Published online Jun 4, 2012. doi: 10.5492/wjccm.v1.i3.67
Revised: May 17, 2012
Accepted: May 25, 2012
Published online: June 4, 2012
Sepsis is one of the leading worldwide causes of morbidity and mortality in critically-ill patients. Prediction of outcome in patients with sepsis requires repeated clinical interpretation of the patients’ conditions, clinical assessment of tissue hypoxia and the use of severity scoring systems, because the prognostic categorization accuracy of severity scoring indices alone, is relatively poor. Generally, such categorization depends on the severity of the septic state, ranging from systemic inflammatory response to septic shock. Now, there is no gold standard for the clinical assessment of tissue hypoxia which can be achieved by both global and regional oxygen extractabilities, added to prognostic pro-inflammatory mediators. Because the technology used to identify the genetic make-up of the human being is rapidly advancing, the structure of 30 000 genes which make-up the human DNA bank is now known. This would allow easy prognostic categorization of critically-ill patients including those suffering from sepsis. The present review spots lights on the main severity scoring systems used for outcome prediction in septic patients. For morbidity prediction, it discusses the Multiple Organ Dysfunction score, the sequential organ failure assessment score, and the logistic organ dysfunction score. For mortality/survival prediction, it discusses the Acute Physiology and Chronic Health Evaluation scores, the Therapeutic Intervention Scoring System, the Simplified acute physiology score and the Mortality Probability Models. An ideal severity scoring system for prognostic categorization of patients with systemic sepsis is far from being reached. Scoring systems should be used with repeated clinical interpretation of the patients’ conditions, and the assessment of tissue hypoxia in order to attain satisfactory discriminative performance and calibration power.
- Citation: Moemen ME. Prognostic categorization of intensive care septic patients. World J Crit Care Med 2012; 1(3): 67-79
- URL: https://www.wjgnet.com/2220-3141/full/v1/i3/67.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v1.i3.67
Prognostic categorization of the intensive care unit (ICU) patients with systemic sepsis may be tried through sequential clinical interpretations, assessment of tissue hypoxia and the use of severity scoring systems. The major prognostic value of scoring systems is mainly to compare the effectiveness of ICU services in different centers or over time. So, to determine patient outcome both the clinical interpretation of patients, the assessment of tissue hypoxia, and the scoring systems are together needed.
In 1991[1], experts from a variety of disciplines met for a Consensus Conference and proposed definitions for sepsis as follows: systemic inflammatory response syndrome (SIRS): It denotes the systemic inflammatory response to a wide variety of severe critical insults, manifested by two or more of the following conditions: temperature > 38 °C or < 36 °C, heart rate > 90 beats/min, respiratory rate > 20 breaths/min or PaCO2 < 32 mmHg, and white blood counts > 12 000/mL, < 4/L, or > 10% immature forms. Sepsis: It denotes the systemic inflammatory response to infection. Severe Sepsis: It denotes sepsis or SIRS associated with organ dysfunction, hypoperfusion or hypotension.
Hypotension and hypoperfusion abnormalities may include lactic acidosis, oliguria or acute alteration in mental status. Systolic blood pressure < 90 mmHg or a reduction of 40 mmHg from the baseline in the absence of other causes of hypotension notify severe sepsis or SIRS. It is usually corrected by fluid loading. Septic Shock: It denotes sepsis or SIRS induced hypotension not corrected by fluid loading and needing inotropic and/or vasopressor support. Perfusion abnormalities to many organs characterize the shock state. Multiple organ dysfunction (MOD) Syndrome: It represents altered organ functions in an acutely-ill patient to the extent that homeostasis cannot be maintained without intervention.
It has been shown that the systemic inflammatory response to severe infection evolves in stages, from sepsis to severe sepsis to septic shock, with corresponding increase in the proportion of patients with positive blood cultures, end-organ failure, and crude mortality[2].
Severe sepsis and septic shock are major reasons for ICU admission. In critically-ill patients in the ICU, who are already compromised because of co-existing serious co-morbidities, septic shock may be associated with higher mortality[3]. In septic patients, the number of organ systems with impaired function is important because it correlates with clinical patient outcome[4].
Sepsis is one of the leading causes of morbidity and mortality worldwide today. It is estimated that there are approximately 700 000 cases of severe sepsis annually in USA and around 400 000 patients die every year as a result of sepsis in both USA and Europe. The incidence of the various degrees of severity of sepsis is not well known but a relatively small Italian study which looked at 1100 ICU admissions as early as 2001, found the following[5] (Table 1):
| Diagnosis | Number (1100) | Deaths (%) |
| Nil | 421 | 101 (24.0) |
| SIRS | 573 | 152 (26.5) |
| Sepsis | 50 | 18 (36.0) |
| Severe sepsis | 23 | 12 (52.2) |
| Septic shock | 33 | 27 (81.8) |
Tissue hypoxia is defined as a decrease in the partial pressure of oxygen in a given tissue or as a condition in which the cells of a tissue have abnormal oxygen utilization such that the tissue is experiencing anaerobic metabolism.
The relationship between whole body oxygen delivery (DO2) and oxygen consumption (VO2) in human sepsis has been extensively studied but remains controversial. The pathological supply dependency is an evidence of occult tissue hypoxia and has been associated with an increased incidence of MODS and poor outcomes in patients with sepsis[6]. Support for this belief comes from some clinical investigators who have demonstrated improved outcomes in patients with septic shock by pharmacologically augmenting systemic oxygenation to supra-normal levels[7,8]. However, other investigators thought that these clinical studies should be criticized because of methodological error from mathematical coupling because DO2 and VO2 were calculated from a common set of measured variables; cardiac output and arterial oxygen content[9]. The author of the present review could not report significant reduction in mortality in septic patients managed by using the supra-normal hemodynamic approach[10].
However, it may be prudent to think that indices of supra-normal oxygenation for management of patients with sepsis may be used for their prognostic categorization. Patients who can attain supra-normal values have decreased morbidity and mortality, mostly due to better physiological reserves. Based on this, it may be concluded that global VO2/DO2 relationship based on good oxygen extractability potentiality may denote that the oxygen extraction ratio is an excellent parameter for prognostic categorization of patients with sepsis.
Mixed venous oxygen saturation (SvO2) determination by pulmonary artery catheterization is a flow-weighted average of venous effluent from all perfused vascular beds. A decrease in SvO2 can be caused by a decrease in DO2 and/or an increase in VO2. An increased value in septic patients denotes tissue hypoxia and its improvement by normal or supra-normal pharmacological interventional therapy may be used as a good prognostic marker[7].
Metabolic lactic acidosis development is one of the most important abnormalities of tissue hypoxia due to the production of hypoxic global lactate during sepsis or septic shock. Plasma lactate has been shown to be a good prognostic indicator of hypo-perfusion in critically-ill patients. Plasma lactate is easy to measure, and lactate clearance can be followed sequentially to assess the prognosis of the response of septic patients to therapy. The more the decrease in pH and the higher the value of base deficit, the more serious the condition of the septic patient is.
Prognostic markers: Procalcitonin (PCT) and pro-inflammatory mediators such as tumour necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 are important clinical prognostic markers in patients with systemic sepsis[11].
There has been strong correlation between serum concentrations of pro-inflammatory mediators and scores of severity of illness[12]. In spite of this, most of these mediators are not established for clinical decision making due to their short half-life[13].
Casey et al[14] designed a biologic score for application in septic patients. It included levels of endotoxin, IL-1B, TNF-α and IL-6. It proved a strong correlation with mortality in septic patients. However, the same goal could be achieved by estimation of blood lactate level as an easier and cheaper test.
Nylen et al[15] presented the first evidence that PCT, one of the best prognostic markers of sepsis[16], may actually be a sepsis mediator and could have an integral role in the inflammatory process and its prognostic categorization.
It has been shown that in vitro and in vivo induction of cytokines leads to the rapid release of PCT which has a long half-life[17,18].
Ugarte et al[19] showed that PCT concentration on the first day of the diagnosis of sepsis, severe sepsis or septic shock was significantly higher in non-survivors than in survivors. Proving a strong correlation between PCT and survival of septic patients.
Using stepwise discriminant analysis, PCT was proved to be the best single predictor of outcome in patients with systemic sepsis, as it allocated survivors in 95.8% and non-survivors in 83.3% of patients, with an overall prediction accuracy of 80%[20].
There has been recent reports of altered outcome in sepsis due to the release of lipo-polysaccharide binding protein, bacterial permeability inducing protein, and other key proteins which may result in altered disease susceptibility and severity: as heat shock protein 70 and nitric oxide synthase[21].
It has long been appreciated that many patients with sepsis demonstrate defects in coagulation and fibrinolytic systems. These are manifested as anti-thrombin III, protein C, and Protein S and the consumption of fibrinogen, together with the appearance of disseminated intravascular coagulation. More recently, there has been a report of a randomized multicenter trial which has examined the use of a novel human activated protein C during the management of patients with severe sepsis[5]. A total of 1690 patients with severe sepsis were enrolled into the study; 850 patients received the protein C preparation and 840 received placebo. The mortality rate was decreased from 30.83% in the control group to 24.71% in the active treatment group, an effect which was statistically significant. This report may clearly have major implication for the prognostic categorization and management of patients with systemic sepsis.
The general interest in genetics culminated in the publication of the findings of the human genome project which appeared in February 2001 issue of Nature. The precise structure of 30 000 genes which make up the human DNA bank is now known and can be downloaded from the USA National Human Genome Project Internet Site. Such knowledge will prove useful because it will increase the understanding of the etiology and pathology of many disease processes. Because the technology used to identify the genetic make-up of individual patients is now advancing so rapidly, it will soon be possible to identify more markers in patients and will allow prognostic stratification of septic patients for future trials of new therapeutic approaches[21].
The technique of gastric or sigmoid tonometry measures intramucosal pH (Phi) by allowing the equilibration of CO2 pressures between fluid or air-filled balloon and the interstitial fluid of the mucosa. Measurement of gut intramucosal CO2 can be also achieved through air introduced directly into the gut (balloonless air tonometry), which equilibrates with the interstitial fluid of the mucosa and is then aspirated from the stomach[22]. Measurement of CO2 content of fluid aspirated from the stomach has been also described by Mohsenifar et al[23]. Both later methods avoid the use of commercial expensive tonometry catheters costing $ 200 each[24].
Phi may decrease due to changes in blood flow to the stomach or sigmoid mucosa due to splanchnic ischemia in shocked patients. Phi appears to be useful for prognostic categorization of ICU patients with systemic sepsis based on serial measurements[25]. The author of the present review has shown that Phi values were significantly lower in septic patients with MODS on admission to the ICU than in patients with no organ dysfunction[10].
Assessment of both global and regional VO2/DO2 relationships can combine both sides of the coin in prognostic categorization of ICU patients with systemic sepsis. However, there is no gold standard for the detection of tissue hypoxia. There are no specific clinical signs and no clearcut threshold for any single laboratory test. But a multitude of tests combined with sequential clinical evaluations of septic patients may be the best way for their prognostic categorization in the ICU. So, management of patients with severe sepsis or septic shock may be through haemodynamic-oriented or splanchnic-directed therapy added to sequential repeated clinical interpretations. This is a gold standard for both therapy and prognostic categorization of ICU patients with systemic sepsis.
Severity scoring systems provide numerical scores that describe the impact of patients’ illnesses on their physiological reserves.
Most of the severity scoring systems include assessment of major organ system functions. A prolonged period of hypoperfusion of critical organ beds, such as the liver, the brain, the heart, and the gastro-intestinal tract, may give rise to MOD and failure, which is associated with a high rate of morbidity and mortality[26]. It has been shown that the pattern and evolution of organ system dysfunction over the first 3 d of sepsis is significantly related to 30 d mortality.
Two main types of scoring systems have been developed for use in ICU patients: those that focus on describing morbidity as it evolves; organ dysfunction systems, and those that focus on a single end point, survival or mortality[27]. So, severity scoring systems are usually designed to help in the prognostic categorization of critically-ill patients as regards their morbidity or survival.
Morbidity prediction systems include a large number of scoring trials by different authors, based on advanced statistical efforts for different populations of critically-ill patients at various centers. We chose to concentrate on three scoring systems that proved useful for clinical applications, namely, the MOD score, the sequential organ failure assessment (SOFA) score, and the logistic organ dysfunction (LOD) score. However, other scoring systems may prove useful and an ideal prediction scoring system has not been reached yet. It should be noted that these systems do not replace serial clinical interpretations of the septic patients.
The MOD score: The MOD scoring system was developed by Marshall et al[4] in 1995 (Table 2).
| Organ system | Score | ||||
| 0 | 1 | 2 | 3 | 4 | |
| Respiratory PaO2/FiO2 | > 300 | 226-300 | 151-225 | 76-150 | ≤ 75 |
| Renal creatinine (μmol/L) | ≤ 100 | 101-200 | 201-350 | 251-500 | > 500 |
| Hepatic bilirubin (μmol/L) | ≤ 20 | 21-60 | 61-120 | 121-240 | > 240 |
| Cardiovascular PAR1 | < 10.0 | 10.1-15 | 15.1-20 | 20.1-30 | > 30.0 |
| Cardiovascular HR (beats/min) | < 120 | 120-140 | > 140 | Dopamine > 3 mg/g per min | Lactate > 5 mmoL/L |
| Hematologic platelet count (/L) | > 120 | 81-120 | 51-80 | 21-50 | ≤ 20 |
| Neurologic Glasgow coma score | 15 | 13-14 | 10-12 | 7-9 | ≤ 6 |
It included six key organ systems and a score of zero to four was given to each organ according to function (zero being normal function and four being the most severe dysfunction), with a maximum score of 24. A mortality rate of 25% was observed for patients with a score of 9-12, 50% for a score of 13-16, 75% for a score of 17-20 and 100% for a score > 20. The detailed analysis of the results of daily scoring demonstrated the prognostic insights gained by adopting this system[28].
A revision[29] of this score has abandoned the cardiovascular parameter (pressure-adjusted heart rate) in favour of a mixed cardiovascular parameter (Table 3) as follows: 0 = heart rate < 120 beat/min; 1 = heart rate > 120 and < 140 beat/min; 2 = heart rate > 140 beat/min; 3 = need for inotrope: (dopamine > 3 μg/kg per minute), and 4 = lactate > 5 mmoL/L. The Revised MOD scoring system proved to be of value, because pressure adjusted heart rate cannot be measured in a significant proportion of ICU patients due to the absence of central venous monitoring. In fact, approximately one half of the patients in the original Marshall et al[4] study could not have a cardiovascular component calculated.
| Organ system | Score | ||||
| 0 | 1 | 2 | 3 | 4 | |
| Respiratory PaO2/FiO2 | > 400 | ≤ 400 | ≤ 300 | ≤ 200 | ≤ 100 |
| Renal creatinine (μmol/L) | ≤ 110 | 110-170 | 171-299 | 300-440 urine output ≤ 500 mL/d | > 440 urine output < 200 mL/d |
| Hepatic bilirubin (μmol/L) | ≤ 20 | 20-32 | 33-101 | 102-204 | > 240 |
| Cardiovascular hypotension | No hypotension | MAP < 70 mmHg | Dopamine ≤ 51 | Dopamine > 51 or epinephrine ≤ 0.11 or norepinephrine ≤ 0.11 | Dopamine > 151 or epinephrine > 0.11 or norepinephrine > 0.11 |
| Dobutamine (any dose) | |||||
| Hemotologic platelet count (/mL) | > 150 | ≤ 150 | ≤ 100 | ≤ 50 | ≤ 20 |
| Neurologic Glasgow coma score | 15 | 13-14 | 10-12 | 6-9 | < 6 |
It is recommended that the MOD score and its Revised form should be measured at the same point in time every day (first morning values). The use of measurements at one particular time avoids capturing momentary physiological changes unrelated to patient condition.
In a small study, Jacobs et al[29] compared daily MOD scores to daily Acute Physiology and Chronic Health Evaluation (APACHE) II scores in 39 septic-shock patients from one Saudi Arabian ICU. The authors found that the maximum MOD score and the maximum change in the score from admission, both discriminated (the ability to predict mortality in one individual patient) very well between survivors and non survivors, whereas APACHE II score did not.
To summarize, the MOD score and its revised form can be used to represent organ dysfunction at baseline and during ICU stay. They can also significantly contribute to the prediction of hospital or ICU mortality.
The SOFA score: The SOFA score (Table 3) was developed in 1994 during a Consensus Conference organized by the European Society of Intensive Care and Emergency Medicine, in an attempt to provide a means of describing the degree of organ failure over time in individuals and groups of ICU septic patients.
It was initially termed the Sepsis-related Organ Failure Assessment score, but it has been realized that it could be applied to non-septic patients as well.
It includes scores for six organ systems where a score of zero is given for normal function and a score of four is given for the most abnormal one. The worst values on each day are recorded and organ function total score can thus be monitored over time[27].
Vincent et al[30] in 1998 working on “sepsis-related”problems published the first evaluation of the SOFA score. They found that infected patients had more severe organ dysfunctions compared to those without infection. Antonelli et al[31] in 1999 proved that the mean total maximum SOFA score was significantly higher for non-survivors than survivors denoting a high discriminative power (the ability to predict mortality in an individual patient). Because the total maximum SOFA score can be easily calculated daily for the patient, no restriction based on the patients’ ICU length of stay is necessary. So, increasing organ dysfunction as measured by the SOFA score consistently correlates with increasing mortality. The SOFA score is also a reliable measure of organ dysfunction at ICU admission.
There were some early published studies that have since examined the utility and accuracy of the SOFA score, which proved that maximum SOFA score and increasing SOFA score are highly prognostic for stratification of critically ill patients including septic patients[32-34].
The LOD score: The LOD score (Table 4) was developed in 1996 using multiple logistic regression applied to selected variables from a large database of ICU patients[35]. The score consists of six organ systems and 12 variables with a maximum of 22 scoring points. If no organ dysfunction is present the score is zero, rising to a maximum of five as the worst severity organ dysfunction.
| Organ system | LOD points | ||||||
| Increasing severity/decreasing values | Organ dysfunction free | Increasing severity/increasing values | |||||
| 5 | 3 | 1 | 0 | 1 | 3 | 5 | |
| Neurologic Glasgow coma score | 3-5 | 6-8 | 9-13 | 14-15 | |||
| Cardiovascular heart rate (min) | < 30 or < 40 | 40-69 | 70-89 | 30-139 | ≥ 140 or 240-269 | ||
| Systolic blood pressure (mmHg) | 90-239 | ≥ 270 | |||||
| Renal | |||||||
| Serum urea (g/L) | < 6 | 6-9.9 | 10-19.9 | ≥ 20 | |||
| Serum urea nitrogen (mmol) | < 6 | 6-9.9 or 106-140 | 10-19.9 or ≥ 141 or ≥ 10 | ≥ 20 | |||
| Creatinine (μmol) | < 106 | ||||||
| Urine output (I/d) | < 0.5 | 0.5-0.74 | 0.75-9.99 | ||||
| Pulmonary PaO2/FiO2 on MV or CPAP PaO2 (kPa)/FiO2 | < 150 (< 19.9) | ≥ 150 (≥ 19.9) | No ventilation, no IPAP or no CPAP | ||||
| Hematologic | |||||||
| White blood cell count (× 109/L) | < 1.0 | 1.0-2.4 or < 50 | 2.5-49.9 | ≥ 50.0 | |||
| Plateltes (× 109/L) | ≥ 50 | ||||||
| Hepatic bilirubin (μmol/L) | < 34.2 | ≥ 34.2 | |||||
| Prothrombin time, seconds above standard (% of standard) | (< 2.5%) | ≤ 3 (≥ 25%) | > 3 | ||||
For maximum dysfunction of the pulmonary and hematologic systems, a maximum of three points can be given for the most severe levels of dysfunction and for the liver, the most severe dysfunction only receives one point. The variables had been recorded as the worst value of each organ dysfunction in the first 24 h of ICU admission. A reference table converts the score to a probability of hospital mortality, the relationship being sigmoid. The score can thus discriminate between survivors and non-survivors.
The LOD score aims to achieve similar goals to the MOD score, namely, to quantitatively and qualitatively describe organ dysfunction. The goal is to provide a tool that can itself provide a useful outcome measure (e.g., improvement/resolution of organ dysfunction) rather than merely predicting mortality. Though, not originally described as a serial measure, it appears that the LOD score may hold the most promise for patient outcome in the future[21].
Mortality/survival prediction scoring systems include a large number of scoring trials by different authors, based on advanced statistical efforts including equations for different populations of critically-ill patients. We chose to concentrate on important examples which are useful for clinical prognostic stratification of mortality/survival of patients namely; the APACHE scores, the therapeutic intervention scoring system, the simplified acute physiology score (APS) and the mortality probability models. However, other scoring systems may prove useful and an ideal scoring system for mortality/survival prediction has not been reached yet. It should be noted that these systems do not replace serial clinical interpretations of the septic patients.
The APACHE II scoring system was developed by Knaus et al[36] in 1985 as a refinement of the original APACHE score. It consists of: APS, Age points, and Chronic Health points. The reduced number of physiological variables of APS from 34 in the original APACHE to 12 in APACHE II was achieved by a multivariate analysis. The total physiological derangement score is the sum of the individual scores (0-4) for each variable, except the Glasgow coma scale (GCS) where the score is 15 min the GCS. The most deranged value in the first 24 h of ICU admission is used as the scoring for each variable (Table 5). The total physiological derangement score is added to a score of age (0 to 6) and a chronic health score for patients with severe organ insufficiency (2 to 5 dependent upon admission status) as shown in Table 5 and Figure 1. The number of disease groups was 56. The total APACHE II score ranges between zero and 71 points. Points of 25 or less denote less than 50% mortality while points of 35 or more denote more than 80% mortality. However, some investigators have used APACHE II scoring over time to assess the prognosis of individual patients.
| Physiological variable | High abnormal range | Low abnormal range | |||||||
| 4 | 3 | 2 | 1 | 0 | 1 | 2 | 3 | 4 | |
| Temperature-rectal (°C ) | ≥ 41 | 39-40.9 | 38.5-38.9 | 36-38.4 | 34-35.9 | 32-33.9 | 30-31.9 | ≤ 29.9 | |
| Mean arterial pressure (mmHg) | ≥ 160 | 130-159 | 110-129 | 70-109 | 50-69 | ≤ 49 | |||
| Heart rate (ventricular response) | ≥ 180 | 140-179 | 110-139 | 70-109 | 50-69 | 40-54 | ≤ 39 | ||
| Respiratory rate (non-ventilated or ventilated) | ≥ 50 | 35-49 | 25-34 | 12-24 | 10-11 | 6-9 | ≤ 5 | ||
| Oxygenation | |||||||||
| A-a DO2 (mmHg) | |||||||||
| FiO2≥ 0.5 | ≥ 500 | 350-499 | 200-349 | < 200 | |||||
| Record | |||||||||
| A-a DO2 (mmHg) | |||||||||
| FiO2 < 0.5 record only PaO2 | PO2 > 70 | PO2 (6-70) | PO2 (55-60) | PO2 < 55 | |||||
| Arterial pH | ≥ 7.7 | 7.6-7.69 | 7.5-7.59 | 7.33-7.49 | 7.25-7.32 | 7.15-7.24 | < 7.15 | ||
| Serum sodium (mmol/L) | ≥ 180 | 160-179 | 155-159 | 150-154 | 130-149 | 120-129 | 111-119 | < 7.15 | |
| Serum potassium (mmol/L) | ≥ 7 | 6-6.9 | 5.5-5.9 | 3.5-5.4 | 3-3.4 | 2.5-2.9 | < 2.5 | ||
| Serum creatinine (10 mg/L) (double point score for acute renal failure) | ≥ 3.5 | 2-3.4 | 1.5-1.9 | 0.6-1.4 | < 0.6 | ||||
| Hematocrit (%) | ≥ 60 | 50-59.9 | 46-49.9 | 30-45.9 | 20-29.9 | < 20 | |||
| White blood count (total/mm3) (in 1000) | ≥ 40 | 20-39.9 | 15-19.9 | 3-14.9 | 1-2.9 | < 1 | |||
| GCS: score = 15 minus actual GCS | |||||||||
| Total APS: Sum of the 12 individual variable points | |||||||||
| Serum HCO3 (venous-mmol/L) (not preferred, use if no ABG) | ≥ 52 | 41-51.9 | 32-40.9 | 22-31.9 | 18-21.9 | 15-17.9 | < 15 | ||
Generally, data of the APACHE II score are computed through the following equation to deliver the final risk of hospital mortality:
(R/1-R) = -3.517 + (APACHE II × 0.146 + S + D)
where: R = Risk of hospital death, S = Risk imposed by emergency surgery, and D = Risk imposed by specific disease.
Under the APACHE II system, the predicted individual death rate is based on a decision criterion of 0.50. Any patient with an estimated risk of death greater than 0.50 is simply expected to die.
Although the APACHE II score provides valuable information about the severity of illness of patient groups, they provide little information about the severity of illness of individual patients[37]. For example, an APACHE II score of 20 does not tell whether the patient has severe renal failure or acute respiratory failure, whereas analysis of component scores of an organ dysfunction score as SOFA will provide an accurate description of the patients’ disease status. This does not mean that organ dysfunction scores as the SOFA score should replace APACHE II score but that the two scores can provide different information and may be used to complement each other[27].
In 1991, Knaus et al[38] published a further refinement to their severity of illness scoring system termed APACHE III (Table 6). Turning first to the APS, they added some variables and eliminated some parameters. Additional weights were assigned to the extremes of physiological measures. For example, the risk associated with extremely high readings is different from that associated with equally low readings. GCS variables were also refined. The authors also re-weighted age and derived an extended chronic health co-morbidity score.The number of disease groups was increased to 94.The APS in APACHE III ranged between zero and 252 points while the total score reached 299 points by adding 24 points for age and 23 points for chronic health evaluation.
| Parameter | Value range | Points | Parameter | Value range | Points |
| Core temperature (°C) | 0-32.9 | 20 | Plasma bilirubin (μmol/L) | 0-34 | 0 |
| 33.0-33.4 | 16 | 35-51 | 5 | ||
| 33.5-33.9 | 13 | 52-85 | 6 | ||
| 34.0-34.9 | 8 | 86-135 | 8 | ||
| 35.0-35.9 | 2 | 136 plus | 16 | ||
| 36.0-36.9 | 0 | ||||
| 40 or more | 4 | ||||
| Heart (r/min) | 0-39 | 8 | Urine volume (mL/24 h) | 0-399 | 15 |
| 40-49 | 5 | 400-599 | 3 | ||
| 50-99 | 0 | 600-899 | 7 | ||
| 100-109 | 1 | 900-1499 | 5 | ||
| 110-119 | 5 | 1500-1999 | 4 | ||
| 120-139 | 7 | 2000-3999 | 0 | ||
| 140-154 | 13 | 4000 plus | 1 | ||
| 155 or more | 17 | ||||
| Mean blood pressure (mmHg) | 0-39 | 23 | Plasma Creatinine (μmol/L) (if no acute renal failure) or in ARF (< 410 mL urine vol/24 h) | 0-43 | 3 |
| 40-59 | 15 | 44-132 | 0 | ||
| 60-69 | 7 | 133-171 | 2 | ||
| 70-79 | 6 | 172 or more | 7 | ||
| 80-99 | 0 | ||||
| 100-119 | 4 | ||||
| 120-129 | 7 | 0-132 | 0 | ||
| 130-139 | 9 | 133 or more | 10 | ||
| 140 or more | 10 | ||||
| Respiratory (r/min) (zero points for 6-12/min rate if on ventilation) | 0-5 | 17 | Arterial PO2 (kPa) (Inspired O2 < 50%) or alveolar/arterial PO2 difference kPa (Pa-PaO2) (Inspired O2 > 50%) | 0-6.66 | 15 |
| 6-11 | 8 | 6.67-9.32 | 5 | ||
| 12-13 | 7 | 9.33-10.6 | 2 | ||
| 14-24 | 0 | 10.7 plus | 0 | ||
| 25-34 | 6 | ||||
| 35-39 | 9 | 0-13.2 | 0 | ||
| 40-49 | 11 | 13.3-33.2 | 7 | ||
| 50 or more | 18 | 33.3-46.5 | 9 | ||
| 46.6-66.6 | 11 | ||||
| 66.7 and over | 14 | ||||
| White cell count (× 109/L) | 0-0.9 | 19 | Age (yr) | 0-44 | 0 |
| 1.0-2.9 | 5 | 45-59 | 5 | ||
| 3.0-19.9 | 0 | 60-64 | 11 | ||
| 20.0-24.9 | 1 | 65-69 | 13 | ||
| 25 or more | 4 | 70-74 | 17 | ||
| 75 or more | 24 | ||||
| Haematocrit (%) | 0-49.9 | 0 | Chronic health evaluation (do not score in elective surgery patients) | Cirrhosis Immunosuppression Leukaemia Multiple myeloma | 4 |
| 50 or more | 3 | 10 | |||
| 10 | |||||
| 10 | |||||
| Plasma sodium (mmol/L) | 0-119 | 3 | Metastaic cancer | 11 | |
| 120-134 | 2 | Lymphoma | 13 | ||
| 135-154 | 0 | Hepatic failure | 16 | ||
| 155 or more | 4 | AIDS | 23 | ||
| Plasma albumin (g/L) | 0-19 | 11 | Neurological score | Use matrix | 0-48 |
| 20-44 | 0 | ||||
| 45 or more | 4 | ||||
| Acid base status | Use matrix | 0-12 |
The equation of hospital prediction mortality by APACHE III differed from that of APACHE II and included a risk of location denoting the condition of transference of the patient from a previous locality, as such: R/1-R = (APACHE III Score × 0.053) + Risk of emergency Surgery + Risk of specific disease category + Risk of patient location.
Similar to APACHE II score, the predicted death rate of the APACHE III score is based on a decision criterion of 0.50 with predicted mortality if R exceeds 0.50.
Independent validation of APACHE III has been undertaken by a number of studies[39-43], which proved acceptable discrimination performance (the ability to predict mortality in individuals as measured by the area under a receiver operating characteristic curve) and inadequate calibration power (the ability to predict mortality in a large population as measured by a goodness-of-fit test).
A critical prognostic importance of APACHE III, may be based on the premise that the changes in APS would reflect the patient response to therapy. The daily APS component of the risk equation would be given by the formula:
Daily risk = day 1 APS + current day APS + change in APS since yesterday
Day 1 APS is a significant predictor of hospital mortality, but its relative influence decreases dramatically over time. The current day APS, as the most important single factor, should be measured retrospectively as scoring values are the most deranged in any 24 h period.
When the daily risk is added to the remaining patient variables included in the APACHE III score, the coefficients of each variable were established resulting in equations for d 1-7 of ICU admission. Research is going on to extend the model beyond day 7.
Changes in protocols and practices within ICUs prompted a full review and updating of all the mortality APACHE III equations[38] by using the same variables as APACHE III with added new variables: mechanical ventilation, thrombolysis, impact of sedation on GCS, together with rescaling of GCS and oxygenation index. Updating used the largest group of patients ever used for APACHE equations modeling care from 104 ICUs in 45 hospitals, with a total of 131 618 observations. The two used statistical techniques were logistic and linear regressions. The result was a new version called APACHE IV[44], whose calculator is shown in Figure 2.
In addition, there were several changes made for the modeling process used in APACHE III. The first involved the laboratory values that were previously considered as “normal”. That is, if a measurement was missing, then the value of the previous day was carried forward. If the previous day value was also missing, then the value from 2 d back was carried forward, etc. the second change excluded patients transferred from another ICU, because extensive clinical interventions and life support before ICU admission biases the prognostic implications of the first ICU day physiologic measures. The third change was measurement of previous length of hospital stay (LOS) as a continuous rather than an integer value. Previous LOS was defined as the square root of (ICU admission date/time-Hospital admission date/time). Fourth, to more precisely determine the impact of neurological derangement, a variable was added indicating whether a GCS could not be assessed due to sedation. The most important change involved the new categorization of disease groups. Based on the frequency of selected diagnosis and their mortality rate, the existing 94 groups were expanded to 116[3,44]. However, the major changes to the equations included the addition of new variables, the recalling of previous LOS, and increasing the number of disease groups from 94 to 116.
The APACHE systems are the only validated ICU risk adjustment models that provide performance information about 2 separate outcomes of care; mortality and ICU LOS, the APACHE IV model is the most recent version. Researches are enthusiastic nowad to discontinue the use of APACHE II and III and move to the more contemporary and accurate APACHE IV, now that both the score and the two predictions are in public[44].
The Therapeutic Intervention scoring system was developed by Cullen et al[45] in 1974 as the earliest severity scoring system. It is composed of 76 monitoring and therapeutic parameters. Each modality is assigned a weighted score, ranging from 1 to 4, depending on the intensity of intervention. For example, a peripheral iv line or a urinary catheter is assigned one point. A central venous line or two peripheral iv catheters are assigned two points. A central iv line for hyperalimentation or the application of a chest tube is assigned three points. A pulmonary artery catheter for vaso-active drug infusion is assigned four points. Each modality is assigned to one of three categories: active therapy, ICU monitoring or standard floor care. Points are totaled and TISS score is obtained by a calculator (Figure 3). Patients can be then stratified into one of four classes based on the number of TISS points. TISS is based on the premise that, regardless of the diagnosis, the amount of therapy based on the amount of monitoring reflects the degree of physiological impairment. The TISS does not predict outcome on patient admission to the ICU. However, trends of the score over the first three d in ICU correlate well with survival. If the TISS points do not improve at the third day, the likelihood of death increases. So, it discriminates between survivors in whom the score falls progressively and non-survivors in whom the score remains static. Moreover, the TISS can identify those patients who require monitoring only.
The TISS is used most frequently in conjunction with the APACHE systems. So, Both together can be used to evaluate concordance between severity of illness and quantity of needed therapy. Either the TISS alone or in conjunction with the APACHE scoring systems can be used for prognostic categorization of patients with systemic sepsis.
In 1984, Le Gall et al[46], published the Simplified Acute Physiology Score. It was designed to overcome some of the problems of APS of the APACHE systems. The authors selected the 13 “most easily measured” physiological variables available in 90% of patients from a previous survey employing the APS that they had conducted. SAPS scores these variables (0-4) in an identical manner to the APS of the APACHE II system, adds a score for age (0-4) and replaces respiratory rate or the P(A-a) O2 which is difficult to measure with a fixed score of 3 for patients receiving mechanical ventilation or CPAP. The most abnormal values from the first 24 h of ICU admission are taken as the total scoring value. Le Gall et al[46] concluded that SAPS performed at least as well if not better than APS of the APACHE system but was more useful as it was much simpler. They stressed that SAPS is applicable to a wide range of pathologies but that its predictive value and performance can only be applied to groups of patients, not to individual patients.
In 1993, Le Gall et al[47] published a refined version of their original SAPS termed SAPS II whose calculator is shown in Figure 4, and the variables were 17 (12 physiological, age, type of admission and 3 chronic health diagnosis).
The main advantage of SAPS II over APACHE III is the ability to accurately predict mortality in stratified groups of patients without recourse to defining a single diagnosis, which is only possible in a minority of patients.
It is clear that SAPS II can be useful for prognostic stratification for groups of critically ill patients including those with systemic sepsis. It can also be useful for guiding therapy, comparing the management of these patients overtime and comparing ICU performance of groups of patients in different ICU’s.
The SAPS II score varies between zero and 163 points: 116 points for physiological variables, 17 points for age and 30 points for previous diagnosis.
SAPS III assesses 12 physiological variables: at the first 24 h of ICU admission as SAPS II, and includes weighing for pre-admission health status and age. It has been poorly studied, with the exception of some formal analysis of data accuracy in the original publication and external validation studies[48,49].
In 1985, Lemeshow et al[50] published their first attempt at an outcome prediction model. They actually developed four models: MPM0 (probability of death from data collected at ICU admission), MPM24 (probability of death from data collected at 24 h), MPM48 (probability of death from data collected at 48 h) and MPM0T (probability of death “overtime” based on MPM0 and the change in probability between MPM0 and MPM24, and between MPM24 and MPM48). Patients whose probability of mortality started high and remained high, or increased by > 10% had a very high actual mortality. It deserves mentioning that for ICU triage purposes, MPMo is the most valid model at present.
In common with APACHE and SAPS systems, MPM had low sensitivity (ability to predict those patients who are going to die) but high specificity (ability to predict those patients who are going to live).
Lemeshow et al[51] published a revision of their MPM termed MPM II. They employed a near identical method to that they had used in developing their original MPM. The authors initially developed MPM II0 and MPM II24, deciding to temporarily abandon the MPM48 and MPM 0T of the original model.MPM II0 was determined by 15 variables. Lemshow et al[52] in 1994, found that patients alive but still requiring to be on ICU at 24 h differed markedly from those who had either died or been discharged. They emphasized that MPM II24, including 13 variables, is a companion model to MPM II0 and represents a different population of patients. The authors argue that this approach exposes one of the main weaknesses of the APACHE and SAPS models, which take the worst data from the first 24 h of ICU admission, and failed to differentiate between the two originally observed populations.
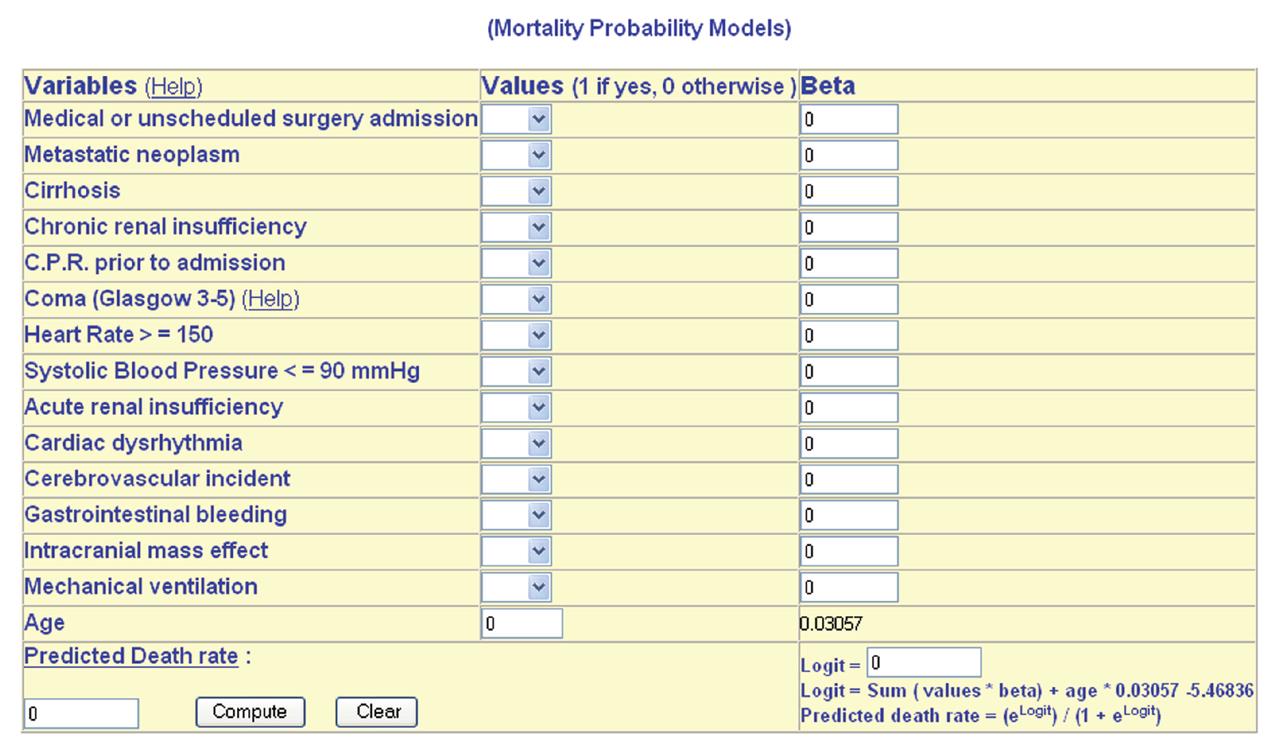
The following year, Lemeshow et al[53] published two further models based upon their data set, MPM II48 and MPM II72. Both these models use the same 13 variables as MPM II24. They pointed out that the probability of death changes with time, while an APACHE or SAPS score is only valid at 24 h of ICU admission. They also emphasized that an ICU patient whose condition failed to improve day after day, was in fact deteriorating and had an increasing risk of death. This well recognized clinical phenomenon is accurately modeled over the first 72 h of their ICU stay by MPM II. The same could not be said for sequential APACHE II scoring. The authors described an on-going process to develop MPM II models for successive time points beyond 72 h. (MPM II OT). Figure 5 shows the calculator of the MPM.
Data-base still continues about the accuracy of scoring systems, their efficiency in assessing the severity of illness, and whether they have a prognostic role in the estimation of illness outcome. Additionally, these tools have to be validated in the population in question before they are adopted for outcome prediction and decision-making[53].
The most important potential limitation of scoring systems is the inappropriate interpretation of the score. Clinicians must be aware that the probability of in-hospital mortality based on a particular score relates to a similar group of patients and not to an individual patient. This is important to understand before attempting to use scoring systems in clinical practice. So, although it can be useful to know the predicted mortality of a group of patients with a similar score, we cannot be sure which patients will die and which will survive. A well calibrated model, applied to an individual patient, may for example predict a hospital mortality of 46% for this patient, which just means that for a group of 100 patients with a similar severity of illness, 46 patients are predicted to die, but it makes no statement if the individual patient is included in the 46% who will die or in the 54% that will survive. Consequently, scoring systems should not be used to make predictions for individual cases. Conversely, scoring systems can appropriately be used to assist the clinical decision making as they do allow an objective assessment of a patient’s severity of illness, and therefore reflect the likelihood of mortality in a similar cohort of patients. Overall, they should be considered as a fact to assist the clinician.
It is now about 30 years since the original APACHE study was published. Tens of thousands of patients have been studied and mortality prediction models and prognostic categorization morbidity models developed on universal ICU critically ill patients.
Severity scoring systems are usually designed to predict morbidity or mortality in critically- ill patients. Examples of general scoring systems are APACHE III; SAPS II and MPM II. Examples of organ dysfunction scoring systems are MODS, SOFA and LODS. Examples of specific severity scoring systems include Acute Pancreatitis and Acute Lung Injury scores. Biological scores include measurements of serum lactate and PHi. Examples of overtime or dynamic severity scoring systems are APACHE III, MPM24-72 and intermediate TISS.
Because general severity scoring systems are developed and validated using admission data from large ICU populations, they are most fitted to predict mortality for groups of ICU patients rather than predicting mortality for individual patients. They are used for determining ICU proficiency (in quality assurance) and treatment efficacy (in clinical practice). Decisions regarding ICU triage are often more dependent on values than probabilities and so, these systems should not determine the utility or futility of ICU for individuals.
Even if a severity scoring index could perfectly predict the mortality of a septic patient from admission data, one should be cautious, because death cannot actually be predicted except just before its occurrence and by that time, there would be little to be gained. By contrast, early prediction of death might be more useful to design patient management. It would be likely to be associated with a greater risk of a false positive result.
Outcome estimates may influence the clinical management. The clinical awareness of the treating physician of a poor outcome for his/her patient may tempt him/her to give less than optimal therapy or to prevent ventilating him or even to withdraw active therapy. To date, however, it is almost impossible to find documented evidence of change in medical practice that have resulted from application of different prognostic scoring systems[21]. There is clearly no “best” severity scoring model, and the performance of such models varies both with time and with the population under study, and so should be periodically addressed. For this, severity scoring systems should be used in conjunction with sequential patient clinical interpretation and clinical assessment of tissue hypoxia for prognostic categorization of critically-ill patients in general and septic patients in particular.
Peer reviewer: Yusuf Kenan Coban, MD, Associated Professor, Burn Unit, Plastic Reconstructive and Aesthetic Surgery Department, Medical Faculty, Inonu Üniversity, Malatya 44280, Turkey
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6211] [Cited by in RCA: 6522] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 2. | Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1047] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 3. | Le Gall JR, Lemeshow S, Leleu G, Klar J, Huillard J, Rué M, Teres D, Artigas A. Customized probability models for early severe sepsis in adult intensive care patients. Intensive Care Unit Scoring Group. JAMA. 1995;273:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 103] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638-1652. [PubMed] |
| 5. | Webster NR. Activated protein C in sepsis. AJAIC. 2001;41-42. |
| 6. | Beal AL, Cerra FB. Multiple organ failure syndrome in the 1990s. Systemic inflammatory response and organ dysfunction. JAMA. 1994;271:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 316] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1330] [Cited by in RCA: 1053] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 8. | Edwards JD, Brown GC, Nightingale P, Slater RM, Faragher EB. Use of survivors' cardiorespiratory values as therapeutic goals in septic shock. Crit Care Med. 1989;17:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 147] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Russell JA, Phang PT. The oxygen delivery/consumption controversy. Approaches to management of the critically ill. Am J Respir Crit Care Med. 1994;149:533-537. [PubMed] |
| 10. | Moemen ME, Omran SA, Rifky MA, Hasan AAS. Tissue oxygenation guided resuscitation in patients with sepsis. Thesis in Anaesthesia and Intensive Care, Faculty of medicine, Zagazig university, Egypt. 2001;. |
| 11. | Dahaba AA, Elawady GA, Rehak PH, List WF. Procalcitonin and proinflammatory cytokine clearance during continuous venovenous haemofiltration in septic patients. Anaesth Intensive Care. 2002;30:269-274. [PubMed] |
| 12. | Montón C, Torres A, El-Ebiary M, Filella X, Xaubet A, de la Bellacasa JP. Cytokine expression in severe pneumonia: a bronchoalveolar lavage study. Crit Care Med. 1999;27:1745-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Wakefield CH, Barclay GR, Fearon KC, Goldie AS, Ross JA, Grant IS, Ramsay G, Howie JC. Proinflammatory mediator activity, endogenous antagonists and the systemic inflammatory response in intra-abdominal sepsis. Scottish Sepsis Intervention Group. Br J Surg. 1998;85:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771-778. [PubMed] |
| 15. | Nylen ES, Whang KT, Snider RH, Steinwald PM, White JC, Becker KL. Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit Care Med. 1998;26:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 211] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1329] [Cited by in RCA: 1360] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 17. | de Werra I, Jaccard C, Corradin SB, Chioléro R, Yersin B, Gallati H, Assicot M, Bohuon C, Baumgartner JD, Glauser MP. Cytokines, nitrite/nitrate, soluble tumor necrosis factor receptors, and procalcitonin concentrations: comparisons in patients with septic shock, cardiogenic shock, and bacterial pneumonia. Crit Care Med. 1997;25:607-613. [PubMed] |
| 18. | Nijsten MW, Olinga P, The TH, de Vries EG, Koops HS, Groothuis GM, Limburg PC, ten Duis HJ, Moshage H, Hoekstra HJ. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med. 2000;28:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 192] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Ugarte H, Silva E, Mercan D, De Mendonça A, Vincent JL. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999;27:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 264] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Fathia HK, Mokhtar MS, Ragab FA, Rizk AF. Role of decisive markers in diagnosis and outcome of patients with septic shock. Thesis in critical care medicine, Faculty of medicine, Cairo University, Egypt. 2000;. |
| 21. | Moemen ME. Prognostic categorization in systemic sepsis. Eg J Anaesth. 2003;19: 183-194. |
| 22. | Salzman AL, Strong KE, Wang H, Wollert PS, Vandermeer TJ, Fink MP. Intraluminal "balloonless" air tonometry: a new method for determination of gastrointestinal mucosal carbon dioxide tension. Crit Care Med. 1994;22:126-134. [PubMed] |
| 23. | Mohsenifar Z, Hay A, Hay J, Lewis MI, Koerner SK. Gastric intramural pH as a predictor of success or failure in weaning patients from mechanical ventilation. Ann Intern Med. 1993;119:794-798. [PubMed] |
| 24. | Corke CF, Prisco G, Gizycki P, Selvakumaran A. A simple method for frequent monitoring of gastric carbon dioxide. Anaesth Intensive Care. 1996;24:590-593. [PubMed] |
| 25. | Gutierrez G, Palizas F, Doglio G, Wainsztein N, Gallesio A, Pacin J, Dubin A, Schiavi E, Jorge M, Pusajo J. Gastric intramucosal pH as a therapeutic index of tissue oxygenation in critically ill patients. Lancet. 1992;339:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 493] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 26. | Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 424] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 27. | Vincent JL, Ferreira F, Moreno R. Scoring systems for assessing organ dysfunction and survival. Crit Care Clin. 2000;16:353-366. [PubMed] |
| 28. | Cook R, Cook D, Tilley J, Lee K, Marshall J. Multiple organ dysfunction: baseline and serial component scores. Crit Care Med. 2001;29:2046-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Jacobs S, Zuleika M, Mphansa T. The Multiple Organ Dysfunction Score as a descriptor of patient outcome in septic shock compared with two other scoring systems. Crit Care Med. 1999;27:741-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2134] [Cited by in RCA: 2402] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 31. | Antonelli M, Moreno R, Vincent JL, Sprung CL, Mendoça A, Passariello M, Riccioni L, Osborn J. Application of SOFA score to trauma patients. Sequential Organ Failure Assessment. Intensive Care Med. 1999;25:389-394. [PubMed] |
| 32. | Oda S, Hirasawa H, Sugai T, Shiga H, Nakanishi K, Kitamura N, Sadahiro T, Hirano T. Comparison of Sepsis-related Organ Failure Assessment (SOFA) score and CIS (cellular injury score) for scoring of severity for patients with multiple organ dysfunction syndrome (MODS). Intensive Care Med. 2000;26:1786-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1597] [Cited by in RCA: 1827] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 34. | Moreno R, Miranda DR, Matos R, Fevereiro T. Mortality after discharge from intensive care: the impact of organ system failure and nursing workload use at discharge. Intensive Care Med. 2001;27:999-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Le Gall JR, Klar J, Lemeshow S, Saulnier F, Alberti C, Artigas A, Teres D. The Logistic Organ Dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA. 1996;276:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 436] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 36. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10902] [Cited by in RCA: 11135] [Article Influence: 278.4] [Reference Citation Analysis (0)] |
| 37. | Chang RW. Individual outcome prediction models for intensive care units. Lancet. 1989;2:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2591] [Cited by in RCA: 2719] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 39. | Beck DH, Taylor BL, Millar B, Smith GB. Prediction of outcome from intensive care: a prospective cohort study comparing Acute Physiology and Chronic Health Evaluation II and III prognostic systems in a United Kingdom intensive care unit. Crit Care Med. 1997;25:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Pappachan JV, Millar B, Bennett ED, Smith GB. Comparison of outcome from intensive care admission after adjustment for case mix by the APACHE III prognostic system. Chest. 1999;115:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Livingston BM, MacKirdy FN, Howie JC, Jones R, Norrie JD. Assessment of the performance of five intensive care scoring models within a large Scottish database. Crit Care Med. 2000;28:1820-1827. [PubMed] |
| 42. | Zimmerman JE, Wagner DP, Draper EA, Wright L, Alzola C, Knaus WA. Evaluation of acute physiology and chronic health evaluation III predictions of hospital mortality in an independent database. Crit Care Med. 1998;26:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 177] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 43. | Sirio CA, Shepardson LB, Rotondi AJ, Cooper GS, Angus DC, Harper DL, Rosenthal GE. Community-wide assessment of intensive care outcomes using a physiologically based prognostic measure: implications for critical care delivery from Cleveland Health Quality Choice. Chest. 1999;115:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34:1297-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 45. | Cullen DJ, Civetta JM, Briggs BA, Ferrara LC. Therapeutic intervention scoring system: a method for quantitative comparison of patient care. Crit Care Med. 1974;2:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 474] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 46. | Le Gall JR, Loirat P, Alperovitch A, Glaser P, Granthil C, Mathieu D, Mercier P, Thomas R, Villers D. A simplified acute physiology score for ICU patients. Crit Care Med. 1984;12:975-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 763] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 47. | Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4314] [Cited by in RCA: 4389] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 48. | Strand K, Søreide E, Aardal S, Flaatten H. A comparison of SAPS II and SAPS 3 in a Norwegian intensive care unit population. Acta Anaesthesiol Scand. 2009;53:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Metnitz PG, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR. SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med. 2005;31:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 467] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 50. | Lemeshow S, Teres D, Pastides H, Avrunin JS, Steingrub JS. A method for predicting survival and mortality of ICU patients using objectively derived weights. Crit Care Med. 1985;13:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 194] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Lemeshow S, Teres D, Klar J, Avrunin JS, Gehlbach SH, Rapoport J. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. JAMA. 1993;270:2478-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 557] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 52. | Lemeshow S, Klar J, Teres D. Outcome prediction for individual intensive care patients: useful, misused, or abused. Intensive Care Med. 1995;21:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 115] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Lemeshow S, Le Gall JR. Modeling the severity of illness of ICU patients. A systems update. JAMA. 1994;272:1049-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 178] [Article Influence: 5.7] [Reference Citation Analysis (2)] |