Published online Jun 9, 2023. doi: 10.5492/wjccm.v12.i3.116
Peer-review started: December 28, 2022
First decision: March 1, 2023
Revised: March 30, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: June 9, 2023
Processing time: 162 Days and 5.5 Hours
Acute neurologic injuries represent a common cause of morbidity and mortality in children presenting to the pediatric intensive care unit. After primary neurologic insults, there may be cerebral brain tissue that remains at risk of secondary insults, which can lead to worsening neurologic injury and unfavorable outcomes. A fundamental goal of pediatric neurocritical care is to mitigate the impact of secondary neurologic injury and improve neurologic outcomes for critically ill children. This review describes the physiologic framework by which strategies in pediatric neurocritical care are designed to reduce the impact of secondary brain injury and improve functional outcomes. Here, we present current and emerging strategies for optimizing neuroprotective strategies in critically ill children.
Core Tip: Acute neurologic injuries are a common cause of morbidity and mortality in critically ill children. A fundamental goal of pediatric neurocritical care is to mitigate the impact of secondary neurologic injury in critically ill children. Here, we discuss strategies for optimizing neuroprotective strategies in critically ill children.
- Citation: Kochar A, Hildebrandt K, Silverstein R, Appavu B. Approaches to neuroprotection in pediatric neurocritical care. World J Crit Care Med 2023; 12(3): 116-129
- URL: https://www.wjgnet.com/2220-3141/full/v12/i3/116.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v12.i3.116
Neurologic injuries represent a substantial component of pediatric intensive care unit (PICU) utilization in the United States. Analyses of admissions to a large tertiary PICU have demonstrated that neurologic diagnoses are present in approximately one quarter of PICU admissions (25.4%)[1] and acute brain injury is the most common proximate cause of death in children admitted to a PICU accounting for up to 65% of PICU mortality[2]. Additionally, children admitted to the PICU are estimated to acquire new long-term functional disability at a rate of 4.8%[3] by hospital discharge with evidence of further decline in functional status after discharge[4]. The significant contribution of neurologic injury to PICU morbidity and mortality has resulted in an increasing emphasis to develop evidence-based practices to prevent acute brain injury in systemically ill patients and to mitigate the impact of such injuries once they occur. Here, we review current approaches to neuroprotective care in commonly seen pediatric critical care conditions as well as ongoing and future research targets that promise individualized, precision-based care.
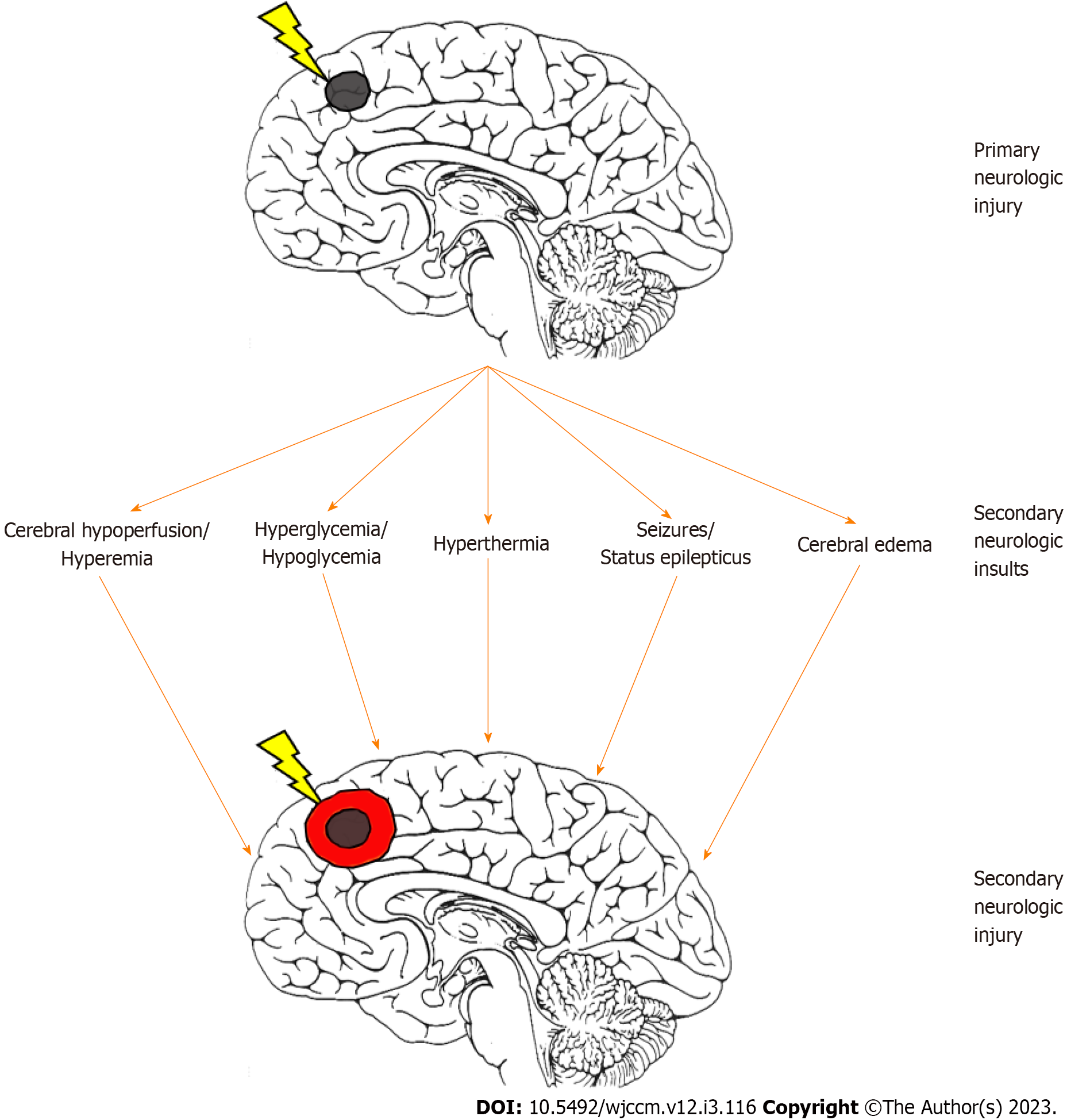
The evolution of neurologic dysfunction after an acute insult is multiphasic. The primary neurologic injury represents an initial inciting event which results in neuronal cell death, for example acute energy failure in the setting of arterial ischemic stroke or direct mechanical shearing of axons in traumatic brain injury (TBI). Some of the damage caused by the primary injury is typically complete at the time of recognition or presentation to care. However, in many situations there remains at-risk brain tissue that can be acutely rescued if brain homeostasis is optimized with appropriate cerebral blood flow (CBF) to meet metabolic demand. A classic example of such an intervention in adult neurocritical care is thrombolytic and other reperfusion therapy that salvages the ischemic penumbra after acute arterial ischemic stroke. Minimizing ongoing or recurrent mismatch between cerebral perfusion and brain metabolic demand during critical care management represents one of the primary goals of neurocritical care.
Following the primary injury, multiple parallel physiologic pathways emerge that result in further cellular injury if not prevented (Figure 1). A fundamental goal in pediatric neurocritical care is to limit secondary brain injury by optimizing cerebral oxygen delivery and its use[5]. Secondary brain injury is the additive cerebral injury which is created by an imbalance of supply and demand in cerebral metabolism.
Neuroprotection generally refers to the preservation of cerebral function by mitigating the above sources of secondary injury[6]. In the PICU, this approach can be broken down into a variety of directives including optimization of cerebral perfusion, limitation of cerebral metabolic demand, and mitigation against cerebral edema.
The process of optimizing cerebral perfusion for a given patient or pathology requires attention to several physiologic and hemodynamic targets for neuroprotection with consideration of both CBF as well as blood content.
CBF is primarily determined by cerebral perfusion pressure (CPP) and cerebral vascular resistance. CPP is calculated as the difference between mean arterial pressure (MAP) and intracranial pressure (ICP). Importantly, however, measurements of MAP vary significantly by site and methodology[7]. Additionally, adult data has demonstrated that invasive arterial blood pressure measurements levelled at the heart underestimates the MAP at the level of the circle of Willis by approximately 15% when a patients head of bed is elevated to 30° or 45° suggesting head of bed positioning is an important consideration in critically ill patients[8]. Hypotension in pediatric ICU patients has been associated with increased mortality after cardiac arrest and worse outcomes after stroke and TBI[9-13]. Consensus-based pediatric guidelines have focused on maintaining MAPs above minimum thresholds and CPP thresholds when invasive ICP monitoring is available (Table 1)[14-16]. While specific thresholds have been proposed for both MAPs and ICPs, some evidence suggests optimal values varies by age and are on average higher than current guidelines-based recommendations[17]. Further research is needed to identify appropriate minimum thresholds in pediatric neurocritical care populations.
| Pathology | Optimize cerebral perfusion | Limit cerebral metabolic demand | Mitigate cerebral edema |
| Severe traumatic brain injury[15] | Maintain age appropriate CPP (Minimum ≥ 40 mmHg) | Targeted normothermias: 35 °C−38 °C | Maintain sodium: ≥ 140 mEq/L |
| If PbtO2 available: ≥ 10 mmHg | Maintain adequate sedation and analgesia | Maintain HOB = 30 °C | |
| Maintain ICP < 20 mmHg | Benzodiazepine + Opiate as initial therapy | Second tier therapies | |
| Targeted normoxemia: SpO2 92%−99% | Consider continuous EEG | Surgical intervention | |
| Maintain PaCo2: 35-40 mmHg | Phenytoin or levetiracetam for seizures | Barbiturate infusion | |
| Target euglycemia: 100–180 mg/dL | Moderate hypothermia (32 °C−34 °C) | ||
| Target euvolemia: CVP 4−10 mmHg | Hyperventilation (PaCO2 28-34 mmHg) | ||
| Maintain hemoglobin: > 7 g/dL | Increased hyperosmolar therapy | ||
| Post-Cardiac arrest[14] | Maintain MAP ≥ 5th percentile for age | Targeted normothermia: 36 °C−37.5 °C | |
| Targeted normoxemia: SpO2 94%−99% | Consider 48 h of T 32 °C− 34 °C for OHCA | ||
| Maintain PaCo2: 35-45 mmHg | Maintain adequate sedation and analgesia | ||
| Target euglycemia: 80−180 mg/dL | Continuous EEG | ||
| Treat seizures if identified | |||
| Acute arterial ischemic stroke[80] | Treat hypertension with caution in patients with intracranial vascular stenosis | Maintain temperature < 38 °C | Consider decompressive surgery for malignant edema |
| Aggressively treat hypotension | For large volume infarcts (> 1/2 MCA territory) | ||
| Treat hyperglycemia to target 140-180 mg/dL | Consider early decompressive hemicraniectomy (< 24 h) | ||
| Treat hypoglycemia: < 60 mg/dL | Serial imaging and frequent assessments for 72 h |
Cerebral vascular resistance is the other major determinant of CBF. Arterial carbon dioxide tension (PaCO2) is the primary modifiable physiologic parameter that impacts cerebral vascular resistance in patients with intact cerebrovascular reactivity, though significant hypoxemia can also play a role in cerebrovascular vasodilation[18]. Increased PaCO2 results in cerebrovascular vasodilation which is often desirable when hypoperfusion is the primary insult as seen with permissive hypercapnia in acute stroke care, though is less desirable in cases where cerebral edema and intracranial hypertension are predominant as it results in a net increase in the intracranial blood volume compartment further contributing to increased ICP. Current pediatric literature supports maintaining normocapnia in most pathologies. Impaired carbon dioxide reactivity to brain tissue oxygenation (PbtO2) can be observed after injuries such as TBI, and recognition of such situations may influence targeting of PaCO2 levels.
Partial pressure of blood oxygenation (PaO2), glucose and hemoglobin content are also important in ensuring adequate cerebral perfusion after acute brain injury. Both hyperoxia and hypoxia are common in pediatric patients after cardiac arrest and TBI, however the impact of oxygen exposure on outcomes remains unclear[19-21]. Arterial hypoxemia in the injured brain results in reduced cerebral oxygen delivery, potentiating injury in ischemic tissue and further contributing to neuronal excitotoxicity. Conversely, hyperoxia is thought to increase oxidative stress through increased production of free radical species and has been associated with increased mortality after cardiac arrest in adult populations[22]. Available data from pediatric investigations has been equivocal on the effect of arterial hypoxia or hyperoxia on morbidity or mortality after cardiac arrest or TBI. One large retrospective review demonstrated increased mortality in pediatric post arrest patients with a PaO2 ≥ 300 mmHg or PaO2 ≤ 60 mmHg on the first arterial blood gas after PICU admission[23]. Other retrospective cohort studies as well as one prospective multicenter observational study of pediatric post-arrest patients have not redemonstrated this association[19,21,24]. Retrospective analysis of pediatric TBI patients has not demonstrated an association between hypoxia and outcome, though extrapolation of this data is limited as hypoxia is often identified and treated rapidly during resuscitation[20,25,26]. A recently published systematic review and meta-analysis did demonstrate an association between arterial hyperoxia (as defined by PaO2 > 250 mmHg) and increased mortality pediatric study populations that included post-cardiac arrest, TBI, extracorporeal membrane oxygenation and general pediatric critical care[27]. Neuroprotective strategies in pediatric critical care generally support maintaining normoxemia while avoiding hyperoxia, though specific thresholds vary. Emerging data in pediatric TBI patients where invasive PbtO2 monitoring is available suggests that episodes of cerebral hypoxia (as measured by PbtO2 < 10 mmHg or 15 mmHg) is associated with unfavorable clinical outcomes as well as reduced performance on neuropsychiatric testing > 1 year post injury[28-30].
Hyperglycemia (serum glucose > 200 mg/dL) on admission after pediatric TBI has been demonstrated to be a predictor of mortality and ICU length of stay suggesting high serum glucose may be a marker of brain injury severity[31-34]. Similarly, persistent hyperglycemia 12-72 h after admission has also been independently associated with mortality and poor clinical outcomes, though prospective data assessing the impact of narrow glycemic control on outcomes is limited[35-37].
Retrospective evaluations of anemia in pediatric TBI patients have not demonstrated a significant association between anemia or need for packed red blood cell transfusion with outcomes to support transfusion thresholds that differ from standard pediatric ICU practices[38,39].
Decreasing the mismatch between cerebral perfusion and metabolic demand is a critical component to neuroprotection after acute brain injury by reducing the amount of tissue experiencing relative ischemia. Mechanisms that limit cerebral metabolism may also slow the pathological processes that contribute to secondary injury such as the enzymatic pathways that result in cell death and the neuro-inflammatory cascade that potentiates vasogenic edema. Physiologically, there are three primary targets for intervention that affect cerebral metabolic activity: Temperature, sedation, and antiseizure medications to combat acute symptomatic seizures.
Optimal temperature management for patients with acute brain injuries has been the subject of extensive research in both adult and pediatric populations. Early animal data demonstrated a linear relationship between temperature and CBF and oxygen consumption suggesting a 6% decrease in cerebral metabolic demand for each decrease of 1 °C compared to normothermia[40]. Conversely, animal studies conducted in the 1980-1990s concluded that mild hypothermia of up 2 °C conferred significant neuroprotective benefits in rat models of focal and global cerebral ischemia[41-43]. Conversely, even brief periods of hyperthermia of 3 h in similar models were associated with increased infarct volume[44-46]. The deleterious effect of fever in neurological injuries has since been redemonstrated in both adult and pediatric populations across multiple neurologic pathologies including stroke, TBI and post cardiac arrest[46-48]. In light of this data, targeted temperature management with the goal of aggressive avoidance of fever has been adopted as standard of care in patients with acute brain injury.
Of greater debate is whether the practice of induced hypothermia (typically within the range of 32-35 °C) improves neurologic outcomes in selected pediatric populations. A recent meta-analysis of eight randomized controlled trials assessing therapeutic hypothermia in pediatric severe TBI found a non-statistically significant trend towards increased mortality in patients who were treated with therapeutic hypothermia compared to normothermic controls[49]. In post-arrest care, two large, multicenter, randomized controlled trials have been conducted to assess the benefit of therapeutic hypothermia after cardiac arrest in children separately evaluating comatose children after in-hospital (THAPCA-IH) and out-of-hospital (THAPCA-OH) arrests. These trials investigated the impact of 48 h of targeted hypothermia (target 33 °C) followed by gradual rewarming and continued targeted temperature management (target 36.8 °C) for a total of 120 h after protocol initiation compared with 120 h of targeted normothermia (target 36.8 °C) on 1-year survival with a good functional outcome (defined as an age corrected standard score of 70 or higher on the Vineland Adaptive Behavior Scales, second edition). The THAPCA-IH trial was terminated during interim analysis for futility as the primary outcome did not differ between groups, though notably the safety analysis did not demonstrate any significant differences in adverse events or 28-d mortality across groups[50]. THAPCA-OH found slightly higher rates of 1-year survival with good functional outcomes in the hypothermia group compared to (20% vs 12%) though this difference was not statistically significant. Secondary analysis found significantly increased survival time in the therapeutic hypothermia group when compared to normothermia (149 d vs 119 d)[51]. Given these findings, investigation into the potential benefit of therapeutic hypothermia in pediatric out-of-hospital cardiac arrest remains ongoing and there remains provider and center dependent variability in practice. The Pediatric Influence of Cooling Duration on Efficacy in Cardiac Arrest Patients trial (NCT05376267) aims to assess the efficacy of cooling and optimal duration of hypothermia in pediatric survivors of out-of-hospital cardiac arrests and is currently enrolling with estimated completion in 2028.
Effective sedation and analgesia play an important role in limiting cerebral metabolic demand and have also been shown to have independent agent-specific effects on CBF, autoregulation and vasomotor reactivity[52]. As such, the optimal selection of anesthetic agents for pathology dependent neuroprotection is a target for ongoing research. In general, for critically ill children in the ICU the most frequently reported anesthetic agents used are benzodiazepines and opiates with frequently used secondary agents including dexmedetomidine, propofol, barbiturates, ketamine and clonidine[53]. Of these, benzodiazepines, dexmedetomidine, propofol and barbiturates have the effect of decreasing both cerebral oxygen consumption and CBF and are often used in patients where there is concern for increased ICP or significant risk for cerebral edema. Ketamine has historically been avoided in patients with acute brain injury as early data suggested its use resulted in direct cerebrovascular vasodilation leading to increased CBF and potentially increased ICP[54]. A more recent prospective pediatric trial suggested that ketamine administration in ventilated patients with intracranial hypertension refractory to initial therapies may in fact reduce ICP by an average of 30% while increasing CPPs and may therefore be safe and effective in patients with acute brain injury[55]. Neuromuscular blockade has also been shown to decrease global oxygen consumption and energy expenditure in mechanically ventilated children. This is an important consideration in children who are shivering when undergoing targeted temperature management and is used extensively in patients with refractory elevations in ICP[56,57]. The use of barbiturate coma to treat acute, refractory intracranial hypertension for pediatric TBI has been shown to be effective in decreasing ICP and is included as a consideration for second-tier therapies in the most recent consensus-based Brain Trauma Foundation guidelines[15,16,58].
The emergence of continuous electroencephalography (cEEG) has allowed for a greater under
Cerebral edema represents an increase in brain volume that is contained within cerebral interstitial tissue, and can manifest as vasogenic, cytotoxic, hydrostatic, or osmotic edema[66,67]. Vasogenic edema manifests with blood brain barrier breakdown and increased water permeability within brain interstitia. Cytotoxic edema results due to metabolic crisis, cell death, and an influx of water and ions into intracellular space. Hydrostatic edema can occur in the setting of obstructive hydrocephalus and is the result of a net influx of spinal fluid from the ventricular space into brain parenchyma. Osmotic edema is a very particular form of cerebral edema in which there is a specific isolated osmotic gradient between brain parenchyma and the cerebrovascular system. Optimizing temperature management, ventilation, and sedative therapy, as described above, remain important elements in mitigating secondary brain insults arising from cerebral edema. Hyperosmolar therapy exists to aid in mitigation of cerebral edema, with common utilization of hypertonic saline and mannitol. A recent comparative effectiveness study of pediatric TBI patients demonstrated bolus dosing of hypertonic saline to be superior to mannitol in reduction of intracranial hypertension[68]. Some evidence suggests that hyperosmolar therapy is more likely to be effective in reducing intracranial hypertension when there is evidence of efficient cerebrovascular pressure reactivity (CVPR)[69-71]. Emerging research has demonstrated several biomarkers that target the blood-brain barrier or receptors of aquaporin-4 or vasopressin V1a to mitigate cerebral edema, although these are not yet standard treatment targets in clinical care[72]. When medical efforts to mitigate against malignant cerebral edema have failed in the setting of refractory intracranial hypertension, therapeutic decompressive craniectomy can be considered[15].
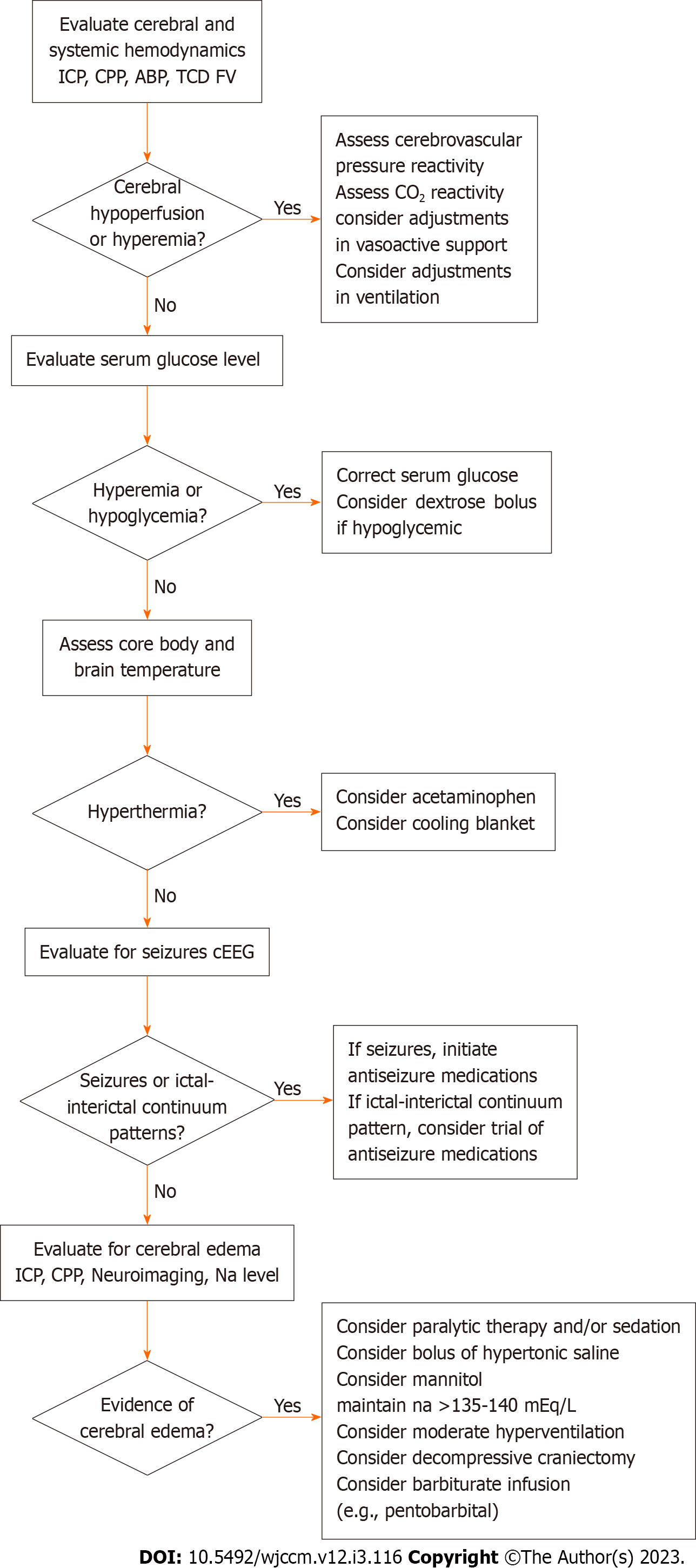
The emergence of neurocritical care as a subspecialty has been strengthened by increasing evidence that clinical care implemented with specialized expertise is associated with improved outcomes for critically ill patients with neurologic injuries. A recent large meta-analysis suggested that adult patients who underwent interventions arising from neurocritical care units, neurointensivists or neurocritical care consulting services had improved survival and functional outcomes as compared to adults with similar conditions who experienced general care in intensive care units[73]. A cohort study of pediatric TBI patients demonstrated that implementation of a pediatric neurocritical care program with a standardized evidence-based approach to neurologic monitoring and clinical care was associated with improved outcomes[74]. Such findings have helped the maturation of several specialized pediatric neurocritical care services across the United States and North America, with an array of diverse models including multidisciplinary consultation services as well as dedicated pediatric neurocritical care units that include involvement from neurologists, pediatric intensivists and neurosurgeons[75-79]. These services work toward providing institutional standardized care pathways for common neurocritical care conditions founded upon the latest evidence-based guidelines, often providing standardized or age-based thresholds for intervention (Figure 2).
Whereas the implementation of standardized institutional pathways for neurocritical care carries an association with improved outcomes at an epidemiological level, there is a severe lack of high-level evidence to demonstrate that specific clinical interventions improve outcomes for commonly seen conditions such as TBI, cardiac arrest, and arterial ischemic and hemorrhagic stroke[14-16,80]. Given the lack of high-level evidence, clinical decisions are often made in context of moderate or low-level evidence alongside fundamental and conceptual knowledge regarding pathophysiological mechanisms of critical care diseases. To this end, there are opportunities to evaluate, at the patient-level, whether individualized targeting of care may aid in optimizing neuroprotection.
Critically ill patients in the ICU often have an abundance of continuous physiologic data collected via various monitoring techniques including heart rate, invasive arterial blood pressure, end-tidal CO2 and respiratory rate or ventilator settings. Patients with acute brain injuries typically warrant additional neurophysiologic monitoring. This includes ICP monitoring using an external ventricular drain or intraparenchymal monitor, intraparenchymal brain tissue oxygenation, regional oxygen saturation via near infrared spectroscopy, cEEG, pupillometry, or information on CBF provided by various imaging techniques including transcranial doppler and thermal diffusion flowmetry[81,82]. Until recently, this information was typically evaluated in isolation or in small subsets. Recent advances in technology have facilitated the development of integrated platforms that aggregate and time-synchronize this information, allowing for easier visualization by the clinician. This approach, known as multimodality neurologic monitoring, has also allowed for investigation of how changes in one physiologic parameter potentially affect others. This allows for a greater understanding of real-time, patient-specific physiology to inform clinical decision making[83].
The utilization of neurologic monitoring is aimed towards recognition of biosignatures of secondary brain injury and initiation of treatment based upon such features. The most common and non-invasive form of this is the recognition and treatment of seizures and IIC patterns based on cEEG. Invasive methods allow for detection of intracranial hypertension and brain tissue hypoxia, with a variety of neuroprotective strategies available to use depending on the underlying source of such insults. A recent survey of pediatric neurocritical care centers in 2020 demonstrated that 20 hospitals use transcranial Doppler ultrasound as part of clinical care for management of pediatric intracranial hemorrhage, arterial ischemic stroke, or TBI, with utilization aimed toward determining when to obtain neuroimaging, how to manipulate CPP, and whether to perform surgical interventions[84]. A single-center cohort of TBI patients undergoing standardized multimodality neurologic monitoring reporting demonstrated that such reporting influenced timing of neuroimaging, ICP monitoring discontinuation, use of paralytic, hyperosmolar and pentobarbital therapies, neurosurgical interventions, use of provocative cerebral autoregulation testing, ventilator and CPP adjustments and neurologic prognostication discussions[85]. Future multicenter work describing use of integrated multimodality neurologic monitoring as a means for detecting biosignatures of secondary brain injury may aid in better understanding benign and malignant neurophysiologic patterns, methods of determining therapeutic efficacy of specific interventions, and comparative effectiveness strategies to determine whether such interventions may improve functional outcomes.
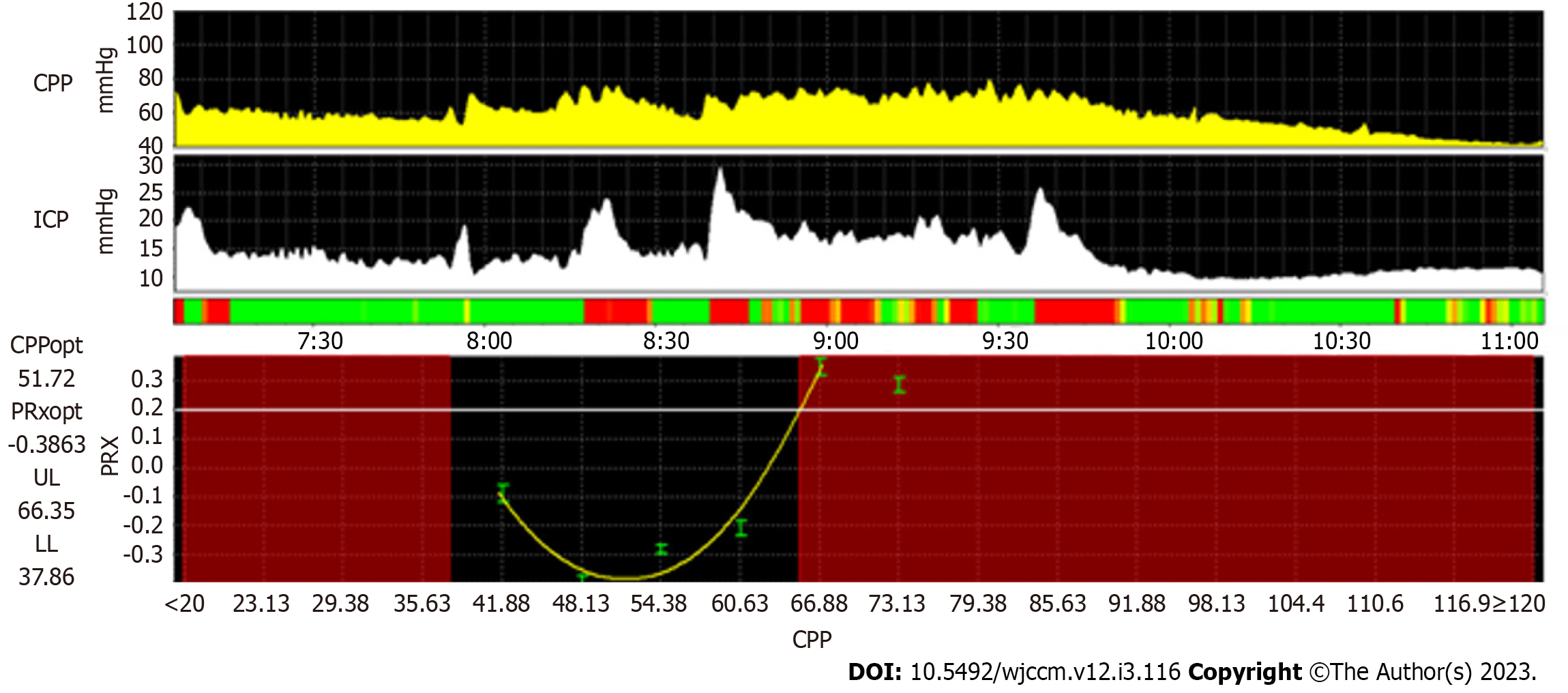
The integration of multiple streams of time-synchronized physiologic data has allowed for the development of real-time biomarkers of key neurophysiologic processes. CVPR can be assessed when integrating arterial blood pressure with neuromonitoring features that may act as surrogates of CBF[83]. Using transcranial Doppler ultrasound, the mean velocity index or systolic velocity index describes CVPR utilizing transcranial doppler ultrasound flow velocity characteristics with arterial blood pressure[86,87]. With patients undergoing continuous ICP monitoring, the pressure reactivity index (PRx) and other similar indices can be utilized with an assumption that slow wave fluctuations in ICP are directly related to changes in cerebral arterial blood volume[83]. The PRx, as an example, represents a moving Pearson correlation coefficient relating slow wave fluctuations in arterial blood pressure with ICP. Elevated PRx values (approaching +1) are postulated to represent inefficient CVPR, whereas lower values (approaching -1) are postulated to represent efficient CVPR[88].
When PRx is plotted with error bars across a range of CPP, parabolic curves can often be extrapolated, with the lowest PRx value, or nadir of the parabolic curve, representing the ‘optimal CPP’ at which CVPR is most efficient. From this, theoretical lower and upper limits of CVPR can be estimated based upon specific thresholds of elevated PRx values[89] (Figure 3). Multiple pediatric TBI studies have linked higher PRx values to worsened outcomes, and there is also evidence that increased time below the lower limit of CVPR is associated with unfavorable outcomes[90-93]. A recent feasibility randomized control trial of adult TBI patients evaluated patients who were treated with CPP targets based upon existing Brain Trauma Foundation guidelines and compared them to patients who were individualized to optimal CPP targets based upon PRx. This trial of 60 patients demonstrated that there were no significant differences in safety endpoints between the two groups, supporting the notion that prospective trials powered for clinical outcomes may be safe and feasible[94]. Other model-based indices of CVPR exist using brain tissue oxygenation, cerebral regional oximetry or other neuromonitoring techniques, with evidence from cardiac arrest, extracorporeal membrane oxygenation and other conditions that suggest that inefficient CVPR or deviations from optimal values of CVPR may be associated with unfavorable outcomes[95-98]. Future prospective work with such techniques may help in determining the efficacy for which they can be used to optimize neuroprotection across a wide range of critical care conditions.
While emerging evidence demonstrates that specific physiologic biomarkers are linked to functional outcomes after pediatric acute brain injuries, there is a severe lack of evidence toward specific neurotherapeutic strategies that improve functional outcomes. Knowledge gaps remain regarding whether biomarkers can be used to better understand whether specific neuroprotective treatments confer potential to benefit for patients stratified toward specific underlying physiologic profiles. Neuroprotective measures optimal toward care in TBI using invasive neuromonitoring may not necessarily translate to other non-traumatic conditions in which invasive monitoring may not be used. It also remains unclear whether implementation of specific strategies, such as vasoactive support for CPP-guided management, may be appropriate for neonates and very young infants where CBF differs from older children[99]. Future comparative effectiveness studies and clinical trials involving different pediatric acute brain injury conditions will be needed to further address these knowledge gaps.
Neuroprotection is a foundational component of pediatric neurocritical care. Standardized clinical approaches that integrate evidence-based guidelines with fundamental and conceptual neurophysiologic knowledge have been associated improved outcomes for patients with acute neurologic injuries. Substantial knowledge gaps remain regarding key clinical interventions that may improve patient outcomes. Multimodality neurologic monitoring demonstrates strong promise toward augmenting a patient-centered approach for optimized neuroprotection.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Govindarajan KK, India S-Editor: Liu XF L-Editor: A P-Editor: Xu ZH
| 1. | Moreau JF, Fink EL, Hartman ME, Angus DC, Bell MJ, Linde-Zwirble WT, Watson RS. Hospitalizations of children with neurologic disorders in the United States. Pediatr Crit Care Med. 2013;14:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Au AK, Carcillo JA, Clark RS, Bell MJ. Brain injuries and neurological system failure are the most common proximate causes of death in children admitted to a pediatric intensive care unit. Pediatr Crit Care Med. 2011;12:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 3. | Pollack MM, Holubkov R, Funai T, Clark A, Berger JT, Meert K, Newth CJ, Shanley T, Moler F, Carcillo J, Berg RA, Dalton H, Wessel DL, Harrison RE, Doctor A, Dean JM, Jenkins TL; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med. 2014;15:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 254] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 4. | Pinto NP, Rhinesmith EW, Kim TY, Ladner PH, Pollack MM. Long-Term Function After Pediatric Critical Illness: Results From the Survivor Outcomes Study. Pediatr Crit Care Med. 2017;18:e122-e130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 5. | Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a "two-hit" model. Crit Care. 2017;21:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 392] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 6. | Germans MR, Boogaarts HD, Macdonald RL. Neuroprotection in Critical Care Neurology. Semin Neurol. 2016;36:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Siaron KB, Cortes MX, Stutzman SE, Venkatachalam A, Ahmed KM, Olson DM. Blood Pressure measurements are site dependent in a cohort of patients with neurological illness. Sci Rep. 2020;10:3382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Lele AV, Wilson D, Chalise P, Nazzaro J, Krishnamoorthy V, Vavilala MS. Differences in blood pressure by measurement technique in neurocritically ill patients: A technological assessment. J Clin Neurosci. 2018;47:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Erickson SL, Killien EY, Wainwright M, Mills B, Vavilala MS. Mean Arterial Pressure and Discharge Outcomes in Severe Pediatric Traumatic Brain Injury. Neurocrit Care. 2021;34:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Topjian AA, Telford R, Holubkov R, Nadkarni VM, Berg RA, Dean JM, Moler FW; Therapeutic Hypothermia After Pediatric Cardiac Arrest (THAPCA) Trial Investigators. Association of Early Postresuscitation Hypotension With Survival to Discharge After Targeted Temperature Management for Pediatric Out-of-Hospital Cardiac Arrest: Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatr. 2018;172:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Topjian AA, French B, Sutton RM, Conlon T, Nadkarni VM, Moler FW, Dean JM, Berg RA. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med. 2014;42:1518-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Grelli KN, Gindville MC, Walker CH, Jordan LC. Association of Blood Pressure, Blood Glucose, and Temperature With Neurological Outcome After Childhood Stroke. JAMA Neurol. 2016;73:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Samant UB 4th, Mack CD, Koepsell T, Rivara FP, Vavilala MS. Time of hypotension and discharge outcome in children with severe traumatic brain injury. J Neurotrauma. 2008;25:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Topjian AA, de Caen A, Wainwright MS, Abella BS, Abend NS, Atkins DL, Bembea MM, Fink EL, Guerguerian AM, Haskell SE, Kilgannon JH, Lasa JJ, Hazinski MF. Pediatric Post-Cardiac Arrest Care: A Scientific Statement From the American Heart Association. Circulation. 2019;140:e194-e233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 15. | Kochanek PM, Tasker RC, Bell MJ, Adelson PD, Carney N, Vavilala MS, Selden NR, Bratton SL, Grant GA, Kissoon N, Reuter-Rice KE, Wainwright MS. Management of Pediatric Severe Traumatic Brain Injury: 2019 Consensus and Guidelines-Based Algorithm for First and Second Tier Therapies. Pediatr Crit Care Med. 2019;20:269-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 16. | Kochanek PM, Tasker RC, Carney N, Totten AM, Adelson PD, Selden NR, Davis-O'Reilly C, Hart EL, Bell MJ, Bratton SL, Grant GA, Kissoon N, Reuter-Rice KE, Vavilala MS, Wainwright MS. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines, Executive Summary. Pediatr Crit Care Med. 2019;20:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Woods KS, Horvat CM, Kantawala S, Simon DW, Rakkar J, Kochanek PM, Clark RSB, Au AK. Intracranial and Cerebral Perfusion Pressure Thresholds Associated With Inhospital Mortality Across Pediatric Neurocritical Care. Pediatr Crit Care Med. 2021;22:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Kety SS, Schmidt CF. The Effects of Altered Arterial Tensions of Carbon Dioxide and Oxygen on Cerebral Blood Flow and Cerebral Oxygen Consumption of Normal Young Men. J Clin Invest. 1948;27:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1288] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 19. | Bennett KS, Clark AE, Meert KL, Topjian AA, Schleien CL, Shaffner DH, Dean JM, Moler FW; Pediatric Emergency Care Medicine Applied Research Network. Early oxygenation and ventilation measurements after pediatric cardiac arrest: lack of association with outcome. Crit Care Med. 2013;41:1534-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Zebrack M, Dandoy C, Hansen K, Scaife E, Mann NC, Bratton SL. Early resuscitation of children with moderate-to-severe traumatic brain injury. Pediatrics. 2009;124:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Guerra-Wallace MM, Casey FL 3rd, Bell MJ, Fink EL, Hickey RW. Hyperoxia and hypoxia in children resuscitated from cardiac arrest. Pediatr Crit Care Med. 2013;14:e143-e148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, Parrillo JE, Trzeciak S; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 603] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 23. | Ferguson LP, Durward A, Tibby SM. Relationship between arterial partial oxygen pressure after resuscitation from cardiac arrest and mortality in children. Circulation. 2012;126:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Del Castillo J, López-Herce J, Matamoros M, Cañadas S, Rodriguez-Calvo A, Cechetti C, Rodriguez-Núñez A, Alvarez AC; Iberoamerican Pediatric Cardiac Arrest Study Network RIBEPCI. Hyperoxia, hypocapnia and hypercapnia as outcome factors after cardiac arrest in children. Resuscitation. 2012;83:1456-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg. 1993;28:310-4; discussion 315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 219] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Kannan N, Wang J, Mink RB, Wainwright MS, Groner JI, Bell MJ, Giza CC, Zatzick DF, Ellenbogen RG, Boyle LN, Mitchell PH, Rivara FP, Rowhani-Rahbar A, Vavilala MS; PEGASUS (Pediatric Guideline Adherence Outcomes) Study. Timely Hemodynamic Resuscitation and Outcomes in Severe Pediatric Traumatic Brain Injury: Preliminary Findings. Pediatr Emerg Care. 2018;34:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Lilien TA, Groeneveld NS, van Etten-Jamaludin F, Peters MJ, Buysse CMP, Ralston SL, van Woensel JBM, Bos LDJ, Bem RA. Association of Arterial Hyperoxia With Outcomes in Critically Ill Children: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5:e2142105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Schrieff-Elson LE, Thomas KG, Rohlwink UK, Figaji AA. Low brain oxygenation and differences in neuropsychological outcomes following severe pediatric TBI. Childs Nerv Syst. 2015;31:2257-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Figaji AA, Zwane E, Thompson C, Fieggen AG, Argent AC, Le Roux PD, Peter JC. Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury. Part 1: Relationship with outcome. Childs Nerv Syst. 2009;25:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Lang SS, Kumar NK, Zhao C, Zhang DY, Tucker AM, Storm PB, Heuer GG, Gajjar AA, Kim CT, Yuan I, Sotardi S, Kilbaugh TJ, Huh JW. Invasive brain tissue oxygen and intracranial pressure (ICP) monitoring vs ICP-only monitoring in pediatric severe traumatic brain injury. J Neurosurg Pediatr. 2022;1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Chong SL, Harjanto S, Testoni D, Ng ZM, Low CY, Lee KP, Lee JH. Early Hyperglycemia in Pediatric Traumatic Brain Injury Predicts for Mortality, Prolonged Duration of Mechanical Ventilation, and Intensive Care Stay. Int J Endocrinol. 2015;2015:719476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Cochran A, Scaife ER, Hansen KW, Downey EC. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma. 2003;55:1035-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Fu YQ, Chong SL, Lee JH, Liu CJ, Fu S, Loh TF, Ng KC, Xu F. The impact of early hyperglycaemia on children with traumatic brain injury. Brain Inj. 2017;31:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Aşılıoğlu N, Turna F, Paksu MS. Admission hyperglycemia is a reliable outcome predictor in children with severe traumatic brain injury. J Pediatr (Rio J). 2011;87:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Elkon B, Cambrin JR, Hirshberg E, Bratton SL. Hyperglycemia: an independent risk factor for poor outcome in children with traumatic brain injury*. Pediatr Crit Care Med. 2014;15:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Smith RL, Lin JC, Adelson PD, Kochanek PM, Fink EL, Wisniewski SR, Bayir H, Tyler-Kabara EC, Clark RS, Brown SD, Bell MJ. Relationship between hyperglycemia and outcome in children with severe traumatic brain injury. Pediatr Crit Care Med. 2012;13:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Seyed Saadat SM, Bidabadi E, Seyed Saadat SN, Mashouf M, Salamat F, Yousefzadeh S. Association of persistent hyperglycemia with outcome of severe traumatic brain injury in pediatric population. Childs Nerv Syst. 2012;28:1773-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Luo HC, Fu YQ, You CY, Liu CJ, Xu F. Comparison of admission serum albumin and hemoglobin as predictors of outcome in children with moderate to severe traumatic brain injury: A retrospective study. Medicine (Baltimore). 2019;98:e17806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Yee KF, Walker AM, Gilfoyle E. The Effect of Hemoglobin Levels on Mortality in Pediatric Patients with Severe Traumatic Brain Injury. Can Respir J. 2016;2016:6803860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | ROSOMOFF HL, HOLADAY DA. Cerebral blood flow and cerebral oxygen consumption during hypothermia. Am J Physiol. 1954;179:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 368] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Busto R, Dietrich WD, Globus MY, Valdés I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1305] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 42. | Minamisawa H, Smith ML, Siesjö BK. The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of forebrain ischemia. Ann Neurol. 1990;28:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 301] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 43. | Meden P, Overgaard K, Pedersen H, Boysen G. The influence of body temperature on infarct volume and thrombolytic therapy in a rat embolic stroke model. Brain Res. 1994;647:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Baena RC, Busto R, Dietrich WD, Globus MY, Ginsberg MD. Hyperthermia delayed by 24 h aggravates neuronal damage in rat hippocampus following global ischemia. Neurology. 1997;48:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Shum-Tim D, Nagashima M, Shinoka T, Bucerius J, Nollert G, Lidov HG, du Plessis A, Laussen PC, Jonas RA. Postischemic hyperthermia exacerbates neurologic injury after deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1998;116:780-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Castillo J, Dávalos A, Marrugat J, Noya M. Timing for fever-related brain damage in acute ischemic stroke. Stroke. 1998;29:2455-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 227] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Suz P, Vavilala MS, Souter M, Muangman S, Lam AM. Clinical features of fever associated with poor outcome in severe pediatric traumatic brain injury. J Neurosurg Anesthesiol. 2006;18:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Bembea MM, Nadkarni VM, Diener-West M, Venugopal V, Carey SM, Berg RA, Hunt EA; American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Temperature patterns in the early postresuscitation period after pediatric inhospital cardiac arrest. Pediatr Crit Care Med. 2010;11:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Du Q, Liu Y, Chen X, Li K. Effect of Hypothermia Therapy on Children with Traumatic Brain Injury: A Meta-Analysis of Randomized Controlled Trials. Brain Sci. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 50. | Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Browning B, Pemberton VL, Page K, Gildea MR, Scholefield BR, Shankaran S, Hutchison JS, Berger JT, Ofori-Amanfo G, Newth CJ, Topjian A, Bennett KS, Koch JD, Pham N, Chanani NK, Pineda JA, Harrison R, Dalton HJ, Alten J, Schleien CL, Goodman DM, Zimmerman JJ, Bhalala US, Schwarz AJ, Porter MB, Shah S, Fink EL, McQuillen P, Wu T, Skellett S, Thomas NJ, Nowak JE, Baines PB, Pappachan J, Mathur M, Lloyd E, van der Jagt EW, Dobyns EL, Meyer MT, Sanders RC Jr, Clark AE, Dean JM; THAPCA Trial Investigators. Therapeutic Hypothermia after In-Hospital Cardiac Arrest in Children. N Engl J Med. 2017;376:318-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 51. | Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Clark AE, Browning B, Pemberton VL, Page K, Shankaran S, Hutchison JS, Newth CJ, Bennett KS, Berger JT, Topjian A, Pineda JA, Koch JD, Schleien CL, Dalton HJ, Ofori-Amanfo G, Goodman DM, Fink EL, McQuillen P, Zimmerman JJ, Thomas NJ, van der Jagt EW, Porter MB, Meyer MT, Harrison R, Pham N, Schwarz AJ, Nowak JE, Alten J, Wheeler DS, Bhalala US, Lidsky K, Lloyd E, Mathur M, Shah S, Wu T, Theodorou AA, Sanders RC Jr, Dean JM; THAPCA Trial Investigators. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372:1898-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 52. | Slupe AM, Kirsch JR. Effects of anesthesia on cerebral blood flow, metabolism, and neuroprotection. J Cereb Blood Flow Metab. 2018;38:2192-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 53. | Curley MA, Wypij D, Watson RS, Grant MJ, Asaro LA, Cheifetz IM, Dodson BL, Franck LS, Gedeit RG, Angus DC, Matthay MA; RESTORE Study Investigators and the Pediatric Acute Lung Injury and Sepsis Investigators Network. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 334] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 54. | Takeshita H, Okuda Y, Sari A. The effects of ketamine on cerebral circulation and metabolism in man. Anesthesiology. 1972;36:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 156] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Bar-Joseph G, Guilburd Y, Tamir A, Guilburd JN. Effectiveness of ketamine in decreasing intracranial pressure in children with intracranial hypertension. J Neurosurg Pediatr. 2009;4:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 56. | Vernon DD, Witte MK. Effect of neuromuscular blockade on oxygen consumption and energy expenditure in sedated, mechanically ventilated children. Crit Care Med. 2000;28:1569-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Chin KH, Bell MJ, Wisniewski SR, Balasubramani GK, Kochanek PM, Beers SR, Brown SD, Adelson PD; Pediatric Traumatic Brain Injury Consortium: Hypothermia Investigators. Effect of administration of neuromuscular blocking agents in children with severe traumatic brain injury on acute complication rates and outcomes: a secondary analysis from a randomized, controlled trial of therapeutic hypothermia. Pediatr Crit Care Med. 2015;16:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Velle F, Lewén A, Howells T, Enblad P, Nilsson P. Intracranial pressure-based barbiturate coma treatment in children with refractory intracranial hypertension due to traumatic brain injury. J Neurosurg Pediatr. 2019;1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Alkhachroum A, Appavu B, Egawa S, Foreman B, Gaspard N, Gilmore EJ, Hirsch LJ, Kurtz P, Lambrecq V, Kromm J, Vespa P, Zafar SF, Rohaut B, Claassen J. Electroencephalogram in the intensive care unit: a focused look at acute brain injury. Intensive Care Med. 2022;48:1443-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 60. | Appavu B, Riviello JJ. Electroencephalographic Patterns in Neurocritical Care: Pathologic Contributors or Epiphenomena? Neurocrit Care. 2018;29:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Hirsch LJ, Fong MWK, Leitinger M, LaRoche SM, Beniczky S, Abend NS, Lee JW, Wusthoff CJ, Hahn CD, Westover MB, Gerard EE, Herman ST, Haider HA, Osman G, Rodriguez-Ruiz A, Maciel CB, Gilmore EJ, Fernandez A, Rosenthal ES, Claassen J, Husain AM, Yoo JY, So EL, Kaplan PW, Nuwer MR, van Putten M, Sutter R, Drislane FW, Trinka E, Gaspard N. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol. 2021;38:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 533] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 62. | Vespa P, Tubi M, Claassen J, Buitrago-Blanco M, McArthur D, Velazquez AG, Tu B, Prins M, Nuwer M. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79:579-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 63. | Witsch J, Frey HP, Schmidt JM, Velazquez A, Falo CM, Reznik M, Roh D, Agarwal S, Park S, Connolly ES, Claassen J. Electroencephalographic Periodic Discharges and Frequency-Dependent Brain Tissue Hypoxia in Acute Brain Injury. JAMA Neurol. 2017;74:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 64. | Claassen J, Perotte A, Albers D, Kleinberg S, Schmidt JM, Tu B, Badjatia N, Lantigua H, Hirsch LJ, Mayer SA, Connolly ES, Hripcsak G. Nonconvulsive seizures after subarachnoid hemorrhage: Multimodal detection and outcomes. Ann Neurol. 2013;74:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 65. | Appavu BL, Fox J, Kuwabara M, Burrows BT, Temkit M', Adelson PD. Association of Cerebral and Systemic Physiology With Quantitative Electroencephalographic Characteristics of Early Posttraumatic Seizures. J Clin Neurophysiol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Silverstein R, Appavu B. Cerebral edema in childhood. In: Roos RP, Editor-in-Chief. Medlink Neurology. San Diego: Medlink, LLC. Available at www.medlink.com. Updated: 06.11.2022.. |
| 67. | Liotta EM. Management of Cerebral Edema, Brain Compression, and Intracranial Pressure. Continuum (Minneap Minn). 2021;27:1172-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Kochanek PM, Adelson PD, Rosario BL, Hutchison J, Miller Ferguson N, Ferrazzano P, O'Brien N, Beca J, Sarnaik A, LaRovere K, Bennett TD, Deep A, Gupta D, Willyerd FA, Gao S, Wisniewski SR, Bell MJ; ADAPT Investigators. Comparison of Intracranial Pressure Measurements Before and After Hypertonic Saline or Mannitol Treatment in Children With Severe Traumatic Brain Injury. JAMA Netw Open. 2022;5:e220891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 69. | Froese L, Dian J, Batson C, Gomez A, Unger B, Zeiler FA. The impact of hypertonic saline on cerebrovascular reactivity and compensatory reserve in traumatic brain injury: an exploratory analysis. Acta Neurochir (Wien). 2020;162:2683-2693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Wellard J, Kuwabara M, Adelson PD, Appavu B. Physiologic Characteristics of Hyperosmolar Therapy After Pediatric Traumatic Brain Injury. Front Neurol. 2021;12:662089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Zipfel J, Engel J, Hockel K, Heimberg E, Schuhmann MU, Neunhoeffer F. Effects of hypertonic saline on intracranial pressure and cerebral autoregulation in pediatric traumatic brain injury. J Neurosurg Pediatr. 2021;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Jha RM, Kochanek PM, Simard JM. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology. 2019;145:230-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 301] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 73. | Pham X, Ray J, Neto AS, Laing J, Perucca P, Kwan P, O'Brien TJ, Udy AA. Association of Neurocritical Care Services With Mortality and Functional Outcomes for Adults With Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurol. 2022;79:1049-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 74. | Pineda JA, Leonard JR, Mazotas IG, Noetzel M, Limbrick DD, Keller MS, Gill J, Doctor A. Effect of implementation of a paediatric neurocritical care programme on outcomes after severe traumatic brain injury: a retrospective cohort study. Lancet Neurol. 2013;12:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 75. | LaRovere KL, Murphy SA, Horak R, Vittner P, Kapur K, Proctor M, Tasker RC. Pediatric Neurocritical Care: Evolution of a New Clinical Service in PICUs Across the United States. Pediatr Crit Care Med. 2018;19:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Murphy SA, Bell MJ, Clark ME, Whalen MJ, Noviski N. Pediatric Neurocritical Care: A Short Survey of Current Perceptions and Practices. Neurocrit Care. 2015;23:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Wainwright MS, Grimason M, Goldstein J, Smith CM, Amlie-Lefond C, Revivo G, Noah ZL, Harris ZL, Epstein LG. Building a pediatric neurocritical care program: a multidisciplinary approach to clinical practice and education from the intensive care unit to the outpatient clinic. Semin Pediatr Neurol. 2014;21:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Bell MJ, Carpenter J, Au AK, Keating RF, Myseros JS, Yaun A, Weinstein S. Development of a pediatric neurocritical care service. Neurocrit Care. 2009;10:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Erklauer JC, Thammasitboon S, Shekerdemian LS, Riviello JJ, Lai YC. Creating a Robust Community of Practice as a Foundation for the Successful Development of a Pediatric Neurocritical Care Program. Pediatr Neurol. 2022;136:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 80. | Ferriero DM, Fullerton HJ, Bernard TJ, Billinghurst L, Daniels SR, DeBaun MR, deVeber G, Ichord RN, Jordan LC, Massicotte P, Meldau J, Roach ES, Smith ER; American Heart Association Stroke Council and Council on Cardiovascular and Stroke Nursing. Management of Stroke in Neonates and Children: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke. 2019;50:e51-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 441] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 81. | Appavu B, Foldes ST, Adelson PD. Clinical trials for pediatric traumatic brain injury: definition of insanity? J Neurosurg Pediatr. 2019;23:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Chang N, Rasmussen L. Exploring Trends in Neuromonitoring Use in a General Pediatric ICU: The Need for Standardized Guidance. Children (Basel). 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Appavu B, Burrows BT, Foldes S, Adelson PD. Approaches to Multimodality Monitoring in Pediatric Traumatic Brain Injury. Front Neurol. 2019;10:1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | LaRovere KL, Tasker RC, Wainwright M, Reuter-Rice K, Appavu B, Miles D, Lidsky K, Vittner P, Gundersen D, O'Brien NF; Pediatric Neurocritical Care Research Group (PNCRG). Transcranial Doppler Ultrasound During Critical Illness in Children: Survey of Practices in Pediatric Neurocritical Care Centers. Pediatr Crit Care Med. 2020;21:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 85. | Appavu B, Burrows BT, Nickoles T, Boerwinkle V, Willyerd A, Gunnala V, Mangum T, Marku I, Adelson PD. Implementation of Multimodality Neurologic Monitoring Reporting in Pediatric Traumatic Brain Injury Management. Neurocrit Care. 2021;35:3-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 86. | Zeiler FA, Cardim D, Donnelly J, Menon DK, Czosnyka M, Smielewski P. Transcranial Doppler Systolic Flow Index and ICP-Derived Cerebrovascular Reactivity Indices in Traumatic Brain Injury. J Neurotrauma. 2018;35:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Budohoski KP, Reinhard M, Aries MJ, Czosnyka Z, Smielewski P, Pickard JD, Kirkpatrick PJ, Czosnyka M. Monitoring cerebral autoregulation after head injury. Which component of transcranial Doppler flow velocity is optimal? Neurocrit Care. 2012;17:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 88. | Zweifel C, Lavinio A, Steiner LA, Radolovich D, Smielewski P, Timofeev I, Hiler M, Balestreri M, Kirkpatrick PJ, Pickard JD, Hutchinson P, Czosnyka M. Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurg Focus. 2008;25:E2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 89. | Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 558] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 90. | Appavu B, Temkit M', Foldes S, Burrows BT, Kuwabara M, Jacobson A, Adelson PD. Association of Outcomes with Model-Based Indices of Cerebral Autoregulation After Pediatric Traumatic Brain Injury. Neurocrit Care. 2021;35:640-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 91. | Brady KM, Shaffner DH, Lee JK, Easley RB, Smielewski P, Czosnyka M, Jallo GI, Guerguerian AM. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics. 2009;124:e1205-e1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 92. | Lewis PM, Czosnyka M, Carter BG, Rosenfeld JV, Paul E, Singhal N, Butt W. Cerebrovascular Pressure Reactivity in Children With Traumatic Brain Injury. Pediatr Crit Care Med. 2015;16:739-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 93. | Young AM, Donnelly J, Czosnyka M, Jalloh I, Liu X, Aries MJ, Fernandes HM, Garnett MR, Smielewski P, Hutchinson PJ, Agrawal S. Continuous Multimodality Monitoring in Children after Traumatic Brain Injury-Preliminary Experience. PLoS One. 2016;11:e0148817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 94. | Tas J, Beqiri E, van Kaam RC, Czosnyka M, Donnelly J, Haeren RH, van der Horst ICC, Hutchinson PJ, van Kuijk SMJ, Liberti AL, Menon DK, Hoedemaekers CWE, Depreitere B, Smielewski P, Meyfroidt G, Ercole A, Aries MJH. Targeting Autoregulation-Guided Cerebral Perfusion Pressure after Traumatic Brain Injury (COGiTATE): A Feasibility Randomized Controlled Clinical Trial. J Neurotrauma. 2021;38:2790-2800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 95. | Appavu B, Foldes S, Burrows BT, Jacobson A, Abruzzo T, Boerwinkle V, Willyerd A, Mangum T, Gunnala V, Marku I, Adelson PD. Multimodal Assessment of Cerebral Autoregulation and Autonomic Function After Pediatric Cerebral Arteriovenous Malformation Rupture. Neurocrit Care. 2021;34:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 96. | Joram N, Beqiri E, Pezzato S, Moscatelli A, Robba C, Liet JM, Chenouard A, Bourgoin P, Czosnyka M, Léger PL, Smielewski P. Continuous Monitoring of Cerebral Autoregulation in Children Supported by Extracorporeal Membrane Oxygenation: A Pilot Study. Neurocrit Care. 2021;34:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 97. | Kirschen MP, Majmudar T, Beaulieu F, Burnett R, Shaik M, Morgan RW, Baker W, Ko T, Balu R, Agarwal K, Lourie K, Sutton R, Kilbaugh T, Diaz-Arrastia R, Berg R, Topjian A. Deviations from NIRS-derived optimal blood pressure are associated with worse outcomes after pediatric cardiac arrest. Resuscitation. 2021;168:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 98. | Lee JK, Brady KM, Chung SE, Jennings JM, Whitaker EE, Aganga D, Easley RB, Heitmiller K, Jamrogowicz JL, Larson AC, Lee JH, Jordan LC, Hogue CW, Lehmann CU, Bembea MM, Hunt EA, Koehler RC, Shaffner DH. A pilot study of cerebrovascular reactivity autoregulation after pediatric cardiac arrest. Resuscitation. 2014;85:1387-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 99. | Paniukov D, Lebel RM, Giesbrecht G, Lebel C. Cerebral blood flow increases across early childhood. Neuroimage. 2020;204:116224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |