Published online Jun 4, 2012. doi: 10.5492/wjccm.v1.i3.61
Revised: October 10, 2011
Accepted: May 25, 2012
Published online: June 4, 2012
Recent advances in the detection of acute kidney injury (AKI) afford the possibility of early intervention. Proteomics and genomics have identified many markers of tubular cell injury, some of which are manifest in the urine. One trial has used novel injury biomarkers to recruit patients to an intervention prior to an elevation in plasma creatinine. This trial and other recent studies have shown that the use of biomarkers of injury will depend on the time the patient presents following insult to the kidney, the likely cause of that insult, and the pre-injury renal function of that patient. The definition of AKI is likely to change in the near future to include a measure of injury. We anticipate novel therapies becoming available following successful trials that utilize the methodology of early intervention following an elevated injury biomarker.
- Citation: Pickering JW, Endre ZH. Challenges facing early detection of acute kidney injury in the critically ill. World J Crit Care Med 2012; 1(3): 61-66
- URL: https://www.wjgnet.com/2220-3141/full/v1/i3/61.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v1.i3.61
Critical care medicine has responded impressively over the last few decades to the challenges of acute lung injury, cardiac arrest, and sepsis, but not so well to the challenge of acute kidney injury (AKI). Recent consensus definitions have helped establish the incidence to be about 35% to 70% in the intensive care unit (ICU)[1,2], yet other than renal replacement therapy (RRT) there are no established treatments.
AKI has several etiologies including renal ischemia, nephrotoxic injury, and AKI complicating sepsis, which complicate detection and treatment. Almost inevitably diagnosis is only at the late stages of the disease, or retrospectively. This is because of the reliance on serum creatinine as a marker of glomerular filtration rate (GFR).
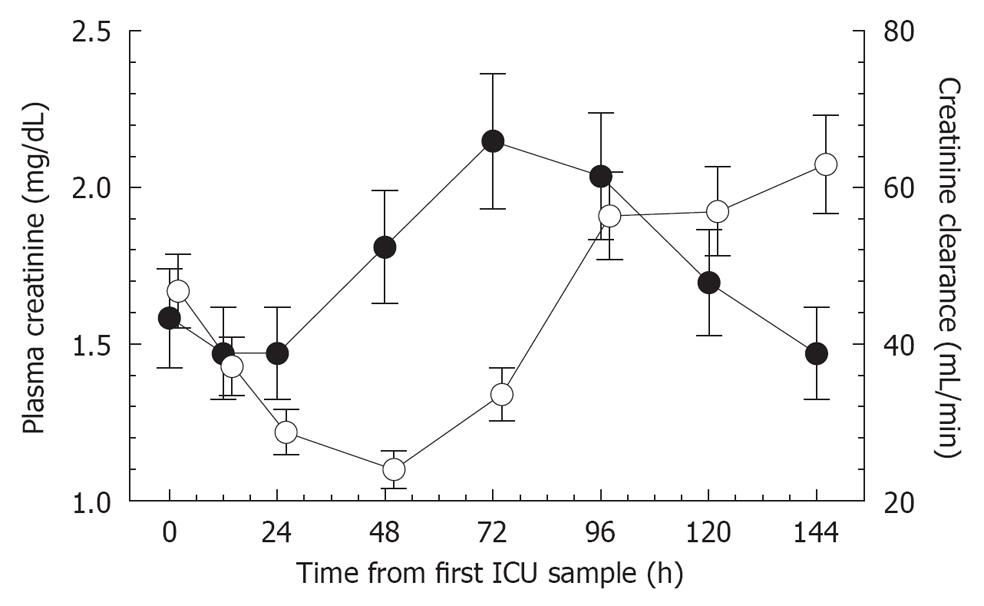
Creatinine is formed from creatine in the muscles, at a constant rate, and with a molecular weight of 113 Da is freely filtered at the glomerulus. When kidney function is normal the rate of production of creatinine is matched by the rate of renal excretion. If GFR decreases, plasma creatinine slowly increases to a new steady state concentration that reflects the new GFR. At normal GFR the half-life of creatinine is about 4 h. This increases as GFR decreases, thus only after 24-72 h (3 to 5 half lives with lower GFR) will a new steady state concentration be reached. The current consensus definition of AKI [risk, injury, failure, loss, end-stage (RIFLE)[3]], of AKI requires at least a 50% increase in plasma creatinine. This results from at least a 33.3% rapid decline in GFR[4]. The delay in a measurable increase in plasma creatinine is further exacerbated by accuracy of plasma creatinine measurements; nephrologists typically look for changes of at least 10% before accepting these. Additionally, the practice of fluid administration in the ICU dilutes creatinine concentration[5]. Figure 1 illustrates a delayed increase in plasma creatinine following a decrease in GFR (estimated by a 4-h creatinine clearance) of a patient from the EARLYARF trial[6].
Inevitably, delayed diagnosis means delayed treatment. In the case of AKI the clinician’s options are limited. They may minimize harm by withdrawing nephrotoxins, and may attempt to replace or increase circulating volume by fluid-loading, or, in worst case, initiate RRT. Recent evidence suggests that fluid-loading (rather than a neutral fluid-balance) may be detrimental rather than supportive, potentially reducing options further[7]. Delayed diagnosis may also contribute to the failure of many interventions that were promising under experimental conditions in animals[8].
Recent advances in proteomics and genomics have breathed new life into the quest for successful treatment of AKI. The search for specific and sensitive injury biomarkers has become a global focus of the nephrology and critical care communities. The paradigm is quite simple; following insult to the kidney a molecule is released into the urine or plasma where it is detected and treatment initiated before, or soon after a decrease in GFR. There has been considerable success in identifying candidate biomarkers[9-12]. In this review we explore whether these new biomarkers will supplant or merely support creatinine in the ICU.
The patient undergoes an immediate decline in GFR (4-h-creatinine clearance; open circles) of 38% in 24 h. The increase in plasma creatinine (closed circles) is not detectable until 48 h and peaks as a 46% increase only at 72 h. The subsequent decline in plasma creatinine suggestive of recovery is also delayed compared with the increase in creatinine clearance 24 h earlier. Error bars are ± 10% indicating measurement uncertainty (Figure 1).
The EARLYARF study was the first trial to employ kidney injury biomarkers of AKI to recruit patients to the intervention arm of a randomized control trial of a novel intervention[6]. Described as a “glimpse of the future”[13], this trial illustrates the challenges faced by this new paradigm.
On entry to the ICU, at 12, 24 h, and then daily for 7 d the urine of at risk patients were monitored for elevated concentrations of the brush border enzymes alkaline phosphatase (AP) and γ-glutamyl-transpeptidase (GGT). These biomarkers were chosen on the basis of a pilot study which had shown them to be highly sensitive and specific for AKI[10] and because they could be measured in a hospital diagnostic lab with rapid turnaround. Since the inception of the EARLYARF trial in 2005 a number of other biomarkers, discussed below, have proven to hold out greater promise as early injury biomarkers, some of which have now entered commercial production and could be used for future early intervention trials. AP and GGT were normalized to urinary creatinine concentration to account for variation in water reabsorption and, in order to avoid false positives, the product GGT × AP > 46.3 was used to recruit patients to an intervention of either two doses of high dose erythropoietin (500 U/kg) or placebo (normal saline) 24 h apart. Erythropoietin was chosen for its anti-apoptotic property and following success in animal studies in ischaemic/repurfusion injury[14,15]. The primary outcome was the difference in the mean relative average value of creatinine (RAVC) of the two groups. The RAVC is the average plasma creatinine increase from baseline as a percentage of baseline creatinine[16]. The difference in the mean RAVC between control and treatment groups is more sensitive to small differences in renal function than a categorical marker such as RIFLE[16,17].
The EARLYARF trial did not show Erythropoietin to be an effective early intervention in AKI, however, it did not preclude this possibility. This is because of the limited utility shown by GGT × AP as a recruitment tool. Whilst GGT × AP > 46.3 did select patients with more severe illness and at greater risk of AKI, needing RRT, and death from the general ICU population there was still a considerable risk of AKI in those not triaged. Analysis of the time profile of GGT × AP taken from a putative time of insult (determined retrospectively) showed that GGT × AP is most likely to be elevated in the first 12 h following insult. For many patients entering the ICU the putative time of insult was more than 12 h earlier, particularly in the case of progressive diseases such as sepsis. An analysis of the timing of the first dose of the study drug showed that it was administered a median 12.9 h following putative insult, outside the experimentally determined optimal treatment window of within 6 h of injury for erythropoietin[14]. Thus, the first lesson is that injury biomarkers have a temporal window of opportunity following injury in which they are diagnostic. If the time from insult is unknown a negative biomarker is not necessarily indicative of no-injury or no change in function. Given the relative short duration of elevation of some of these biomarkers the second lesson is that repeated measures of biomarkers about 3-6 h apart will be necessary to avoid false negatives by missing the temporal window of opportunity.
In addition to AP and GGT four other urinary injury biomarkers were measured, namely: kidney injury molecule-1, neutrophil-gelatinase-associated-lipocalin (NGAL), interleukin-18 and cystatin C (CysC). Each demonstrated a unique temporal profile[18]. Furthermore, as had been demonstrated with NGAL[19], the diagnostic performance was shown to depend on the underlying baseline (normal) renal function. Optimal diagnostic ability for each biomarker depended on the combination of both time from insult and pre-injury renal function. For example, CysC was diagnostic of AKI when measured 6 to 12 h from insult in those with estimated baseline GFR (eGFR) of 90 to 120 mL/min with an area under the receiver operator curve, AUC, of 0.89 (95% CI: 0.70-1), but was not diagnostic of those with lower eGFR during the same time period. The third lesson is that biomarkers must be chosen according to each patient’s pre-injury renal function.
There have been a proliferation of studies identifying potential AKI biomarkers in addition to those already described, including liver-fatty acid binding protein[20], albumin[21], netrin[22], α- and π-glutathione-S-transferase[10,23], and β2-microglobulin[24]. There are several recent reviews which cover the potential of several biomarkers to be early markers of AKI and describe their pathophysiology[11,12,25,26]. The most studied of biomarkers is plasma and urinary NGAL. A meta-analysis of 19 clinical studies involving more than 2500 patients resulted in an overall AUC of 0.82 (0.73-0.89) for diagnosis of AKI. The AUC in critically ill patients was lower, 0.73 (0.62-0.83)[27]. The AUC for prediction of RRT was 0.78 (0.65-0.92). This performance is good without being spectacular, however, as the authors report, it is similar to the AUC range for troponin detection of myocardial infraction during its clinical implementation.
AKI injury biomarkers have been assessed almost exclusively on the basis of their ability to detect or predict a rise in plasma creatinine. This injury-function method is potentially misleading. It assumes that a change in function that results in an observable change in plasma creatinine is more important than an increase in injury biomarkers per-se. This remains to be seen. The subcategory of biomarker positive/creatinine negative patients has received scant attention. Only one study has addressed this directly. Haase et al[28] analysed plasma and urinary NGAL data from 10 studies and concluded that NGAL-positive/creatinine-negative patients were more likely to require RRT, more likely to die in hospital and had longer lengths of ICU and hospital stay than NGAL-negative/creatinine-negative patients. This illustrates the potential for an injury biomarker to stand alone from creatinine as a marker of AKI. In this study patients with both an elevated NGAL and elevated creatinine were more likely to require RRT or die in hospital than those with only biomarker elevated. The fourth lesson is that future diagnosis will involve biomarkers of both injury and function. Before this goal can be realized appropriate cutpoints for the various AKI injury biomarkers must be determined.
Injury biomarkers may loosely be classified as pre-formed, such as brush border-enzymes AP and GGT, or induced (upregulated) through some injury mechanism, such as KIM-1 from tubular epithelial cells during the process of dedifferentiation and re-proliferation[29,30]. Biomarkers pre-formed in the plasma (e.g., CysC and albumin) or absorbed into the plasma following tubular injury (e.g., NGAL) may also be present in the urine due to failure of the tubular transport mechanisms to reabsorb them from the tubular fluid[31]. Potentially, an improved understanding of disease pathways may lead to utilization of biomarkers according to suspected cause of injury rather than the one biomarker fits all approach of the EARLYARF trial. The recent work of the Predictive Safety Testing Consortium on pre-clinical nephrotoxic biomarkers is revealing in this respect, as not all biomarkers responded to all nephrotoxins. For example, urinary CysC and β2-microglobulin were elevated for Purcomycin and Doxorubin but not Cisplatin or Gentamicin whereas Clusterin was elevated for all these toxins[32]. The fifth lesson is that a panel of biomarkers that respond to the range of possible different AKI causes of patients entering intensive care will be required to capture all cases.
We have argued that the removal of a change in GFR (leaving only a change in the surrogate plasma creatinine) from the AKIN consensus definition of AKI was a mistake[33,34]. Short duration creatinine clearance potentially provides an earlier indicator of GFR than plasma creatinine (Figure 1) and new technologies are under development which may lead to a direct, near real-time, measure of GFR. We have reviewed these recently[30]. For example, a device attached to a patient’s arm, the ambulatory renal monitor (ARM) measures the decay of 99mTc-DTPA for up to 24 h following a single injection. A change in GFR could be detected within 5-10 min[35,36]. A second technique involves two fluorescent markers, one cleared by the kidney and one not. The ratio between these markers when observed with imaging of blood vessels in the skin of rats provided a measure of GFR[37,38]. The sixth lesson is that rapid measures of GFR may provide an adjunct to injury biomarkers for the early detection of AKI.
Plasma CysC has been proposed as an alternative surrogate of GFR to plasma creatinine. CysC is a low molecular weight protein, 13.3 kDa, produced at a constant rate and freely filtered through the glomerulus[39]. Unlike creatinine it is reabsorbed in the proximal tubule by megalin-facilitated endocytosis[31]. CysC has one-third the volume of distribution of creatinine meaning that any loss of renal function will be reflected by a more rapid rise in plasma CysC than plasma creatinine. Herget-Rosenthal et al[40] first demonstrated the potential of plasma CysC in the ICU. In a group of 44 patients the mean time to an increase of 50% in CysC was 1.5 ± 0.6 d earlier than the mean time to an increase of 50% in creatinine. In the larger EARLYARF trial the difference in time for each individual was calculated resulting in a mean difference of 5.8 ± 13 h[41]. While these differences appear modest, sampling was still 12 to 24 h apart and investigations with more frequent measurement will determine if CysC may detect loss of GFR on shorter time scales. Because it is likely to respond to changes in GFR more quickly than creatinine, sampling of CysC is likely to need to be more frequent to observe these changes. CysC also has the advantage that it is less influenced by muscle mass (and hence, sex and age) than plasma creatinine, however it is influenced by thyroid dysfunction, some cancers and glucocorticoids[42-44]. The assumption of a constant production rate of CysC has yet to be investigated in the critically ill. Any switch to CysC would involve considerable expense, partly because of the more complex assay methods and also because to be effective it would need to become widely measured outside of the ICU as well. The final lesson is that plasma CysC has the potential to supplant creatinine as a surrogate measure of renal function, although there is insufficient evidence to justify the expenditure of replacing creatinine.
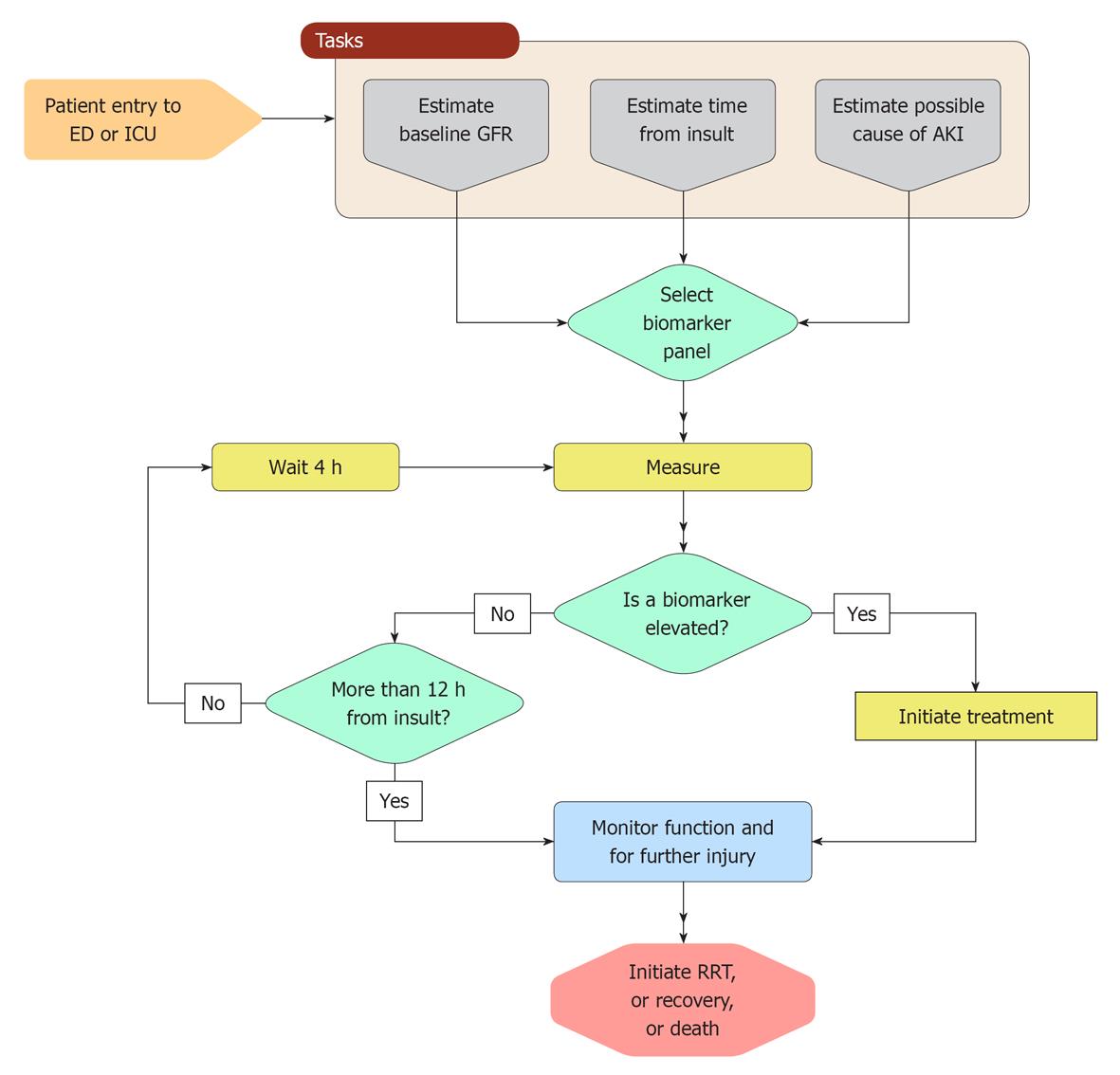
AKI will be diagnosed using multiple definitions incorporating injury biomarkers, functional measurement, and surrogates of function (Table 1). The earliest diagnosis will be by either an elevated injury biomarker or a change in a functional measure of GFR. Injury biomarkers will be measured on arrival in an emergency department of ICU. One elevated biomarker (e.g., 2 times above the normal upper limit[45]) may be sufficient to diagnose AKI. However, the absence of an elevated injury biomarker would not exclude the possibility of AKI because of the possibility that the time between the injury and the measurement is outside of the window of opportunity for that biomarker. As with the use of troponins to monitor for myocardial infarction, repeated measurements of AKI injury biomarkers a few hours apart will be necessary. A measurement of function, either through a brief creatinine clearance, possible now, or a device such as the ARM monitor in the future, may be used to aid diagnosis. However, definitive diagnosis will only be possible if a baseline GFR has been measured (e.g., prior to surgery) or can be estimated with reasonable accuracy. For many critically ill patients the value of monitoring function will be as an early indication of hospital acquired AKI and will continue to be important for deciding when to initiate RRT. Urine output will continue to be used, although recent evidence suggests that it is often associated with physiological changes other than AKI[46]. Surrogate markers of function, particularly creatinine, which are the current primary tool for diagnosing AKI will still have a place, but because they are a very much later diagnosis their value will be limited to when the window for the particular injury biomarkers has been missed, and when the first plasma surrogate measurement is within the normal range. Increases in the plasma surrogate would suggest an earlier loss of function. Figure 2 is a possible algorithm for implementation of injury biomarker of AKI in the ICU.
| AKI diagnosis | Timing | Staging | Examples |
| Injury biomarkers | Within 0-12 h of injury and every 3 to 6 h | Cutpoints of concentrations | Urinary NGAL, cystain C, IL-18, KIM-1, GGT, L-FABP, Plasma NGAL |
| Functional measurements | Any time following injury | Change from a baseline | 4 h creatinine clearance, ARM urine output |
| Surrogates of function | 12 h to 7 d post injury | Change from a baseline | Plasma creatinine, plasma cystatin C |
Novel injury biomarkers will soon be incorporated into the definition of AKI alongside current surrogates of renal function. These biomarkers will change practice in the ICU once efficacious early intervention treatments are discovered. These will require randomized control trials which utilize the trial methodology of the EARLYARF trial, namely recruitment following elevation of an injury biomarker. Plasma creatinine will continue to play a role as a functional marker until replaced by a more responsive functional marker such as plasma CysC or a rapid measure of GFR.
Peer reviewers: Dr. Athanasios Marinis, First Department of Surgery, Tzaneion General Hospital, 54 Dimokritou, 13671 Athens, Greece; Huang-Xian Ju, Professor, State Key Laboratory of Analytical Chemistry for Life Science, Nanjing University, Nanjing 210093, China
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 531] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 3. | Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4448] [Cited by in RCA: 4702] [Article Influence: 223.9] [Reference Citation Analysis (0)] |
| 4. | Pickering JW, Endre ZH. GFR shot by RIFLE: errors in staging acute kidney injury. Lancet. 2009;373:1318-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Macedo E, Bouchard J, Soroko SH, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14:R82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 6. | Endre ZH, Walker RJ, Pickering JW, Shaw GM, Frampton CM, Henderson SJ, Hutchison R, Mehrtens JE, Robinson JM, Schollum JB. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial). Kidney Int. 2010;77:1020-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 7. | Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6:966-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 8. | Pickering JW, Ralib AM, Nejat M, Endre ZH. New considerations in the design of clinical trials of acute kidney injury. Clin Investig (Lond). 2011;1:637-650. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1694] [Cited by in RCA: 1696] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 10. | Westhuyzen J, Endre ZH, Reece G, Reith DM, Saltissi D, Morgan TJ. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol Dial Transplant. 2003;18:543-551. [PubMed] |
| 11. | Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 474] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 12. | Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 496] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 13. | Mehta RL. Timed and targeted therapy for acute kidney injury: a glimpse of the future. Kidney Int. 2010;77:947-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Johnson DW, Pat B, Vesey DA, Guan Z, Endre Z, Gobe GC. Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int. 2006;69:1806-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Vesey DA, Cheung C, Pat B, Endre Z, Gobé G, Johnson DW. Erythropoietin protects against ischaemic acute renal injury. Nephrol Dial Transplant. 2004;19:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Pickering JW, Frampton CM, Endre ZH. Evaluation of trial outcomes in acute kidney injury by creatinine modeling. Clin J Am Soc Nephrol. 2009;4:1705-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol. 2011;22:810-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, Frampton CM, Bennett MR, Ma Q, Sabbisetti VS. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011;79:1119-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 21. | Bolisetty S, Agarwal A. Urine albumin as a biomarker in acute kidney injury. Am J Physiol Renal Physiol. 2011;300:F626-F627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Ramesh G, Krawczeski CD, Woo JG, Wang Y, Devarajan P. Urinary netrin-1 is an early predictive biomarker of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2010;5:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O'Connor MF, Devarajan P. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5:2154-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 24. | Häring N, Mähr HS, Mündle M, Strohal R, Lhotta K. Early detection of renal damage caused by fumaric acid ester therapy by determination of urinary β2-microglobulin. Br J Dermatol. 2011;164:648-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245:182-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 26. | Endre ZH, Westhuyzen J. Early detection of acute kidney injury: emerging new biomarkers. Nephrology (Carlton). 2008;13:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 912] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 28. | Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 509] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 29. | Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 449] [Cited by in RCA: 400] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 30. | Endre ZH, Pickering JW, Walker RJ. Clearance and beyond: the complementary roles of GFR measurement and injury biomarkers in acute kidney injury (AKI). Am J Physiol Renal Physiol. 2011;301:F697-F707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Nejat M, Hill JV, Pickering JW, Edelstein CL, Devarajan P, Endre ZH. Albuminuria increases cystatin C excretion: implications for urinary biomarkers. Nephrol Dial Transplant. 2011;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl A. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 33. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 4989] [Article Influence: 277.2] [Reference Citation Analysis (0)] |
| 34. | Pickering JW, Endre ZH. RIFLE and AKIN--maintain the momentum and the GFR! Crit Care. 2009;13:416; author reply 416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Rabito C, Halpern EF, Scott J, Tolkoff-Rubin N. Accurate, fast, and convenient measurement of glomerular filtration rate in potential renal transplant donors. Transplantation. 2010;90:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Rabito CA, Panico F, Rubin R, Tolkoff-Rubin N, Teplick R. Noninvasive, real-time monitoring of renal function during critical care. J Am Soc Nephrol. 1994;4:1421-1428. [PubMed] |
| 37. | Yu W, Sandoval RM, Molitoris BA. Rapid determination of renal filtration function using an optical ratiometric imaging approach. Am J Physiol Renal Physiol. 2007;292:F1873-F1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Wang E, Sandoval RM, Campos SB, Molitoris BA. Rapid diagnosis and quantification of acute kidney injury using fluorescent ratio-metric determination of glomerular filtration rate in the rat. Am J Physiol Renal Physiol. 2010;299:F1048-F1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Grubb A. Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol. 1992;38 Suppl 1:S20-S27. [PubMed] |
| 40. | Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, Philipp T, Kribben A. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 550] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 41. | Nejat M, Pickering JW, Walker RJ, Endre ZH. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant. 2010;25:3283-3289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 42. | Fricker M, Wiesli P, Brändle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63:1944-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 260] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 43. | Wegiel B, Jiborn T, Abrahamson M, Helczynski L, Otterbein L, Persson JL, Bjartell A. Cystatin C is downregulated in prostate cancer and modulates invasion of prostate cancer cells via MAPK/Erk and androgen receptor pathways. PLoS One. 2009;4:e7953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47:2055-2059. [PubMed] |
| 45. | Murugan R, Kellum JA. Acute kidney injury: what's the prognosis. Nat Rev Nephrol. 2011;7:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 311] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 46. | Prowle JR, Liu YL, Licari E, Bagshaw SM, Egi M, Haase M, Haase-Fielitz A, Kellum JA, Cruz D, Ronco C. Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care. 2011;15:R172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |