Published online Jun 9, 2023. doi: 10.5492/wjccm.v12.i3.92
Peer-review started: December 28, 2022
First decision: January 31, 2023
Revised: February 13, 2023
Accepted: March 22, 2023
Article in press: March 22, 2023
Published online: June 9, 2023
Processing time: 162 Days and 7.9 Hours
Sleep is a complex process influenced by biological and environmental factors. Disturbances of sleep quantity and quality occur frequently in the critically ill and remain prevalent in survivors for at least 12 mo. Sleep disturbances are associated with adverse outcomes across multiple organ systems but are most strongly linked to delirium and cognitive impairment. This review will outline the predisposing and precipitating factors for sleep disturbance, categorised into patient, environmental and treatment-related factors. The objective and subjective methodologies used to quantify sleep during critical illness will be reviewed. While polysomnography remains the gold-standard, its use in the critical care setting still presents many barriers. Other methodologies are needed to better understand the pathophysiology, epidemiology and treatment of sleep dis
Core Tip: Disturbed sleep is common among the critically ill and contributes to adverse physiological and psychological outcomes. Multiple contributory factors have been identified, including environmental, care-related and patient elements. Assessing sleep in the ICU is challenging, and objective and subjective methods are required to evaluate the disruption to sleep architecture and the patient’s experience of this. Both pharmacological and non-pharmacological interventions to improve sleep quality and quantity have been studied with mixed results, however, a multimodal approach to sleep optimisation is likely necessary to improve outcomes.
- Citation: Showler L, Ali Abdelhamid Y, Goldin J, Deane AM. Sleep during and following critical illness: A narrative review. World J Crit Care Med 2023; 12(3): 92-115
- URL: https://www.wjgnet.com/2220-3141/full/v12/i3/92.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v12.i3.92
Sleep is an essential biological process that is frequently disturbed in patients with critical illness[1,2]. Sleep deprivation in healthy adults is associated with adverse effects on neuropsychiatric, cognitive, cardiovascular, respiratory and endocrine systems and with acute and long-term detrimental effects[3].
There are concerns that an inadequate quantity and quality of sleep during critical illness contributes to increased delirium, depression, and a lesser quality of life in survivors and, potentially, increased mortality, with the detrimental effects of sleep deprivation compounded among those with prolonged admission to the intensive care unit (ICU)[4]. In addition, sleep disturbance is frequently reported as a source of patient distress and has been proposed to have financial implications related to longer ICU admission and increased risk of delirium[5].
Sleep disturbance in the ICU is multifactorial, with pre-morbid diagnoses, acute pathology, treatment and environment all contributing[6,7]. Given the complex pathophysiology, it should be expected that the studied interventions, including pharmacological and non-pharmacological strategies, have had mixed results on sleep[7,8].
This review aims to describe the current understanding of sleep disruption during and after critical illness, current strategies to measure sleep in the ICU, and provide an overview of interventions to improve the quality and quantity of sleep in this population.
A narrative review of the literature was performed. Relevant articles were identified by searching Medline, Embase and the Cochrane database. Search terms included “intensive care unit”, “high dependency unit”, “critical illness”, “sleep”, “sleep disturbance”, “sleep deprivation”, “sleep-wake disorder”, and “sleep fragmentation”. Searches were limited to human adult subjects and English language articles. No restrictions on the date of publication were imposed. Abstracts were reviewed for relevance, and the reference list of these articles was searched for related articles. The full text of relevant articles was reviewed for inclusion.
Sleep is a complex and active process, recognized by reversible perceptual disengagement from, and unresponsiveness to, the environment[9]. The initiation and maintenance of the sleep state are controlled by the coordinated interplay of circadian and homeostatic mechanisms[10-13]. On the basis of polygraphic recordings of brain, muscle and eye activity, normal sleep can be divided into distinct periods, which are recognized as non-rapid eye movement (NREM) and rapid eye movement (REM) sleep[11]. Characteristic features of each sleep stage are described in Table 1[14,15]. NREM sleep is further subdivided into three stages, N1, N2 and N3, reflecting an increasing depth of sleep[16,17]. The N2 phase has characteristic K-complexes and sleep spindles, electrical features which are believed to represent important functions, including the promotion of deeper sleep and memory consolidation[11,18,19]. The N3 phase is synonymous with slow wave sleep, during which many of the physiologically restorative processes of sleep occur[11,20]. REM sleep is when dreaming occurs and is important for memory consolidation and learning[11,21,22]. The brain normally cycles through each phase of sleep over 90-120 min, with 4-5 cycles occurring over the course of the night[11,23]. While the total amount of time spent asleep varies significantly, observational studies indicate that adverse outcomes are associated with sleeping less than seven hours or greater than nine hours per day over the long term[24-26]. In summary, both the architecture, or quality, and duration of sleep are important to mediate its beneficial effects.
| Sleep stage | Electroencephalogram | Electrooculogram | Chin electromyogram |
| Wake | Alpha activity (sinusoidal 8-13 Hz) | Rapid eye movements; Reading eye movements; Slow eye movements; Blinks | Normal or high tone |
| N1 | < 50% alpha activity; > 50% low amplitude mixed frequency activity (4-7 Hz) | Slow eye movements | Variable, usually lower than wake |
| N2 | Sleep spindles; K-complexes | None | Variable tone |
| N3 | Slow (delta) wave (0.5-2 Hz) ≥ 20%; Sleep spindles may occur | None | Variable tone |
| REM | Low amplitude mixed frequency activity; No sleep spindles or K-complexes | Rapid eye movements | Low tone |
Disturbed sleep in the ICU is a near-universal phenomenon. Subjective perception of poor sleep determined using a variety of questionnaires has been reported by 47%-59% of patients[27-30]. Studies using objective measures, including polysomnography and actigraphy, estimate that 67%-100% of patients experience abnormal sleep quality[29,31,32].
Following discharge from the ICU, sleep disturbances persist in 10%-61%[33]. Both objective and subjective measures indicate that sleep disruption improves over time but is still present in up to 61% of ICU survivors 6-12 mo after discharge[34]. In a single-centre, prospective cohort study of 347 patients, Combes et al[35] identified sleep disturbance as far as three years after ICU discharge. Women appear to be more affected by persistent sleep disturbances than men[36]. Sleep disruption was associated with other adverse features, including persistent post-traumatic stress disorder, depression, weakness, fatigue, pain and reduced quality of life, although these associations are likely bidirectional[37-42].
Studies that assess sleep using objective methodologies report improvements in sleep architecture between one week and six months post-discharge. Sleep fragmentation, with a high number of arousals, was prominent up to three months, and sleep efficiency remained impaired out to six months[39,43,44]. Objective sleep disturbances correlated with subjectively measured patient perception.
There is a high prevalence of sleep disturbances among ICU patients and survivors that persists for at least 12 mo following discharge and appear to be associated with other long-term, adverse patient outcomes and reduced quality of life.
The cause of sleep disruption in the critically ill is multi-factorial and can be divided into environmental, therapy-related and patient factors.
Patient factors, including increasing age, male sex, and poor sleep quality at home, have been associated with worse ICU sleep parameters[36,45]. The relationship between acute illness severity and sleep disruption is biologically plausible but has been inconsistently demonstrated. Two small studies, including a total of 35 patients, found a correlation between greater illness severity, determined by Acute Physiology and Chronic Health Evaluation (APACHE III) score and Simplified Acute Physiology Score (SAPS II) respectively, and greater sleep disruption[46,47]. In contrast, illness severity, as measured by the patient’s Acute Physiology and Chronic Health Evaluation (APACHE III) score, was not found to be correlated with total sleep time, sleep fragmentation or subjective perception of sleep quality from four studies involving 264 patients[31,36,45,48].
Patients report that distress, anxiety, and pain are factors that impair their ability to sleep[49-53]. Sleep deprivation has, in turn, been identified as a stressor contributing to patient anxiety and distress and creating a positive feedback loop[51,54-56].
Loss of diurnal variation and circadian entrainment: Critically ill patients have been shown to have temporally recognized circadian rhythmicity, likely due to the absence or disruption of normal external entraining cues, such as light exposure, changes in ambient temperature and eating patterns[13,42,57,58]. In health, circadian rhythms are crucial for sleep regulation, and disrupted sleep during critical illness is likely to be part of the circadian dysfunction that occurs in these patients[13,58,59].
Ambient light: Diurnal variation in light is an important entrainer of the circadian rhythm. Light intensity, wavelength and spectral distribution all affect the physiological response to light exposure[60]. ICU patients rate ambient light as a common contributing factor to poor sleep[30,61-63]. Both low levels of daytime light and peak light levels in the early evening have been reported, which pose a risk to circadian rhythms and maintenance of normal sleep-wake patterns[6]. Prolonged light exposures have been documented to occur frequently during the nocturnal sleep period[64].
Noise: Patients perceive noise as a significant factor leading to poor sleep in the ICU, with talking, equipment alarms, the television, and use of the bedside phone by staff being common causes[36,46,65]. The World Health Organisation recommends that noise levels within hospital environments should not exceed 35 decibels (dB) during the day and 30 dB at night[66]. Multiple studies report noise levels are frequently greater than this, with equivalent continuous sound levels of 50-75 dB and peaks up to 96 dB[67-69]. This noise level is associated with sleep disruption[45,70]. Polysomnography detected sleep disturbances were observed when sound thresholds exceeded 63 and 59 dB during daytime and nighttime, respectively. Estimates of noise-related sleep disturbance in the ICU vary from 11% to 58%[31,46,62,63,71-74].
Critically ill patients require intensive monitoring and care 24 h a day. Nursing and medical interventions, including mouth and eye care, decubitus ulcer care, change of dressings, medication administration, blood sampling, endotracheal tube suctioning, clinical examination, and procedures may interfere with patient sleep[46,75]. Patients perceive these care activities as a substantial contributor to sleep disruption[30,62,74]. It has been reported that over the course of a night, patients were subjected to an average of 42.6 to 51 care interactions, with approximately 20% of these resulting in a clinically evident sleep disruption[46,75,76]. One study even identified increased care activities occurring between 02:00 and 05:00[75].
A proportion of nocturnal care activities are essential in the ICU. Whether the frequency and intrusiveness of nocturnal care activities are excessive and lead to harm due to sleep fragmentation and sleep deprivation, such as neurological observations performed and recorded at one-hourly intervals, remains uncertain[77,78].
Mode of mechanical ventilation: Critically ill patients frequently require respiratory support, and mechanical ventilation contributes to sleep disruption. Patient-ventilator dyssynchrony, abnormal gas exchange, and mechanical ventilation-related central apnoeas are all considered contributory[6,79,80]. Mechanically ventilated patients experience disturbed sleep architecture with frequent arousals and decreased amounts of slow wave and REM sleep[48,57,81]. The effect of the mode of ventilation on sleep has been studied, but due to the limited number of patients observed and methodological limitations, the impact of ventilator mode remains to be determined.
Studies comparing pressure support ventilation (PSV) to assist-control ventilation report point estimates suggesting assist-control decreases fragmentation, increases total sleep time, slow wave sleep and REM sleep, and reduces central apneas, but the wide confidence intervals are indicative of considerable uncertainty about this effect[79,81,82].
A single study comparing pressure control ventilation to pressure support ventilation reported statistically significant improvements in sleep efficiency and proportion of time in N2, N3 and REM sleep with a pressure control mode[83]. Notably, all 26 patients included in the study had chronic respiratory disease, which limits the application of these findings to a broader patient population, and whether nocturnal pressure control ventilation delays liberation from ventilation is also unknown.
Several proportional assist ventilatory modes have been compared to pressure support ventilation with mixed results[84-86]. Details of these studies have been recognized in Table 2.
| Ref. | Study Design | n | Treatment arms | Sedation | Outcomes | ||
| Studies comparing pressure support ventilation against assist control ventilation | |||||||
| Parthasarathy et al[79], 2002 | Single centre, randomised, cross over study | 11 | 2 h each of: | ACV; Vt: 8 mL/kg; f: Set as patient RR minus 4/min | Yes | Sleep efficiency: Arousal index, mean (SD) | Not reported; ACV 39 (6); PSV 35 (7); No statistically significant difference between ventilation modes |
| Toublanc et al[81], 2007 | Single centre, randomised, cross over study | 20 | 4 h each of: | ACV; Vt: 10 ml/kg; f: 12/min; Increased until no spontaneous inspiratory effort | Free from sedation for 48 h | Sleep efficiency: Arousal index, mean (SD) | No difference, values not reported; ACV 7 (SD 5); PSV 7 (SD 5); No statistically significant difference between ventilation modes |
| Cabello et al[82], 2008 | Single centre, randomised, cross over study | 15 | 6 h each of: cPSV; PS to achieve Vt 6-8 ml/kg (PBW); RR < 35/min; aPSV | ACV; Vt: 8 mL/kg; f: 10/min (back up) | Free from sedation for 24 h | Sleep efficiency, median [IQR]: Arousal index, median [IQR] | ACV 58 [48-82], cPSV 44 [29-30], aPSV 63 [29-80]; ACV 30 [17-41], PSV 28 [17-53], aPSV 23 [21-45]; No statistically significant difference between ventilation modes |
| Studies comparing pressure support ventilation against pressure control ventilation | |||||||
| Andréjak et al[83], 2013 | Single centre, randomised,cross over study | 26 | 4 h each of: | PCV; PS = 20 cmH2O; f: Greater than patient RR I/E ratio: 1/1.2 to 1/1.5 | Not reported | Sleep efficiency, median [IQR]: Arousal index | PCV 63 [9-100]; PSV 37 [0-96] Not reported; Significantly improved sleep efficiency with PCV |
| Studies comparing pressure support ventilation against proportional assist ventilation | |||||||
| Bosma et al[84], 2007 | Single centre, randomised, cross over study | 13 | 1 night each of: PSV | PAV | Propofol, midazolam or lorazepam | Sleep efficiency, mean (SD): Arousal index, median [IQR]: Patient-ventilator asynchrony per hour, mean (SD) | PSV 58% (25); PAV 60 (23); PSV 16 (2-74); PAV 9 (1-41); PSV 53 (59); PAV 24 (15); PAV associated with statistically significantly fewer arousals and episodes of asynchrony |
| Alexopoulou et al[85], 2007 | Single centre, randomised, cross over study | 17 | 1 night each of: | PAV+base; Set to achieve mean inspiratory pressure similar to PSVbase; PAV+high; Percentage of unloading increased by 40%-50% from PSVbase or until it reached 85% | Group A; n = 11; Propofol; Group B; n = 9; Non-sedated | Group A; Sleep efficiency, mean (SD): Arousal index, mean (SD): Group B; Sleep efficiency, mean (SD): Arousal index, mean (SD) | PAV+base 99 (2); PAVhigh 98 (5); |
| Alexopoulou et al[86], 2013 | Single centre, randomised,cross over study | 14 | Alternating 4-h blocks over 24 h of: | Free from sedation and opioids for 24 h | Sleep efficiency, median [IQR]: Arousal index, median [IQR] | PAV+ 51 [13-66]; PSV 27 [6-22]; PAV+ 11 [4-25]; PSV 12 [3-16]; No statistically significant improvement found with PAV+ | |
| PSV; PS maintained at pre-study level | PAV+; % of unloading set to achieve a mean inspiratory pressure similar to PSV | ||||||
| Studies comparing pressure support ventilation against neurally adjusted ventilatory assist | |||||||
| Delisle et al[236], 2011 | Single centre, randomised, cross over study | 14 | 2 non-consecutive 4-h blocks (d/night) of: | Free from sedation and opioids for 24 h | Sleep efficiency, median [IQR]: Fragmentation index, median [IQR] | NAVA 74 [52-77]; PSV 44 [29-74]; NAVA 18 [8-22]; PSV 34 [25-54]; NAVA statistically significant improvement in the efficiency and reduced fragmentation of sleep | |
| PSV; PS to achieve Vt 8 mL/kg; RR < 35/min | NAVA | ||||||
The association between non-invasive ventilation use and sleep quality has also been evaluated. Using an ICU ventilator, rather than a dedicated non-invasive ventilator, to provide non-invasive respiratory support is associated with reduced patient-ventilator dyssynchrony and number of arousals[87]. In addition, detection of early abnormal sleep architecture in patients with hypercapnoeic respiratory failure was associated with late NIV failure[88].
In the immediate period following discharge from ICU and at both 6 and 12 mo following discharge, exposure to mechanical ventilation during a patient’s ICU stay does not seem to be associated with subsequent sleep disturbance[34,47].
In summary, there appears to be some effect of ventilatory mode on sleep quality and quantity, however, a consistent physiological rationale remains elusive. In addition, the included studies are hindered by small sample sizes, and further larger-scale studies are required to elaborate on the relationship between ventilation mode and sleep.
Feeding and nutrition: Nutritional support is an essential ICU treatment and would commonly be administered as a continuous infusion over 24 h in those that cannot eat[89]. The timing of meals and the associated release of nutritional hormones is an important entraining cue for circadian rhythms. Continuous delivery of nutrition may contribute to circadian rhythm and sleep disruption, and intermittent feeding may reduce this effect[90]. However, intermittent feeding regimens have not been shown to improve patient outcomes, possibly because of delayed gastric emptying[91,92]. Hitherto, there have been no trials evaluating intermittent enteral nutrition on circadian rhythm and sleep parameters, but a randomised clinical trial will soon be completed (ClinicalTrials.gov Identifier: NCT04737200).
Pharmacological: Critically ill patients are exposed to multiple drug classes that may affect sleep quantity and quality. However, very little published research directly quantifies this, and much of the information below is extrapolated from drug effects in other patient populations.
Several studies have demonstrated that mechanically ventilated patients receiving sedation have longer total sleep time and higher sleep efficiency but more atypical sleep than patients who are not intubated and sedated[57,93,94]. Propofol is one of the most frequently used sedative agents in the ICU, but there is conflicting evidence of its effect on sleep. Propofol is reported to disrupt REM sleep and delay sleep onset latency, however, in animal models there is evidence that propofol-induced sedation may confer some of sleep’s restorative effects[95,96]. A single-centre, prospective cohort study of 50 intubated patients found that sedation with propofol as a single agent was associated with increased sleep duration and decreased fragmentation when compared to fentanyl, propofol and fentanyl, or no sedation[97]. In contrast, a small crossover study of 12 mechanically ventilated patients reported that propofol, compared to no sedation, did not significantly affect total sleep duration or fragmentation, but adversely impacted the duration of REM sleep[98].
Benzodiazepine use is associated with increased total sleep time, resulting from decreased sleep latency and prolongation of the N2 sleep phase, at the cost of reduced slow wave and REM sleep[99]. Opioids, even as a single dose, have been shown to reduce the duration of slow wave and REM sleep[100-102]. The central alpha-2 adrenoreceptor agonist, dexmedetomidine, is associated with increased sleep efficiency and proportions of N3 sleep but decreased REM sleep[96,103,104].
Adrenergic catecholamines can cause suppression of REM and slow wave sleep[105,106]. Both amiodarone and lipid soluble beta-blockers may theoretically have adverse effects on sleep that include decreased REM sleep and nightmares[99]; however, whether these drugs have any effect during critical illness has not been evaluated.
In other patient groups, sedating tricyclic antidepressants such as amitriptyline decrease sleep latency, increase the proportion of slow wave sleep and decrease the proportion of REM[99,107]. Venlafaxine is recognised to suppress REM sleep and cause nightmares, while selective serotonin inhibitors can cause increased wakefulness, reduced total sleep time and decreased REM sleep[99,107,108]. Antipsychotic medications are of particular interest due to their use in the management of delirium and have been observed to have variable effects on sleep architecture. Haloperidol has been shown to increase sleep efficiency, whereas the atypical agents, olanzapine and risperidone, have the additional effect of promoting slow wave sleep[99,109-111].
Corticosteroid use is associated with multiple neurocognitive, behavioural and circadian changes that may contribute to poor sleep[99,112]. Exogenous steroid use may cause misalignment of the hypopituitary adrenal axis with adverse effects on the circadian rhythm, which may be further exacerbated by steroid-induced suppression of melatonin secretion[112].
Multiple pharmacological agents may diminish sleep in the ICU. Sedation is frequently necessary to facilitate treatment and reduce patient distress. The true impact of current sedative regimes on sleep quantity and quality remains incompletely defined. Multiple pharmacological agents suppress slow wave and REM sleep, which may contribute to sleep deficit-related morbidity.
Sleep disturbance may be characterised by abnormalities, including difficulties falling asleep (sleep initiation), staying asleep (sleep maintenance), frequent awakenings or arousals (fragmentation), and atypical sleep architecture. Patients with critical illness largely preserve their total time asleep, or total sleep time, however, this sleep is highly fragmented and spread over 24-h[63,113-118]. Instead of being consolidated in a single nocturnal sleep period, approximately 50% of sleep in critically ill patients occurs during daytime hours[63,114,115].
Sleep architecture during critical illness is frequently abnormal[31,32]. Polysomnographic studies demonstrate a lack of variability in the electroencephalogram (EEG), with a predominance of the ‘lighter’ N1 and N2 phases, paucity or absence of N3 and REM sleep, and frequent arousals[119,120]. Additional features of atypical sleep include the relative absence of K-complexes and sleep spindles, as well as dissociation of the EEG from behavioural findings. Such dissociations manifest as either pathologic wakefulness, characterised by an EEG frequency consistent with sleep in awake patients or unresponsive patients with EEG frequencies associated with being awake[119]. These EEG abnormalities mean that 16-85% of polysomnographic data in observational studies were not able to be qualified using standard scoring systems[113-115,117,121]. Amended criteria have been proposed that recognise this atypical sleep pattern[113,115]. Watson et al[115] proposed an additional seven criteria for sleep scoring in the critically ill with robust reported interrater reliability (weighted kappa 0.80; bootstrapped 95% confidence interval 0.48, 0.89) but this has not been externally validated. Notably, the development of an atypical sleep pattern was strongly associated with the subsequent development of delirium, a longer ICU length of stay, and higher odds of death[116].
In summary, critically ill patients display multiple and severe perturbations in their sleep that are not well described by current sleep scoring classifications. Several of these abnormalities are associated with a worse prognosis, yet it remains unclear if these are modifiable endpoints or markers of disease severity.
Measuring sleep in the critically ill poses many challenges and is frequently confounded by sedation, encephalopathy, primary neurological insults, and prioritisation of more imminently life-threatening issues[6]. Both objective and subjective measurement tools have been used independently or in combination[59].
Polysomnography uses polygraphic recording of electroencephalographic, electromyographic, and electro-oculographic data to measure sleep and is considered the gold-standard technique. There are two predominant systems for scoring polysomnographic sleep data. The Rechtschaffen and Kales (R&K) criteria, first published in 1968, describe five phases of sleep in healthy individuals but were superseded in 2007 by the American Academy of Sleep Medicine’s (AASM) sleep scoring rules[16]. The AASM and R&K scoring rules share many similarities (Table 3) but show relatively low concordance when scoring NREM phases[15-17,122,123]. Moreover, both lack accuracy in quantifying the atypical sleep seen in the critically ill[124]. Logistical, technical, and financial barriers to the use of polysomnography in ICU have been described, including access to specialist equipment and the support of a sleep service for set-up and analysis[119,125,126]. The device itself is reported to interfere with the delivery of patient care, is tolerated poorly by up to 25% of patients, and patient discomfort from the device may worsen sleep[119,127]. Accordingly, while polysomnography remains the gold-standard technique for ambulant patients, there is a need for other methodologies to quantify sleep during critical illness.
| AASM | R&K | |
| Wake | Stage W | Stage W |
| NREM sleep | Stage N1 | Stage 1 |
| Stage N2 | Stage 2 | |
| Stage N3 | Stage 3 | |
| Stage 4 | ||
| REM sleep | Stage R | Stage REM |
The electroencephalogram used in polysomnography provides invaluable information about sleep stages. Multiple attempts to simplify this element of sleep analysis have been described, using a reduced number of EEG leads, spectral analysis of the EEG frequencies, and automated scoring algorithms. Several studies have attempted to analyse limited EEG leads using different techniques. Bispectral Index (BIS) was developed as a depth of anaesthesia monitor for use in the operating theatre. A limited channel EEG signal is acquired using a single strip of electrodes applied to the forehead. Bispectral and power spectral analysis of the EEG is used to generate a numerical score to indicate depth of sedation[128]. While BIS has been used to investigate sleep in the critically ill, studies of BIS for sleep monitoring in both healthy volunteers and critically ill adults have reported that BIS is inaccurate for the detection of various sleep stages, particularly in differentiating REM from N1/N2 sleep phases, and correlates weakly with multiple domains on the Richards-Campbell Sleep Questionnaire[129,130].
Alternative attempts to use spectral EEG analysis to monitor sleep in the critically ill, including the odds-ratio product index and ICU depth of sleep index, offer potentially useful alternatives[131]. Spectral EEG analysis using fast Fourier transformation showed perfect inter-observer and intra-observer agreement, however, the sample size of only 14 patients limits the generalizability of this finding[124]. These techniques do not rely on traditional scoring parameters, such as the presence of sleep spindles, and consequently are not affected by the absence or atypia of these features as reported by other authors[113]. The use of spectral analysis has the potential to simplify sleep assessment in the ICU, however, correlation with standard polysomnography parameters, as well as standardization and external validation, will be necessary before it can be more widely applied.
To reduce the complexity associated with the use of polysomnography, several ‘simplified’ proprietary devices have been trialled. The SedlineTM is a portable monitor that is able to acquire limited lead EEG using bifrontal electrodes to derive a Patient State Index, which represents varying levels of cons
Actigraphy devices, commonly worn on the wrist or ankle, use omnidirectional accelerometers to detect limb movement; these limb movements are interpreted using automated algorithms to estimate sleep-wake state[125,134]. These devices are minimally invasive, relatively straightforward to use, and have been used to assess sleep in outpatient settings[135]. Given the frequency and magnitude of critical illness weakness, studies of actigraphy in the critical care setting have identified poor overall accuracy, with over-estimation of total sleep time and sleep efficiency, when compared to polysomnography, nurse observation, or BIS[136]. Actigraphy has been used to evaluate sleep-promoting interventions in ICU, however, the poor correlation with other validated measures of sleep limits inferences from these studies[136,137].
Novel devices: The Nemuri SCANTM, an under-bed mattress sensor, has been evaluated to measure sleep in a total of 29 ICU patients in two prospective observational studies[138,139]. When compared to polysomnography, moderate agreement but poor specificity was reported. In addition, there was no correlation with subjective sleep, quantified using the Richards-Campbell Sleep Questionnaire.
The most frequently used research methods to objectively measure sleep in the critically ill have been summarised in Table 4. There is no methodology available that provides clinicians with real-time objective information each morning regarding the quantity and quality of a patient’s sleep the night before. Such information has the capacity to transform clinical care.
| Method | Benefits | Limitations |
| Full polysomnography (PSG) | Gold standard technique; Provides polygraphic data on EEG, eye movements and chin tone; Established guidelines for interpreting data for normal sleep | Complex set up; Relatively expensive; Poorly tolerated in 25% of patients; Interferes with nursing care; May interfere with patient sleep; Interpretation requires sleep specialist; No validated criteria for atypical EEG found commonly in critically ill |
| Bispectral index (BIS) monitor | Small anatomic footprint; Simplified set up compared to PSG; Does not require sleep specialist for interpretation; Less affected by atypical EEG common in critically ill | Inaccurate differentiation of REM from N1/N2 sleep; Correlates weakly with RCSQ; No validated criteria for interpretation of results; Primarily designed to monitor depth of sedation |
| Limited lead EEG | Small anatomic footprint; Simplified set up compared to PSG; May not require sleep specialist for interpretation | Accuracy dependent on device and auto-staging software; Interpretation dependent on sleep specialist if not using auto-staging |
| Actigraphy | Minimally invasive; Simple set up; Easy to perform serial measures; Established use in outpatient setting | Poor accuracy compared to PSG and nurse observation, including over-estimation of total sleep time and sleep efficiency; Confounded by immobility, weakness, sedation, and neurological injury |
| Under mattress sensor | Non-invasive modality; Simple set up | Moderate agreement, but poor specificity compared to PSG; No correlation with RCSQ |
Understanding the subjective quality of patients’ sleep is an important component of a holistic assessment. Direct patient self-report is of greatest interest, however, due to factors such as delirium and administration of sedation and analgesic drugs, it is estimated that only around 50% of the ICU population can participate in such efforts[73].
Thirteen different questionnaires have been used to quantify sleep in the ICU, of which 10 were reported by patients and three reported by ICU nurses[119,140]. Several tools allow for either the patient or nurse to complete them, although accuracy is inconsistent[140].
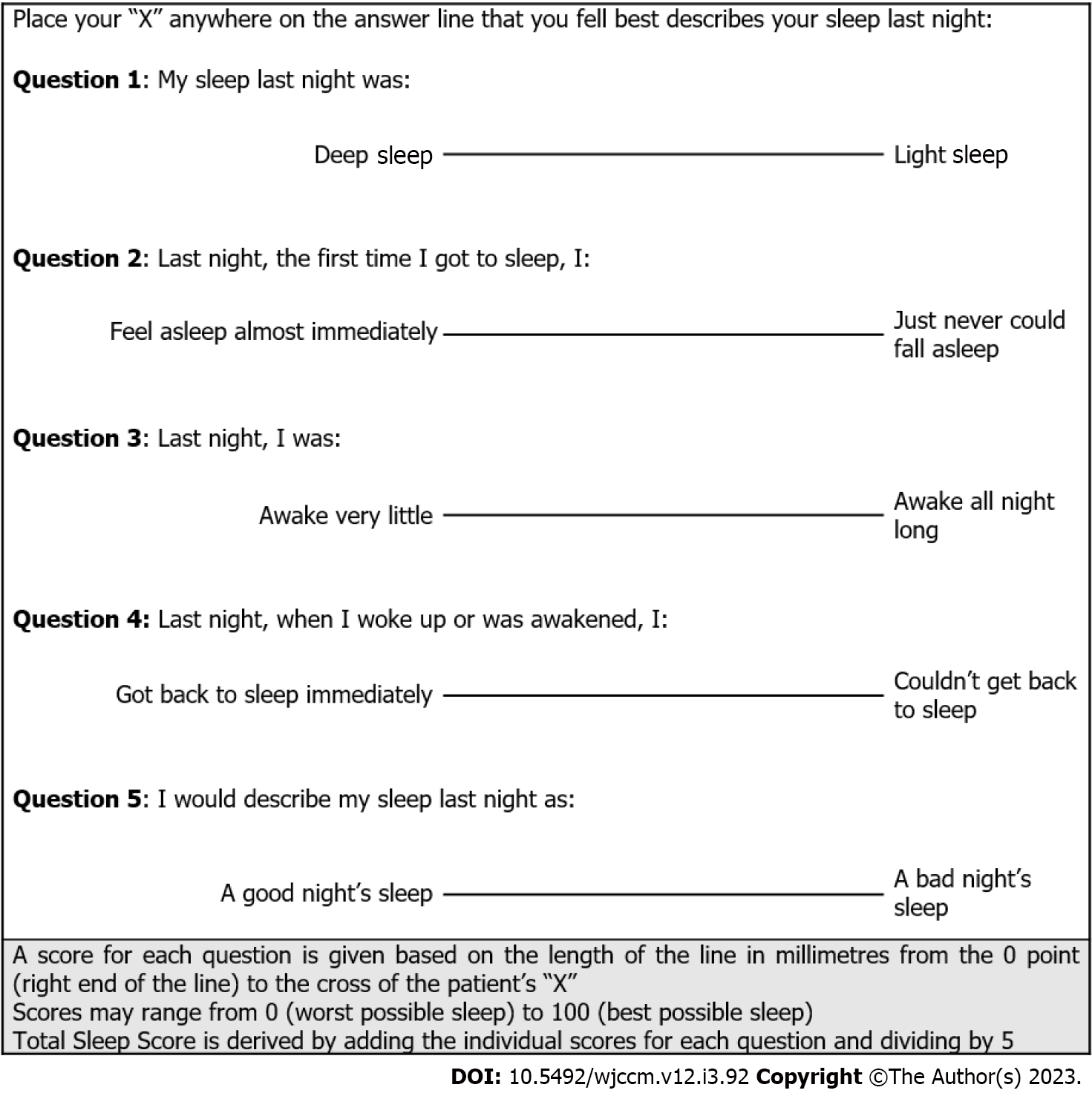
Of the 13 sleep questionnaires used, the most rigorously studied is the Richards-Campbell Sleep Questionnaire (RCSQ). The RCSQ was specifically designed for use in the ICU population and uses five visual analogue scales to assess the domains of sleep latency, sleep efficiency, sleep depth, number of awakenings and overall sleep quality (Figure 1)[141]. Individual domain scores can be interpreted respectively or combined into a global score, with a score of ≥ 63 out of 100 reported as the optimal cut-off for self-reported ‘good sleep’[142]. Both content and criterion validity have been established against polysomnography[143]. While the RCSQ was designed as a patient self-assessment tool, it may also be completed by clinical staff. The accuracy of clinician-completed RCSQ remains unclear with a reported strength of agreement including slight to moderate, moderate, and strong[73,144]. The use of the RCSQ in the outpatient setting has also been established, allowing serial assessments to be continued following ICU discharge[145]. The RCSQ has been translated and validated in multiple languages[146].
The Verran Snyder-Halpern (VSH) sleep scale is an 8-15 visual analogue scale, self-reported sleep questionnaire that assesses similar domains to the RCSQ but, due to its higher number of questions, is considered more labour intensive[125]. The VSH sleep scale was designed to assess sleep in hospitalised patients without known sleep disorder[125,147,148]. The VSH has been validated for use in the ICU in several studies, but the association between patient and clinician-reported sleep was low[120,149-152].
The Pittsburgh sleep quality index (PSQI) is a nine-item, self-reported sleep questionnaire initially developed for use in the psychiatric population[153]. However, the use of the PSQI in critical care has mainly been to assess sleep following ICU discharge and has no association with objective sleep parameters[154].
Integrating sleep assessment into a daily patient assessment is hindered by the complexity of current tools. The Numeric Rating Scale for sleep (NRS-Sleep) is a single-item assessment tool that requires patients to rank their sleep on a scale of 0 to 10. It was developed in a prospective, multicentre study of 456 ICU patients and using receiver operator curves, a score greater than five was determined as the threshold for good sleep. The NRS-sleep is significantly correlated with mean RCSQ score (Pearson’s correlation coefficient 0.88, P < 0.01)[155].
The sleep observation tool (SOT) requires an observer to assess and document the patient’s sleep or wake status every 15 min and has been found to correctly identify sleep 81.9% of the time compared to polysomnography. It has been used in its standard format to assess the effect of therapeutic interventions and in an amended format that uses 30-min intervals[156-158].
The use of subjective measurement tools alongside objective measures is vital to ensure that future research maintains a patient-focused outcome. The RCSQ is promising as a tool for the measurement of sleep both during and after ICU admission. It may be beneficial for researchers to use a core subjective methodology to facilitate comparisons between studies.
The effects of disrupted sleep in the critically ill remain poorly understood. In healthy adults, short-term sleep deprivation is associated with multi-system physiologic disturbances, and longer term is associated with increased risks of obesity, type 2 diabetes, malignancy and death[3].
Delirium occurs in up to 80% of mechanically ventilated patients and is independently associated with increased mortality[159]. There are suggestions of a bidirectional relationship with sleep deprivation contributing to the development of delirium, and delirium worsening sleep disturbances[160]. A causal link between sleep deprivation and delirium has not been established, but several studies support an association. The detection of atypical sleep on EEG, commonly seen in critically ill patients, was associated with a significantly increased risk of developing delirium in the following 48 h[113]. A prospective observational before and after study of the introduction of a quality improvement intervention to promote sleep in 300 ICU patients reported a marked reduction in the incidence of delirium (odds ratio 0.46; 95% confidence intervals, 0.23-0.89), however, improvements in RCSQ measured sleep did not reach statistical significance[161]. A similar study on the introduction of a multicomponent, multidisciplinary bundle of interventions in 338 ICU patients reported improved sleep efficiency, decreased daytime sleepiness, and reduced incidence and duration of delirium[162]. The results of a meta-regression conducted by Kakar et al[94] reported a somewhat unexpected relationship between total sleep time and delirium, where each hour increase in total sleep time per night was associated with a 5.8% increase in the risk of delirium. This counterintuitive result may be due to confounders, such as duration of mechanical ventilation, depth of sedation or disease severity.
Seizures are exacerbated by sleep deprivation and in focal epilepsy, the risk of seizure has been shown to correlate with day-to-day variations in daily sleep[163,164]. In animal models, REM sleep seems to play an important role in enhancing the seizure threshold[165]. However, the impact of sleep deprivation on seizures during critical illness is yet to be described or quantified.
Sleep deprivation in healthy adults is associated with cognitive dysfunction, including impaired attention, memory and situational awareness[166]. Critical illness survivors frequently report troublesome short- and long-term impairments of cognitive function[167]. For example, a multicentre observational study of 102 ICU survivors reported that sleep fragmentation was associated with cognitive impairment at seven days post discharge from ICU in patients who had been mechanically ventilated[168]. No measured sleep parameters were associated with cognitive outcomes at 6 or 12 mo.
Sleep and circadian disruption during critical illness have been proposed to result in endocrine abnormalities, including decreased secretion of anabolic hormones, including testosterone, growth hormone and insulin-like growth factor, as well as increased secretion of catabolic hormones that results in reduced protein synthesis and increased protein breakdown[169]. This net loss of protein contributes to muscle atrophy and critical illness weakness, which may be more marked in older populations and contribute to adverse outcomes, including increased frailty and functional decline in ICU survivors[169,170].
A single night of sleep deprivation in healthy adults causes impaired glucagon secretion, elevated evening cortisol, and insulin resistance[171,172]. In the critically ill, these endocrine disturbances may conceivably contribute to the development of impaired glucose tolerance and hyperglycaemia[173].
Melatonin is a circadian regulating hormone produced by the pineal gland[174]. Critically ill patients may experience reduced plasma melatonin concentrations due to loss of light-related physiological regulation of melatonin secretion and lack of normal diurnal variation[175-177]. These abnormalities likely contribute to sleep disturbances in the ICU population and have been associated with increased morbidity and mortality in animal models[178].
Immune upregulation, including immune cell proliferation and production of pro-inflammatory cytokines, is typical during the early phases of sleep[179]. Natural killer cell activity is reduced by 28% after one night of sleep deprivation, and a significant increase in total white blood cell count is seen after 3-5 d of sleep restriction[180,181]. A reduced response to influenza and hepatitis A vaccination is seen with sleep deprivation, which does not improve with catch-up sleep[182,183]. A retrospective cohort study of 135 patients with coronavirus disease 2019 (COVID-19) reported that poor sleep was linked to more severe lymphopaenia and a more frequent need for ICU admission[184].
Sleep deprivation is associated with an impaired ventilatory response to hypercapnia and hypoxaemia, reduced cortical respiratory motor output, and decreased inspiratory muscle endurance[185]. In addition, sleep fragmentation, but not sleep deprivation, has been found to increase the risk of upper airway collapsibility, which may predispose to extubation failure[186].
A prospective observational study of 45 patients evaluating sleep alterations and duration of mechanical ventilation, reported that the detection of atypical sleep on polysomnography was associated with a longer period of invasive respiratory support[187]. This relationship remained after multivariate logistic regression. Furthermore, a separate study reported that each percentage increase in slow wave sleep was associated with 0.58 d increase in the duration of mechanical ventilation[94]. Slow wave sleep is usually considered a deeper, restorative sleep phase and is typically reduced or absent during critical illness. Consequently, confounding variables, such as sedation, are influencing these associations.
The relationship between sleep deprivation and psychiatric disorders may be bidirectional[188]. Total sleep deprivation in healthy adults disrupts affective functioning[189]. In contrast, one night of total sleep deprivation has been shown to improve depressive symptoms in up to 60% of depressed patients. However, this improvement is not evident in the majority after recovery sleep[190]. Anxiety and depression frequently occur in ICU survivors, occurring in up to 43% and 48% respectively[191]. ICU survivors with depressive symptoms three months after discharge were observed to have a higher likelihood of sleep disturbance, yet the direction of causality is unclear[161].
Given the prevalence, persistence and impact of sleep disturbance during critical illness, there is considerable interest in improving patients’ sleep duration and quality. In 2018, the Society of Critical Care Medicine published its clinical practice guidelines for the prevention and management of pain, agitation, delirium, immobility and sleep disruption to summarise the contemporary evidence on this subject[192]. Sleep optimisation strategies can be categorised into non-pharmacological and pharmacological interventions.
Several authors have reported on implementing nurse-led or multi-disciplinary, multi-component, intervention bundles to improve patient sleep. Eight domains that could be included in an intervention bundle were described by Beck Edvardsen et al[193] including noise reduction; use of earplugs and eye masks; use of music; promotion of natural circadian rhythms; managing pain; use of “quiet time”; clustering of nursing activities, and optimising mechanical ventilation. However, evidence regarding such sleep-promoting intervention bundles is mixed. Improved objective and subjective measures of sleep have been reported in two studies[162,194]. Bundles from each study were implemented by a multi-disciplinary team and contained over 10 interventions, including the offer of eye masks and ear plugs. In contrast, no significant benefit of a sleep promotion bundle was reported in two further studies that had fewer interventions and did not include the provision of ear plugs and eye masks[195,196]. Studies of bundled care assess the net effect of multiple interventions, obscuring the magnitude and direction of effect from the individual components. Consequently, it is unclear which interventions contained in the reported studies are mediating the benefit[197].
Several strategies have been described to reduce the effect of noise disturbance on sleep. For example, Walder et al[198] reported the implementation of five policy steps, including the closure of doors, reducing monitor alarm volumes and, between 23:00 and 05:00, limiting nursing care, conversational noise and direct light in patients’ rooms. These interventions successfully reduced nocturnal noise and light. The implementation of a behavioural modification program for nursing staff reported similar results that such measures could reduce ambient noise and light in the ICU to provide a better sleeping environment[199]. However, neither study measured patients’ sleep, making it impossible to assess the impact of these environmental interventions on sleep outcomes.
‘Quiet time’ protocols designate a 1–2-h period during the day during which ambient noise and light are reduced to facilitate patient sleep. Three prospective studies of quiet time, involving 361 patients and using once or twice daily two hour sessions, report that patients are more likely to be reported as asleep during quiet time than during the control period[156,157,200]. Sleep was determined using a novel subjective nurse assessment or the Sleep Observation Tool[201]. Given the short available sleep period, the highly subjective nature of the assessments, and the inability to interpret reported sleep in the context of total sleep time, the inferences are limited. A quasi-experimental, non-randomised, post-test-only study of a once-daily session of quiet time in 129 patients did not detect a significant improvement in sleep measured by RCSQ with increasing numbers of quiet time sessions[202].
While quiet time is a simple, safe and low-cost intervention, methodological issues in the few available studies mean the impact of quiet time on sleep in the ICU remains uncertain.
Earplugs and eye masks offer an inexpensive and potentially low-risk intervention to reduce or diminish the impact of nocturnal ambient noise and light. Despite the intuitive appeal, the available literature reports mixed results (Supplementary Table 1).
Studies evaluating earplugs as a single intervention include a total of 276 patients but are methodologically heterogeneous with respect to duration of the intervention, inclusion of intubated patients, use of sedation, and choice of sleep measurement tool[152,203,204]. One study reported a statistically significant improvement in sleep satisfaction with earplugs but had a 12% dropout rate[152]. Van Rompaey et al[204] reported that earplugs were associated with improved sleep on the first study night, but this improvement lessened on the second night and reported sleep was worse than the intervention group by the third night. Litton et al[203] proved that using earplugs for noise abatement in the ICU setting was feasible but did not demonstrate a statistically significant benefit to sleep quality.
The combination of ear plugs and eye masks has been assessed together. Several single-centre studies report an improved subjective perception of sleep compared to usual care[195,205-214]. Earplugs and eye masks have also been reported to significantly improve sleep compared to relaxing ocean sounds played for 30 min around the onset of the sleep period[215].
Within the methodological limitations (single centre and lack of blinding), there is increasing evidence that combined eye masks and ear plugs improve self-reported sleep. In contrast, the available literature does not support using earplugs alone.
The use of non-commercial music as a sleep-promoting therapy has been evaluated. In a prospective, quasi-experimental, randomised study, 96 patients who were post-op following coronary artery bypass grafting were exposed to either a daily 30 minute session of music or a rest period[216]. Patients receiving the music intervention were reported to have significantly improved sleep, as measured by RCSQ, on postoperative day three. Further studies on music to improve sleep in the ICU were unable to identify clear evidence of benefit. A small, randomised, controlled trial of 28 ICU patients receiving either 45 min of music prior to sleep or usual care did not identify a difference in total sleep time or subjective sleep assessment[120]. An increased duration of N3 sleep was reported in the first two hours, however, the polysomnogram was not assessed beyond this window and the significance of this finding is obscured by this methodological choice. A cross-over, randomised, experimental study evaluated the effect of 20 min of music therapy against uninterrupted rest on the BIS[217]. The bispectral index was reduced during the music session; however, no assessment of nocturnal sleep quantity or quality was made, obfuscating any association with improved sleep.
The use of music therapy to improve sleep is not well supported by the published literature. Factors including the type, volume, duration and timing of the intervention are likely all important but have not been well explored to date.
Studies of massage or therapeutic touch to aid sleep in the ICU have conflicting results. A case series of 53 patients receiving therapeutic touch from a trained nurse could not identify any statistically significant change in physiologic variables[218]. Patients were reported to fall asleep frequently during treatments, but no effect on nocturnal sleep was reported. A quasi-experimental study in 60 patients compared the efficacy of a 10-minute back massage on three consecutive days against usual care and reported improvements in self-reported sleep and actigraphy-determined total sleep on the second and third days of the intervention.
A randomised controlled trial of acupressure for three hours on two consecutive nights was compared to usual care and reported a statistically significant difference in actigraphy-derived total sleep time and sleep quality, as per the Stanford Sleepiness Scale[137]. However, the use of actigraphy, which overestimates total sleep time and is not accurate in the ICU setting, and the Stanford Sleepiness Scale, which has not been validated for use in the ICU, raises questions about the internal validity of this result.
About half of the ICU survivors asked about their sleep believe a sleeping pill would have improved their sleep, but there is scant evidence to support the use of pharmacological sleep aids in this setting[30]. Cohort studies indicate that pharmacological sleep aids are frequently administered to ICU patients[219,220].
Because of the disturbed secretion of melatonin (described above), there is a biologically plausible rationale to support the use of exogenous melatonin. However, a meta-analysis of four studies reported that melatonin, at doses of between 3 and 10 mg per day, had uncertain effects on objective and subjective measures of sleep quantity and quality (Table 5)[136,158,221-223].
| Ref. | Design | Patients | Intervention & control | Sedation | Outcome |
| Ibrahim et al[158], 2006 | Single centre, double-blind, randomised trial | 32 pts | I: Melatonin 4 mg; C: placebo; For ≥ 48 h | Infusions ceased for ≥ 12 h | No significant difference in total sleep time by modified SOT |
| Bourne et al[136], 2008 | Single centre, double-blind, randomised trial | 24 pts | I: Melatonin 10 mg; C: Placebo; For 4 nights | Ceased for ≥ 30 h | No significant difference in total RCSQ or sleep efficiency by BIS |
| Foreman et al[222], 2015 | Single centre, pilot, randomised trial | 12 pts | I: Melatonin 3 mg plus eye masks and headphonesC: Standard care; For 1-7 d | Propofol allowed. Opiates ceased > 24 h | Primary outcome not determined in 65% due to uninterpretable PSG |
| Mistraletti et al[221], 2015 | Single centre, double-blind, randomised trial | 82 pts | I: Melatonin 3+3 mg; C: Placebo; From day 3 of ICU until ICU discharge | Enteral hydroxyzine and lorazepam allowed | No significant difference in total sleep time by nurse observation |
| Gandolfi et al[224], 2020 | Double centre, double-blind, randomised trial | 203 pts | I: Melatonin 10 mg; C: Placebo For 7 d or until hospital discharge | As per treating clinician | Statistically improved total RCSQ, mean (SD): I: 61 (26) C: 70 (21) (P = 0.03); No significant difference in total sleep time by nurse observation |
| Wibrow et al[225], 2021 | Multicentre (12), double blind, randomised, trial | 841 pts | I: Melatonin 4 mg; C: Placebo; For 14 d or until ICU discharge | As per treating clinician | No significant difference in total RCSQ |
More recently, a blinded, parallel-group, placebo-controlled, randomised clinical trial compared 10 mg melatonin to placebo in 203 ICU patients reported a statistically significant improvement in sleep with melatonin represented by an increase in RCSQ by nine points, but no difference in nurse-observed total sleep time[224]. Finally, the Pro-MEDIC study was a multicenter, parallel-group, placebo-controlled randomised clinical trial that included 841 patients and assessed a 4 mg dose of melatonin[225]. While the primary outcome was the incidence of delirium, sleep was recorded using RSCQ. The investigators identified no effect of melatonin on sleep and, as the largest trial to date, provides the greatest certainty as to the effect of melatonin on sleep in the ICU.
Accordingly, while there is a physiological rationale that melatonin should be an effective pharmacological sleep aid in the critically ill, there is a lack of clinical trial data to support its use.
The melatonin receptor agonist, Ramelteon, has been assessed in a single centre, blinded, randomised, placebo-controlled trial using 8 mg ramelteon per day in 88 ICU patients[226]. While the primary outcome was delirium, the use of ramelteon was associated with fewer awakenings and a higher proportion of nights without awakenings but no difference in mean hours of sleep. Determination of sleep status was performed by non-validated, retrospective means, creating uncertainty regarding this tertiary outcome.
There is no clinical trial data to guide the use of temazepam in the critically ill. A single-centre, placebo-controlled, blinded, randomised trial evaluating temazepam is currently recruiting (ANZCTR registration number: ACTRN12621000742875).
Propofol is an intravenous anaesthetic agent that enhances GABA-ergic inhibition in the brain and is frequently administered in the ICU for patient sedation[227]. Engelmann et al[228] conducted a single-centre, blinded trial comparing an intravenous infusion of 2 mg/kg/h propofol against a single bolus of intravenous 0.015 mg/kg flunitrazepam for a single night. Sleep quantity was measured using BIS, and the investigators reported a statistically significant improvement in the propofol group. However, the comparison of a continuously infused agent against a single bolus, and the use of BIS to monitor sleep undermine the validity of this result. A randomised cross-over trial of nocturnal propofol infusion in 12 mechanically ventilated ICU patients reported no difference in total sleep time or NREM sleep distribution using polysomnography[98]. A prospective clinical study of 30 mechanically ventilated patients sedated with propofol and morphine evaluated additional doses of propofol to achieve a diurnal sedation pattern[229]. The authors report that 60% of patients receiving additional nocturnal propofol developed a diurnal rhythmicity, which they attributed to natural sleep, rather than deeper anaesthesia, despite using increased sedation in this group. An open-label, randomised, comparative study of 0.3-3 mg/h propofol infusion compared to 0.03-0.2 mg/hr midazolam infusion was performed in 40 conscious ICU patients overnight to assess sleep quality, anxiety and depression[230]. Using the Hospital Anxiety and Depression Scale, no significant difference in sleep quality could be detected. Notably, the Hospital Anxiety and Depression scale is not validated for sleep assessment and is likely to be insufficiently sensitive or specific to measure this outcome accurately.
Overall, there is no convincing published evidence that propofol is able to improve sleep quality or quantity in critically ill patients.
Two, small pilot experimental studies have assessed the effect of dexmedetomidine on sleep quality and polysomnographic appearances in critically ill patients[85,231]. Subsequent randomised trials have shown that when compared to placebo, dexmedetomidine increases sleep efficiency, total sleep time and percentage of N2 sleep phase in intubated and non-intubated patients[103,133,232]. Subjective measures of sleep have infrequently been assessed but have not reached statistical significance when reported[103]. A single, non-randomised clinical trial of non-intubated, post-abdominal surgery ICU patients compared dexmedetomidine and sufentanil infusion against sufentanil infusion alone[104]. BIS monitoring showed increased total sleep time in the dexmedetomidine group. Although this result is consistent with prior data, the outcome must be interpreted in the context of the significant limitations created by non-random allocation, small sample size and use of BIS monitoring. A blinded, parallel-group, placebo-controlled clinical trial evaluated the effect of nocturnal dexmedetomidine in 100 delirium-free, critically ill patients[233]. The secondary outcome of sleep quality, measured by the Leeds Sleep Evaluation Questionnaire, reported no significant difference in sleep quality with dexmede
Studies of dexmedetomidine report objective improvements in sleep duration and architecture. However, many of the studies of dexmedetomidine do not have sleep as a primary outcome; therefore, interpreting these findings should be undertaken with cautious curiosity.
Suvorexant is an orexin receptor antagonist used as a novel hypnotic agent[234]. A single randomised, placebo-controlled trial of 15 mg/d of suvorexant for the prevention of delirium reported a significantly decreased incidence of delirium in the suvorexant group[235]. No other measured parameters, including time to sleep onset, number of awakenings, subjective quality of sleep, or total sleep time, were statistically different.
There are no currently available pharmacological sleep aids that have a robust evidence base to support their use in the ICU population.
Sleep is an important issue for the critically ill. Observational studies report that sleep disturbance is common during critical illness, and a growing body of evidence reports that this is subjectively distressing for the patient, causes physiological derangements and is associated with adverse outcomes. The causes for disrupted sleep in this population are multifactorial and, while not unique to the ICU, may be exacerbated by the treatment modalities, the intensity of care delivery, and the severity of illness that is synonymous with the management of critical illness in this setting.
Measuring sleep in the ICU for clinical and research purposes poses many issues. Polysomnography remains the gold-standard technique but is hindered by logistical issues and the frequent occurrence of atypical electroencephalographic findings. Other objective modalities, including actigraphy and BIS, have not proven sufficiently accurate and do not have a clear role in the ICU setting. Validated, subjective measures of sleep provide an important, patient-centred perspective. However, future research may benefit from adopting a core subjective methodology that would facilitate comparisons between studies.
Many interventions have been assessed to improve sleep during critical illness. When used together, earplugs and eye masks seem to improve sleep. However, a clear and reproducible benefit from other non-pharmacologic strategies has been hard to demonstrate. The use of pharmacological sleep aids to improve sleep is common, yet the currently available evidence does not demonstrate consistent, patient-oriented benefits from any agent. Sleep is a complex physiological process, and successful management of sleep disturbance will likely require a multimodal approach to benefit this vulnerable patient group.
The primary author, Laurie Showler, was supported through an Australian Government Research Training Program Scholarship.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arunachala Murthy T, Australia; Jha P, United States; Sánchez JIA, Colombia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Elliott R, Chawla A, Wormleaton N, Harrington Z. Short-term physical health effects of sleep disruptions attributed to the acute hospital environment: a systematic review. Sleep Health. 2021;7:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |
| 2. | Honkus VL. Sleep deprivation in critical care units. Crit Care Nurs Q. 2003;26:179-89; quiz 190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 844] [Cited by in RCA: 869] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 4. | Miranda-Ackerman RC, Lira-Trujillo M, Gollaz-Cervantez AC, Cortés-Flores AO, Zuloaga-Fernández Del Valle CJ, García-González LA, Morgan-Villela G, Barbosa-Camacho FJ, Pintor-Belmontes KJ, Guzmán-Ramírez BG, Bernal-Hernández A, Fuentes-Orozco C, González-Ojeda A. Associations between stressors and difficulty sleeping in critically ill patients admitted to the intensive care unit: a cohort study. BMC Health Serv Res. 2020;20:631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Simini B. Patients' perceptions of intensive care. Lancet. 1999;354:571-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 7. | Pulak LM, Jensen L. Sleep in the Intensive Care Unit: A Review. J Intensive Care Med. 2016;31:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Chang VA, Owens RL, LaBuzetta JN. Impact of Sleep Deprivation in the Neurological Intensive Care Unit: A Narrative Review. Neurocrit Care. 2020;32:596-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Carskadon MA, Dement WC. Chapter 2 - Normal Human Sleep: An Overview2005. |
| 10. | Oh J, Petersen C, Walsh CM, Bittencourt JC, Neylan TC, Grinberg LT. The role of co-neurotransmitters in sleep and wake regulation. Mol Psychiatry. 2019;24:1284-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Carley DW, Farabi SS. Physiology of Sleep. Diabetes Spectr. 2016;29:5-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 583] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 13. | Daou M, Telias I, Younes M, Brochard L, Wilcox ME. Abnormal Sleep, Circadian Rhythm Disruption, and Delirium in the ICU: Are They Related? Front Neurol. 2020;11:549908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Berry RB, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughan BV. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.2. In: Medicine AAoS, editor. Darien, Illinois: American Academy of Sleep Medicine; 2015. |
| 15. | Berry RB, Wagner M. Fundamentals 3. Sleep Staging in Adults 1. In: Sleep medicine pearls 3rd ed [Internet]. United States of America: Elsevier Saunders. 2015. |
| 16. | Moser D, Anderer P, Gruber G, Parapatics S, Loretz E, Boeck M, Kloesch G, Heller E, Schmidt A, Danker-Hopfe H, Saletu B, Zeitlhofer J, Dorffner G. Sleep classification according to AASM and Rechtschaffen & Kales: effects on sleep scoring parameters. Sleep. 2009;32:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Novelli L, Ferri R, Bruni O. Sleep classification according to AASM and Rechtschaffen and Kales: effects on sleep scoring parameters of children and adolescents. J Sleep Res. 2010;19:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Forget D, Morin CM, Bastien CH. The role of the spontaneous and evoked k-complex in good-sleeper controls and in individuals with insomnia. Sleep. 2011;34:1251-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Fernandez LMJ, Lüthi A. Sleep Spindles: Mechanisms and Functions. Physiol Rev. 2020;100:805-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 360] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 20. | Roth T. Slow wave sleep: does it matter? J Clin Sleep Med. 2009;5:S4-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Peever J, Fuller PM. Neuroscience: A Distributed Neural Network Controls REM Sleep. Curr Biol. 2016;26:R34-R35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Peever J, Fuller PM. The Biology of REM Sleep. Curr Biol. 2017;27:R1237-R1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 23. | Chokroverty S. Overview of Normal Sleep. In: Chokroverty S, editor. Sleep Disorders Medicine: Basic Science, Technical Considerations and Clinical Aspects. New York, NY: Springer New York; 2017; 5-27. [DOI] [Full Text] |
| 24. | Chaput JP, Dutil C, Sampasa-Kanyinga H. Sleeping hours: what is the ideal number and how does age impact this? Nat Sci Sleep. 2018;10:421-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 25. | Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1626] [Cited by in RCA: 1481] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 26. | Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 802] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 27. | Nelson JE, Meier DE, Oei EJ, Nierman DM, Senzel RS, Manfredi PL, Davis SM, Morrison RS. Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med. 2001;29:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 306] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 28. | Little A, Ethier C, Ayas N, Thanachayanont T, Jiang D, Mehta S. A patient survey of sleep quality in the Intensive Care Unit. Minerva Anestesiol. 2012;78:406-414. [PubMed] |
| 29. | Naik RD, Gupta K, Soneja M, Elavarasi A, Sreenivas V, Sinha S. Sleep Quality and Quantity in Intensive Care Unit Patients: A Cross-sectional Study. Indian J Crit Care Med. 2018;22:408-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Martinez FE, Poulter AL, Seneviratne C, Chrimes A, Havill K, Balogh ZJ, Paech GM. ICU Patients' Perception of Sleep and Modifiable versus Non-Modifiable Factors That Affect It: A Prospective Observational Study. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 31. | Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 444] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 32. | Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. 2013;17:R46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 33. | Altman MT, Knauert MP, Pisani MA. Sleep Disturbance after Hospitalization and Critical Illness: A Systematic Review. Ann Am Thorac Soc. 2017;14:1457-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 34. | Orwelius L, Nordlund A, Nordlund P, Edéll-Gustafsson U, Sjöberg F. Prevalence of sleep disturbances and long-term reduced health-related quality of life after critical care: a prospective multicenter cohort study. Crit Care. 2008;12:R97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Combes A, Costa MA, Trouillet JL, Baudot J, Mokhtari M, Gibert C, Chastre J. Morbidity, mortality, and quality-of-life outcomes of patients requiring >or=14 days of mechanical ventilation. Crit Care Med. 2003;31:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 237] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 36. | Bihari S, Doug McEvoy R, Matheson E, Kim S, Woodman RJ, Bersten AD. Factors affecting sleep quality of patients in intensive care unit. J Clin Sleep Med. 2012;8:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | McKinley S, Fien M, Elliott R, Elliott D. Sleep and psychological health during early recovery from critical illness: an observational study. J Psychosom Res. 2013;75:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | McKinley S, Aitken LM, Alison JA, King M, Leslie G, Burmeister E, Elliott D. Sleep and other factors associated with mental health and psychological distress after intensive care for critical illness. Intensive Care Med. 2012;38:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Solverson KJ, Easton PA, Doig CJ. Assessment of sleep quality post-hospital discharge in survivors of critical illness. Respir Med. 2016;114:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Choi J, Hoffman LA, Schulz R, Tate JA, Donahoe MP, Ren D, Given BA, Sherwood PR. Self-reported physical symptoms in intensive care unit (ICU) survivors: pilot exploration over four months post-ICU discharge. J Pain Symptom Manage. 2014;47:257-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Parsons EC, Kross EK, Caldwell ES, Kapur VK, McCurry SM, Vitiello MV, Hough CL. Post-discharge insomnia symptoms are associated with quality of life impairment among survivors of acute lung injury. Sleep Med. 2012;13:1106-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Benítez ID, Moncusí-Moix A, Vaca R, Gort-Paniello C, Minguez O, Santisteve S, Carmona P, Torres G, Fagotti J, Labarca G, Torres A, González J, de Gonzalo-Calvo D, Barbé F, Targa ADS. Sleep and Circadian Health of Critical COVID-19 Survivors 3 Months After Hospital Discharge. Crit Care Med. 2022;50:945-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Lee CM, Herridge MS, Gabor JY, Tansey CM, Matte A, Hanly PJ. Chronic sleep disorders in survivors of the acute respiratory distress syndrome. Intensive Care Med. 2009;35:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Alvaro PK, Roberts RM, Harris JK. A Systematic Review Assessing Bidirectionality between Sleep Disturbances, Anxiety, and Depression. Sleep. 2013;36:1059-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1005] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 45. | Wang CY, Shang M, Feng LZ, Zhou CL, Zhou QS, Hu K. Correlation between APACHE III score and sleep quality in ICU patients. J Int Med Res. 2019;47:3670-3680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, Hanly PJ. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 349] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 47. | Fanfulla F, Ceriana P, D'Artavilla Lupo N, Trentin R, Frigerio F, Nava S. Sleep disturbances in patients admitted to a step-down unit after ICU discharge: the role of mechanical ventilation. Sleep. 2011;34:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Hardin KA, Seyal M, Stewart T, Bonekat HW. Sleep in critically ill chemically paralyzed patients requiring mechanical ventilation. Chest. 2006;129:1468-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Wong FY, Arthur DG. Hong Kong patients' experiences of intensive care after surgery: nurses' and patients' views. Intensive Crit Care Nurs. 2000;16:290-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Johansson L, Bergbom I, Lindahl B. Meanings of being critically ill in a sound-intensive ICU patient room - a phenomenological hermeneutical study. Open Nurs J. 2012;6:108-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Chahraoui K, Laurent A, Bioy A, Quenot JP. Psychological experience of patients 3 months after a stay in the intensive care unit: A descriptive and qualitative study. J Crit Care. 2015;30:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 52. | Ding Q, Redeker NS, Pisani MA, Yaggi HK, Knauert MP. Factors Influencing Patients' Sleep in the Intensive Care Unit: Perceptions of Patients and Clinical Staff. Am J Crit Care. 2017;26:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 53. | Mattiussi E, Danielis M, Venuti L, Vidoni M, Palese A. Sleep deprivation determinants as perceived by intensive care unit patients: Findings from a systematic review, meta-summary and meta-synthesis. Intensive Crit Care Nurs. 2019;53:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? BMC Med Res Methodol. 2010;10:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 867] [Cited by in RCA: 870] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 55. | Tembo AC, Parker V, Higgins I. The experience of sleep deprivation in intensive care patients: findings from a larger hermeneutic phenomenological study. Intensive Crit Care Nurs. 2013;29:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Novaes MA, Knobel E, Bork AM, Pavão OF, Nogueira-Martins LA, Ferraz MB. Stressors in ICU: perception of the patient, relatives and health care team. Intensive Care Med. 1999;25:1421-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 57. | Gehlbach BK, Chapotot F, Leproult R, Whitmore H, Poston J, Pohlman M, Miller A, Pohlman AS, Nedeltcheva A, Jacobsen JH, Hall JB, Van Cauter E. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35:1105-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 58. | Telias I, Wilcox ME. Sleep and Circadian Rhythm in Critical Illness. Crit Care. 2019;23:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 59. | Nilius G, Richter M, Schroeder M. Updated Perspectives on the Management of Sleep Disorders in the Intensive Care Unit. Nat Sci Sleep. 2021;13:751-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Blume C, Garbazza C, Spitschan M. Effects of light on human circadian rhythms, sleep and mood. Somnologie (Berl). 2019;23:147-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 61. | Elliott R, Rai T, McKinley S. Factors affecting sleep in the critically ill: an observational study. J Crit Care. 2014;29:859-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Freedman NS, Kotzer N, Schwab RJ. Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 1999;159:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 274] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 63. | Hilton BA. Quantity and quality of patients' sleep and sleep-disturbing factors in a respiratory intensive care unit. J Adv Nurs. 1976;1:453-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 123] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Dunn H, Anderson MA, Hill PD. Nighttime lighting in intensive care units. Crit Care Nurse. 2010;30:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Darbyshire JL, Müller-Trapet M, Cheer J, Fazi FM, Young JD. Mapping sources of noise in an intensive care unit. Anaesthesia. 2019;74:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 66. | Berglund B, Lindval T, Schwela DH. Guidelines for community noise. World Health Organisation. Geneva. 1999. |
| 67. | Xie H, Kang J, Mills GH. Clinical review: The impact of noise on patients' sleep and the effectiveness of noise reduction strategies in intensive care units. Crit Care. 2009;13:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 68. | Jaiswal SJ, Garcia S, Owens RL. Sound and Light Levels Are Similarly Disruptive in ICU and non-ICU Wards. J Hosp Med. 2017;12:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Balogh D, Kittinger E, Benzer A, Hackl JM. Noise in the ICU. Intensive Care Med. 1993;19:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Elbaz M, Léger D, Sauvet F, Champigneulle B, Rio S, Strauss M, Chennaoui M, Guilleminault C, Mira JP. Sound level intensity severely disrupts sleep in ventilated ICU patients throughout a 24-h period: a preliminary 24-h study of sleep stages and associated sound levels. Ann Intensive Care. 2017;7:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Lawson N, Thompson K, Saunders G, Saiz J, Richardson J, Brown D, Ince N, Caldwell M, Pope D. Sound intensity and noise evaluation in a critical care unit. Am J Crit Care. 2010;19:e88-98; quiz e99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Jones J, Hoggart B, Withey J, Donaghue K, Ellis BW. What the patients say: A study of reactions to an intensive care unit. Intensive Care Med. 1979;5:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 98] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Frisk U, Nordström G. Patients' sleep in an intensive care unit--patients' and nurses' perception. Intensive Crit Care Nurs. 2003;19:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Uğraş GA, Oztekin SD. Patient perception of environmental and nursing factors contributing to sleep disturbances in a neurosurgical intensive care unit. Tohoku J Exp Med. 2007;212:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Celik S, Oztekin D, Akyolcu N, Işsever H. Sleep disturbance: the patient care activities applied at the night shift in the intensive care unit. J Clin Nurs. 2005;14:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Tamburri LM, DiBrienza R, Zozula R, Redeker NS. Nocturnal care interactions with patients in critical care units. Am J Crit Care. 2004;13:102-12; quiz 114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | McLaughlin DC, Hartjes TM, Freeman WD. Sleep Deprivation in Neurointensive Care Unit Patients From Serial Neurological Checks: How Much Is Too Much? J Neurosci Nurs. 2018;50:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 78. | Stone JJ, Childs S, Smith LE, Battin M, Papadakos PJ, Huang JH. Hourly neurologic assessments for traumatic brain injury in the ICU. Neurol Res. 2014;36:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 188] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 80. | Boyko Y, Ording H, Jennum P. Sleep disturbances in critically ill patients in ICU: how much do we know? Acta Anaesthesiol Scand. 2012;56:950-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 81. | Toublanc B, Rose D, Glérant JC, Francois G, Mayeux I, Rodenstein D, Jounieaux V. Assist-control ventilation vs. low levels of pressure support ventilation on sleep quality in intubated ICU patients. Intensive Care Med. 2007;33:1148-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Cabello B, Thille AW, Drouot X, Galia F, Mancebo J, d'Ortho MP, Brochard L. Sleep quality in mechanically ventilated patients: comparison of three ventilatory modes. Crit Care Med. 2008;36:1749-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 83. | Andréjak C, Monconduit J, Rose D, Toublanc B, Mayeux I, Rodenstein D, Jounieaux V. Does using pressure-controlled ventilation to rest respiratory muscles improve sleep in ICU patients? Respir Med. 2013;107:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Bosma K, Ferreyra G, Ambrogio C, Pasero D, Mirabella L, Braghiroli A, Appendini L, Mascia L, Ranieri VM. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. 2007;35:1048-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 85. | Alexopoulou C, Kondili E, Vakouti E, Klimathianaki M, Prinianakis G, Georgopoulos D. Sleep during proportional-assist ventilation with load-adjustable gain factors in critically ill patients. Intensive Care Med. 2007;33:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Alexopoulou C, Kondili E, Plataki M, Georgopoulos D. Patient-ventilator synchrony and sleep quality with proportional assist and pressure support ventilation. Intensive Care Med. 2013;39:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Córdoba-Izquierdo A, Drouot X, Thille AW, Galia F, Roche-Campo F, Schortgen F, Prats-Soro E, Brochard L. Sleep in hypercapnic critical care patients under noninvasive ventilation: conventional versus dedicated ventilators. Crit Care Med. 2013;41:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Roche-Campo F, Thille AW, Drouot X, Galia F, Margarit L, Córdoba-Izquierdo A, Mancebo J, d'Ortho MP, Brochard L. Comparison of sleep quality with mechanical versus spontaneous ventilation during weaning of critically III tracheostomized patients. Crit Care Med. 2013;41:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 89. | Heffernan AJ, Talekar C, Henain M, Purcell L, Palmer M, White H. Comparison of continuous versus intermittent enteral feeding in critically ill patients: a systematic review and meta-analysis. Crit Care. 2022;26:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 90. | Kouw IWK, Heilbronn LK, van Zanten ARH. Intermittent feeding and circadian rhythm in critical illness. Curr Opin Crit Care. 2022;28:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 91. | McNelly AS, Bear DE, Connolly BA, Arbane G, Allum L, Tarbhai A, Cooper JA, Hopkins PA, Wise MP, Brealey D, Rooney K, Cupitt J, Carr B, Koelfat K, Damink SO, Atherton PJ, Hart N, Montgomery HE, Puthucheary ZA. Effect of Intermittent or Continuous Feed on Muscle Wasting in Critical Illness: A Phase 2 Clinical Trial. Chest. 2020;158:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 92. | Kar P, Jones KL, Horowitz M, Chapman MJ, Deane AM. Measurement of gastric emptying in the critically ill. Clin Nutr. 2015;34:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 93. | Finan PH, Richards JM, Gamaldo CE, Han D, Leoutsakos JM, Salas R, Irwin MR, Smith MT. Validation of a Wireless, Self-Application, Ambulatory Electroencephalographic Sleep Monitoring Device in Healthy Volunteers. J Clin Sleep Med. 2016;12:1443-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 94. | Kakar E, Priester M, Wessels P, Slooter AJC, Louter M, van der Jagt M. Sleep assessment in critically ill adults: A systematic review and meta-analysis. J Crit Care. 2022;71:154102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 95. | Tung A, Lynch JP, Mendelson WB. Prolonged sedation with propofol in the rat does not result in sleep deprivation. Anesth Analg. 2001;92:1232-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Song J, Um YH, Kim TW, Kim SM, Kwon SY, Hong S-C. Sleep and Anesthesia. Sleep Med Res. 2018;9:11-19. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | Jean R, Shah P, Yudelevich E, Genese F, Gershner K, Levendowski D, Martillo M, Ventura I, Basu A, Ochieng P, Gibson CD. Effects of deep sedation on sleep in critically ill medical patients on mechanical ventilation. J Sleep Res. 2020;29:e12894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Kondili E, Alexopoulou C, Xirouchaki N, Georgopoulos D. Effects of propofol on sleep quality in mechanically ventilated critically ill patients: a physiological study. Intensive Care Med. 2012;38:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 99. | Bourne RS, Mills GH. Sleep disruption in critically ill patients--pharmacological considerations. Anaesthesia. 2004;59:374-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 100. | Cronin A, Keifer JC, Baghdoyan HA, Lydic R. Opioid inhibition of rapid eye movement sleep by a specific mu receptor agonist. Br J Anaesth. 1995;74:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Shaw IR, Lavigne G, Mayer P, Choinière M. Acute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: a preliminary study. Sleep. 2005;28:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 102. | Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3:33-36. [PubMed] |
| 103. | Sun YM, Zhu SN, Zhang C, Li SL, Wang DX. Effect of low-dose dexmedetomidine on sleep quality in postoperative patients with mechanical ventilation in the intensive care unit: A pilot randomized trial. Front Med (Lausanne). 2022;9:931084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 104. | Lu W, Fu Q, Luo X, Fu S, Hu K. Effects of dexmedetomidine on sleep quality of patients after surgery without mechanical ventilation in ICU. Medicine (Baltimore). 2017;96:e7081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 105. | Litton E, Elliott R, Thompson K, Watts N, Seppelt I, Webb SAR; ANZICS Clinical Trials Group and The George Institute for Global Health. Using Clinically Accessible Tools to Measure Sound Levels and Sleep Disruption in the ICU: A Prospective Multicenter Observational Study. Crit Care Med. 2017;45:966-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 106. | Monti JM. Catecholamines and the sleep-wake cycle. I. EEG and behavioral arousal. Life Sci. 1982;30:1145-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 107. | Wichniak A, Wierzbicka A, Walęcka M, Jernajczyk W. Effects of Antidepressants on Sleep. Curr Psychiatry Rep. 2017;19:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 287] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 108. | Miljatovic AM. Comparative effects of venlafaxine and mirtazapine on sleep physiology measures in patients with major depresive disorder and insomnia. European Psychiatry. 2012;27:1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Moreno RA, Hanna MM, Tavares SM, Wang YP. A double-blind comparison of the effect of the antipsychotics haloperidol and olanzapine on sleep in mania. Braz J Med Biol Res. 2007;40:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 110. | Yamashita H, Morinobu S, Yamawaki S, Horiguchi J, Nagao M. Effect of risperidone on sleep in schizophrenia: a comparison with haloperidol. Psychiatry Res. 2002;109:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 111. | Giménez S, Clos S, Romero S, Grasa E, Morte A, Barbanoj MJ. Effects of olanzapine, risperidone and haloperidol on sleep after a single oral morning dose in healthy volunteers. Psychopharmacology (Berl). 2007;190:507-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 112. | Cole JL. Steroid-Induced Sleep Disturbance and Delirium: A Focused Review for Critically Ill Patients. Fed Pract. 2020;37:260-267. [PubMed] |
| 113. | Drouot X, Roche-Campo F, Thille AW, Cabello B, Galia F, Margarit L, d'Ortho MP, Brochard L. A new classification for sleep analysis in critically ill patients. Sleep Med. 2012;13:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 114. | Knauert MP, Yaggi HK, Redeker NS, Murphy TE, Araujo KL, Pisani MA. Feasibility study of unattended polysomnography in medical intensive care unit patients. Heart Lung. 2014;43:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 115. | Watson PL, Pandharipande P, Gehlbach BK, Thompson JL, Shintani AK, Dittus BS, Bernard GR, Malow BA, Ely EW. Atypical sleep in ventilated patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med. 2013;41:1958-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 116. | Knauert MP, Gilmore EJ, Murphy TE, Yaggi HK, Van Ness PH, Han L, Hirsch LJ, Pisani MA. Association between death and loss of stage N2 sleep features among critically Ill patients with delirium. J Crit Care. 2018;48:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 117. | Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 314] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 118. | Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed). 1985;290:1029-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 233] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 119. | Weinhouse GL, Kimchi E, Watson P, Devlin JW. Sleep Assessment in Critically Ill Adults: Established Methods and Emerging Strategies. Crit Care Explor. 2022;4:e0628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Su CP, Lai HL, Chang ET, Yiin LM, Perng SJ, Chen PW. A randomized controlled trial of the effects of listening to non-commercial music on quality of nocturnal sleep and relaxation indices in patients in medical intensive care unit. J Adv Nurs. 2013;69:1377-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 121. | Darbyshire JL, Borthwick M, Edmonds P, Vollam S, Hinton L, Young JD. Measuring sleep in the intensive care unit: Electroencephalogram, actigraphy, or questionnaire? J Intensive Care Soc. 2020;21:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Fallmann S, Chen L. Computational Sleep Behavior Analysis: A Survey. IEEE Access. 2019;7:142421-142440. [DOI] [Full Text] |
| 123. | Quereshi S, Karilla S, Vanichayobon S. Human sleep scoring based on K-nearest neighbors. Turk J Elec Eng & Comp Sci. 2018;26:2802-2818. [DOI] [Full Text] |
| 124. | Ambrogio C, Koebnick J, Quan SF, Ranieri M, Parthasarathy S. Assessment of sleep in ventilator-supported critically III patients. Sleep. 2008;31:1559-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 125. |
Delaney LJ Van Harren F, Currie M, Huang H-CC, Lopez V Sleep Monitoring Techniques within Intensive Care.
|
| 126. | Bourne RS, Minelli C, Mills GH, Kandler R. Clinical review: Sleep measurement in critical care patients: research and clinical implications. Crit Care. 2007;11:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 127. | Richards KC, Wang YY, Jun J, Ye L. A Systematic Review of Sleep Measurement in Critically Ill Patients. Front Neurol. 2020;11:542529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 128. | Lewis SR, Pritchard MW, Fawcett LJ, Punjasawadwong Y. Bispectral index for improving intraoperative awareness and early postoperative recovery in adults. Cochrane Database Syst Rev. 2019;9:CD003843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 129. | Nicholson T, Patel J, Sleigh JW. Sleep patterns in intensive care unit patients: a study using the bispectral index. Crit Care Resusc. 2001;3:86-91. [PubMed] |
| 130. | Pedrão RAA, Riella RJ, Richards K, Valderramas SR. Viability and validity of the bispectral index to measure sleep in patients in the intensive care unit. Rev Bras Ter Intensiva. 2020;32:535-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 131. | Reinke L, van der Hoeven JH, van Putten MJ, Dieperink W, Tulleken JE. Intensive care unit depth of sleep: proof of concept of a simple electroencephalography index in the non-sedated. Crit Care. 2014;18:R66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 132. | Vacas S, McInrue E, Gropper MA, Maze M, Zak R, Lim E, Leung JM. The Feasibility and Utility of Continuous Sleep Monitoring in Critically Ill Patients Using a Portable Electroencephalography Monitor. Anesth Analg. 2016;123:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 133. | Romagnoli S, Villa G, Fontanarosa L, Tofani L, Pinelli F, De Gaudio AR, Ricci Z. Sleep duration and architecture in non-intubated intensive care unit patients: an observational study. Sleep Med. 2020;70:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Schwab KE, Ronish B, Needham DM, To AQ, Martin JL, Kamdar BB. Actigraphy to Evaluate Sleep in the Intensive Care Unit. A Systematic Review. Ann Am Thorac Soc. 2018;15:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 135. |
Acker JG, Becker-Carus C, Büttner-Teleaga A, Cassel W, Danker-Hopfe H, Dück A, et al The role of actigraphy in sleep medicine.
|
| 136. | Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12:R52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 137. | Chen JH, Chao YH, Lu SF, Shiung TF, Chao YF. The effectiveness of valerian acupressure on the sleep of ICU patients: a randomized clinical trial. Int J Nurs Stud. 2012;49:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 138. | Nagatomo K, Masuyama T, Iizuka Y, Makino J, Shiotsuka J, Sanui M. Validity of an under-mattress sensor for objective sleep measurement in critically ill patients: a prospective observational study. J Intensive Care. 2020;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 139. | Matsui K, Sato N, Idei M, Arakida M, Seino Y, Ishikawa JY, Nakagawa M, Akaho R, Nishimura K, Nomura T. An Automated Algorithm for Determining Sleep Using Single-Channel Electroencephalography to Detect Delirium: A Prospective Observational Study in Intensive Care Units. Healthcare (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 140. | Jeffs EL, Darbyshire JL. Measuring Sleep in the Intensive Care Unit: A Critical Appraisal of the Use of Subjective Methods. J Intensive Care Med. 2019;34:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 141. | Richards K. Techniques for measurement of sleep in critical care. Focus Crit Care. 1987;14:34-40. [PubMed] |
| 142. | Elliott R, Axelin A, Richards KC, Vahlberg T, Ritmala-Castren M. Sensitivity and specificity of proposed Richards-Campbell Sleep Questionnaire cut-off scores for good quality sleep during an ICU stay. J Clin Nurs. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 143. | Richards KC, O'Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Meas. 2000;8:131-144. [PubMed] |
| 144. | Louis M, Treger K, Ashby T, Smotherman C, Gautum S, Seeram V, Cury J, Jones L. Patient-related factors may influence nursing perception of sleep in the Intensive Care Unit. PLoS One. 2020;15:e0226323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 145. | Ritmala-Castren M, Axelin A, Richards KC, Mitchell ML, Vahlberg T, Leino-Kilpi H. Investigating the construct and concurrent validity of the Richards-Campbell Sleep Questionnaire with intensive care unit patients and home sleepers. Aust Crit Care. 2022;35:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 146. | Murata H, Oono Y, Sanui M, Saito K, Yamaguchi Y, Takinami M, Richards KC, Henker R. The Japanese version of the Richards-Campbell Sleep Questionnaire: Reliability and validity assessment. Nurs Open. 2019;6:808-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 147. | Snyder-Halpern R, Verran JA. Instrumentation to describe subjective sleep characteristics in healthy subjects. Res Nurs Health. 1987;10:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 174] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 148. | Reishtein JL. Sleep in mechanically ventilated patients. Crit Care Nurs Clin North Am. 2005;17:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 149. | Richardson S. Effects of relaxation and imagery on the sleep of critically ill adults. Dimens Crit Care Nurs. 2003;22:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 150. | Richardson A, Crow W, Coghill E, Turnock C. A comparison of sleep assessment tools by nurses and patients in critical care. J Clin Nurs. 2007;16:1660-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 151. | Hsu WC, Guo SE, Chang CH. Back massage intervention for improving health and sleep quality among intensive care unit patients. Nurs Crit Care. 2019;24:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 152. | Scotto CJ, McClusky C, Spillan S, Kimmel J. Earplugs improve patients' subjective experience of sleep in critical care. Nurs Crit Care. 2009;14:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 153. | Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17520] [Cited by in RCA: 21901] [Article Influence: 608.4] [Reference Citation Analysis (0)] |
| 154. | Zitser J, Allen IE, Falgàs N, Le MM, Neylan TC, Kramer JH, Walsh CM. Pittsburgh Sleep Quality Index (PSQI) responses are modulated by total sleep time and wake after sleep onset in healthy older adults. PLoS One. 2022;17:e0270095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 155. | Rood P, Frenzel T, Verhage R, Bonn M, van der Hoeven H, Pickkers P, van den Boogaard M. Development and daily use of a numeric rating score to assess sleep quality in ICU patients. J Crit Care. 2019;52:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 156. | Dennis CM, Lee R, Woodard EK, Szalaj JJ, Walker CA. Benefits of quiet time for neuro-intensive care patients. J Neurosci Nurs. 2010;42:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 157. | Olson DM, Borel CO, Laskowitz DT, Moore DT, McConnell ES. Quiet time: a nursing intervention to promote sleep in neurocritical care units. Am J Crit Care. 2001;10:74-78. [PubMed] |
| 158. | Ibrahim MG, Bellomo R, Hart GK, Norman TR, Goldsmith D, Bates S, Egi M. A double-blind placebo-controlled randomised pilot study of nocturnal melatonin in tracheostomised patients. Crit Care Resusc. 2006;8:187-191. [PubMed] |
| 159. | Aitken LM, Elliott R, Mitchell M, Davis C, Macfarlane B, Ullman A, Wetzig K, Datt A, McKinley S. Sleep assessment by patients and nurses in the intensive care: An exploratory descriptive study. Aust Crit Care. 2017;30:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 160. | Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, Padilla G, Puntillo KA. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Med. 2009;35:781-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 161. | Kamdar BB, Needham DM, Collop NA. Sleep deprivation in critical illness: its role in physical and psychological recovery. J Intensive Care Med. 2012;27:97-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 162. | Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69:540-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 163. | Dell KL, Payne DE, Kremen V, Maturana MI, Gerla V, Nejedly P, Worrell GA, Lenka L, Mivalt F, Boston RC, Brinkmann BH, D'Souza W, Burkitt AN, Grayden DB, Kuhlmann L, Freestone DR, Cook MJ. Seizure likelihood varies with day-to-day variations in sleep duration in patients with refractory focal epilepsy: A longitudinal electroencephalography investigation. EClinicalMedicine. 2021;37:100934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 164. | Razavi B, Fisher RS. Chapter 7 - Sleep and Epilepsy. In: Miglis MG, editor. Sleep and Neurologic Disease. San Diego: Academic Press; 2017; 129-140. [DOI] [Full Text] |
| 165. | Kumar P, Raju TR. Seizure susceptibility decreases with enhancement of rapid eye movement sleep. Brain Res. 2001;922:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 166. | Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 812] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 167. | Wilcox ME, Brummel NE, Archer K, Ely EW, Jackson JC, Hopkins RO. Cognitive dysfunction in ICU patients: risk factors, predictors, and rehabilitation interventions. Crit Care Med. 2013;41:S81-S98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 168. | Wilcox ME, McAndrews MP, Van J, Jackson JC, Pinto R, Black SE, Lim AS, Friedrich JO, Rubenfeld GD. Sleep Fragmentation and Cognitive Trajectories After Critical Illness. Chest. 2021;159:366-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 169. | Elías MN, Munro CL, Liang Z, Calero K, Ji M. Sleep and Intensive Care Unit-Acquired Weakness in Critically Ill Older Adults. Dimens Crit Care Nurs. 2019;38:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 170. | Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020;46:637-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 363] [Article Influence: 72.6] [Reference Citation Analysis (1)] |
| 171. | Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 2011;5:31-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 172. | Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW, Corssmit EP, Romijn JA. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95:2963-2968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 173. | Deane AM, Horowitz M. Dysglycaemia in the critically ill - significance and management. Diabetes Obes Metab. 2013;15:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 174. | Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, Fougerou C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr Neuropharmacol. 2017;15:434-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 505] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 175. | Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, Balaum H, Shenkman L. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci. 1999;317:278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 176. | Perras B, Meier M, Dodt C. Light and darkness fail to regulate melatonin release in critically ill humans. Intensive Care Med. 2007;33:1954-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 177. | Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. 2004;48:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 178. | Reynolds FD, Dauchy R, Blask D, Dietz PA, Lynch D, Zuckerman R. The pineal gland hormone melatonin improves survival in a rat model of sepsis/shock induced by zymosan A. Surgery. 2003;134:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 179. | Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 593] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 180. | Lasselin J, Rehman JU, Åkerstedt T, Lekander M, Axelsson J. Effect of long-term sleep restriction and subsequent recovery sleep on the diurnal rhythms of white blood cell subpopulations. Brain Behav Immun. 2015;47:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 181. | Boudjeltia KZ, Faraut B, Stenuit P, Esposito MJ, Dyzma M, Brohée D, Ducobu J, Vanhaeverbeek M, Kerkhofs M. Sleep restriction increases white blood cells, mainly neutrophil count, in young healthy men: a pilot study. Vasc Health Risk Manag. 2008;4:1467-1470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 182. | Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2347] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 183. | Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65:831-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 220] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 184. | Zhang J, Xu D, Xie B, Zhang Y, Huang H, Liu H, Chen H, Sun Y, Shang Y, Hashimoto K, Yuan S. Poor-sleep is associated with slow recovery from lymphopenia and an increased need for ICU care in hospitalized patients with COVID-19: A retrospective cohort study. Brain Behav Immun. 2020;88:50-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 185. | Chen HI, Tang YR. Sleep loss impairs inspiratory muscle endurance. Am Rev Respir Dis. 1989;140:907-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 186. | Sériès F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 158] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 187. | Thille AW, Reynaud F, Marie D, Barrau S, Rousseau L, Rault C, Diaz V, Meurice JC, Coudroy R, Frat JP, Robert R, Drouot X. Impact of sleep alterations on weaning duration in mechanically ventilated patients: a prospective study. Eur Respir J. 2018;51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 188. | Krystal AD. Psychiatric disorders and sleep. Neurol Clin. 2012;30:1389-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 264] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 189. | Grèzes J, Erblang M, Vilarem E, Quiquempoix M, Van Beers P, Guillard M, Sauvet F, Mennella R, Rabat A. Impact of total sleep deprivation and related mood changes on approach-avoidance decisions to threat-related facial displays. Sleep. 2021;44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 190. | Giedke H, Schwärzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6:361-377. [PubMed] |
| 191. | Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 192. | Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, Balas MC, van den Boogaard M, Bosma KJ, Brummel NE, Chanques G, Denehy L, Drouot X, Fraser GL, Harris JE, Joffe AM, Kho ME, Kress JP, Lanphere JA, McKinley S, Neufeld KJ, Pisani MA, Payen JF, Pun BT, Puntillo KA, Riker RR, Robinson BRH, Shehabi Y, Szumita PM, Winkelman C, Centofanti JE, Price C, Nikayin S, Misak CJ, Flood PD, Kiedrowski K, Alhazzani W. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46:e825-e873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 2141] [Article Influence: 356.8] [Reference Citation Analysis (0)] |
| 193. | Beck Edvardsen J, Hetmann F. Promoting Sleep in the Intensive Care Unit. SAGE Open Nurs. 2020;6:2377960820930209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 194. | Andrews JL, Louzon PR, Torres X, Pyles E, Ali MH, Du Y, Devlin JW. Impact of a Pharmacist-Led Intensive Care Unit Sleep Improvement Protocol on Sleep Duration and Quality. Ann Pharmacother. 2021;55:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 195. | Kamdar BB, King LM, Collop NA, Sakamuri S, Colantuoni E, Neufeld KJ, Bienvenu OJ, Rowden AM, Touradji P, Brower RG, Needham DM. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med. 2013;41:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 196. | Faraklas I, Holt B, Tran S, Lin H, Saffle J, Cochran A. Impact of a nursing-driven sleep hygiene protocol on sleep quality. J Burn Care Res. 2013;34:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 197. | Lavallée JF, Gray TA, Dumville J, Russell W, Cullum N. The effects of care bundles on patient outcomes: a systematic review and meta-analysis. Implement Sci. 2017;12:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 198. | Walder B, Francioli D, Meyer JJ, Lançon M, Romand JA. Effects of guidelines implementation in a surgical intensive care unit to control nighttime light and noise levels. Crit Care Med. 2000;28:2242-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 199. | Monsén MG, Edéll-Gustafsson UM. Noise and sleep disturbance factors before and after implementation of a behavioural modification programme. Intensive Crit Care Nurs. 2005;21:208-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 200. | McAndrew NS, Leske J, Guttormson J, Kelber ST, Moore K, Dabrowski S. Quiet time for mechanically ventilated patients in the medical intensive care unit. Intensive Crit Care Nurs. 2016;35:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 201. | Edwards GB, Schuring LM. Pilot study: validating staff nurses' observations of sleep and wake states among critically ill patients, using polysomnography. Am J Crit Care. 1993;2:125-131. [PubMed] |
| 202. | Maidl CA, Leske JS, Garcia AE. The influence of "quiet time" for patients in critical care. Clin Nurs Res. 2014;23:544-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 203. | Litton E, Elliott R, Ferrier J, Webb SAR. Quality sleep using earplugs in the intensive care unit: the QUIET pilot randomised controlled trial. Crit Care Resusc. 2017;19:128-133. [PubMed] |
| 204. | Van Rompaey B, Elseviers MM, Van Drom W, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012;16:R73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 205. | Yazdannik AR, Zareie A, Hasanpour M, Kashefi P. The effect of earplugs and eye mask on patients' perceived sleep quality in intensive care unit. Iran J Nurs Midwifery Res. 2014;19:673-678. [PubMed] |
| 206. | Dave K, Qureshi A, Gopichandran L. Effects of Earplugs and Eye Masks on Perceived Quality of Sleep during Night among Patients in Intensive Care Units. AJNER. 2015;5:319-322. [DOI] [Full Text] |
| 207. | Bajwa N Saini P, Kaur H, Kalra S, Kaur J. Effect of ear plugs and eye mask on sleep among ICU patients: a randomized control trial. Int J Curr Res. 2015;7:23741-23745. |
| 208. | Akpinar RB, Aksoy M, Kant E. Effect of earplug/eye mask on sleep and delirium in intensive care patients. Nurs Crit Care. 2022;27:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 209. | Obanor OO, McBroom MM, Elia JM, Ahmed F, Sasaki JD, Murphy KM, Chalk S, Menard GA, Pratt NV, Venkatachalam AM, Romito BT. The Impact of Earplugs and Eye Masks on Sleep Quality in Surgical ICU Patients at Risk for Frequent Awakenings. Crit Care Med. 2021;49:e822-e832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 210. | Demoule A, Carreira S, Lavault S, Pallanca O, Morawiec E, Mayaux J, Arnulf I, Similowski T. Impact of earplugs and eye mask on sleep in critically ill patients: a prospective randomized study. Crit Care. 2017;21:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 211. | Richardson A, Allsop M, Coghill E, Turnock C. Earplugs and eye masks: do they improve critical care patients' sleep? Nurs Crit Care. 2007;12:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 212. | Jones C, Dawson D. Eye masks and earplugs improve patient's perception of sleep. Nurs Crit Care. 2012;17:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 213. | Hu RF, Jiang XY, Hegadoren KM, Zhang YH. Effects of earplugs and eye masks combined with relaxing music on sleep, melatonin and cortisol levels in ICU patients: a randomized controlled trial. Crit Care. 2015;19:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 214. | Arttawejkul P, Reutrakul S, Muntham D, Chirakalwasan N. Effect of Nighttime Earplugs and Eye Masks on Sleep Quality in Intensive Care Unit Patients. Indian J Crit Care Med. 2020;24:6-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 215. | Chaudhary A, Kumari V, Neetu N. Sleep Promotion among Critically Ill Patients: Earplugs/Eye Mask versus Ocean Sound-A Randomized Controlled Trial Study. Crit Care Res Pract. 2020;2020:8898172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 216. | Zimmerman L, Nieveen J, Barnason S, Schmaderer M. The effects of music interventions on postoperative pain and sleep in coronary artery bypass graft (CABG) patients. Sch Inq Nurs Pract. 1996;10:153-170; discussion 171. [PubMed] |
| 217. | Jaber S, Bahloul H, Guétin S, Chanques G, Sebbane M, Eledjam JJ. [Effects of music therapy in intensive care unit without sedation in weaning patients versus non-ventilated patients]. Ann Fr Anesth Reanim. 2007;26:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 218. | Cox C, Hayes J. Physiologic and psychodynamic responses to the administration of therapeutic touch in critical care. Complement Ther Nurs Midwifery. 1999;5:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 219. | Wong C, Ho J, Ankravs MJ, Sharrock L, Kee K, Goldin J, MacIsaac C, Presneill JJ, Ali Abdelhamid Y, Deane AM. Administration of pharmacological sleep aids prior to, during and following critical illness. Intern Med J. 2022;52:1962-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 220. | Hamidi A, Roberts RJ, Weinhouse GL, Szumita PM, Degrado JR, Dube KM, Kovacevic MP, Choi M, Sevinsky R, Duprey MS, Devlin JW. Characterization of Nocturnal Neuroactive Medication Use and Related Sleep Documentation in Critically Ill Adults. Crit Care Explor. 2021;3:e0367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 221. | Mistraletti G, Umbrello M, Sabbatini G, Miori S, Taverna M, Cerri B, Mantovani ES, Formenti P, Spanu P, D'Agostino A, Salini S, Morabito A, Fraschini F, Reiter RJ, Iapichino G. Melatonin reduces the need for sedation in ICU patients: a randomized controlled trial. Minerva Anestesiol. 2015;81:1298-1310. [PubMed] |
| 222. | Foreman B, Westwood AJ, Claassen J, Bazil CW. Sleep in the neurological intensive care unit: feasibility of quantifying sleep after melatonin supplementation with environmental light and noise reduction. J Clin Neurophysiol. 2015;32:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 223. | Lewis SR, Pritchard MW, Schofield-Robinson OJ, Alderson P, Smith AF. Melatonin for the promotion of sleep in adults in the intensive care unit. Cochrane Database Syst Rev. 2018;5:CD012455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 224. | Gandolfi JV, Di Bernardo APA, Chanes DAV, Martin DF, Joles VB, Amendola CP, Sanches LC, Ciorlia GL, Lobo SM. The Effects of Melatonin Supplementation on Sleep Quality and Assessment of the Serum Melatonin in ICU Patients: A Randomized Controlled Trial. Crit Care Med. 2020;48:e1286-e1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 225. | Wibrow B, Martinez FE, Myers E, Chapman A, Litton E, Ho KM, Regli A, Hawkins D, Ford A, van Haren FMP, Wyer S, McCaffrey J, Rashid A, Kelty E, Murray K, Anstey M. Prophylactic melatonin for delirium in intensive care (Pro-MEDIC): a randomized controlled trial. Intensive Care Med. 2022;48:414-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 226. | Nishikimi M, Numaguchi A, Takahashi K, Miyagawa Y, Matsui K, Higashi M, Makishi G, Matsui S, Matsuda N. Effect of Administration of Ramelteon, a Melatonin Receptor Agonist, on the Duration of Stay in the ICU: A Single-Center Randomized Placebo-Controlled Trial. Crit Care Med. 2018;46:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 227. | Williams DB, Akabas MH. Structural evidence that propofol stabilizes different GABA(A) receptor states at potentiating and activating concentrations. J Neurosci. 2002;22:7417-7424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 228. | Engelmann C, Wallenborn J, Olthoff D, Kaisers UX, Rüffert H. Propofol versus flunitrazepam for inducing and maintaining sleep in postoperative ICU patients. Indian J Crit Care Med. 2014;18:212-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 229. | McLeod G, Wallis C, Dick J, Cox C, Patterson A, Colvin J. Use of 2% propofol to produce diurnal sedation in critically ill patients. Intensive Care Med. 1997;23:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 230. | Treggiari-Venzi M, Borgeat A, Fuchs-Buder T, Gachoud JP, Suter PM. Overnight sedation with midazolam or propofol in the ICU: effects on sleep quality, anxiety and depression. Intensive Care Med. 1996;22:1186-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 231. | Oto J, Yamamoto K, Koike S, Onodera M, Imanaka H, Nishimura M. Sleep quality of mechanically ventilated patients sedated with dexmedetomidine. Intensive Care Med. 2012;38:1982-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 232. | Wu XH, Cui F, Zhang C, Meng ZT, Wang DX, Ma J, Wang GF, Zhu SN, Ma D. Low-dose Dexmedetomidine Improves Sleep Quality Pattern in Elderly Patients after Noncardiac Surgery in the Intensive Care Unit: A Pilot Randomized Controlled Trial. Anesthesiology. 2016;125:979-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 233. | Skrobik Y, Duprey MS, Hill NS, Devlin JW. Low-Dose Nocturnal Dexmedetomidine Prevents ICU Delirium. A Randomized, Placebo-controlled Trial. Am J Respir Crit Care Med. 2018;197:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 234. | Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 235. | Hatta K, Kishi Y, Wada K, Takeuchi T, Ito S, Kurata A, Murakami K, Sugita M, Usui C, Nakamura H; DELIRIA-J Group. Preventive Effects of Suvorexant on Delirium: A Randomized Placebo-Controlled Trial. J Clin Psychiatry. 2017;78:e970-e979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 236. | Delisle S, Ouellet P, Bellemare P, Tétrault JP, Arsenault P. Sleep quality in mechanically ventilated patients: comparison between NAVA and PSV modes. Ann Intensive Care. 2011;1:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |