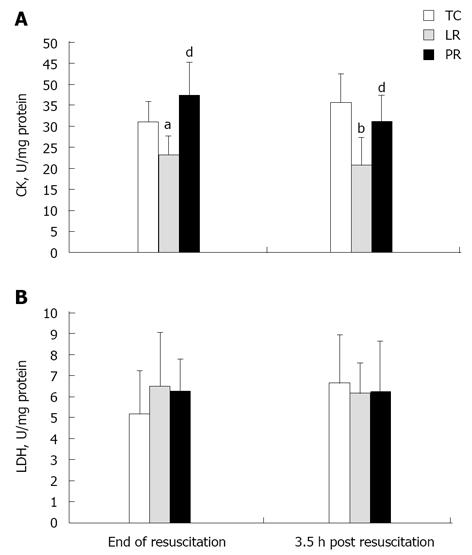
Creatine kinase activity
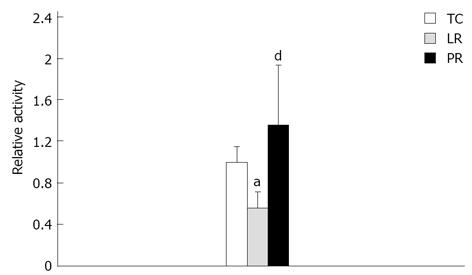
CK plays a pivotal role in myocardial energy metabolism by shuttling high energy phosphates from the mitochondria to the myofilaments and membrane ion pumps[41]. Myocardial CK activity fell 25% (P < 0.05) during LR resuscitation vs TC (Figure 5A), but PR resuscitation preserved CK activity (P < 0.01 vs LR). These treatment effects persisted for 3.5 h after resuscitation: CK activity post-LR was 42% below the respective TC value (P < 0.001), but remained robust 3.5 h after PR resuscitation (P < 0.01 vs LR). Examination of CKMB isoenzyme activity (Figure 6) revealed that LR resuscitation was unable to protect the cardiac specific isoenzyme from inactivation, but PR resuscitation preserved CKMB activity (P < 0.001 vs LR). Activities of LDH, which is released from damaged cardiomyocytes[42], did not differ among the treatments, and remained stable throughout the protocol (Figure 5B). Thus, loss of CK activity after LR resuscitation probably was not due to CK leakage from damaged cardiomyocytes.
Figure 5 Myocardial creatine kinase (A) and lactate dehydrogenase (B) activities.
Enzyme activities were measured in 8 time control (TC), 9 lactate Ringer’s (LR) and 10 pyruvate Ringer’s (PR) experiments. aP < 0.05, bP < 0.01 vs TC; dP < 0.01 vs LR. CK: Creatine kinase; LDH: Lactate dehydrogenase.
Figure 6 Myocardial creatine kinase MB isoenzyme activities.
Creatine kinase-MB activities, measured in 8 time control (TC), 9 lactate Ringer’s (LR) and 10 pyruvate Ringer’s (PR) experiments, are expressed relative to the TC value. aP < 0.05 vs TC; dP < 0.01 vs LR.
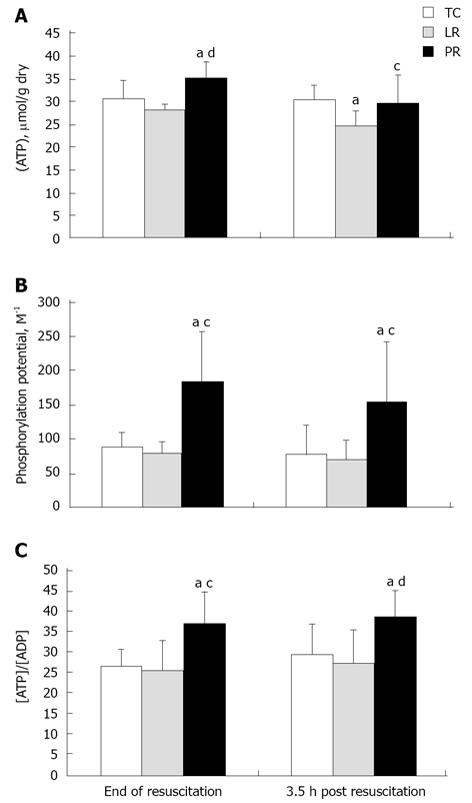
Myocardial energy metabolites
The combination of hemorrhage, hindlimb ischemia-reperfusion, and LR resuscitation slightly depleted myocardial ATP (Figure 7A) at 3.5 h post-resuscitation (P < 0.05 vs TC). PR resuscitation not only stabilized but even increased ATP content (P < 0.01 vs LR and P < 0.05 vs TC). At 3.5 h post-PR, ATP content remained 20% above the post-LR value (P < 0.05; Figure 7A). Resuscitation with PR sharply increased myocardial phosphocreatine phosphorylation potential, i.e. [PCr]/{[Cr] [Pi]}, vs LR-resuscitated and TC myocardium (P < 0.05), an effect that persisted 3.5 h after resuscitation (Figure 7B). Another measure of myocardial energetics, the ATP/free ADP concentration ratio (Figure 7C) defines the poise of the cytosolic free adenylate system[31]. PR resuscitation increased ATP/ADP ratio by 30%-40% (P < 0.05) vs LR-resuscitated and TC myocardium. Enhancement of ATP/ADP, like [PCr]/{[Cr] [Pi]}, persisted 3.5 h after PR resuscitation. Thus, PR resuscitation stabilized myocardial energetics more effectively than LR; importantly, these favorable effects persisted for 3.5 h after pyruvate administration.
Figure 7 Myocardial ATP content (A), phosphorylation potential (B) and ATP/ADP ratio (C).
Metabolites were measured in the same biopsies [8 time control (TC), 9 lactate Ringer’s (LR), and 10 pyruvate Ringer’s (PR)]. aP < 0.05 vs TC; cP < 0.05 , dP < 0.01 vs LR.
DISCUSSION
This study tested the hypothesis that resuscitation with a pyruvate-fortified Ringer’s solution during hypovolemia and hindlimb ischemia-reperfusion would stabilize cardiac electrical function and preserve creatine kinase activity in the face of oxidative stress, and maintain myocardial energy reserves in a manner superior to Ringer’s lactate. In addition, we proposed that these beneficial effects would be evident not only during pyruvate administration, but also would persist another 3.5 h. Compared to LR, PR resuscitation stabilized cardiac electrical activity, suppressed lipid peroxidation, prevented CK inactivation, preserved ATP content and augmented myocardial phosphorylation potential and [ATP]/[ADP] ratio. Importantly, these myriad favorable effects of PR persisted at least 3.5 h after its administration. PR also restored arterial pressure more effectively than LR, although this effect was no longer statistically significant 3.5 h post-resuscitation.
Pyruvate provides antioxidative protection against ROS
The combination of hemorrhage, systemic hypotension, hindlimb ischemia-reperfusion and LR resuscitation imposed oxidative stress on the myocardium. By chemically modifying cellular components, ROS can impair cellular metabolism and compromise function[6,16,43]. A natural antioxidant, pyruvate dampened myocardial ROS formation both during and for 3.5 h after its administration. LR resuscitation was comparatively ineffective at suppressing ROS. Previous studies of pyruvate-enriched resuscitative fluids for treatment of hemorrhagic shock also have documented pyruvate’s antioxidant capabilities[12,13,44]. Pyruvate detoxifies ROS in direct, non-enzymatic reactions[15,45]. In addition, pyruvate carboxylation generates Krebs cycle intermediates, producing citrate which, by inhibiting phosphofructokinase, diverts glucose-6-phosphate into the hexose-monophosphate pathway, the source of NADPH to maintain the endogenous antioxidant glutathione[15].
Pyruvate protects creatine kinase
Studies of myocardial ischemia-reperfusion have implicated ROS in the inhibition of metabolic enzymes and have shown that pyruvate preserves these enzymes in parallel with enhanced glutathione redox state[8,46]. Sharma et al[8] demonstrated that ROS inactivated key glycolytic and Krebs cycle enzymes in canine myocardium during cardiac arrest, but pyruvate infusion restored these enzyme activities. Cardioplegia-arrested swine myocardium reperfused for 3 min with whole blood had lower glutathione redox state and activities of CK and other enzymes than myocardium reperfused with pyruvate-enriched blood[7]. In the present study, PR protected myocardial CK activity more effectively than LR, and this effect persisted 3.5 h after PR administration. Creatine kinase is physically associated with SUR2A subunits of sarcolemmal KATP channels[10]. CK inactivation could open these channels and promote potassium efflux, thereby shortening the action potential and favoring proarrhythmic afterdepolarizations. Thus, preservation of CK by PR may have helped to stabilize action potential duration and cardiac electrical function. Loss of the cytosolic enzyme LDH indicates sarcolemmal rupture. Because LDH activity was stable, the loss of myocardial CK activity in LR-resuscitated goats likely was not due to sarcolemmal rupture but rather inactivation by ROS. In that case, PR preservation of CK activity could be ascribed specifically to pyruvate’s antioxidant properties.
Pyruvate stabilizes cardiac electrical function: role of ATP: ADP ratio
Victims of hemorrhage are at risk of developing cardiac electrical instability[47] which can be monitored by assessing QTc variability[18,48-50]. ROS have been implicated in the pathogenesis of arrhythmias, and it has been suggested that the antioxidative properties of certain medications could suppress ROS-mediated arrhythmogenesis[40]. Several studies have identified K+ channels that are sensitive to oxyradical attack[4,51]. Zhang et al[52] elegantly demonstrated that ROS could dampen the delayed rectifier and HERG K+ channel activities, thereby prolonging the action potential. Inactivation of the transient outward K+ channel by hydrogen peroxide[53,54] could prolong the QT interval and action potential duration. However, S-nitrosylation of cardiac ion channels can either attenuate or prolong the action potential[52]. Thus, the temporal variability of repolarization produced by oxyradical attack on various cardiac ion channels can place the myocardium in a proarrhythmic state. Pyruvate supplementation can prevent ST segment elevation in myocardial ischemia-reperfusion[55,56], but pyruvate’s influence on cardiac repolarization has not been reported. In this study, pyruvate-enriched resuscitation prevented the increased instability of repolarization produced by lactated Ringer’s resuscitation following hypovolemia and hindlimb ischemia-reperfusion. Importantly, this anti-arrhythmic protection persisted at least 3.5 h after pyruvate administration.
Resuscitation with PR augmented ATP content and PCr phosphorylation potential vs. LR, both during and 3.5 h after resuscitation. These favorable energetic effects are likely due, in large part, to pyruvate’s antioxidative properties as a ROS scavenger and stabilizer of glutathione redox state[16], thereby protecting metabolic enzymes, combined with its energy-yielding capabilities as a readily oxidized fuel. Indeed, pyruvate- but not lactate-enriched perfusion bolstered PCr phosphorylation potential of post-ischemic guinea-pig myocardium[57]. Studies of the energy-generating properties of pyruvate have focused on responses during pyruvate supplementation[6,16]. This study is the first to demonstrate that pyruvate’s enhancement of myocardial energy state persists at least 3.5 h after pyruvate administration.
The dynamic balance between ATP and ADP modulates the gating properties of the sarcolemmal KATP channel, a sensor of cellular energy state[58]. Zhou et al[5] demonstrated that a decrease in ATP/ADP ratio opened KATP channels and shortened action potentials; restoring ATP/ADP inactivated the channels. Crawford et al[10] demonstrated that ATP dampened KATP channel activity, but its conversion to ADP activated the channel. Accordingly, increased myocardial ATP/ADP ratio may have contributed importantly to PR-induced stabilization of cardiac electrical activity. This study provides the first in vivo evidence that pyruvate administration can minimize the cardiac electrophysiological consequences of hemorrhagic shock and tourniquet-imposed ischemia-reperfusion of extremities.
Limitations
This project aimed to decipher the mechanism linking pyruvate-enriched resuscitation to cardiac electrical stabilization. In vitro experiments are required to define the contributions of the KATP channel and possibly other membrane ion channels and pumps to the heart’s electrophysiological responses to PR vs LR. CK is present in various subcellular locations within cardiomyocytes, including the mitochondrial inter-membrane space[41], adjacent to the myofilaments[41] and in association with membrane ion pumps and KATP channels[9,10]. Although total CK activity was assessed in this study, the enzyme could not be measured at its different subcellular locations, so the specific impact of LR vs PR resuscitation on KATP-associated CK is undefined. The cardiac effects of PR vs LR resuscitation beyond the first 4 h recovery, pyruvate-enrichment of other resuscitative media, including colloidal fluids, and the efficacy of esterified pyruvate derivatives, e.g., ethyl pyruvate[59,60] have not been examined in this model of hemorrhagic shock.
This study revealed several beneficial effects of pyruvate-enriched crystalloid resuscitation during central hypovolemia and hindlimb ischemia-reperfusion. Pyruvate-enriched Ringer’s effectively scavenged ROS, protecting CK and augmenting myocardial energy state during and 3.5 h after PR resuscitation. These actions may have contributed to the observed pyruvate stabilization of cardiac electrical function. Fluid resuscitation is an important therapeutic strategy for central hypovolemia. These findings support development of resuscitative fluids enriched with pyruvate or its derivatives, e.g., ethyl pyruvate, to improve treatment and outcomes in combat casualties and civilian trauma victims.
ACKNOWLEDGMENTS
The skillful technical assistance of Arthur G Williams, BS, Linda Howard, Shirley Nelson, RVAT, Diana R Schulz, MS, Sheldon Gaines, BS and Luis Dlouhy, BS, is gratefully acknowledged. This study was conducted in partial fulfillment of the requirements for the PhD degree for HAG.
COMMENTS
Background
Central hypovolemia and systemic hypotension can compromise myocardial perfusion, thereby depleting cellular energy reserves and generating cytotoxic reactive oxygen species (ROS). Creatine kinase (CK), which catalyzes high energy phosphate shuttling from the mitochondria to the cytosol, can be reversibly inactivated by ROS[6-8], potentially producing a pro-arrhythmic state[7].
Research frontiers
Ringer’s lactate ranks among the mainstay fluids for resuscitation of trauma victims. A non-antioxidant, lactate does not protect myocardium from oxidative stress. In contrast, pyruvate is a potent physiological antioxidant. Accordingly, fluid resuscitation with pyruvate-enriched Ringer’s solution could dampen ROS formation in the myocardium, protecting CK and ameliorating cardiac electrical instability during hypovolemia and tourniquet application and release.
Innovations and breakthroughs
This study tested the hypothesis that substituting pyruvate resuscitation for lactate can suppress myocardial ROS formation, preserve CK activity and ATP metabolism and, thus, maintain cardiac electrical stability in goats subjected to hemorrhage and hindlimb ischemia-reperfusion.
Applications
This study revealed several beneficial effects of pyruvate-enriched crystalloid resuscitation during central hypovolemia and hindlimb ischemia-reperfusion. Pyruvate-enriched Ringer’s effectively scavenged ROS, protecting CK and augmenting myocardial energy state during and 3.5 h after pyruvate Ringer’s resuscitation.
Peer review
This was a well written manuscript and suitable for publication. The hypothesis is one of constant debate in the clinical literature and I think their experiment was well performed in a good/appropriate animal model. Their methods and results appear well supported.