Published online Nov 9, 2022. doi: 10.5492/wjccm.v11.i6.375
Peer-review started: March 13, 2022
First decision: May 31, 2022
Revised: June 13, 2022
Accepted: September 9, 2022
Article in press: September 9, 2022
Published online: November 9, 2022
Processing time: 235 Days and 15.9 Hours
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) is a comprehensive treatment option performed for peritoneal surface malignancies. Postoperatively almost all patients are transferred to the intensive care unit electively.
To describe the common and rare postoperative complications, postoperative mortality and their critical care management after CRS-HIPEC.
The authors assessed 54 articles for eligibility. Full text assessment identified 14 original articles regarding postoperative complications and critical care mana
There is an exaggerated metabolic and inflammatory response after surgery which may be termed as physiological in view of the nature of surgery combined with the use of heated intraperitoneal chemotherapy with/out early postoperative intravenous chemotherapy. The expected postoperative course is further dis
CRS-HIPEC is associated with a varying rate of postoperative complications including post
Core Tip: Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy is a complex procedure with some life-threatening complications in the immediate postoperative period, reported morbidity rates between 12%-60% and a mortality rate of 0.9%-5.8%. There is an exaggerated metabolic and inflammatory response after surgery which may be termed as physiological in view of the nature of surgery combined with use of heated intraperitoneal chemotherapy.
- Citation: Wajekar AS, Solanki SL, Patil VP. Postoperative complications and critical care management after cytoreduction surgery and hyperthermic intraperitoneal chemotherapy: A systematic review of the literature. World J Crit Care Med 2022; 11(6): 375-386
- URL: https://www.wjgnet.com/2220-3141/full/v11/i6/375.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i6.375
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) is a comprehensive treatment option performed for peritoneal surface malignancies (PSM), both primary peritoneal cancers and peritoneal metastasis secondary to colorectal, appendiceal, ovarian, gastric and other malignancies. CRS comprises the surgical removal of visible tumour from peritoneal surfaces as well as abdomino-pelvic organs. CRS includes a wide spectrum which ranges from excision of a single per
CRSHIPEC is a complex procedure with some life-threatening complications in the immediate post
The present article reviews the early postoperative management and common complications after CRS-HIPEC, reported in the last decade.
An electronic literature search was conducted using the databases of ‘PubMed’ and ‘Google Scholar’. The ‘Reference Citation Analysis’, an artificial intelligence technology-based open citation analysis database was employed. The period of the search was from 2010 to 2021. The search terms included, “Peritoneal Cancer”, “Hyperthermic”, “Intraperitoneal”, “HIPEC”, Critical Care, Intensive Care, Postoperative Care, Perioperative Care, Postoperative Complications and their synonyms in various combinations. The extracted articles were further reviewed in a step-wise manner for identification of relevant studies. The titles and abstracts were inspected independently by two authors.
Only full text articles published in English were included for review. Only articles which reported postoperative critical care management and complications were included. Articles regarding only preoperative and intraoperative management were excluded. Only original research articles were included for analysis. Meta-analyses and review articles were excluded.
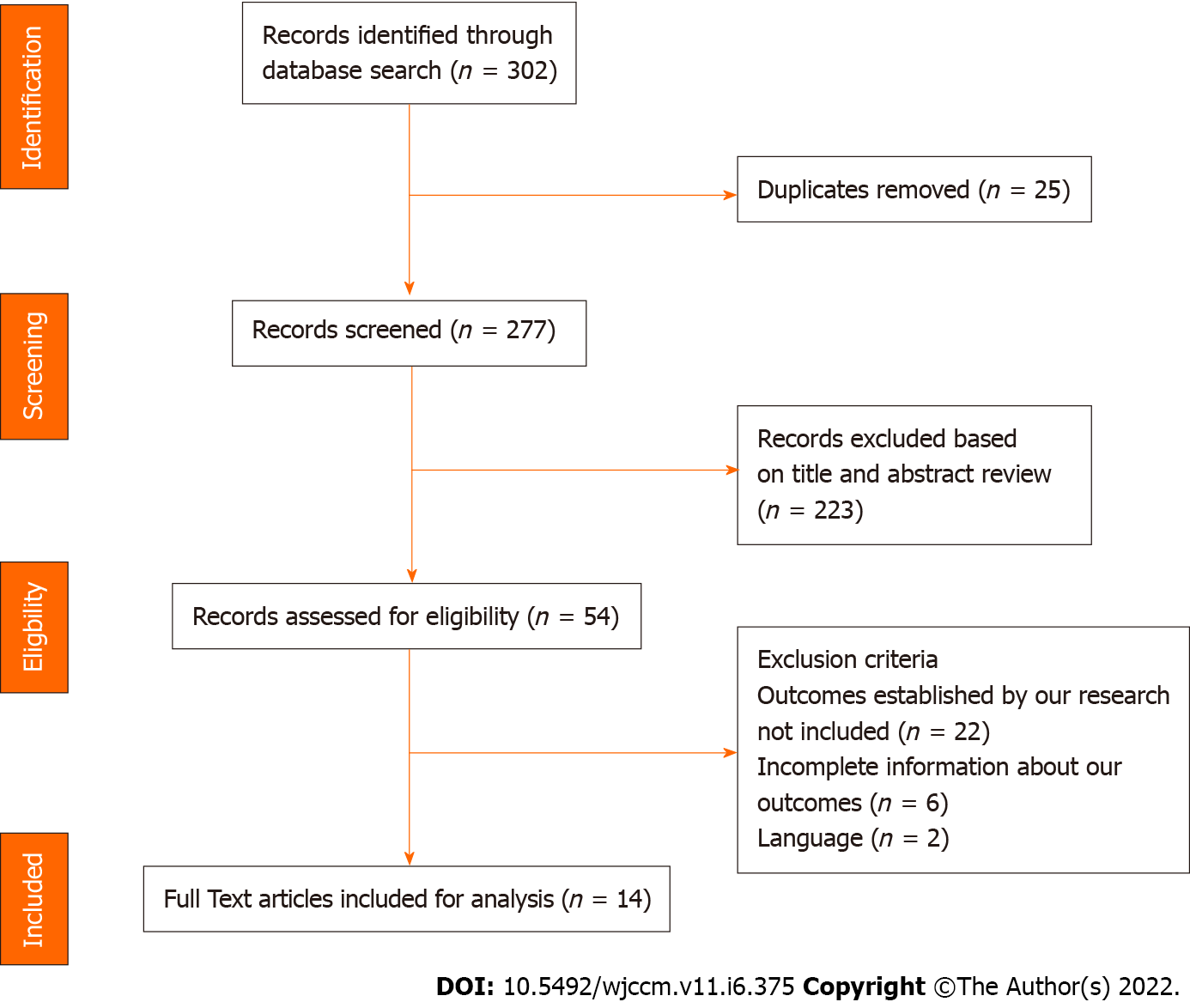
A total of 277 articles were identified after the initial literature search. Initial review included screening of article titles for relevance and identifying duplicates. A further screening of abstracts identified articles for full text review. Full text assessment identified 14 original articles regarding postoperative complications and critical care management for inclusion into the final review article (Table 1, Figure 1).
| Ref. | Data duration | Type of Cohort/Study | No of Institutes (Country) | PSM | No of procedures | Age | PCI | ||
| Cavaliere et al[35], 2011 | 1995-2007 | Prospective | Five (Italy) | Colorectal | 146 | 56 (19-76) | median (range) | < 11-48, 11-20-72, > 20-26 | Range |
| Glehen et al[36], 2010 | 1989-2007 | Retrospective | Twenty-five (Europe and Canada) | Non-ovarian | 1154, 190(EPIC) | 52 (12) | mean (SD) | 13.1 (8.9) | mean (SD) |
| Cooksley et al[7], 2011 | 2009-2010 | Retrospective | Single (England) | Mixed | 69 | 53.3 (30-73) | mean (range) | 10.5 | Mean |
| Mizumoto et al[37], 2012 | 2007-2011 | Retrospective | Single (Japan) | Mixed | 284 | 57 (13) (23–88) | mean (SD) (range) | 20 (13) (0–39) | mean (SD) (range) |
| Bakrin et al[1], 2012 | 1991-2008 | Retrospective | Two (France) | Ovarian | 246 | 57.5 (28.6-77.6) | Mean (range) | 10.8 (1-31) | Mean (range) |
| Baratti et al[17], 2012 | 1995-2011 | Prospective | Single (Italy) | Mixed | 426 | 53.4 (12.7) | mean (SD) | 18.7 (10.8) | mean (SD) |
| Bakrin et al[16], 2013 | 1991-2010 | Retrospective | Thirteen (France) | Ovarian | 566 | 57.89 (22-77) | Median (range) | 8.5 (0-31) | Median (range) |
| Canda et al[27], 2013 | 2007-2012 | Retrospective | Single (Turkey) | Mixed | 118 | 53.4 (20-82) | Mean (range) | 14.7 (3-28) | Mean (range) |
| Jafari et al[15], 2014 | 2005-2011 | Retrospective | > 500 (USA) | Mixed | 694 | 55 (10) | mean (SD) | NA | |
| Levine et al[30], 2014 | 1991-2013 | Prospective | Single (USA) | Mixed | 1000 | 52.9 (12.4) | mean (SD) | 12 | Mean |
| Cascales-Campos et al[24], 2016 | 2008-2014 | Prospective | Single (Spain) | Mixed | 156 | 57 (33-79) | Median (range) | 8 (0-13) | Median (range) |
| Martin et al[25], 2016 | 1991-2014 | Retrospective | Single (USA) | Mixed | 302 | 54% (40-60) | Percent (range) | 13 (6-18) | Median (IQR) |
| Elekonawo et al[38], 2019 | 2010-2015 | Case matched RCT | Two centres in Netherlands | Colorectal | 223 | 61.4(10.7) | mean (SD) | 9.0 (0–24) | Median (range) |
| Kelly et al[39], 2018 | 2007-2014 | Retrospective | Single (USA) | Mixed | 226 | 53 (20-66) | Median (range) | 14 (0-27) | Median (range) |
Postoperatively almost all the patients were transferred to the intensive care unit (ICU) electively. Only a few selected patients with limited CRS and short duration HIPEC may be amenable for high dependency unit (HDU) management. There is an exaggerated metabolic and inflammatory response after surgery which may be termed as physiological in view of the nature of surgery combined with use of heated intraperitoneal chemotherapy with/out early postoperative intravenous chemotherapy.
At the end of surgery, the decision to extubate or electively ventilate depends upon patient comorbidities, duration of surgery, degree of cytoreduction, haemodynamic instability, vasopressor use, blood loss and the need for massive blood transfusion, and metabolic derangement. Even in the ICU, it is quite common to extubate the patients to a high flow nasal cannula or non-invasive ventilation depending upon the extent of diaphragmatic peritonectomy, breathing efforts of the patients and site of gastrointestinal anastomosis. Preoperative malnutrition and anaemia, long duration of surgery, fluid overload, poorly controlled pain leading to diaphragmatic splinting, lithotomy with steep Tr
Massive fluid shifts, third spacing and blood loss are quite common in the CRS phase of the surgery whereas the HIPEC phase can lead to extensive vasodilatation necessitating use of vasopressors. The fluid losses, both external and internal (third space), continue in the immediate postoperative period. The abdominal drain losses can be as high as 40% of the total output, in the first 72 h after surgery[3,8]. Continuous monitoring and assessment of fluid status guided by various static and dynamic parameters such as cardiac output monitoring, central venous pressure, serum lactate, urine output, abdominal drain and nasogastric losses need to be conducted. Adequate and timely resuscitation with crystalloids, colloids, blood and blood products helps reduce postoperative morbidity and mortality. In view of the increased risk of postoperative sepsis, acute kidney injury and coagulopathy, it is advisable to avoid use of hydroxylethyl starches in the perioperative period. There is a significant protein loss secondary to the exudating ascitic fluid and extensive surgical dissection. Postoperative decline in albumin levels is common, which starts intraoperatively and continues postoperatively, with the need for exogenous replacement. The routine use of furosemide, mannitol or low doses of dopamine to prevent renal injury is no longer recommended.
Malfroy et al[8] found that abdominal drain output more than 1500 mL, postoperative fluid re
Coagulopathy during the perioperative period is multifactorial which includes the length of surgery, extent of resection, both hypothermia and hyperthermia, blood loss and massive blood transfusion. There may be prolongation of prothrombin time, activated partial thromboplastin time and/or reduction in platelet count. Monitoring viscoelastic properties of clots with the use of thromboelastography both intra- and postoperatively can help with management. The coagulation profile generally normalises by the third to sixth postoperative day. Platelet transfusion is rarely required and should only be considered when platelet levels fall below 50000 with associated bleeding or additional surgical procedures become imminent.
Electrolyte abnormalities may be common due to perioperative massive fluid shifts. Sodium, chlo
Extensive CRS and HIPEC can cause wide fluctuations in temperature. The hyperdynamic alterations secondary to hyperthermia generally reverse once the temperature normalises. Hyperthermia can also cause coagulopathies, renal tubulopathy, liver dysfunction, neuropathies and seizures. Delta temperature (difference between lowest and highest temperatures) during CRSHIPEC was found to be a significant predictor of ICU stay > 5 d[3]. This is highest in patients with a high peritoneal carcinomatosis index (PCI) necessitating longer, aggressive resection. Hypothermia during the CRS phase is associated with cardiac morbidity, decreased humoral and cellmediated immunity and worsen metabolic acidosis and may be responsible for increased ICU stay. The lactate levels after HIPEC should be interpreted with caution and along with other markers of perfusion as the inflammatory state itself can be responsible for hyperlactatemia.
Perioperative fluid shifts and hypoperfusion combined with nephrotoxic chemotherapy especially cisplatin predisposes to acute kidney injury. The critical time for renal perfusion is generally the first 2 postoperative days. Transient severe hypophosphatemia may be observed on the first two-three postoperative days due to hyperthermia-related renal tubulopathy. It can lead to decreased diaphragm mobility leading to atelectasis and increased insulin requirements. Transaminitis (2to 3fold rise) is common during the first four postoperative days. Diarrhoea can occur in the first week due to digestive hypersecretion secondary to the hyper inflammatory status.
Initiation of enteral feed should depend on the extent of bowel resection, presence or absence of inflammation and haemodynamic stability. Parenteral nutrition should be initiated early and switched to enteral nutrition as soon as possible. The decisions regarding nutrition should consider patients baseline nutritional status, and surgical and medical concerns. Dieticians should be actively involved from the preoperative phase. Preoperative nutritional status may predict length of stay, risk of in
The anticipated postoperative course includes lowgrade fever up to 38oC, even in the absence of infection, during the first 7-10 postoperative days. Leukocyte counts and platelet counts progressively decrease in the first two weeks followed by a progressive increase. Inflammatory markers such as Creactive protein, interleukins and elastase increase during surgery and return to normal within 12-24 h. Hyperglycaemia can be a common finding due to surgical stress and hypercatabolic state, nece
Moderate to severe pain is quite common. Use of thoracic epidural anaesthesia (TEA) is desirable in these patients for management of postoperative analgesia, prevention of respiratory complications and reduction in rates of paralytic ileus. Thoracic epidural analgesia with local anaesthetics and short acting opioids up to 72-96 h after surgery have been found to be useful. Owusu-Agyemang et al[10] in their study of 215 patients reported that intraoperative initiation of continuous epidural infusions pre-HIPEC was associated with significantly less blood loss and decreased intraoperative fluid requirements. Despite common postoperative coagulation abnormalities and an increased incidence of sepsis, no epidural hematomas or abscesses were reported in their study. A single centre retrospective analysis reported improved survival and reduced grade III/IV postoperative morbidity after HIPEC when TEA was used compared to patient-controlled opioid analgesia[11]. Along with thoracic epidural analgesia, adjuncts such as paracetamol as a component of multimodal analgesia are recommended. Opioid usage needs to be minimised. The use of truncal blocks such as transversus abdominis block or quadratus lumborum blocks in the absence of epidurals are encouraged.
Adherence to ERAS protocols in the perioperative period have helped to considerably decrease the grade III/IV complications and associated morbidity, length of ICU and hospital stays and improve the survival rates[3,12-14]. Mechanical and pharmacological deep vein thrombosis prophylaxis should be considered as appropriate during the entire perioperative period if not contraindicated. The first dose of low molecular weight heparin is generally given the previous night as part of ERAS and continued postoperatively. Implementation of ERAS protocols in the postoperative period such as early ex
Compliance to ERAS protocols have been found to reduce the major postoperative complication rate from 33% to 21% due to early detection and reversal of the pathophysiological cascade after this major surgery, consequently reducing the length of stay from 13.1 ± 9.5 d to 8.6 ± 4.9 d[12]. A more recent National Surgical Quality Improvement Program review reported an average length of stay of 13 d[15].
The extent of peritoneal disease as scored by the PCI, the completeness of the cytoreduction (CC) score and dose of intraperitoneal platinum chemotherapy are important prognostic factors of both morbidity and survival[1,8,16]. PCI > 8-10 and CC-1/CC-2 have been found to have an increased incidence of postoperative grade III/IV complications. The risk of complications increased by 3.5% for every single point increase in PCI[17]. Additionally, initial indication of surgery, ECOG score, number of organ resections etc may help further prognostication[1,17]. Tao et al[18] in their meta-analysis, reported a similar incidence of anastomotic leaks and duration of hospital stay between younger (< 65 years) and elderly (> 65 years) patients but the morbidity outcomes and mortality were higher in elderly patients. Cooksley et al[7] found that the higher the vasopressor requirement intra- and postoperatively, the higher the risk of postoperative complications.
In recent years, a gamut of studies investigated the utility of inflammatory markers to predict the postoperative course as well as survival. Inflammation plays an important role not only in carcinogenesis but also during CRS-HIPEC surgery. Some inflammatory biomarkers have been found to have an increased association with postoperative infective complications. Kim et al[19] reported that higher values of preoperative neutrophil to lymphocyte ratio (NLR) and mean platelet volume (MPV), platelet to lymphocyte ratio (PLR), and MPV on postoperative days 2, 3, and 5 were associated with decreased 1-year survival after CRS-HIPEC. C-reactive protein (CRP), an acute phase inflammation protein, is a highly sensitive but nonspecific biomarker of systemic inflammatory response. van Kooten et al[20] reported an increasing value of CRP after postoperative day (POD) 2 or CRP > 166 mg/L at POD3 or > 116 mg/L at POD4, had a predictive value for early detection of severe adverse events. Saeed et al[21] studied the dynamics of precalcitonin (PCT) pre and postoperative in CRS-HIPEC patients and compared them to CRP and white cell counts (WCC) in patients who developed infective complications postoperatively. They found a trend for faster rise in serum PCT on POD1 as compared to CRP and WCC, along with a faster PCT decline following appropriate therapy on POD3 and 6 when infected cases were clinically resolving while WCC and CRP continued to rise, particularly in non-splenectomised patients. Splenectomised patients had an increase in PCT postoperatively even in the absence of infection. Although all three, namely PCT, WCC and CRP showed an increase postoperatively consequent to systemic inflammatory response syndrome (SIRS) post CRS-HIPEC surgery, PCT had the highest negative predictive value to rule out bacterial infectious complications. Finally, they cautioned the interpretation of postoperative PCT in predicting infectious complications only in association with other clinical, biochemical, microbiological and radiological findings. Viyuela García et al[22] reported that CRP on POD7 and 8 had best accuracy, with an optimal cut-off value of 88 mg/L and 130 mg/L, respectively, to predict postoperative infective complications in ovarian cancer patients who underwent CRS-HIPEC.
The complications are commonly graded on two main classification systems – Clavien Dindo classification and National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCICTCAE). Major surgical complications generally include those requiring interventional endoscopy or CT-scan/ultrasound-guided procedures (grade 3), return to the operating room or ICU (grade 4), and death (grade 5). It has been found that conventional 30-d mortality underestimates post-operative mortality by 50% in CRS-HIPEC patients[5]. In their study, Alyami et al[5] found that most major complications occurred within 30 d, but more than 50% of deaths related to postoperative complications occurred after 30 d. Various studies have suggested evaluating morbidity and mortality related to complex surgical procedures such as CRS-HIPEC, using a 90-d time period for its definition[5,17,23].
CRS-HIPEC, being a major abdominal surgery, is associated with a gamut of postoperative complications. Grade III/IV complications are most common in the first 2 wk after surgery (Table 2). Malfroy et al[8] reported a median time to complications post-surgery of 2.5 d.
| Ref. | N | Mortality (%) | Days | Morbidity (Grade III/IV), % | Complication classification | Commonest complications | Re-operations (%) | ||
| First (%) | Second (%) | Third (%) | |||||||
| Cavaliere et al[35], 2011 | 146 | 2.7 | 30 | 27.4 | WHO | GI perforation/anastomotic leak (7.4) | Sepsis (4.1) | Pancreatitis/pancreatic fistula (1.4) | NA |
| Glehen et al[36], 2010 | 1154, 190 (EPIC) | 4.1 | 30 | 33.6 | NCICTCAE | GI perforation/anastomotic leak (9.7) | Pneumonia (9.1) | Intraabdominal bleeding (7.7) | 14 |
| Cooksley et al[7], 2011 | 69 | 0 | 30 | 5.79 | NA | Pneumonia (2.9) | Central line infection (1.5) | Uncontrolled hypertension (1.5) | NA |
| Mizumoto et al[37], 2012 | 284 | 3.5 | 30 | 17 | NCICTCAE | GI perforation/anastomotic leak (6.7) | Sepsis (4.6) | Intraabdominal bleeding (2.1) | 11 |
| Bakrin et al[1], 2012 | 246 | 0.37 | 30 | 11.6 | NCICTCAE | GI perforation/anastomotic leak (4.9) | Intraabdominal bleeding (2.4) | 4.9 | |
| Baratti et al[17], 2012 | 426 | 2.6 | 90 | 25.3 | NCICTCAE | GI perforation/anastomotic leak (11.03) | Sepsis (3.76) | Intraabdominal bleeding (3.3) | 10.7 |
| Bakrin et al[16], 2013 | 566 | 0.8 | 30 | 31.3 | NCICTCAE | Intraabdominal bleeding (8) | GI perforation/anastomotic leak (3) | 8 | |
| Canda et al[27], 2013 | 118 | 7.6 | 30 | 31.35 | NCICTCAE | Sepsis (7.6) | Pneumonia (2.5) | Ileus (2.5) | 5.08 |
| Jafari et al[15], 2014 | 694 | 2.3 | 30 | 32.9 | NA | Intraabdominal bleeding (17) | Sepsis (15.9) | Pneumonia (4.8) | 9.8 |
| Levine et al[30], 2014 | 1000 | 3.8 | 30 | 42 | NA | Sepsis | GI perforation/anastomotic leak | Pneumonia | NA |
| Cascales-Campos et al[24], 2016 | 156 | 0.6 | 30 | 11.5 | NCICTCAE | Pleural effusion (4.49) | Sepsis (3.8) | GI perforation/anastomotic leak (1.9) | NA |
| Martin et al[25], 2016 | 302 | 3 | 30 | NA | Pleural effusion (10.8) | Thrombosis (6.8) | Sepsis (5.4) | NA | |
| Elekonawo et al[38], 2019 | 223 | 1.5 | 30 | 17.6 | Clavien Dindo | Sepsis (14.7) | GI perforation/anastomotic leak | NA | |
| Kelly et al[39], 2018 | 226 | 30 | NA | NA | Ileus (31) | Sepsis (21) | Thrombosis (15) | 16 | |
CRS with HIPEC involves extensive abdominal surgery with major handling of small bowel, several visceral resections, anastomosis and peritonectomy. The major complications include anastomotic leaks, gastrointestinal perforations distant from the suture line, abdominal abscess, sepsis, haemorrhage, biliary, pancreatic or ureteral leakage, pancreatitis, paralytic ileus, diarrhoea etc. An important consideration is the timing of the anastomosis vis-à-vis HIPEC. There is no evidence in the literature to suggest an increased risk of anastomotic leaks or isolated disease recurrence on suture lines if anastomosis is performed at the completion of the cytoreduction and prior to HIPEC[6]. Some authors prefer bowel anastomoses to be performed before HIPEC in closed procedures to avoid reopening the patient but after HIPEC in cases of open procedure[16]. Malfroy et al[8] found that septic shock was the commonest factor for postoperative ICU re-admission (28.1%) with gastrointestinal origin of sepsis to be the highest (64.3%). Paralytic ileus is the commonest morbidity observed postoperatively, classified as Grade I-III morbidity[24]. One meta-analysis showed an incidence of prolonged postoperative ileus of 10.2% following elective colonic surgery, with potential higher rates with added effects of the hyperthermic bath, chemotherapy and peritoneal carcinomatosis[14]. The use of thoracic epidural analgesia, postoperative use of prokinetics, laxatives and adjuncts such as coffee or chewing gum, and early mobilisation have all been recommended to hasten gut recovery after such major surgery. ICU readmissions occur in 11%-25% of patients and in one study, ileus/dehydration was responsible for one third of readmissions[14]. The rate of re-operations increases in patients with postoperative complications due to sepsis, anastomotic leaks, etc.
Sepsis both abdominal and unrelated to the surgical site is the commonest complication post-surgery. It is also the commonest cause of mortality. Infections with resistant organisms are also common[8].
Martin et al[25] reported 30- and 90-d readmission rates after CRS-HIPEC to be 14.9% (n = 32), and 21.4% (n = 46), respectively. The main factor implicated in re-admissions was the presence of enterocutaneous fistula. They did not find any association between factors such as age, sex, race, intraoperative blood loss, pancreatic or hepatic resection at the index operation, and postoperative complications of surgical site infection, line infection, and thromboembolic events with higher re-admission rates.
Common postoperative grade III/IV respiratory complications include pneumonia, pleural effusions, respiratory failure, and pulmonary embolism[8,23,26]. These can prolong the ICU stay or cause ICU re-admissions. Respiratory sepsis is the second most common cause of septic shock at 28.6%[8]. The massive fluid shifts during CRS-HIPEC are most commonly responsible for the increased incidence of unplanned intubations, prolonged ventilations and pulmonary interventions. Preti et al[26] reported an incidence of pulmonary adverse events of 10% which included 4.6% pleural effusions, 4.2% respiratory distress necessitating oxygen supplementation and intubations and 3.2% pneumonia. Martin et al[25] reported pleural effusions in 10.8% of patients postoperatively and mortality in two patients secondary to pulmonary embolism.
Hypovolemic shock especially in the first 48 h post-surgery secondary to exuding peritoneal surfaces and systemic inflammatory response can lead to higher rates of grade III/IV complications. The incidence of acute myocardial infarction and arrythmias is similar to any major gastrointestinal surgery. Jafari et al[15] reported a 0.3% incidence of postoperative myocardial infarction. Martin et al[25] reported a 4.4% incidence of cardiac arrythmias (atrial fibrillation, supraventricular tachycardia and pulseless electrical activity) and attributed one patient’s mortality to cardiac dysrhythmia.
Sepsis (unrelated to abdominal complications), central line infections as well as urinary tract infection are common[6,17,25,27]. Some case reports have mentioned rare complications such as non-cirrhotic, non-total parenteral nutrition hyperammonia etc[28]. Prolonged postoperative acidosis has also been observed[8]. Multi-organ failure is common. The risk of pulmonary embolism, deep venous thrombosis and superior mesenteric vein thrombosis is in the range of 5%-10%[29]. The significant risk factors associated with the development of venous thromboembolism include advanced cancer stage at the time of diagnosis, prolonged immobilization, extensive surgical procedures, mucinous tumours of the gastrointestinal tract and the use of central venous catheters.
Depending on the cancer histology, high concentrations of different chemotherapeutic agents (20-1000 times greater than plasma levels) are delivered into the abdominal cavity. Drugs which have a synergistic effect with heat, namely, mitomycinC and the platinumbased drugs, cisplatin, carboplatin, and oxaliplatin are used for intraperitoneal (IP) administration. The less commonly used drugs are doxorubicin, 5fluorouracil, docetaxel, paclitaxel and irinotecan.
Intraperitoneal chemotherapy is sometimes combined with concomitant or early postoperative administration of intravenous chemotherapy, aiming to create a bidirectional diffusion gradient through the cancer cells.
Most of the PSM are platinum-sensitive, with cisplatin being the commonest chemotherapeutic agent used for HIPEC. Common toxicities include nephropathy and haematological toxicity (Table 3). A cisplatin dose more than 240 mg was demonstrated to increase both surgical morbidity and systemic toxicity[17]. Some centres have used sodium thiosulphate for the prevention of cisplatininduced nephrotoxicity with promising results[3,14,30]. One of the considerations for patients with a second recurrence is platinum sensitivity. The progression-free interval since the most recent course of platinum chemotherapy may differentiate between platinum sensitive and platinum resistant disease[16]. Few studies have reported an increased rate of systemic complications with the combined use of cisplatin and mitomycin for IP chemotherapy[1,31]. Canda et al[27] found that patients with pre
| Ref. | HIPEC drugs | EPIC | Nephrotoxicity, % | Haematological toxicity, % |
| Glehen et al[36], 2010 | MMC + CDDP/Ox + 5FU/leucovorin | MMC+5FU | 1 | 13.3 |
| Bakrin et al[1], 2012 | CDDP + MMC/DX | 3 | ||
| Baratti et al[17], 2012 | CDDP + MMC/DX | 5.4 | 5.9 | |
| Bakrin et al[16], 2013 | CDDP/MMC/DX | 11 | 11 | |
| Canda et al[27], 2013 | CDDP + /MMC | 25.8 | 19.8 | |
| Jafari et al[15], 2014 | NA | 3.7 | 0 |
Haematological complications secondary to chemotherapeutic agents are also commonly reported in various studies[1,7,32]. Leukopenia and neutropenia have been frequently reported. Mitomycin-C (MMC), when dosed by body surface area or weight, has been attributed to leukopenia to the tune of 20%-40%[32]. In a study by Feferman et al[32], the use of MMC-HIPEC produced an incidence of 7% severe leukopenia and 4.5% neutropenia, with some patients requiring therapeutic granulocyte colony stimulating factor (GCSF). They reported that the risk of myelosuppression was reduced with a fixed 40 mg dose of MMC in HIPEC and routine use of GCSF for prophylaxis is not indicated. Bakrin et al[16] reported an 11% incidence of grade III/IV leukopenia in their cohort of 566 epithelial ovarian cancer patients undergoing CRS-HIPEC.
The data provided in the included studies in this systematic review lacks standardisation in reporting of methodology, postoperative complications etc[33-37]. There is variance in the classification of complications, drugs used in HIPEC, etc. Although the first ERAS protocols for major abdominal surgery were developed in 2010, ERAS guidelines for CRS-HIPEC were recently published[14,38,39]. Hence the degree of adherence to ERAS in the studies included in our review and its effect on the rate of complications may vary in the future.
CRS-HIPEC for PSM has advantageous survival outcomes, and has become a common surgery in oncological centres all over the world. Being a complex surgery, with proven postoperative systemic inflammatory response, the focus in recent years has shifted to understanding the immediate post
CRS-HIPEC is an aggressive option for the comprehensive management of all peritoneal surface malignancies. It can result in some life-threatening complications in the immediate postoperative period and reported higher morbidity and mortality rates. Postoperative morbidity and survival have sig
The number of patients undergoing CRS-HIPEC has increased in the last decade as have improvements in surgical techniques, surgical skills and perioperative management strategies. All these have led to improvements in post-surgical outcomes and survival rates. The present article reviews the early postoperative management and common complications after CRS-HIPEC, reported in the last decade.
To review early postoperative management after CRS-HIPEC. To review common im
An electronic literature search was conducted using the databases of ‘PubMed’ and ‘Google Scholar’, during the period from 2010 to 2021. Postoperative complications and their synonyms in various combinations were searched. The extracted articles were further reviewed in a step-wise manner for the identification of relevant studies. The full-text assessment identified 14 original articles regarding postoperative complications and critical care management for inclusion in the final review article.
This article reviewed the early postoperative critical care management of such patients and the immediate post-surgical complications as reported in the gamut of studies included in the final review.
CRS-HIPEC is a complex surgery, with a proven postoperative systemic inflammatory response. The focus in recent years has shifted to understanding the immediate postoperative pathophysiology and its management, early detection of complications and the institution of appropriate treatment to reduce morbidity and improve survival. The implementation of ERAS guidelines specific to CRS-HIPEC should help to further reduce postoperative complications.
There are two major avenues for research in this area. One is the early prediction of postoperative complications and early intervention to reduce morbidity and mortality. Although numerous inflammatory markers such as mean platelet volume, CRP, procalcitonin etc have been studied, no single test is foolproof and they should be utilized in association with the clinical scenario, microbiological and biochemical investigations. The second avenue is the implementation of ERAS guidelines for CRS-HIPEC and its impact on postoperative outcomes and survival.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gao W, China; Menendez-Menendez J, Spain; Zhu Z, China S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Bakrin N, Cotte E, Golfier F, Gilly FN, Freyer G, Helm W, Glehen O, Bereder JM. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for persistent and recurrent advanced ovarian carcinoma: a multicenter, prospective study of 246 patients. Ann Surg Oncol. 2012;19:4052-4058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Padmakumar AV. Intensive Care Management of Patient After Cytoreductive Surgery and HIPEC - A Concise Review. Indian J Surg Oncol. 2016;7:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Solanki SL, Mukherjee S, Agarwal V, Thota RS, Balakrishnan K, Shah SB, Desai N, Garg R, Ambulkar RP, Bhorkar NM, Patro V, Sinukumar S, Venketeswaran MV, Joshi MP, Chikkalingegowda RH, Gottumukkala V, Owusu-Agyemang P, Saklani AP, Mehta SS, Seshadri RA, Bell JC, Bhatnagar S, Divatia JV. Society of Onco-Anaesthesia and Perioperative Care consensus guidelines for perioperative management of patients for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC). Indian J Anaesth. 2019;63:972-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Solanki SL, Jhingan MAK, Saklani AP. Rebound hypothermia after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) and cardiac arrest in immediate postoperative period: a report of two cases and review of literature. Pleura Peritoneum. 2020;5:20200126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Alyami M, Kim BJ, Villeneuve L, Vaudoyer D, Képénékian V, Bakrin N, Gilly FN, Cotte E, Glehen O, Passot G. Ninety-day post-operative morbidity and mortality using the National Cancer Institute's common terminology criteria for adverse events better describe post-operative outcome after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia. 2018;34:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Baratti D, Kusamura S, Laterza B, Balestra MR, Deraco M. Early and long-term postoperative management following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Gastrointest Oncol. 2010;2:36-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Cooksley TJ, Haji-Michael P. Post-operative critical care management of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC). World J Surg Oncol. 2011;9:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Malfroy S, Wallet F, Maucort-Boulch D, Chardonnal L, Sens N, Friggeri A, Passot G, Glehen O, Piriou V. Complications after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis: Risk factors for ICU admission and morbidity prognostic score. Surg Oncol. 2016;25:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Colantonio L, Claroni C, Fabrizi L, Marcelli ME, Sofra M, Giannarelli D, Garofalo A, Forastiere E. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg. 2015;19:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Owusu-Agyemang P, Soliz J, Hayes-Jordan A, Harun N, Gottumukkala V. Safety of epidural analgesia in the perioperative care of patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2014;21:1487-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Lorimier G, Seegers V, Coudert M, Dupoiron D, Thibaudeau E, Pouplin L, Lebrec N, Dubois PY, Dumont F, Guérin-Meyer V, Capitain O, Campone M, Wernert R. Prolonged perioperative thoracic epidural analgesia may improve survival after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: A comparative study. Eur J Surg Oncol. 2018;44:1824-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Webb C, Day R, Velazco CS, Pockaj BA, Gray RJ, Stucky CC, Young-Fadok T, Wasif N. Implementation of an Enhanced Recovery After Surgery (ERAS) Program is Associated with Improved Outcomes in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol. 2020;27:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Mao F, Huang Z. Enhanced Recovery After Surgery for Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Systematic Review and Meta-Analysis. Front Surg. 2021;8:713171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hübner M, Kusamura S, Villeneuve L, Al-Niaimi A, Alyami M, Balonov K, Bell J, Bristow R, Guiral DC, Fagotti A, Falcão LFR, Glehen O, Lambert L, Mack L, Muenster T, Piso P, Pocard M, Rau B, Sgarbura O, Somashekhar SP, Wadhwa A, Altman A, Fawcett W, Veerapong J, Nelson G. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced Recovery After Surgery (ERAS®) Society Recommendations - Part II: Postoperative management and special considerations. Eur J Surg Oncol. 2020;46:2311-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 15. | Jafari MD, Halabi WJ, Stamos MJ, Nguyen VQ, Carmichael JC, Mills SD, Pigazzi A. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: analysis of the american college of surgeons national surgical quality improvement program. JAMA Surg. 2014;149:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Bakrin N, Bereder JM, Decullier E, Classe JM, Msika S, Lorimier G, Abboud K, Meeus P, Ferron G, Quenet F, Marchal F, Gouy S, Morice P, Pomel C, Pocard M, Guyon F, Porcheron J, Glehen O; FROGHI (FRench Oncologic and Gynecologic HIPEC) Group. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013;39:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 17. | Baratti D, Kusamura S, Mingrone E, Balestra MR, Laterza B, Deraco M. Identification of a subgroup of patients at highest risk for complications after surgical cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2012;256:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Tao J, Ji PT, Shen JJ, Lu Y. Survival and complications of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in elderly patients: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25:5330-5348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Kim NY, Chun DH, Kim SY, Kim NK, Baik SH, Hong JH, Kim KS, Shin CS. Prognostic Value of Systemic Inflammatory Indices, NLR, PLR, and MPV, for Predicting 1-Year Survival of Patients Undergoing Cytoreductive Surgery with HIPEC. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | van Kooten JP, Oemrawsingh A, de Boer NL, Verhoef C, Burger JWA, Madsen EVE, Brandt-Kerkhof ARM. Predictive Ability of C-Reactive Protein in Detecting Short-Term Complications After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Retrospective Cross-Sectional Study. Ann Surg Oncol. 2021;28:233-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Saeed K, Dale AP, Leung E, Cusack T, Mohamed F, Lockyer G, Arnaudov S, Wade A, Moran B, Lewis G, Dryden M, Cecil T, Cepeda JA. Procalcitonin levels predict infectious complications and response to treatment in patients undergoing cytoreductive surgery for peritoneal malignancy. Eur J Surg Oncol. 2016;42:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Viyuela García C, Medina Fernández FJ, Arjona-Sánchez Á, Casado-Adam Á, Sánchez Hidalgo JM, Rufián Peña S, Briceño Delgado J. Systemic inflammatory markers for the detection of infectious complications and safe discharge after cytoreductive surgery and HIPEC. Surg Oncol. 2020;34:163-167. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Sinukumar S, Mehta S, Damodaran D, Rajan F, Zaveri S, Ray M, Katdare N, Sethna K, Patel MD, Kammer P, Peedicayil A, Bhatt A. Failure-to-Rescue Following Cytoreductive Surgery with or Without HIPEC is Determined by the Type of Complication-a Retrospective Study by INDEPSO. Indian J Surg Oncol. 2019;10:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Cascales-Campos PA, Sánchez-Fuentes PA, Gil J, Gil E, López-López V, Rodriguez Gomez-Hidalgo N, Fuentes D, Parrilla P. Effectiveness and failures of a fast track protocol after cytoreduction and hyperthermic intraoperative intraperitoneal chemotherapy in patients with peritoneal surface malignancies. Surg Oncol. 2016;25:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Martin AS, Abbott DE, Hanseman D, Sussman JE, Kenkel A, Greiwe P, Saeed N, Ahmad SH, Sussman JJ, Ahmad SA. Factors Associated with Readmission After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis. Ann Surg Oncol. 2016;23:1941-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Preti V, Chang D, Sugarbaker PH. Pulmonary Complications following Cytoreductive Surgery and Perioperative Chemotherapy in 147 Consecutive Patients. Gastroenterol Res Pract. 2012;2012:635314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Canda AE, Sokmen S, Terzi C, Arslan C, Oztop I, Karabulut B, Ozzeybek D, Sarioglu S, Fuzun M. Complications and toxicities after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2013;20:1082-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Sharma V, Solanki SL, Saklani AP. Hyperammonemia after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Report of Three Cases with Unusual Presentation. Indian J Crit Care Med. 2021;25:590-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Lanuke K, Mack LA, Temple WJ. A prospective evaluation of venous thromboembolism in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Can J Surg. 2009;52:18-22. [PubMed] |
| 30. | Levine EA, Stewart JH 4th, Shen P, Russell GB, Loggie BL, Votanopoulos KI. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218:573-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 31. | Tan GHC, Shannon NB, Chia CS, Soo KC, Teo MCC. Platinum agents and mitomycin C-specific complications in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Int J Hyperthermia. 2018;34:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Feferman Y, Bhagwandin S, Kim J, Aycart SN, Feingold D, Labow DM, Sarpel U. Conflicting Data on the Incidence of Leukopenia and Neutropenia After Heated Intraperitoneal Chemotherapy with Mitomycin C. Ann Surg Oncol. 2017;24:3831-3836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Bhandoria G, Solanki SL, Bhavsar M, Balakrishnan K, Bapuji C, Bhorkar N, Bhandarkar P, Bhosale S, Divatia JV, Ghosh A, Mahajan V, Peedicayil A, Nath P, Sinukumar S, Thambudorai R, Seshadri RA, Bhatt A. Enhanced recovery after surgery (ERAS) in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): a cross-sectional survey. Pleura Peritoneum. 2021;6:99-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Hübner M, Kusamura S, Villeneuve L, Al-Niaimi A, Alyami M, Balonov K, Bell J, Bristow R, Guiral DC, Fagotti A, Falcão LFR, Glehen O, Lambert L, Mack L, Muenster T, Piso P, Pocard M, Rau B, Sgarbura O, Somashekhar SP, Wadhwa A, Altman A, Fawcett W, Veerapong J, Nelson G. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced recovery after surgery (ERAS®) Society Recommendations - Part I: Preoperative and intraoperative management. Eur J Surg Oncol. 2020;46:2292-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 35. | Cavaliere F, De Simone M, Virzì S, Deraco M, Rossi CR, Garofalo A, Di Filippo F, Giannarelli D, Vaira M, Valle M, Pilati P, Perri P, La Pinta M, Monsellato I, Guadagni F. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol. 2011;37:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S, Elias D; French Surgical Association. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608-5618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 400] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 37. | Mizumoto A, Canbay E, Hirano M, Takao N, Matsuda T, Ichinose M, Yonemura Y. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy at a single institution in Japan. Gastroenterol Res Pract. 2012;2012:836425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Elekonawo FMK, van der Meeren MMD, Simkens GA, de Wilt JHW, de Hingh IH, Bremers AJA. Comparison of 2 Perioperative Management Protocols and Their Influence on Postoperative Recovery after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Standard Parenteral Nutrition, Selective Bowel Decontamination and Suprapubic Catheters? Dig Surg. 2019;36:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Kelly KJ, Cajas L, Baumgartner JM, Lowy AM. Factors Associated with 60-Day Readmission Following Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol. 2018;25:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |