Published online Sep 9, 2025. doi: 10.5492/wjccm.v14.i3.103458
Revised: March 4, 2025
Accepted: March 11, 2025
Published online: September 9, 2025
Processing time: 241 Days and 1 Hours
Sepsis is a life-threatening organ dysfunction associated with a robust systemic inflammatory and immune response to infection. Its pathological consequences lead to multiple organ deficits. Klotho was initially introduced as an antiaging molecule. Its deficiency significantly reduces lifespan, and its overexpression protects against organ injury. It reduces oxidative stress and apoptosis and has anti-inflammatory and antifibrotic properties. In this review, we discuss the un
Core Tip: Klotho, originally identified as an anti-aging protein, plays a critical role in mitigating sepsis-induced organ dysfunction by reducing oxidative stress, inflammation, and apoptosis. This review highlights klotho's potential as a therapeutic target, exploring its ability to modulate key signaling pathways like nuclear factor-kappa β, mitogen activated protein kinase, and nuclear factor erythroid-related factor 2. Reduced klotho levels correlate with increased severity of sepsis, while its supplementation shows promise in improving survival rates and alleviating organ damage. Understanding the mechanisms of klotho's cytoprotective effects may pave the way for novel treatments in managing sepsis-related organ failure.
- Citation: Al-Kadi A, Anter A, Rofaeil RR, Sayed-Ahmed MM, Ahmed ASF. Klotho: A multifaceted protector in sepsis-induced organ damage and a potential therapeutic target. World J Crit Care Med 2025; 14(3): 103458
- URL: https://www.wjgnet.com/2220-3141/full/v14/i3/103458.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i3.103458
Sepsis is defined as “life-threatening organ dysfunction caused by a dysregulated host response to infection”[1]. Sepsis is a devastating clinical problem and the most prevalent cause of end-stage organ dysfunction and remains one of the largest contributors to health loss worldwide[2,3]. In 2020, it was estimated to affect 48.9 million people of whom 11 million died representing 20% of all global deaths[4]. Sepsis occurs in people of all ages, however, sepsis has been described as an illness of the elderly[5]. Earlier research showed over 60% of septic cases were patients over 65 years of age[3,6]. In addition to the high incidence, sepsis-related mortality has been reported to be almost 80% in patients above 80 years of age admitted to the intensive care unit[7,8].
The influence of old age on sepsis risk and outcome is evidenced clinically, and they have greater comorbidities and are more vulnerable to severe organ dysfunction[7,9]. When the integrity of the anti-inflammatory mechanism during sepsis is affected by aging, it results in a dysregulated innate immune system with excessive, long-lasting inflammatory response, and uncontrolled oxidative stress conditions. Such a phenomenon is defined as “inflamm-aging”[10,11]. Also, it was observed in different animal studies of sepsis that aged animals had a lower survival rate and exhibited excessive multi-organ dysfunction, as well as exaggerated inflammation[12,13].
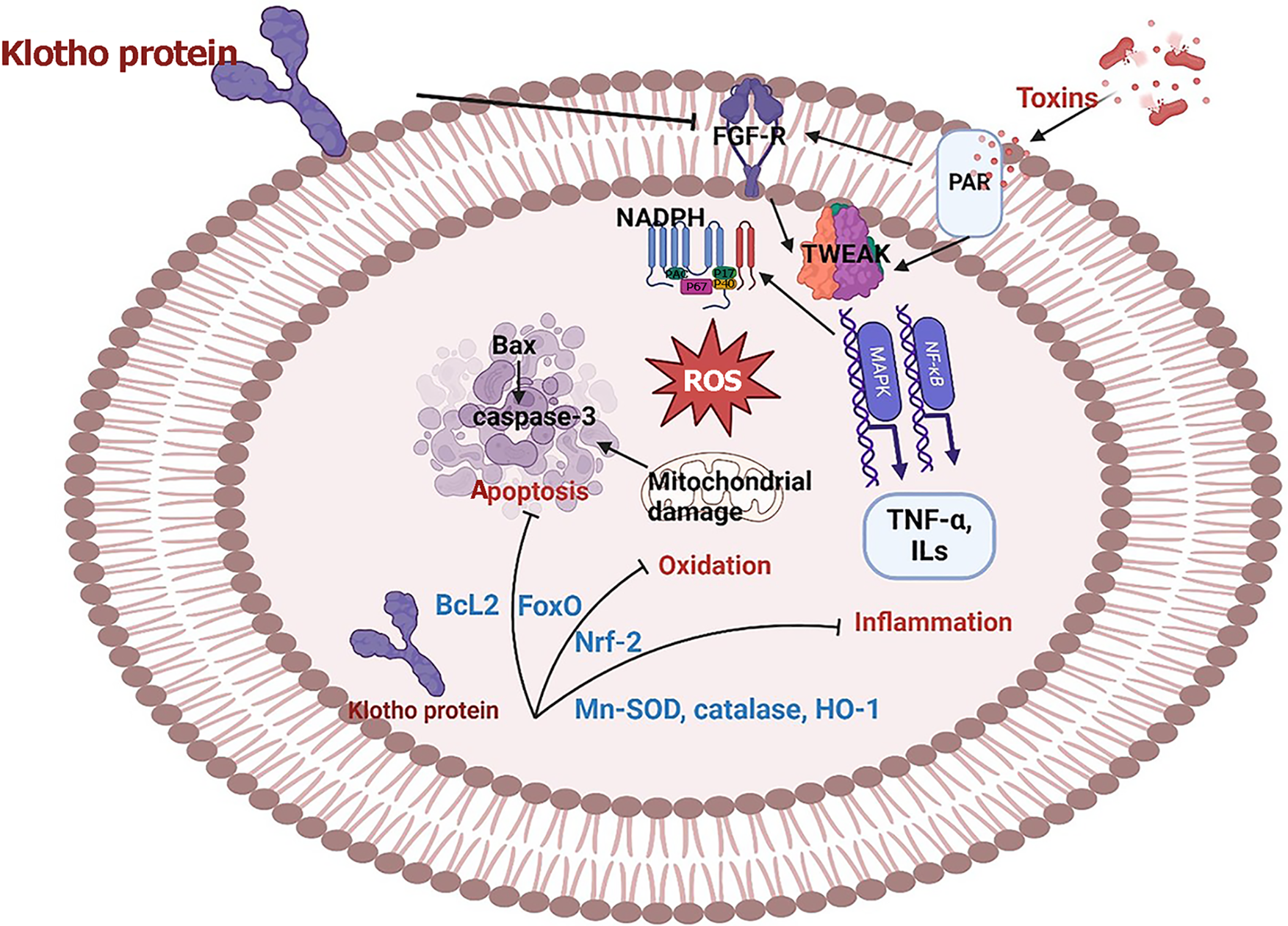
Klotho is a protein that suppresses oxidative stress and inflammation[14]. In animal models, klotho was first proposed to induce premature-aging syndromes and shortened life span[15], whereas overexpression appeared to extend life span by 20%–30%[16]. Among a nationally representative sample of American adults, they found that low serum klotho concentration below 666 pg/mL was associated with a 31% higher risk of mortality[17]. Also, a prospective cohort study of patients with chronic kidney disease showed that serum klotho concentration of less than 760 pg/mL was associated with a higher mortality rate[18]. Given that sepsis and low klotho protein expression are independent predictors of mortality in sepsis, deficiency of klotho before or during the onset of sepsis appears to worsen the prognosis. The purpose of this review is to gain a deeper understanding of the protective role of klotho in sepsis (Figure 1).
In 1997, Kuro-o et al[15] identified “klotho", the aging suppressor gene whose mutation manifests in a syndrome mimicking advanced aging. The name was taken from the mythological Greek figure responsible for spinning the thread of life[15]. Klotho predominantly originates in renal distal and proximal convoluted tubules, parathyroid glands, and the choroid plexus. Recent findings also show its presence in cardiovascular tissues, liver, urine, blood, and cerebrospinal fluid[19-23]. The klotho gene family comprises three subtypes: (1) Α-klotho, the most prevalent subtype; (2) β-klotho; and (3) γ-klotho[20]. Klotho functions as a protective factor against aging by promoting antioxidants[24], anti-inflammatory effects[25], and protects against cellular apoptosis, fibrosis, and senescence[26,27]. Low expression levels can lead to accelerated aging, a syndrome resembling human aging that includes skin and muscle atrophy, osteoporosis, cognitive impairment, and atherosclerosis—together, increased oxidative stress production and upregulation of inflammatory cytokines increased the risk of multisystem dysfunction[23,26-28]. Klotho exhibits alternatively spliced transcripts that yield either a secreted soluble form known as soluble klotho or a full-length single-pass transmembrane protein referred to as membrane klotho. The latter functions as a coreceptor regulator for fibroblast growth factor (FGF)-23[29,30].
Interestingly, klotho depletion contributes to acute kidney injury[14] and cardiac damage[31], and is found in indi
Klotho deficiency results in the development of renal, cardiovascular, and brain injuries and death in septic animals and is associated with endothelial dysfunction, oxidative stress activation, inflammatory responses, impaired immunity, and apoptosis[25,28,38]. Klotho protein exerts its function as a humoral factor and influences the pathophysiological process by modulating different intracellular signal pathways[39]. Research suggests a complex relationship between klotho expression and sepsis. Klotho deficiency aggravates sepsis-related multiple organ dysfunction[28]. The severity of septic shock influences the low expression of klotho and correlates with disease severity. Mild sepsis was associated with slight klotho reduction[40]. Klotho levels start to decline within 4 hours following the onset of systemic hyperinflammation.
Furthermore, research indicates that administering recombinant klotho 1 hour or 4 hours after lipopolysaccharide (LPS) or cecal ligation and puncture (CLP) surgery is linked to reduced organ damage[28]. The administration of klotho is currently in the preclinical stage and has not yet received approval for clinical use. If the clinical application of klotho begins successfully, the dosage will be determined based on the preclinical findings, with early administration within the first 24 hours.
Low klotho mRNA expression was reported in an experimental model of gram-negative sepsis induced by LPS injection[25]. In addition, the CLP model in klotho knockout mice demonstrated significantly higher mortality, higher concentrations of cytokines, and high oxidative stress[28]. It has been reported that renal klotho expression was significantly reduced in patients and animals with sepsis[13,38]. Klotho deficiency has been reported to increase the production of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6, aggravating sepsis-induced multiple organ dysfunction syndrome[23,25,28]. In contrast, recombinant klotho ameliorates sepsis-induced multiple organ dysfunction[13,23,41]. Also, its administration increases the survival rate and decreases lung injury, neutrophil influx, and inflammatory factors[42].
Reduction of klotho protein expression physiologically (by natural aging) or by using genetic models or pharmacological agents (experimental) was shown to elicit multiple detrimental effects in various previous studies. Table 1 below summarizes these studies[22,25,28,38,43-46].
| Klotho regulator | Sepsis model | Mechanism | Effect | Ref. |
| Aged mice | LPS | Klotho downregulation/upregulation of NF-κB | Cardiovascular dysfunction. Higher myocardial levels of cytokines TNF-α, IL-6, and IL-1β | Hui et al[25] |
| Aged mice | LPS | Exacerbated inflammation associated with aging/klotho downregulation | More severe and persistent cardiac dysfunction. Higher myocardial IL-6, ICAM-1, and vascular cell adhesion molecule levels | Li et al[22] |
| Aged mice | CLP | Serum TNF-α and IL-6 were elevated. Caspase-3 tissue expression. The bacterial count increased | The survival rate was reduced. Apoptosis activation in the thymus and spleen. Impairment of innate and adaptive immunity | Inoue et al[43] |
| Genetically deficient Kl/+ mice | CLP | Klotho downregulation/upregulation of NF-κB | Low survival rate. Increase in the production of TNF-α, IL-1β, IL-6. Increase in thiobarbituric acid reactive substances and lactate with a reduction in reduced glutathione. The autonomic response in sepsis was much more harmful through impairment of the vasopressor effect and baroreflex sensitivity | Jorge et al[28] |
| Klotho-siRNA fibroblasts. In-vitro study | LPS | Upregulation of superoxide production/downregulation of glutathione antioxidant. Upregulation of high mobility group box-1. Increase in NF-κB activation | Reduction in wound healing. Apoptotic cell death induction. Imbalance in intracellular zinc and calcium amounts. Increased secretion of TNF-α, IL-6, and IL-Iβ, concurrently with suppression of anti-inflammatory cytokine IL-10 | Mytych et al[44] |
| Haploinsufficient mice (Kl+/–) | LPS | Down-regulation in renal and brain klotho gene expression. Elevation in plasma plasma-neutrophil gelatinase-associated lipocalin mRNA level. High mRNA levels of E-selectin, ICAM-1, and retinoic acid-inducible gene-I | Renal and brain damage were observed. Endothelial activation promotes immune cell infiltration into the tissue, which increases tissue damage | Jou-Valencia et al[38] |
| Klotho knockout mice | CLP | Reduction in klotho level/Hyper-cytokinemia. Impairment of bacterial clearance | Decrease in survival rate when compared to the wild-type. Activation of innate immune cells. Elevation of apoptosis in lymphocytes. Increasing serum concentrations of TNF-α, IL-6, and monocyte chemoattractant protein | Inoue et al[45] |
| Adult mice | CLP | Reduction in klotho level | Increase in serum creatine and blood urea nitrogen. Damage to renal tissue. Autophagy activation | Chen et al[46] |
As shown in the previous section, the reduction in klotho level had detrimental effects on sepsis, thus attempts were made to use different pharmacological techniques to rescue animals with sepsis and its associated organ damage. Table 2 summarizes some of these studies and their findings[13,14,22,23,25,31,35,38,41].
| Klotho regulator | Sepsis model | Mechanism | Effect | Ref. |
| Treated mice with klotho protein (0.02 mg/kg) for 4 days | LPS | Klotho administration/attenuates the reactive oxygen species/p38-mitogen activated protein kinase signaling pathway | Reversal of myocardial injury. Reduction in atrial and brain natriuretic peptide (atrial natriuretic peptide and BNP). Reduction in apoptosis | Yan et al[31] |
| Recombinant klotho protein | CLP | Restoration of endogenous klotho expression | Alleviated CLP-induced acute renal injury by reducing serum creatinine and BUN levels | Chen et al[13] |
| Recombinant klotho protein | LPS | Klotho administration/suppression of NF-κB activation | Cardiac function was improved. Lower myocardial levels of chemokines and cytokines TNF-α, IL-6, and IL-1β | Hui et al[25] |
| Recombinant klotho protein | LPS | Anti-inflammatory effect of klotho administration/suppression of IL-6 and adhesion molecules | Return to the baseline levels for myocardial ICAM-1, VCAM-1, and IL-6. Recovery from cardiac dysfunction | Li et al[22] |
| Recombinant IL-37 | LPS | Anti-inflammatory effect of IL-37/klotho upregulation/suppression of IL-6 and adhesion molecules | Improvement of cardiac functions | Li et al[22] |
| Pretreatment-recombinant α-klotho protein | LPS | Antiapoptotic mechanism through caspase-3 reduction. Anti-inflammatory by shifting the balance towards an anti-inflammatory environment. Antioxidant activities | Amelioration of septic cardiorenal injury. Reduction in serum levels of troponin, NGAL, BNP, and creatinine. Reduction in the renal cytokines IL-6, IL-1, and TNF-α levels, with marked elevation in IL-10. Reduction in malondialdehyde and nitric oxide production | Liu et al[41] |
| Recombinant human-klotho protein | CLP. LPS in-vitro study in the human HK2 epithelial cell line | Klotho exerts its protective effects by upregulating nuclear factor erythroid related factor 2 to suppress the ferroptosis signaling pathway | Alleviated kidney injury and increased HK2 cell viability. Administration of klotho upregulates klotho expression in blood, renal tissue, and HK2 cells. Low levels of the inflammatory factors TNF-α and IL-6 and oxidative stress responses. Improvement in mitochondrial number, structure, and function in HK2 cells | Zhou et al[14] |
| Recombinant α-klotho protein | In-vitro LPS administration to HPAEpiCs. CLP in-vivo | Prevents activation of the Bcl-2/Bax/caspase-3 signaling pathway | Klotho increased mouse survival and decreased IL-1β, IL-6, and TNF-α levels. Reducing the percentage of apoptotic cells in lung tissue and HPAEpiCs exposed to LPS | Li et al[42] |
| Treatment with human Wharton’s Jelly-Derived Mesenchymal Stem Cells | CLP | Klotho protein expression was high. Reduction in NF-κB tissue expression. Expression of Bax was reduced, whereas Bcl-X was increased | Survival was improved. Glomerular filtration rate and renal and liver functions were improved. Reduction in apoptosis. Levels of the proinflammatory cytokines IL-1α, IL-6, interferon-γ, and TNF-α were reduced. Endothelial function was improved | Cóndor et al[23] |
| Resveratrol and Recombinant murine α-klotho protein | CLP | Increased tissue expression of klotho protein in both treated groups. The expression of Bax and caspase-3 was reduced, whereas that of Bcl-2 was increased. The reno-protective effect exerted by klotho is through the antiapoptotic effect | Resveratrol has the same effect as exogenous administration of the klotho protein. Improvement in renal function and structure, decrease in serum creatinine and BUN | Chen et al[35] |
| Recombinant klotho protein | LPS | Reduced level of myeloid peroxidase. Attenuation of renal and brain E-selectin, VCAM-1, ICAM-1 mRNA, endothelial adhesion molecule expression, and neutrophil infiltration | Renal and brain inflammation were reduced. Low plasma BUN and plasma NGAL levels. Decreased renal and brain levels of IL-6, IL-1β, IL-8 and TNF-α | Jou-Valencia et al[38] |
Sepsis is associated with increased production of inflammatory cytokines, including TNF-α, IL-6, IL-1 β, and TNF-related weak inducer of apoptosis (TWEAK)[47]. From a pathophysiological view, impaired heart, kidney, or multiple organ functions during sepsis are consequences of inflammation and metabolic disturbance[2]. TWEAK is a TNF-superfamily member that activates the FGF-14 receptor to trigger multiple signaling cascades. FGF-14 levels are relatively low in normal tissue but increase rapidly following tissue injury and inflammatory conditions[48]. Upon TWEAK engagement, trimerization of FGF-14 promotes intracellular signal transduction through canonical and non-canonical nuclear factor-kappa β (NF-κB) pathways and protein kinase cascades including extracellular signal-regulated kinases, c-Jun N-terminal kinase, p38-mitogen activated protein kinase (MAPK), and phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt)[49]. Following sepsis, TWEAK-dependent canonical NF-κB activation and TNF-α production induce multiple responses including cell proliferation, expression of other inflammatory cytokines, and downregulation of protective factors such as klotho[25,28,50]. Targeting NF-κB by administering klotho improved cardiac, renal, and liver functions and reduced TNF-α, IL-6, and IL-1β[23,25].
The antiaging functions of klotho are closely associated with its antioxidative stress effect, inhibiting reactive oxygen species (ROS) production and promoting ROS clearance[51]. The antioxidants superoxide dismutase (SOD) and catalase are reduced with aging. This reduction is associated with activating nicotinamide adenine dinucleotide phosphate and increasing production of hydrogen peroxide[52]. Previous findings showed that increased oxidative stress inhibits klotho expression while low klotho expression in tissues aggravates oxidative damage[28,31]. Studies revealed that overexpression of klotho alleviated oxidative stress-mediated cell injuries[38,41], including heart[31], kidney[41], and endo
The previous findings indicated that oxidative stress arises after sepsis exposure, which harms intracellular organs[53].
It is reported that MAPKs have an important role in regulating oxidative stress-related signaling and participate in the pathogenesis of sepsis[54]. MAPKs are involved in regulating a variety of physiological processes in cells, including proliferation, differentiation, and apoptosis[54]. Klotho treatment inhibited P38-MAPK activation post-sepsis exposure[31]. Klotho prevents oxidative injury and apoptosis through activation of the Forkhead transcription factor O (FoxO) family of transcription factors by inhibition of its phosphorylation and stimulation of manganese SOD (Mn-SOD)[55,56]. The antioxidant effect of klotho protein is mediated by modulating antioxidant signaling pathways involving Mn-SOD, PI3K/Akt/FoxO, and nuclear factor erythroid-related factor 2 (Nrf-2) pathways[57].
Previous reports showed that excessive intracellular ROS can cause oxidative damage to membrane proteins and mitochondrial DNA, leading to structural changes and mitochondrial impairments[14,58]. Moreover, ROS-induced mitochondrial dysfunction, including oxidative injury, could lead to an aggravated imbalance between ROS generation and elimination, causing ROS overload and ultimately triggering the initiation of apoptosis[59]. The activation of apoptosis is characterized by the decline of Bcl and the presence of Bax and caspase-3 activity[23,35,40]. An imbalance between Bax and Bcl-2 plays an important role in the mitochondria-mediated caspase cascade, which was confirmed by an increased Bax/Bcl-2 ratio, release of cytochrome-c from the mitochondria to the cytoplasm, cleavage of caspase-3, and subsequent induction of apoptosis[60]. Klotho increased Bcl and reduced the activity of caspase-3[23,35]. Accumulating evidence indicated that klotho exerts an antiapoptotic effect by suppressing endothelial apoptosis via MAPK and mitochondria-dependent apoptosis pathways as klotho administration inhibited caspase-3 activation[42,61].
The biological effects of FGF-23 are strictly dependent on the cognate coreceptor klotho, which is fundamental for FGF-23 exerting its action at the organ level[29]. Regarding inflammation, FGF-23 has been associated directly with high levels of C-reactive protein and IL-6 in patients with sepsis[37,49]. In addition, klotho was inversely related to IL-6[62]. There is information from biological knowledge in experimental models showing binding between the cytokines and circulating FGF-23 and klotho in proinflammatory conditions. In CLP mice[63], injection of IL-1β as an inflammatory cytokine doubles the serum concentration of intact FGF-23. Contrary to FGF-23, α-klotho protein expression is reduced in response to inflammatory stimuli[25,38].
Nrf-2, the core transcription factor of the endogenous cellular antioxidant stress system, translocates to the nucleus and binds to specific gene loci, thereby attenuating the cellular oxidative stress response and protecting cellular function[64]. In addition, different studies confirmed that increasing Nrf-2 levels were associated with klotho elevation, effectively reducing the related organ damage[65,66]. Klotho protein administration inhibits ferroptosis by activating the Nrf-2 signaling pathway[14]. Klotho overexpression protects cardiac function by regulating the Wnt signaling pathway and activating the AKT/Nrf-2/heme oxygenase-1 pathway to slow cellular senescence and antioxidant activity[67,68]. Studies have shown that klotho promotes angiogenesis and maintains endothelial cell integrity by regulating apoptosis and autophagy of endothelial cells[23,38].
The fall in klotho level after sepsis is of pathophysiological and clinical importance[36,37]. In different sepsis studies, the administration of recombinant klotho protein resulted in considerable improvement in cardiac, liver, and renal functions and structure, with a diminution of oxidation, inflammation, and apoptosis[23,35,38]. Moreover, klotho gene delivery might be a prophylactic agent against sepsis-induced multiple organ injuries.
When a single dose of klotho protein was intraperitoneally injected into septic rats 30-60 minutes after CLP or LPS, it considerably preserved endogenous klotho levels and suppressed the increase in creatinine and blood urea nitrogen[13,25]. Thus, the earlier klotho administration, the better the outcome. Young adult mice expressing more significant plasma klotho levels than adult mice were more resistant to myocardial inflammation[22]. If these results are translated into a clinical setting, klotho would be promising as a prophylactic and therapeutic agent for patients with a high risk of sepsis-induced multiple organ injuries.
Clinical sepsis studies noted that the serum concentration of α-klotho was over-expressed, and FGF-23 was downregulated as a counter-regulatory mechanism in response to acute inflammation[40,62]. The acute increase of serum α-klotho during septic shock may be an attempt to modulate proinflammatory pathways, which then decrease as sepsis resolves[40]. This finding contrasts with other clinical settings that showed lower klotho levels in septic patients[37,38,69]. These findings are summarized in Table 3[37,38,40,62,69].
| Sepsis model | Mechanism | Effect | Ref. |
| Sepsis patients. Collection of warm postmortem biopsies from patients who died of sepsis and renal failure in the ICU | Reduction in renal klotho level. Increased mRNA levels of kidney damage markers neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 | Correlated to renal damage | Jou-Valencia et al[38] |
| Sepsis patients | Reduced serum klotho level | An early predictor of AKI to allow immediate interventions | Pei et al[69] |
| A prospective cohort of ICU patients with sepsis and previously normal renal function | Low klotho and high erythropoietin plasma levels are cofactors for activating FGF-23 | The klotho level is an early predictor of the development of AKI, mortality, and long-term CKD progression in sepsis patients | Toro et al[37] |
| Patients with CKD are admitted to the ICU with acute inflammation/sepsis | At the peak of infection, suppressing the active form of FGF-23 and activating α-klotho | Counter-regulatory response to acute inflammation | Dounousi et al[62] |
| Patients admitted to the ICU with septic shock within the previous 72 hours | Increasing serum level of α-klotho | Higher mortality rate | Abdelmalik et al[40] |
Data from animal or clinical studies show decreased klotho levels in the kidney, heart, and plasma following sepsis. These findings suggest that sepsis is a klotho-deficient state, and this deficiency might have a role in organ dysfunction. The importance of klotho are as follows: First, klotho is a potential early biomarker for diagnosing acute organ injury; Second, early administration of exogenous klotho protein, or enhancement of endogenous klotho by resveratrol or IL-37, may prevent sepsis-induced organ damage; Third, administration of klotho protein and upregulation of endogenous klotho expression after sepsis may accelerate recovery; and Fourth, even if klotho does not change the course of damage, treating klotho deficiency could decrease the risk of progression to a severe prognosis. Elucidating the molecular mechanisms of how klotho functions as a cytoprotective protein will involve its cell regeneration, antioxidant, anti-inflammatory, and antiapoptotic effects.
| 1. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17142] [Article Influence: 1904.7] [Reference Citation Analysis (2)] |
| 2. | Nedeva C, Menassa J, Puthalakath H. Sepsis: Inflammation Is a Necessary Evil. Front Cell Dev Biol. 2019;7:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 223] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 3. | Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1296] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 4. | Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2870] [Cited by in RCA: 4096] [Article Influence: 819.2] [Reference Citation Analysis (4)] |
| 5. | Wardi G, Tainter CR, Ramnath VR, Brennan JJ, Tolia V, Castillo EM, Hsia RY, Malhotra A, Schmidt U, Meier A. Age-related incidence and outcomes of sepsis in California, 2008-2015. J Crit Care. 2021;62:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 663] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 7. | Jump RLP, Canaday DH. Aging Has Unique Effects on the Risks, Presentation, Diagnosis, Treatment, and Prognosis of Infectious Diseases. Infect Dis Clin North Am. 2017;31:xiii-xixv. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Haas L, van Dillen L, de Lange D, van Dijk D, Hamaker M. Outcome of very old patients admitted to the ICU for sepsis: A systematic review. Eur Geriatr Med. 2017;8:446-453. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Martín S, Pérez A, Aldecoa C. Sepsis and Immunosenescence in the Elderly Patient: A Review. Front Med (Lausanne). 2017;4:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Santoro A, Bientinesi E, Monti D. Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Res Rev. 2021;71:101422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 283] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 11. | Kumar NR, Balraj TA, Shivashankar KK, Jayaram TC, Prashant A. Inflammaging in Multidrug-Resistant Sepsis of Geriatric ICU Patients and Healthcare Challenges. Geriatrics (Basel). 2024;9:45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Stortz JA, Hollen MK, Nacionales DC, Horiguchi H, Ungaro R, Dirain ML, Wang Z, Wu Q, Wu KK, Kumar A, Foster TC, Stewart BD, Ross JA, Segal M, Bihorac A, Brakenridge S, Moore FA, Wohlgemuth SE, Leeuwenburgh C, Mohr AM, Moldawer LL, Efron PA. Old Mice Demonstrate Organ Dysfunction as well as Prolonged Inflammation, Immunosuppression, and Weight Loss in a Modified Surgical Sepsis Model. Crit Care Med. 2019;47:e919-e929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Chen X, Tong H, Chen Y, Chen C, Ye J, Mo Q, Zhao G, Hong G, Zheng C, Lu Z. Klotho ameliorates sepsis-induced acute kidney injury but is irrelevant to autophagy. Onco Targets Ther. 2018;11:867-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Zhou P, Zhao C, Chen Y, Liu X, Wu C, Hu Z. Klotho activation of Nrf2 inhibits the ferroptosis signaling pathway to ameliorate sepsis-associated acute kidney injury. Transl Androl Urol. 2023;12:1871-1884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2556] [Cited by in RCA: 2841] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 16. | Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1402] [Cited by in RCA: 1451] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 17. | Kresovich JK, Bulka CM. Low Serum Klotho Associated With All-cause Mortality Among a Nationally Representative Sample of American Adults. J Gerontol A Biol Sci Med Sci. 2022;77:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Han S, Zhang X, Wang X, Wang Y, Xu Y, Shang L. Association between Serum Klotho and All-Cause Mortality in Chronic Kidney Disease: Evidence from a Prospective Cohort Study. Am J Nephrol. 2024;55:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Kim JH, Hwang KH, Park KS, Kong ID, Cha SK. Biological Role of Anti-aging Protein Klotho. J Lifestyle Med. 2015;5:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Olejnik A, Franczak A, Krzywonos-Zawadzka A, Kałużna-Oleksy M, Bil-Lula I. The Biological Role of Klotho Protein in the Development of Cardiovascular Diseases. Biomed Res Int. 2018;2018:5171945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011;60:1907-1916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 22. | Li X, Zhai Y, Yao Q, The E, Ao L, Fullerton DA, Yu KJ, Meng X. Up-regulation of Myocardial Klotho Expression to Promote Cardiac Functional Recovery in Old Mice following Endotoxemia. Res Sq. 2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Cóndor JM, Rodrigues CE, Sousa Moreira Rd, Canale D, Volpini RA, Shimizu MH, Camara NO, Noronha Ide L, Andrade L. Treatment With Human Wharton's Jelly-Derived Mesenchymal Stem Cells Attenuates Sepsis-Induced Kidney Injury, Liver Injury, and Endothelial Dysfunction. Stem Cells Transl Med. 2016;5:1048-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 25. | Hui H, Zhai Y, Ao L, Cleveland JC Jr, Liu H, Fullerton DA, Meng X. Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget. 2017;8:15663-15676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Buchanan S, Combet E, Stenvinkel P, Shiels PG. Klotho, Aging, and the Failing Kidney. Front Endocrinol (Lausanne). 2020;11:560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 27. | Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36:174-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 399] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 28. | Jorge LB, Coelho FO, Sanches TR, Malheiros DMAC, Ezaquiel de Souza L, Dos Santos F, de Sá Lima L, Scavone C, Irigoyen M, Kuro-O M, Andrade L. Klotho deficiency aggravates sepsis-related multiple organ dysfunction. Am J Physiol Renal Physiol. 2019;316:F438-F448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Abolghasemi M, Yousefi T, Maniati M, Qujeq D. The interplay of Klotho with signaling pathway and microRNAs in cancers. J Cell Biochem. 2019;120:14306-14317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Rotondi S, Pasquali M, Tartaglione L, Muci ML, Mandanici G, Leonangeli C, Sales S, Farcomeni A, Mazzaferro S. Soluble α -Klotho Serum Levels in Chronic Kidney Disease. Int J Endocrinol. 2015;2015:872193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Yan F, Feng Y, Chen J, Yan J. Klotho downregulation contributes to myocardial damage of cardiorenal syndrome in sepsis. Mol Med Rep. 2020;22:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Flotyńska J, Uruska A, Araszkiewicz A, Zozulińska-Ziółkiewicz D. Klotho protein function among patients with type 1 diabetes. Endokrynol Pol. 2018;69:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Hu MC, Shi M, Gillings N, Flores B, Takahashi M, Kuro-O M, Moe OW. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 2017;91:1104-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 34. | Xu J, Lin E, Hong X, Li L, Gu J, Zhao J, Liu Y. Klotho-derived peptide KP1 ameliorates SARS-CoV-2-associated acute kidney injury. Front Pharmacol. 2023;14:1333389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Chen Y, Ye X, Jin S, Huang W, Chen C, Chen X. Resveratrol protects against sepsis induced acute kidney injury in mice by inducing Klotho mediated apoptosis inhibition. Trop J Pharm Res. 2022;21:1615-1623. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240-1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 37. | Toro L, Rojas V, Conejeros C, Ayala P, Parra-Lucares A, Ahumada F, Almeida P, Silva MF, Bravo K, Pumarino C, Tong AM, Pinto ME, Romero C, Michea L. A Combined Biomarker That Includes Plasma Fibroblast Growth Factor 23, Erythropoietin, and Klotho Predicts Short- and Long-Term Morbimortality and Development of Chronic Kidney Disease in Critical Care Patients with Sepsis: A Prospective Cohort. Biomolecules. 2023;13:1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Jou-Valencia D, Molema G, Popa E, Aslan A, van Dijk F, Mencke R, Hillebrands JL, Heeringa P, Hoenderop JG, Zijlstra JG, van Meurs M, Moser J. Renal Klotho is Reduced in Septic Patients and Pretreatment With Recombinant Klotho Attenuates Organ Injury in Lipopolysaccharide-Challenged Mice. Crit Care Med. 2018;46:e1196-e1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Sopjani M, Rinnerthaler M, Kruja J, Dermaku-Sopjani M. Intracellular signaling of the aging suppressor protein Klotho. Curr Mol Med. 2015;15:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 40. | Abdelmalik PA, Stevens RD, Singh S, Skinner J, Carhuapoma JR, Noel S, Johns R, Fuchs RJ. Anti-aging factor, serum alpha-Klotho, as a marker of acute physiological stress, and a predictor of ICU mortality, in patients with septic shock. J Crit Care. 2018;44:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Liu X, Niu Y, Zhang X, Zhang Y, Yu Y, Huang J, Li J, Yu C. Recombinant α-Klotho Protein Alleviated Acute Cardiorenal Injury in a Mouse Model of Lipopolysaccharide-Induced Septic Cardiorenal Syndrome Type 5. Anal Cell Pathol (Amst). 2019;2019:5853426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Li XB, Liu JL, Zhao S, Li J, Zhang GY, Tang Q, Chen WY. Recombinant Klotho protein protects pulmonary alveolar epithelial cells against sepsis-induced apoptosis by inhibiting the Bcl-2/Bax/caspase-3 pathway. Adv Clin Exp Med. 2025;34:123-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Inoue S, Suzuki-Utsunomiya K, Suzuki-Utsunomiya K, Sato T, Chiba T, Hozumi K. Abstracts of the 32nd International Symposium on Intensive Care and Emergency Medicine. March 20-23, 2012. Brussels, Belgium. Crit Care. 2012;16 Suppl 1:P1-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Mytych J, Solek P, Koziorowski M. Klotho modulates ER-mediated signaling crosstalk between prosurvival autophagy and apoptotic cell death during LPS challenge. Apoptosis. 2019;24:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Inoue S, Sato T, Suzuki-Utsunomiya K, Komori Y, Hozumi K, Chiba T, Yahata T, Nakai K, Inokuchi S. Sepsis-induced hypercytokinemia and lymphocyte apoptosis in aging-accelerated Klotho knockout mice. Shock. 2013;39:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Chen XX, Zhu XQ, LV W, Tong H, Chen Y, Zhao GJ, Hong GL, Chen ZS; LV ZQ. [The trend of changes in expression of Klotho and autophagy in sepsis-induced acute kidney injury mice model]. Zhonghua Jizhen Yixue Zazhi. 2017;370-376. [DOI] [Full Text] |
| 47. | Guo Y, Ren M, Ge L, Sun C, Li R, Ma C, Sui S. Increased Serum Concentrations of TNF-Like Weak Inducer of Apoptosis Predict Higher 28-Day Mortality in Patients with Sepsis. Emerg Med Int. 2019;2019:7238705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Poveda J, Vázquez-Sánchez S, Sanz AB, Ortiz A, Ruilope LM, Ruiz-Hurtado G. TWEAK-Fn14 as a common pathway in the heart and the kidneys in cardiorenal syndrome. J Pathol. 2021;254:5-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 49. | Wang M, Xie Z, Xu J, Feng Z. TWEAK/Fn14 axis in respiratory diseases. Clin Chim Acta. 2020;509:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Moreno JA, Izquierdo MC, Sanchez-Niño MD, Suárez-Alvarez B, Lopez-Larrea C, Jakubowski A, Blanco J, Ramirez R, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A, Sanz AB. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol. 2011;22:1315-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 331] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 51. | Lin YT, Wu PH, Tsai YC, Hsu YL, Wang HY, Kuo MC, Kuo PL, Hwang SJ. Indoxyl Sulfate Induces Apoptosis Through Oxidative Stress and Mitogen-Activated Protein Kinase Signaling Pathway Inhibition in Human Astrocytes. J Clin Med. 2019;8:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Poljsak B, Milisav I. Aging, Oxidative Stress and Antioxidants. Oxidative Stress and Chronic Degenerative Diseases-A Role for Antioxidants. In: Morales-Gonzalez JA, editor. United Kingdom: IntechOpen, 2013. [DOI] [Full Text] |
| 53. | Prauchner CA. Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy. Burns. 2017;43:471-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 54. | Zhang YY, Ning BT. Signaling pathways and intervention therapies in sepsis. Signal Transduct Target Ther. 2021;6:407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 181] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 55. | Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029-38034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 587] [Cited by in RCA: 563] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 56. | Xing L, Fang J, Zhu B, Wang L, Chen J, Wang Y, Huang J, Wang H, Yao X. Astragaloside IV protects against podocyte apoptosis by inhibiting oxidative stress via activating PPARγ-Klotho-FoxO1 axis in diabetic nephropathy. Life Sci. 2021;269:119068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 57. | Donate-Correa J, Martín-Carro B, Cannata-Andía JB, Mora-Fernández C, Navarro-González JF. Klotho, Oxidative Stress, and Mitochondrial Damage in Kidney Disease. Antioxidants (Basel). 2023;12:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 58. | Supinski GS, Wang L, Schroder EA, Callahan LAP. MitoTEMPOL, a mitochondrial targeted antioxidant, prevents sepsis-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol. 2020;319:L228-L238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 59. | Yao X, Carlson D, Sun Y, Ma L, Wolf SE, Minei JP, Zang QS. Mitochondrial ROS Induces Cardiac Inflammation via a Pathway through mtDNA Damage in a Pneumonia-Related Sepsis Model. PLoS One. 2015;10:e0139416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 60. | Li J, Liu F, Jiang S, Liu J, Chen X, Zhang S, Zhao H. Berberine hydrochloride inhibits cell proliferation and promotes apoptosis of non-small cell lung cancer via the suppression of the MMP2 and Bcl-2/Bax signaling pathways. Oncol Lett. 2018;15:7409-7414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Maekawa Y, Ohishi M, Ikushima M, Yamamoto K, Yasuda O, Oguro R, Yamamoto-Hanasaki H, Tatara Y, Takeya Y, Rakugi H. Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr Gerontol Int. 2011;11:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 62. | Dounousi E, Torino C, Pizzini P, Cutrupi S, Panuccio V, D'Arrigo G, Abd ElHafeez S, Tripepi G, Mallamaci F, Zoccali C. Intact FGF23 and α-Klotho during acute inflammation/sepsis in CKD patients. Eur J Clin Invest. 2016;46:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Bayer J, Vaghela R, Drechsler S, Osuchowski MF, Erben RG, Andrukhova O. The bone is the major source of high circulating intact fibroblast growth factor-23 in acute murine polymicrobial sepsis induced by cecum ligation puncture. PLoS One. 2021;16:e0251317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20:689-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1299] [Cited by in RCA: 1452] [Article Influence: 363.0] [Reference Citation Analysis (0)] |
| 65. | Chen K, Wang S, Sun QW, Zhang B, Ullah M, Sun Z. Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway. Circ Res. 2021;128:492-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 66. | Li W, Yu W, Xu W, Xiong J, Zhong X, Hu S, Yu J. Death-Associated Protein Kinase 1 Regulates Oxidative Stress in Cardiac Ischemia Reperfusion Injury. Cells Tissues Organs. 2021;210:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 67. | Xu Z, Zheng S, Feng X, Cai C, Ye X, Liu P. Klotho gene improves oxidative stress injury after myocardial infarction. Exp Ther Med. 2021;21:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Cui W, Leng B, Wang G. Klotho protein inhibits H(2)O(2)-induced oxidative injury in endothelial cells via regulation of PI3K/AKT/Nrf2/HO-1 pathways. Can J Physiol Pharmacol. 2019;97:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 69. | Pei Y, Zhou G, Wang P, Shi F, Ma X, Zhu J. Serum cystatin C, kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, klotho and fibroblast growth factor-23 in the early prediction of acute kidney injury associated with sepsis in a Chinese emergency cohort study. Eur J Med Res. 2022;27:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |