Published online Jun 9, 2023. doi: 10.5492/wjccm.v12.i3.153
Peer-review started: February 27, 2023
First decision: March 28, 2023
Revised: April 18, 2023
Accepted: May 17, 2023
Article in press: May 17, 2023
Published online: June 9, 2023
Processing time: 100 Days and 9.5 Hours
Interstitial lung disease (ILD) is typically managed on an outpatient basis. Critical care physicians manage patients with ILD in the setting of an acute exacerbation (ILD flare) causing severe hypoxia. The principles of management of acute exacerbation of ILD are different from those used to manage patients with acute respiratory distress syndrome from sepsis, etc. Selected patients may be cand
Core Tip: Interstitial lung disease (ILD) refers to a heterogeneous group of parenchymal lung disorders. Most patients with ILD receive management in outpatient clinics. Patients with acute exacerbation of ILD may experience significant respiratory distress, requiring urgent management in an intensive care unit. Timely diagnosis and management of these patients using a multimodality team approach may improve both morbidity and mortality. When acute exacerbation of ILD progresses to irreversible end-stage respiratory failure, lung transplantation and/or palliative care may be appropriate treatment options depending on the individual patient’s clinical presentation.
- Citation: Hayat Syed MK, Bruck O, Kumar A, Surani S. Acute exacerbation of interstitial lung disease in the intensive care unit: Principles of diagnostic evaluation and management. World J Crit Care Med 2023; 12(3): 153-164
- URL: https://www.wjgnet.com/2220-3141/full/v12/i3/153.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v12.i3.153
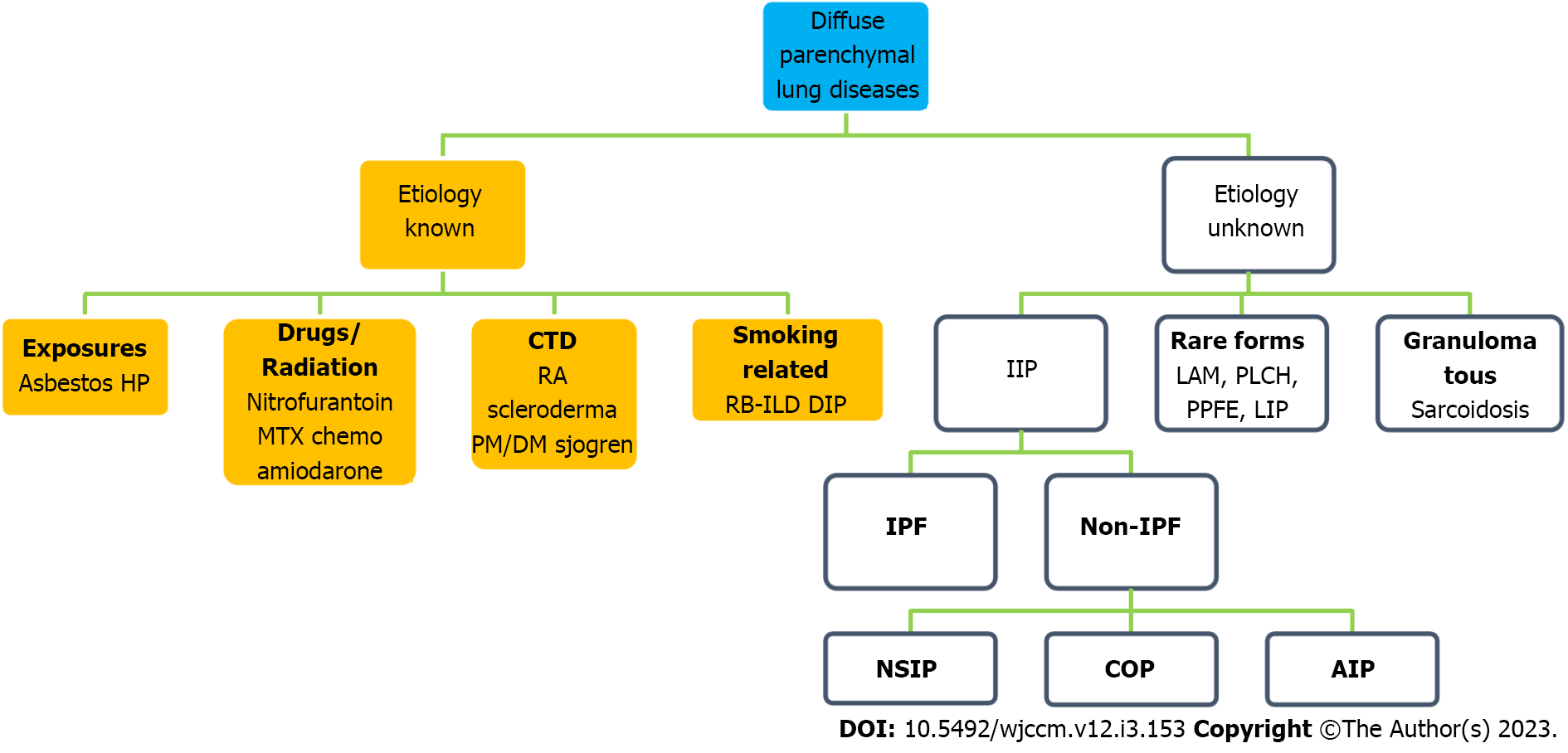
Interstitial lung disease (ILD) is a heterogeneous group of approximately 200 disorders affecting lung parenchyma. The classification schema of this group of diseases has changed over time, with the most current iteration agreed upon in 2013 in a consensus statement from the American Thoracic Society and European Respiratory Society[1]. This classification system will be the basis for the nomenclature used in this review (Figure 1).
ILD can be more broadly categorized into those with a known cause and those without an identifiable etiology, also known as “idiopathic.” Those ILDs with known causes can be due to drugs and toxins, rheumatic disease, granulomatous diseases such as sarcoidosis, and other forms such as lymphangioleiomyomatosis, eosinophilic pneumonia, or pulmonary Langerhans cell histiocytosis. The major idiopathic ILDs can be distributed into three categories: Chronic fibrosing [idiopathic pulmonary fibrosis (IPF), non-specific interstitial pneumonia (NSIP)], acute/subacute fibrosing [cryptogenic organizing pneumonia, acute interstitial pneumonia (AIP)], and lastly smoking-related (delta sleep-inducing peptide, respiratory bronchiolitis-ILD)[1]. Notably, not all ILDs have the propensity to present as an acute exacerbation (AE). Moreover, common conditions like pulmonary edema, pneumonia, pulmonary embolism, and aspiration should be considered in the differential diagnosis of acute worsening. Furthermore, patients may present to the intensive care unit (ICU) with no known history of ILD.
An abnormal chest radiograph may be the initial finding in these patients[2]. Much attention is paid to the specific patterns noted on chest computed tomography (CT) that, when coupled with clinical history, can sometimes obviate the need for tissue sampling[3]. It is important to note that the lung has a limited and predictable response to injury; thus, a variety of disease processes may produce similar imaging findings[4]. Patients will almost universally present in a similar fashion, with dyspnea and acute hypoxemic respiratory failure. CT becomes an essential tool in the differentiation of the diagnosis.
The definition of AE-ILD has evolved over time. AE-ILD was defined by 2007 IPFnet[5] as an “acute, clinically significant respiratory deterioration characterized by evidence of new widespread alveolar abnormality typically less than 1-mo’s duration with exclusion of alternative etiologies”[2]. The revised criteria in 2016[2] did not set a definite 30-d duration of onset of symptoms. The critical component of establishing a diagnosis of AE-ILD is excluding other etiologies that could be causing increased respiratory distress such as heart failure, pulmonary embolism, pneumothorax, and infection, etc (Table 1). However, in some cases infections and other pulmonary insults might act as a trigger for AE-ILD. It is difficult to lump all ILDs into one framework, and it is important to acknowledge that some of the acute presentations may be exacerbations of a previously unrecognized process[6]. AE-ILD symptoms tend to be shared across the spectrum of the disease. Commonly patients will present with worsening cough and dyspnea, but presentations may be more fulminant in the case of some AIP. However, given the non-specific nature of these symptoms, chest imaging and clinical history play an important role in discerning the diagnosis and thus the appropriate management.
| No. | Potentially reversible (partial or complete) causes of worsening ILD |
| 1 | Pulmonary edema |
| 2 | Pneumonia |
| 3 | Aspiration |
| 4 | Pulmonary hemorrhage |
| 5 | Pulmonary embolism |
The incidence of AE-IPF ranges from 2% to 15% per year, depending on the cohort studied[7-10]. Nonetheless, AEs appear at a lower rate in non-IPF ILDs[11]. AEs are often devastating, with a median survival of usually about 4 mo[12]. If an IPF patient requires mechanical ventilation, mortality exceeds 75%[13]. Given that patients with ILD exist on the fringe between functionality and fulminant worsening, even the most minor perturbations in homeostasis can lead to a significant decline. Intuitively it makes sense that any of the common causes of respiratory failure in the general population also occur in the ILD population, with the implications of such in the latter being far more pronounced.
Risk factors for AE-IPF have been described in several studies but have not been validated in independent cohorts[11]. Whether the AE represents a progression of the underlying ILD or an aberrant response to external insults such as aspiration of gastric content, infection, or mechanical stretch remains unknown. Song et al[12] demonstrated that patients with lower baseline lung function as measured by forced vital capacity and those who never smoked had a greater risk for AE-IPF[12]. They described a subset of patients with AE-ILD as rapid deterioration of ILD, where the respiratory failure occurs in shorter duration (days to weeks) requiring hospitalization, with the presence of new radiographic abnormalities. Interestingly, within this cohort, many patients were found to have an infection as a trigger; more than half of those patients had opportunistic infections. The demonstration that AE is the most common cause of clinic deterioration in IPF patients is not a novel discovery[14]; thus, acceptance that disease progression may explain the patient’s presentation can obviate the need for expensive and possibly morbid diagnostic investigations.
Other previously described exposures that can precipitate AE include bronchoalveolar lavage, cryobiopsy, lung resection surgery, pollution, aspiration, vaccination, and infection[15-21]. Churg et al[22] described the pathologic features found on lung biopsy in patients with fibrotic lung disease who were admitted with worsening respiratory failure. They noted three microscopic patterns found in these patients: Diffuse alveolar damage; organizing pneumonia; and a pattern of numerous very large fibroblast foci superimposed on underlying fibrosis. Ultimately, the onus on the admitting team is to differentiate idiopathic exacerbations from secondary ones, potentially more amenable to treatment. It has been shown to be related to mortality; that is, patients with suspected AE had worse in-hospital mortality as compared to those patients who had other causes for respiratory worsening[23].
The diagnostic evaluation of patients with ILD admitted to the ICU is largely the same as that for all other patients admitted with acute hypoxemic respiratory failure. A caveat to this approach is for the patient with previously undiagnosed ILD, in which case, they will require a more thoughtful appraisal. It is reasonable to exclude all the common, secondary causes of worsening that can be seen in ILD patients (Table 1).
Chest CT is now routinely utilized in all patients admitted to the ICU with AE-ILD. In those patients without a known diagnosis of ILD, a basic working knowledge of some of the classic radiographic findings can be helpful. As previously noted, ILDs are both a radiographic and histopathologic diagnosis.
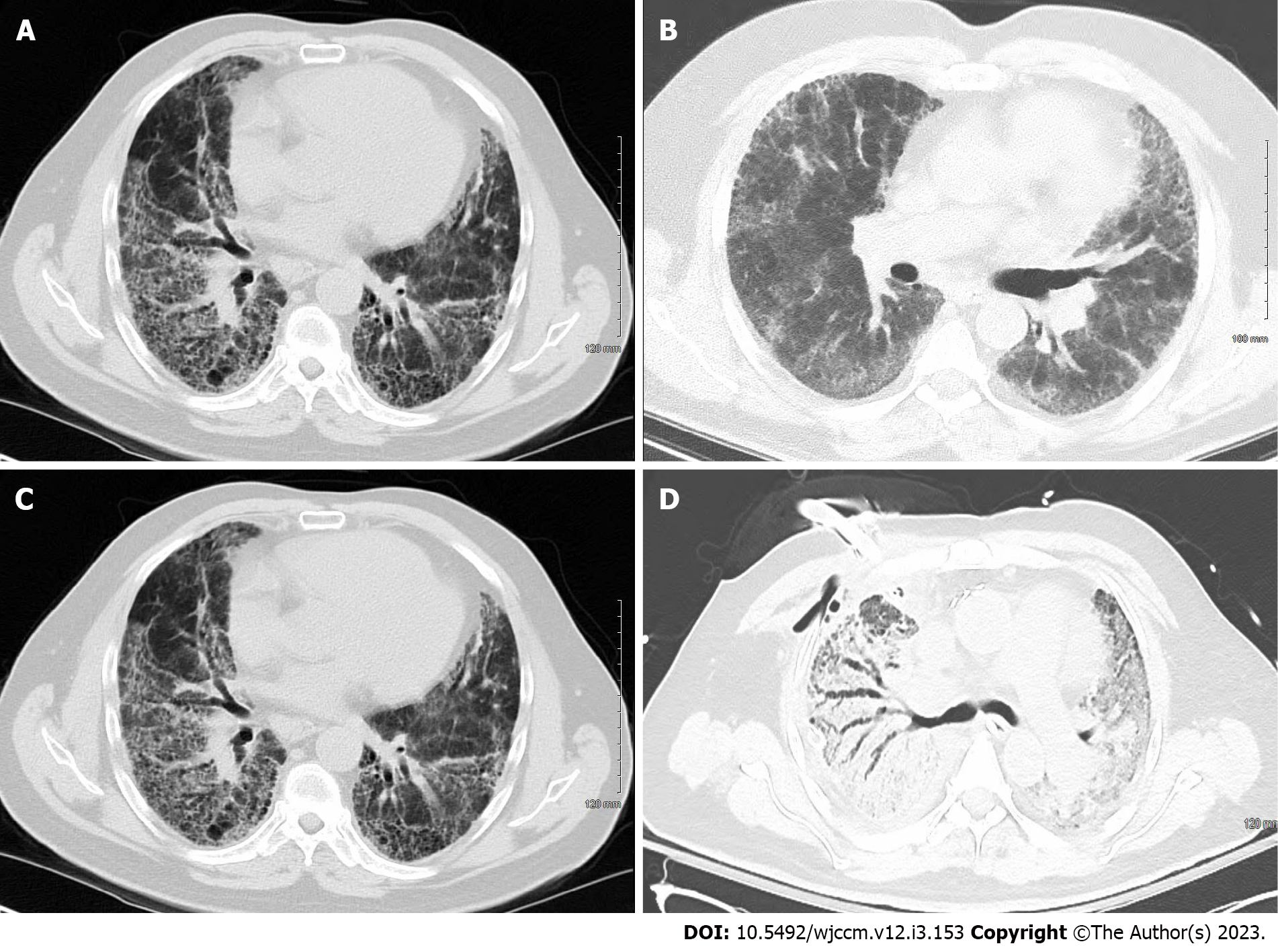
There are several characteristics that can be used to quickly narrow the diagnosis: (1) Is increased attenuation present? i.e. reticulation, ground-glass, consolidation, nodules, or linear opacities; (2) Are cysts present?; and (3) Are areas of decreased attenuation (as is seen with honeycombing) a sign of lung fibrosis? While ILD is considered a rare family of diseases, IPF is the most common archetype of these rare diseases. The terms NSIP and usual interstitial pneumonia (UIP) are often encountered in the impression of CT scans of these patients. It should be mentioned that these are both descriptive of radiographic and histologic processes. With the advent of high-resolution chest CT, many ILDs can be diagnosed based on imaging alone. Radiologically, in UIP, honeycombing is the prototypical appearance of IPF (which represents areas of the destroyed and fibrotic lung) and is a predominant feature with an apical to basal gradient. It classically involves the subpleural region and is more often in the middle and lower lungs (Figure 2A). Ground glass opacities are minimal or absent, and traction bronchiectasis is often seen, which signifies architectural changes secondary to fibrosis[4]. NSIP refers to a pattern that is predominantly composed of diffuse, bilateral, ground-glass opacities and, at times, associated with peripheral irregular linear or reticular opacities. The distribution is mainly peripheral and basal and typically spares the subpleural region. Honeycombing, if present, is generally mild in comparison to UIP (Figure 2B).
However, there are some radiographic findings that are nearly pathognomonic and obviating the need for a tissue diagnosis[24]. While tissue sampling does provide a definitive diagnosis, surgical lung biopsy is generally not pursued in the setting of AE-ILD as it does not alter the course of treatment[25], and the procedure itself carries with it significant morbidity[26]. The typical UIP and NSIP CT patterns were outlined above. The findings seen during an AE may be of prognostic value. In AEs, the CT scan may show areas of consolidation, ground glass opacification, or a combination of the two (Figure 2C and D). In fact, Akira et al[27] were able to demonstrate Kaplan-Meir survival curve differences as it relates to the patterns found on CT scans in patients admitted with AE-UIP[27]. The chest CT will also exclude pulmonary embolism (if protocoled correctly) and pneumothorax.
Laboratory workups should follow the same standard of care as for patients admitted to the ICU with respiratory failure. Sputum and blood cultures should be drawn at the time of admission. Urine antigens for Legionella and Streptococcus and respiratory samples should be sent for PCR to assess for viral infection. Evidence exists that serum procalcitonin may be a helpful marker in differentiating bacterial pneumonia from AE-ILD[28,29]. Bronchoscopy likely has limited utility in the evaluation of these patients and if performed should be conducted with anticipation that further respiratory decompensation may occur. In the single-center cohort, it was demonstrated that bronchoscopy revealed potential causes of respiratory decline in 13% of patients and a change in management from the initial empiric regimen in 25% of patients. There was no difference in mortality between those with and without bronchoscopy findings. However, bronchoscopy in non-ICU patients led to immediate respiratory decompensation and the need for a higher level of care or invasive mechanical ventilation (IMV) in 25% of patients[30].
In patients without previously diagnosed ILD, there may be a role of assessing autoimmune serology for the purpose of differentiating between IPF and connective tissue disease-related ILD or pulmonary alveolar hemorrhage syndromes. The latter two are more likely to respond to high doses of immuno
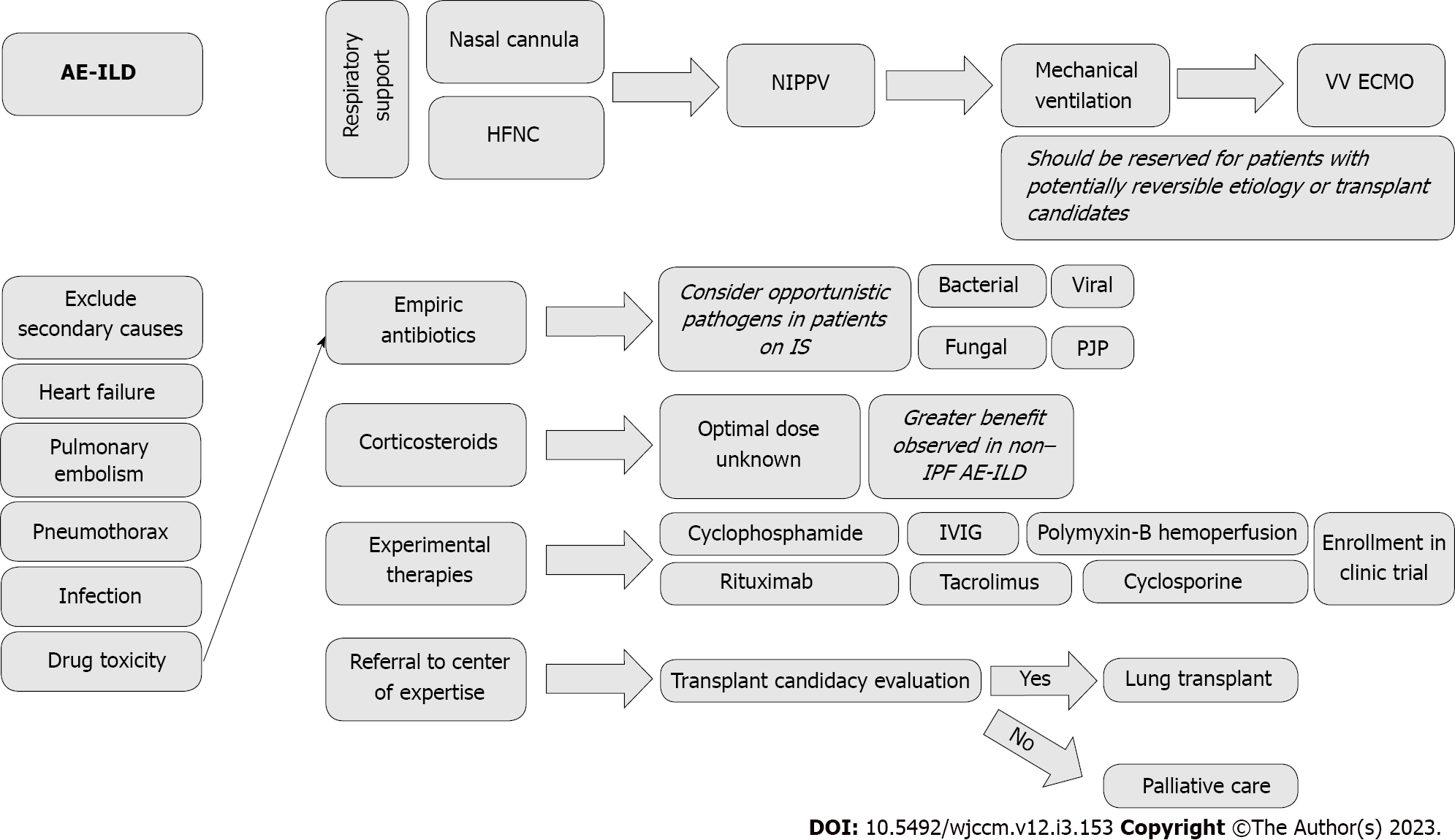
Management can be viewed as a multifaceted approach that is mainly comprised of supportive care for respiratory failure. Given the relative heterogeneity of disease processes, there is not one treatment plan that can be universally applied. Below we will outline the different considerations and treatment modalities that have been applied to patients with AE-ILD. It should be noted that much of this practice is done with a paucity of high-quality evidence and ultimately relies on expert opinion on consensus guidelines. Figure 3 is a generalized flowsheet that can be used to guide through the various steps of management of AE-ILD patients.
Presently there are no proven, effective therapies for the treatment of AE-IPF. Despite this, many patients with AE-IPF receive corticosteroids in accordance with the guidelines, which admit there are no controlled trials to judge efficacy and that the recommendation comes largely from anecdotal evidence of benefit[3]. Given the high mortality associated with AE-IPF, it is reasonable to administer corticosteroids to these patients. However, the dose, route, and duration are of unknown amounts. The same can be said for AIP, which is a rapidly progressing, lethal form of ILD with a dismal prognosis and is not responsive to steroids[6]. A general approach adopted is to use daily corticosteroid dosage in the 1-2 mg/kg range in divided doses. In patients who respond to this treatment, a gradual taper is attempted over the course of weeks.
A recent trial looked at the addition of cyclophosphamide to corticosteroids for the management of AE-IPF and showed that 3-mo mortality increased in the treatment arm, which provides further evidence against its use in this setting[32,33]. Another drug of interest is cyclosporine, which has been investigated in conjunction with corticosteroids in several non-randomized, retrospective studies in patients with AE-IPF and has shown potential benefits. However, larger randomized controlled studies are needed to confirm this[34]. It has been postulated that immune dysregulation may lead to autoantibody production, which may drive the progression of IPF or the development of AE. As such, rituximab, in conjunction with plasma exchange, has been investigated as a potential therapeutic with promising results[35].
Currently, there are ongoing, prospective, randomized controlled studies, STRIVE-IPF [NCT03
Currently, there are two medications available on the market, nintedanib and pirfenidone, which are classified as antifibrotics that are approved for the treatment of IPF. Nintedanib is a tyrosine kinase inhibitor that blocks the processes that propagate fibrosis. In the clinical trial, which ultimately led to its approval, it was shown to reduce the decline of lung function and thus slow disease progression[36]. Pirfenidone works by inhibiting transforming growth factor beta, which plays a role in collagen-directed fibroblast formation. While there is no reversal of disease, this medication has also shown the ability to slow the progression of lung decline. Both medications have been shown to reduce the rate of exacerbations[3]. Many patients with IPF will be on these medications at the time of hospitalization. Polke et al[33] looked at the management practices of AE-IPF in specialized and non-specialized ILD centers worldwide. They found that 80% of physicians in specialized centers continue antifibrotics during hospitalization, and 66% initiate therapy during the AE[33]. These therapies have not been investigated specifically for use in the setting of AE-IPF or other ILDs, for that matter. There is, however, a growing body of evidence for the use of these drugs in other fibrosing lung diseases other than IPF[37].
Antimicrobial agents are used routinely in patients with AE-ILD in conjunction with a thorough inf
It is postulated that microaspiration might play a role in the incitement of lung fibrosis. Studies have frequently demonstrated a higher prevalence of gastroesophageal reflux disease in ILD patients; however, this association cannot be interpreted as causation[43,44]. The role of acid-reducing therapy in AE has not been formally investigated. However, many physicians will continue antacids during hospitalization, and at the very least, the patients will qualify for alimentary prophylaxis based on mechanical ventilation and corticosteroid administration.
The coronavirus disease 2019 pandemic has changed the way non-invasive ventilation (NIV) is viewed and utilized. With the popularization of high-flow nasal cannula (HFNC), the oxygen demands of severely hypoxemic patients can be met without the need for endotracheal intubation. IMV has a dismal prognosis for patients with AE-ILD[45], and a trial of HFNC is warranted. The benefit and feasibility of using HFNC for acute hypoxemic respiratory failure has been shown. However, patients with chronic hypoxemic respiratory failure were excluded from this study. While the patients that were randomized to HFNC vs continuous positive airway pressure (CPAP) or bi-level positive airway pressure devices did not result in a decreased need for intubation, there was a reduction in mortality at 90 d in the HFNC group. HFNC can deliver a fraction of inspired oxygen up to 100% with high flow rates that can match the patients’ respiratory demands. Given the high flow rates, there is also a degree of dead-space washout that results in decreased work breathing. There is limited high-quality evidence regarding the matter, but it has been shown in several retrospective studies that it is a safe and well-tolerated modality with comparable outcomes to CPAP and bi-level positive airway pressure[46,47]. HFNC has been shown to have salutatory effects in IPF patients without an AE, specifically decreased minute ventilation and respiratory rate, and capillary carbon dioxide was seen. The minor increase in positive end-expiratory pressure seen with it is also considered beneficial[48,49]. Finally, there is the added advantage of the patient being able to eat and communicate with HFNC vs IMV.
NIV is often applied in patients with acute hypoxemic respiratory failure in the hopes of staving off endotracheal intubation. There is robust evidence for the utility of its use in respiratory failure secondary to chronic obstructive lung disease and congestive heart failure. However, the benefit is less certain in AE-ILD. Yokoyama et al[50] demonstrated that NIV might have potential benefits in patients with acute hypoxemic respiratory failure secondary to AE-ILD. Eleven patients received CPAP therapy. Of those patients, 6 failed and required IMV but did not survive. The remaining 5 patients survived and were alive at the 3-mo follow-up. Similar results were shown in another study that also used NIV for patients with IPF[51,52]. These studies are not without limitations, given small sample sizes and retrospective natures that may not be representative of the general AE-ILD population. Further, it may reflect a selection bias that patients who benefitted from the use of NIV may have had less severe diseases. Nonetheless, it is proof of concept that perhaps for a subset of AE-ILD patients, a trial of NIV is a potential alternative to an otherwise morbid intervention that in some cohorts see a mortality rate of up to 90% for ICU patients[51].
The outcomes for ILD patients that require IMV are so poor that outside of transplant candidates, some experts have advised against endotracheal intubation for these patients apart from those patients that have clearly reversible causes for respiratory decompensation[53]. The lungs of ILD patients are plagued by two detrimental factors: Significant V/Q mismatch and poor compliance. No specific guidelines dictate the optimal way to provide IMV to this subset of patients. The intrinsic substrate properties of ILD lungs make them especially prone to ventilator-induced lung injury with decreased compliance making the lung more prone to both barotraumas as well as atelecatrauma[54,55]. Therefore, it is reasonable to utilize strategies from the ARDSNET group for the management of patients with acute lung injury and acute respiratory distress syndrome (ARDS)[56]. Some authors use this fact as a caution against absolute denial of IMV for ILD patients, given that some of the data were collected before the publication of the landmark trial in 2000, which demonstrated the mortality benefit of low tidal volume ventilation[13].
There have been studies that looked at the lung mechanics of stable ILD patients and found that based on both static and dynamic lung compliance, the lung is less distensible than normal. Furthermore, it was demonstrated that the elastance of the mechanically ventilated IPF patient was four times higher than even those patients with ARDS[57]. This is further corroborated by retrospective studies that have shown that higher levels of positive end-expiratory pressure have been associated with higher mortality in AE-ILD patients[54].
In conclusion, the outcomes of ILD patients requiring IMV are extremely poor, with cohorts that report up to 100% mortality[58]. As such, the decision to proceed with endotracheal intubation should not be made lightly and should only occur after thoughtful discussion with the patient and family members. In the case of transplant candidates, IMV can be seen as a bridge therapy. Otherwise, early involvement in palliative services are advised.
Veno-venous extracorporeal membrane oxygenation (VV ECMO) is presently viewed as a salvage tool in the management of patients with refractory hypoxemic respiratory failure. When the known strategies, such as low-tidal volume ventilation, prone positioning, and neuromuscular blockade, all fail to improve oxygenation and/or refractory acidemia due to impossible ventilation in the capable centers, VV ECMO is often employed. ECMO helps to diminish ventilator-induced lung injury in these patients. There is no consensus recommendation on the use of VV ECMO for ARDS, and the data supporting its use leaves most intensivists with a great deal of uncertainty regarding the degree of and who will benefit[59,60].
There is even less certainty regarding its use in AE-ILD patients. Trudzinski et al[61] showed that ECMO is a viable option for patients who are suitable for a lung transplant. However, it should not be offered to those who are not, given that it does not reverse the otherwise poor prognosis faced by patients who are not transplant candidates. In an international poll, roughly 50% of physicians at specialized centers offered ECMO as a bridge to patients who were suitable for transplantation[33]. To summarize, ECMO should be considered for patients with AE-ILD with a clear reversible cause (e.g., infection or pulmonary embolism) or as a bridging therapy for patients appropriate for lung transplant.
ILD, particularly IPF, is now the most common indication for lung transplantation worldwide[62]. The median post-transplant survival for patients with idiopathic interstitial pneumonia, which includes IPF, is 5.2 years and 6.7 years for all other ILDs transplanted between 1992 and 2017[63]. Current guidelines recommend that transplant only be offered to those patients with a > 80% likelihood of 5-year post-transplant survival[64]. There are several relative contraindications to transplant, such as severe psychosocial problems or life-threatening extrapulmonary organ dysfunction. The latter may frequently be encountered in AE-ILD patients who require ICU admission. The need for IMV and/or ECMO for respiratory failure before the transplantation are associated with adverse post-transplant outcomes, which may prevent some of the sickest patients from undergoing transplantation[64]. For those patients who were already listed for transplantation and who experienced an AE, ECMO as a bridge to transplantation could be considered on a case-by-case basis. The emphasis in these patients is to avoid the development of critical illness myopathy and extrapulmonary organ damage. For de novo patients, performing invasive tests like heart catheterization and screening colonoscopies can be perilous. Ultimately, candidacy for an organ transplant will be a decision made in a multidisciplinary fashion incorporating the values of the patient and family, transplant surgeons, and transplant pulmonologists.
Palliative care (PC) medicine specialists are dedicated to improving patient quality of life during serious illnesses. In a recent survey, it was shown that the majority of ILD providers use PC and are comfortable discussing PC with their patients[65]. Given the poor prognosis that ILD patients face when they are admitted to the ICU, it warrants early involvement of PC services. Furthermore, the median life expectancy for all patients with IPF is between 2-7 years, which hastens in the AE setting. The American Thoracic Society/European Respiratory Society guidelines recommend that advanced directives and end-of-life issues be addressed in the ambulatory setting in all patients with IPF[3]. A subset of patients will require ICU level of care due to AE-ILD that was not previously diagnosed and will require more nuanced discussions given the emotional burden of a new, life-limiting diagnosis. In one IPF cohort, it was found that the hospital was the place of death for 80% of the patients, and most of these patients (93%) were hospitalized for an average of 30 d during the last 6 mo of their life. More striking was the statistic that 42% had a do not resuscitate order that was decided upon ≤ 3 d prior to their death[66]. This further confirms the importance of early and thoughtful communication with these patients and their families to ease the emotional and physical suffering they may endure at the end of their lives.
Flares or AEs of ILD have feared complications, owing both to their poor prognosis and uncertain management. The mainstay of therapy is supportive care and early recognition of a potentially reversible cause. Immunosuppression plays a role in a subset of patients; however, the optimal dosage and duration have not yet been defined. It behooves the intensivist to trial NIV forms on respiratory support to avoid the highly morbid effects of mechanical ventilation in these patients. The most aggressive therapies, such as IMV and ECMO, should be reserved for those patients who are potential transplant candidates. Early involvement in PC will assist in managing complex discussions and minimize suffering for patients with a high propensity for mortality.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of CHEST Physician; Society of Critical Care Medicine.
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Juneja D, India; Wu J, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D; ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2232] [Cited by in RCA: 2935] [Article Influence: 244.6] [Reference Citation Analysis (0)] |
| 2. | Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, Behr J, Brown KK, Cottin V, Flaherty KR, Fukuoka J, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kolb M, Lynch DA, Myers JL, Raghu G, Richeldi L, Taniguchi H, Martinez FJ. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med. 2016;194:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 997] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 3. | Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5557] [Cited by in RCA: 5299] [Article Influence: 378.5] [Reference Citation Analysis (0)] |
| 4. | Jawad H, Chung JH, Lynch DA, Newell JD Jr. Radiological approach to interstitial lung disease: a guide for the nonradiologist. Clin Chest Med. 2012;33:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr, Lasky JA, Loyd JE, Noth I, Olman MA, Raghu G, Roman J, Ryu JH, Zisman DA, Hunninghake GW, Colby TV, Egan JJ, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kondoh Y, Lynch DA, Müller-Quernheim J, Myers JL, Nicholson AG, Selman M, Toews GB, Wells AU, Martinez FJ; Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 917] [Cited by in RCA: 817] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 6. | Taniguchi H, Kondoh Y. Acute and subacute idiopathic interstitial pneumonias. Respirology. 2016;21:810-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Idiopathic Pulmonary Fibrosis Clinical Research Network, Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1181] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 8. | Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G, Brun M, Gupta A, Juhel N, Klüglich M, du Bois RM. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 819] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 9. | King TE Jr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, Thomeer M, Valeyre D, du Bois RM; INSPIRE Study Group. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet. 2009;374:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 389] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 10. | King TE Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, Valeyre D, Leconte I, Morganti A, Roux S, Behr J. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 345] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 11. | Ryerson CJ, Collard HR. Acute exacerbations complicating interstitial lung disease. Curr Opin Pulm Med. 2014;20:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 569] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 13. | Gaudry S, Vincent F, Rabbat A, Nunes H, Crestani B, Naccache JM, Wolff M, Thabut G, Valeyre D, Cohen Y, Mal H. Invasive mechanical ventilation in patients with fibrosing interstitial pneumonia. J Thorac Cardiovasc Surg. 2014;147:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Panos RJ, Mortenson RL, Niccoli SA, King TE Jr. Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med. 1990;88:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 220] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Sakamoto K, Taniguchi H, Kondoh Y, Wakai K, Kimura T, Kataoka K, Hashimoto N, Nishiyama O, Hasegawa Y. Acute exacerbation of IPF following diagnostic bronchoalveolar lavage procedures. Respir Med. 2012;106:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Casoni GL, Tomassetti S, Cavazza A, Colby TV, Dubini A, Ryu JH, Carretta E, Tantalocco P, Piciucchi S, Ravaglia C, Gurioli C, Romagnoli M, Chilosi M, Poletti V. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 2014;9:e86716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 230] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 17. | Johannson KA, Vittinghoff E, Lee K, Balmes JR, Ji W, Kaplan GG, Kim DS, Collard HR. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J. 2014;43:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 18. | Lee JS, Song JW, Wolters PJ, Elicker BM, King TE Jr, Kim DS, Collard HR. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Sugino K, Ono H, Saito M, Ando M, Tsuboi E. Coronavirus disease 2019 vaccination-induced acute exacerbation in idiopathic pulmonary fibrosis. Respirol Case Rep. 2022;10:e01051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 20. | Sugiura H, Takeda A, Hoshi T, Kawabata Y, Sayama K, Jinzaki M, Kuribayashi S. Acute exacerbation of usual interstitial pneumonia after resection of lung cancer. Ann Thorac Surg. 2012;93:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Wootton SC, Kim DS, Kondoh Y, Chen E, Lee JS, Song JW, Huh JW, Taniguchi H, Chiu C, Boushey H, Lancaster LH, Wolters PJ, DeRisi J, Ganem D, Collard HR. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:1698-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 22. | Churg A, Müller NL, Silva CI, Wright JL. Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol. 2007;31:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Moua T, Westerly BD, Dulohery MM, Daniels CE, Ryu JH, Lim KG. Patients With Fibrotic Interstitial Lung Disease Hospitalized for Acute Respiratory Worsening: A Large Cohort Analysis. Chest. 2016;149:1205-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Hunninghake GW, Zimmerman MB, Schwartz DA, King TE Jr, Lynch J, Hegele R, Waldron J, Colby T, Müller N, Lynch D, Galvin J, Gross B, Hogg J, Toews G, Helmers R, Cooper JA Jr, Baughman R, Strange C, Millard M. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest. 2005;128:3310-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States. 2000 to 2011. Am J Respir Crit Care Med. 2016;193:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 310] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 27. | Akira M, Kozuka T, Yamamoto S, Sakatani M. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Nagata K, Tomii K, Otsuka K, Tachikawa R, Nakagawa A, Takeshita J, Tanaka K, Matsumoto T, Monden K, Kawamura T, Tamai K. Serum procalcitonin is a valuable diagnostic marker in acute exacerbation of interstitial pneumonia. Respirology. 2013;18:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Sim JK, Oh JY, Lee EJ, Hur GY, Lee SH, Lee SY, Kim JH, Shin C, Shim JJ, In KH, Kang KH, Min KH. Serum Procalcitonin for Differential Diagnosis of Acute Exacerbation and Bacterial Pneumonia in Patients With Interstitial Lung Disease. Am J Med Sci. 2016;351:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Arcadu A, Moua T. Bronchoscopy assessment of acute respiratory failure in interstitial lung disease. Respirology. 2017;22:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 32. | Naccache JM, Jouneau S, Didier M, Borie R, Cachanado M, Bourdin A, Reynaud-Gaubert M, Bonniaud P, Israël-Biet D, Prévot G, Hirschi S, Lebargy F, Marchand-Adam S, Bautin N, Traclet J, Gomez E, Leroy S, Gagnadoux F, Rivière F, Bergot E, Gondouin A, Blanchard E, Parrot A, Blanc FX, Chabrol A, Dominique S, Gibelin A, Tazi A, Berard L, Brillet PY, Debray MP, Rousseau A, Kerjouan M, Freynet O, Dombret MC, Gamez AS, Nieves A, Beltramo G, Pastré J, Le Borgne-Krams A, Dégot T, Launois C, Plantier L, Wémeau-Stervinou L, Cadranel J, Chenivesse C, Valeyre D, Crestani B, Cottin V, Simon T, Nunes H; EXAFIP investigators and the OrphaLung network. Cyclophosphamide added to glucocorticoids in acute exacerbation of idiopathic pulmonary fibrosis (EXAFIP): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 33. | Polke M, Kondoh Y, Wijsenbeek M, Cottin V, Walsh SLF, Collard HR, Chaudhuri N, Avdeev S, Behr J, Calligaro G, Corte TJ, Flaherty K, Funke-Chambour M, Kolb M, Krisam J, Maher TM, Molina Molina M, Morais A, Moor CC, Morisset J, Pereira C, Quadrelli S, Selman M, Tzouvelekis A, Valenzuela C, Vancheri C, Vicens-Zygmunt V, Wälscher J, Wuyts W, Bendstrup E, Kreuter M. Management of Acute Exacerbation of Idiopathic Pulmonary Fibrosis in Specialised and Non-specialised ILD Centres Around the World. Front Med (Lausanne). 2021;8:699644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Sakamoto S, Homma S, Miyamoto A, Kurosaki A, Fujii T, Yoshimura K. Cyclosporin A in the treatment of acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2010;49:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Donahoe M, Valentine VG, Chien N, Gibson KF, Raval JS, Saul M, Xue J, Zhang Y, Duncan SR. Autoantibody-Targeted Treatments for Acute Exacerbations of Idiopathic Pulmonary Fibrosis. PLoS One. 2015;10:e0127771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 36. | Mazzei ME, Richeldi L, Collard HR. Nintedanib in the treatment of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2015;9:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, Richeldi L, Kolb M, Tetzlaff K, Stowasser S, Coeck C, Clerisme-Beaty E, Rosenstock B, Quaresma M, Haeufel T, Goeldner RG, Schlenker-Herceg R, Brown KK; INBUILD Trial Investigators. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med. 2019;381:1718-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1431] [Article Influence: 238.5] [Reference Citation Analysis (0)] |
| 38. | Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, Make B, Marchetti N, Martinez FJ, Madinger NE, McEvoy C, Niewoehner DE, Porsasz J, Price CS, Reilly J, Scanlon PD, Sciurba FC, Scharf SM, Washko GR, Woodruff PG, Anthonisen NR; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 843] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 39. | Martinez FJ, Curtis JL, Albert R. Role of macrolide therapy in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:331-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Kawamura K, Ichikado K, Suga M, Yoshioka M. Efficacy of azithromycin for treatment of acute exacerbation of chronic fibrosing interstitial pneumonia: a prospective, open-label study with historical controls. Respiration. 2014;87:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Kawamura K, Ichikado K, Yasuda Y, Anan K, Suga M. Azithromycin for idiopathic acute exacerbation of idiopathic pulmonary fibrosis: a retrospective single-center study. BMC Pulm Med. 2017;17:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Peng JM, Du B, Wang Q, Weng L, Hu XY, Wu CY, Shi Y. Dermatomyositis and Polymyositis in the Intensive Care Unit: A Single-Center Retrospective Cohort Study of 102 Patients. PLoS One. 2016;11:e0154441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Hershcovici T, Jha LK, Johnson T, Gerson L, Stave C, Malo J, Knox KS, Quan S, Fass R. Systematic review: the relationship between interstitial lung diseases and gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:1295-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Lee JS, Collard HR, Anstrom KJ, Martinez FJ, Noth I, Roberts RS, Yow E, Raghu G; IPFnet Investigators. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med. 2013;1:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 293] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 45. | Rangappa P, Moran JL. Outcomes of patients admitted to the intensive care unit with idiopathic pulmonary fibrosis. Crit Care Resusc. 2009;11:102-109. [PubMed] |
| 46. | Koyauchi T, Hasegawa H, Kanata K, Kakutani T, Amano Y, Ozawa Y, Matsui T, Yokomura K, Suda T. Efficacy and Tolerability of High-Flow Nasal Cannula Oxygen Therapy for Hypoxemic Respiratory Failure in Patients with Interstitial Lung Disease with Do-Not-Intubate Orders: A Retrospective Single-Center Study. Respiration. 2018;96:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Koyauchi T, Yasui H, Enomoto N, Hasegawa H, Hozumi H, Suzuki Y, Karayama M, Furuhashi K, Fujisawa T, Nakamura Y, Inui N, Yokomura K, Suda T. Pulse oximetric saturation to fraction of inspired oxygen (SpO(2)/FIO(2)) ratio 24 h after high-flow nasal cannula (HFNC) initiation is a good predictor of HFNC therapy in patients with acute exacerbation of interstitial lung disease. Ther Adv Respir Dis. 2020;14:1753466620906327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Ito J, Nagata K, Morimoto T, Kogo M, Fujimoto D, Nakagawa A, Otsuka K, Tomii K. Respiratory management of acute exacerbation of interstitial pneumonia using high-flow nasal cannula oxygen therapy: a single center cohort study. J Thorac Dis. 2019;11:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Bräunlich J, Beyer D, Mai D, Hammerschmidt S, Seyfarth HJ, Wirtz H. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration. 2013;85:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 50. | Yokoyama T, Kondoh Y, Taniguchi H, Kataoka K, Kato K, Nishiyama O, Kimura T, Hasegawa R, Kubo K. Noninvasive ventilation in acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2010;49:1509-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Vianello A, Arcaro G, Battistella L, Pipitone E, Vio S, Concas A, Paladini L, Gallan F, Marchi MR, Tona F, Iliceto S. Noninvasive ventilation in the event of acute respiratory failure in patients with idiopathic pulmonary fibrosis. J Crit Care. 2014;29:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Yokoyama T, Tsushima K, Yamamoto H, Koizumi T, Kubo K. Potential benefits of early continuous positive pressure ventilation in patients with rapidly progressive interstitial pneumonia. Respirology. 2012;17:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Al-Hameed FM, Sharma S. Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J. 2004;11:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Fernández-Pérez ER, Yilmaz M, Jenad H, Daniels CE, Ryu JH, Hubmayr RD, Gajic O. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest. 2008;133:1113-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Syed MKH, Selickman J, Evans MD, Dries D, Marini JJ. Elastic Power of Mechanical Ventilation in Morbid Obesity and Severe Hypoxemia. Respir Care. 2021;66:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8487] [Cited by in RCA: 8335] [Article Influence: 333.4] [Reference Citation Analysis (3)] |
| 57. | Nava S, Rubini F. Lung and chest wall mechanics in ventilated patients with end stage idiopathic pulmonary fibrosis. Thorax. 1999;54:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Mollica C, Paone G, Conti V, Ceccarelli D, Schmid G, Mattia P, Perrone N, Petroianni A, Sebastiani A, Cecchini L, Orsetti R, Terzano C. Mechanical ventilation in patients with end-stage idiopathic pulmonary fibrosis. Respiration. 2010;79:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A; EOLIA Trial Group, REVA, and ECMONet. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018;378:1965-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1417] [Cited by in RCA: 1485] [Article Influence: 212.1] [Reference Citation Analysis (0)] |
| 60. | Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2740] [Cited by in RCA: 2332] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 61. | Trudzinski FC, Kaestner F, Schäfers HJ, Fähndrich S, Seiler F, Böhmer P, Linn O, Kaiser R, Haake H, Langer F, Bals R, Wilkens H, Lepper PM. Outcome of Patients with Interstitial Lung Disease Treated with Extracorporeal Membrane Oxygenation for Acute Respiratory Failure. Am J Respir Crit Care Med. 2016;193:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 62. | Kapnadak SG, Raghu G. Lung transplantation for interstitial lung disease. Eur Respir Rev. 2021;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 63. | Chambers DC, Cherikh WS, Harhay MO, Hayes D Jr, Hsich E, Khush KK, Meiser B, Potena L, Rossano JW, Toll AE, Singh TP, Sadavarte A, Zuckermann A, Stehlik J; International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019;38:1042-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 632] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 64. | Leard LE, Holm AM, Valapour M, Glanville AR, Attawar S, Aversa M, Campos SV, Christon LM, Cypel M, Dellgren G, Hartwig MG, Kapnadak SG, Kolaitis NA, Kotloff RM, Patterson CM, Shlobin OA, Smith PJ, Solé A, Solomon M, Weill D, Wijsenbeek MS, Willemse BWM, Arcasoy SM, Ramos KJ. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2021;40:1349-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 471] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 65. | Gersten RA, Seth B, Arellano L, Shore J, O'Hare L, Patel N, Safdar Z, Krishna R, Mageto Y, Cochran D, Lindell K, Danoff SK; Pulmonary Fibrosis Foundation. Provider Perspectives on and Access to Palliative Care for Patients With Interstitial Lung Disease. Chest. 2022;162:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 66. | Rajala K, Lehto JT, Saarinen M, Sutinen E, Saarto T, Myllärniemi M. End-of-life care of patients with idiopathic pulmonary fibrosis. BMC Palliat Care. 2016;15:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |