BACKGROUND
In 1925, Dr. Felix Mandl performed the first excision of a parathyroid tumor in Vienna on patient Albert Jahne, a 34-year-old tramcar conductor suffering from osteitis fibrosa cystica who was admitted for a femur fracture[1]. Although he initially experienced a benefit from the parathyroidectomy, Jahne subsequently developed recurrent disease, possibly due to parathyroid carcinoma. He underwent reoperation in 1933, but ultimately died of uremia three years after this second surgical exploration[1]. Despite failing to achieve the desired clinical outcome, Jahne’s case shifted the practice dogma towards surgery as the management of choice for primary hyperparathyroidism (pHPT). For most of the 19th century, the surgical treatment of pHPT was based on locating the four parathyroid glands intraoperatively and the excision of any grossly enlarged parathyroid glands while leaving all normal-sized glands in situ[2,3]. This qualitative approach that requires bilateral neck exploration (BNE) can be problematic, however, since parathyroid gland size and/or color does not always directly correlate to its secretory function[4,5]. If hypersecreting gland(s) are left behind, hypercalcemia will persist. Conversely, if all normal parathyroid glands are excised or their blood supply compromised during extensive BNE, postoperative hypocalcemia and tetany may occur. Today, when performed by experienced endocrine surgeons, BNE yields success rates of 95% to 99%[2,3].
With the advent of preoperative imaging modalities for the localization of hyperfunctioning glands, targeted or focused parathyroidectomy guided by intraoperative parathormone monitoring (IPM) is currently the standard treatment for patients with pHPT at numerous specialized centers both nationally and internationally[6-12]. This focused approach incorporates the common aspects of minimally invasive surgery resulting in limited surgical exploration, reduced operative time and less morbidity for patients with pHPT while maintaining comparable operative success rates to traditional BNE which ranges from 97% to 99%[6-10]. In general, focused parathyroidectomy is performed by creating a transverse cervical incision along the anterior neck which measures from 2 to 4 cm in those patients with one hyperfunctioning parathyroid gland identified by preoperative localization studies, sestamibi (MIBI) and/or ultrasound. When the offending parathyroid gland(s) is excised, an intraoperative parathormone (PTH) assay is used to confirm that there is no remaining hyperfunctioning tissue. When IPM levels drop by > 50%, usually at 10 min following abnormal parathyroid gland removal, the operation is concluded[13]. Focused parathyroidectomy guided by IPM can be achieved with either general or local anesthesia and can be performed in an ambulatory setting.
THE MIAMI CRITERION
In 1990, Irvin et al[14] refined and applied the intraoperative PTH immunoradiometric assay for the surgical management of pHPT after an unsuccessful parathyroid operation. His patient, who was the supervisor of the operating rooms, at the University of Miami/Jackson Memorial Hospital, had pHPT, and she approached Irvin to perform the operation. She underwent traditional BNE during which one large parathyroid gland was excised, and a second contralateral parathyroid gland was biopsied and preserved. Postoperatively, however, her serum calcium failed to normalize. Irvin spent the next 4 mo refining an intraoperative PTH assay to allow for results to be obtained within 15 min. He then took her back to the operating room and, by measuring intact PTH levels intraoperatively, was able to confirm removal of any remaining hyperfunctioning parathyroid glands and predict curative resection in this reoperative patient who had an intrathyroidal parathyroid gland in the contralateral lobe that was not appreciated in her initial operation[14].
In 1991, Irvin et al[15] would begin using IPM as a routine adjunct to focused parathyroidectomy at the University of Miami to reduce failure rates due to missed multiglandular disease (MGD). Having performed over 700 parathyroidectomies at that time, he attributed his failure rate of 7% to misdiagnosis or inability to excise all hyperfunctioning parathyroid gland tissue[15]. This intraoperative adjunct often termed the “quick PTH assay” takes advantage of the half-life of PTH which is approximately 3 to 5 min. Irvin further refined the PTH assay in 1993 to address the issue of long turnaround time for PTH results, which made previous attempts at intraoperative monitoring less practical[16,17]. Since then, the intraoperative “quick PTH assay” has undergone many modifications since the original immunoradiometric assay developed by Dr. Irvin. In current practice, intraoperative PTH is measured using a rapid immunochemiluminescence assay.
With the success and practicality of the intraoperative quick PTH assay, Irvin went on to describe the Miami criterion, a protocol that uses a “> 50% PTH drop” from either the highest pre-incision or pre-excision PTH measurement in a sample taken 10 min following complete resection of the hyperfunctioning glands. Following removal of the hyperfunctioning parathyroid gland, a > 50% PTH drop at 10 min indicates removal of the abnormal parathyroid glands, predicting operative success at 6 mo[13]. As a result, IPM allows for a focused or targeted approach to parathyroidectomy that involves surgical excision of the offending gland through smaller incisions with equal curative rates of > 97% which is comparable to BNE[6-10]. The focused approach is also associated with fewer comorbidities including permanent hypoparathyroidism that may result from iatrogenic ischemia or injury to the remaining parathyroids during BNE.
At the University of Miami, the intraoperative PTH assay permits the surgeon to confirm excision of all abnormal parathyroid glands while preserving the remaining normally functioning parathyroid glands before the operation is finished; guide the surgeon to continue neck exploration for additional abnormal glands when the intraoperative PTH levels do not drop sufficiently; distinguish parathyroid from non-parathyroid tissue by measurement of intraoperative PTH levels in fine needle aspiration (FNA) samples; and lateralize hypersecreting parathyroid(s) to either side of the neck through differential jugular venous sampling when preoperative localization studies are equivocal.
IPM IN CURRENT PRACTICE
Surgeons must understand that the intraoperative PTH assay only measures the circulating amount of hormone from the location where blood samples are obtained and direct the sampling times related to the stages of the operative procedure. The “Miami criterion”, which uses a “> 50% PTH drop” from either the greatest pre-incision or pre-excision PTH measurement in a sample of blood drawn 10 min following complete resection of a hyperfunctioning gland, requires peripheral venous or arterial access for blood collection at specific times during parathyroidectomy[13,16-18]. Intravenous access is maintained with a slow saline infusion that is discarded from the line to prevent dilution before any blood sample is quantified. Intraoperatively, at least 4 mL of peripheral whole blood sample in an EDTA specimen tube is collected at the following times: (1) a “pre-incision” level prior to skin incision; (2) a “pre-excision” level collected prior to clamping the blood supply to the abnormal gland; (3) a 5-min level; and (4) 10-min level after excision of the abnormal tissue. The samples should be promptly delivered to the laboratory for processing. With the efficiency and speed of the intraoperative PTH assay, point of care testing which measures PTH at the bedside is not performed at this institution.
When the PTH levels drop > 50% from the highest pre-incision or pre-excision value 10 min following the removal of the hyperfunctioning gland, this criterion predicts normal or low calcium measurements postoperatively with an overall accuracy of 98%[13]. After this “> 50% PTH drop” occurs, the surgeon terminates the operation without further identification of the normal parathyroid glands that remain. In the event that the PTH level at 10 min does not meet this criterion, an additional level may be obtained at 20 min and/or additional neck exploration can be performed until the removal of the remaining hyperfunctioning glands is determined by > 50% PTH drop from the highest subsequent pre-excision PTH measurement[19].
INTERPRETATION OF IPM DYNAMICS
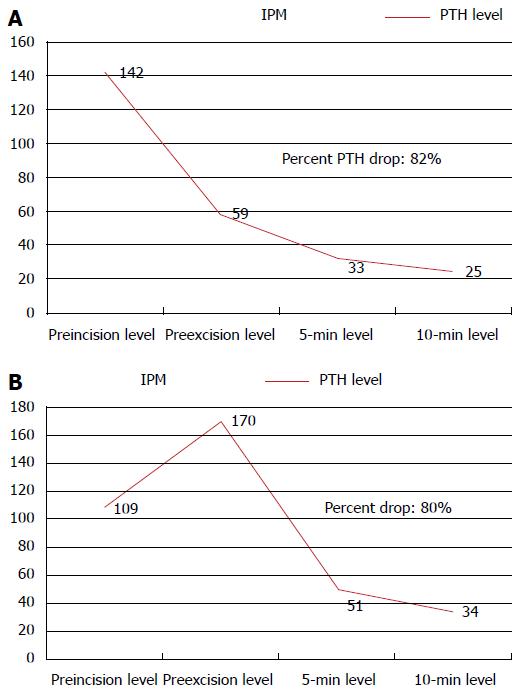
A thorough knowledge of the disease process and careful interpretation of intraoperative PTH dynamics is required to effectively guide the surgeon during parathyroidectomy. The first example is of a 58-years-old woman with biochemical evidence confirming pHPT who presented with a PTH measurement of 107 pg/mL and a calcium level of 11.1 mg/dL on routine blood testing (Figure 1A). Her Tc-99m-sestamibi and ultrasound scans were concordant and suspicious for a right inferior parathyroid gland. An abnormal right inferior parathyroid was visualized intraoperatively, and this gland was carefully removed. Intraoperative PTH levels were drawn with the following measured values: Pre-incision 142 pg/mL; pre-excision 59 pg/mL; at 5 min 33 pg/mL, and at 10 min 25 pg/mL. The drop in Pre-excision level suggests the surgeon has identified the hyperfunctioning parathyroid gland as reflected in the > 50% PTH drop, which predicts operative success.
Figure 1 Intraoperative parathormone monitoring dynamics demonstrating a > 50% drop when compared to the pre-incision parathormone level using the Miami criterion.
A: The drop of pre-excision PTH level suggests that the surgeon identified the hyperfunctioning gland during dissection reflected in the drop of PTH level; B: During dissection, manipulation of the abnormal gland may result in a release of PTH into the bloodstream, reflected by a surge in PTH level. It is important in this scenario to observe a drop in the PTH level on the subsequent 5 and 10 min samples from the higher pre-excision PTH level. IPM: Intraoperative parathormone monitoring; PTH: Parathormone.
The next example is of a 45-year-old gentleman with biochemical confirmation of pHPT who presented with a calcium level of 10.8 mg/dL and PTH level of 125 pg/mL on routine blood tests (Figure 1B). His MIBI and ultrasound studies were concordant for a suspicious left inferior parathyroid. Intraoperatively, an abnormal left inferior parathyroid gland was located and excised with intraoperative PTH levels measured as follows: Pre-incision 109 pg/mL; pre-excision 170 pg/mL; at 5 min 51 pg/mL, and at 10 min 34 pg/mL. Unlike in the first case, the dramatic rise in pre-excision level, which was not observed in the previous example, suggests the surgeon has identified the hyperfunctioning parathyroid gland. During dissection, manipulation of the abnormal gland by the surgeon may have resulted in a sudden surge of PTH into the bloodstream reflected by a dramatic rise of pre-excision PTH level, it is important in this scenario to witness a drop in the PTH level on the subsequent 5 and 10 min samples. The patient’s values ultimately reflect a > 50% PTH drop when compared to the pre-incision PTH level.
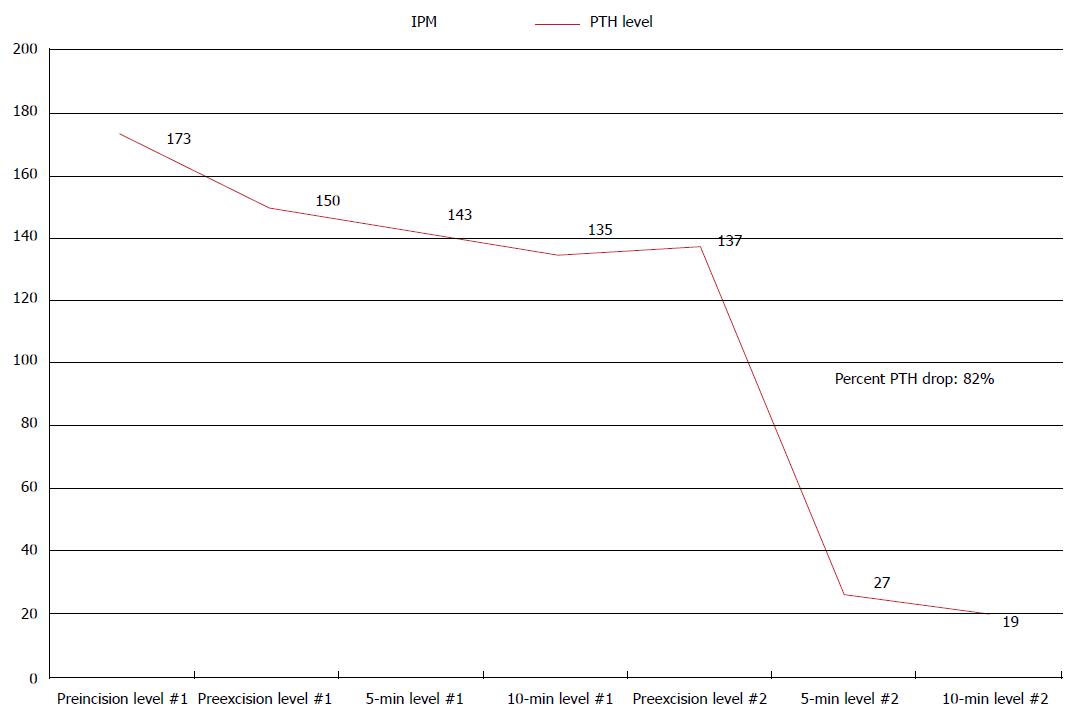
The final scenario is of a 34-years-old man who arrived to the emergency room with kidney stones (Figure 2). As a part of his evaluation, an elevated calcium level of 11 mg/dL and parathyroid hormone level of 119 pg/mL were measured. A preoperative MIBI scan did not localize an abnormal parathyroid gland. Following the excision of a right inferior parathyroid gland, intraoperative PTH levels drawn were: Pre-incision 173 pg/mL; pre-excision 150 pg/mL; at 5 min 143 pg/mL, and at 10 min 135 pg/mL. Without a > 50% PTH drop, exploration continued contralaterally and an abnormal left inferior parathyroid gland was discovered and excised. Intraoperative PTH levels were again measured and were as follow: Pre-excision 137 pg/mL; at 5 min 27 pg/mL; and at 10 min 19 pg/mL, confirming removal of hyperplastic parathyroid tissue with a > 50% PTH drop. As demonstrated in this case, when the PTH level fails to decrease > 50% from either highest pre-incision or pre-excision level, there should be a suspicion for MGD.
Figure 2 Intraoperative parathormone monitoring dynamics demonstrating > 50% drop using the Miami criterion when more than one abnormal parathyroid gland is removed.
When the PTH level fails to drop > 50% from either pre-incision or pre-excision levels, there should be a suspicion for MGD. IPM: Intraoperative parathormone monitoring; MGD: Multiglandular disease; PTH: Parathormone.
OTHER USES OF INTRAOPERATIVE PTH MEASUREMENT
Biochemical FNA
FNA of tissue for PTH measurement has valuable use in differentiating parathyroid glands from other tissues. During BNE or focused parathryoidectomy, biochemical FNA may be of value in identifying parathyroid tissue vs other tissues within the neck. When trying to differentiate between parathyroid from thyroid tissue or lymph nodes, this technique may be very helpful to the surgeon. A sample is obtained using a 25 gauge needle and diluted in 1 mL of normal saline. The sample is then sent to the laboratory where it is centrifuged. The PTH level is measured from the remaining supernatant after centrifugation[20]. As biochemical FNA has 100% specificity, this intraoperative technique can confirm parathyroid tissue more expeditiously than frozen section.
Internal jugular venous sampling
In the setting of discordant or negative preoperative localization imaging, differential venous sampling using the intraoperative PTH assay may allow surgeons to perform unilateral neck exploration in patients rather than BNE[21-23]. In order to lateralize the hyperfunctioning gland, bilateral internal jugular venous sampling of PTH is effective in directing surgical exploration. This procedure can be safely performed with ultrasound guided sampling of the inferior right and left internal jugular veins prior to skin incision. When there is a greater than 5% to 10% difference in PTH level, laterality to the side of the hyperfunctioning gland can be determined[21,22]. The surgeon may begin the operation by first exploring the identified side of the neck. The sensitivity of differential venous sampling approaches 80% according to published studies[21,22].
IPM and discordant localization studies
It has been argued that with the advancements in imaging modalities, combined preoperative localization with technetium Tc 99m sestamibi and ultrasound may eliminate need for IPM. In one retrospective cohort study of 569 patients with pHPT who underwent both MIBI and ultrasound, only 57% (n = 322) of patients had preoperative concordant localization studies and, in this group, there was a 99% success rate in achieving postoperative eucalcemia[24]. However, in 35% (n = 201) of patients with only one of two localization studies identifying an abnormal gland, neither MIBI nor ultrasound alone were able to correctly predict the location or extent of disease in 38% (76/201) patients in this discordant group. While there was marginal benefit among patients who had concordant preoperative localization imaging studies, IPM remained vital for patients with discordant studies undergoing limited parathyroidectomy[24]. In a retrospective series of 225 patients with pHPT where operative success was 97%, IPM remained an important adjunct for performing targeted parathyroidectomy in patients with discordant localization studies[25]. In a subgroup of 85 patients (38%) with discordant preoperative imaging, where IPM altered operative management and helped the surgeon during parathyroidectomy, operative success was 93%. In this series, IPM allowed surgeons to perform unilateral operation in 66% of patients, and confirmed excision of hyperfunctioning parathyroid glands in 7 patients with MGD[25].
LONG TERM OUTCOMES FOR IPM GUIDED PARATHYROIDECTOMY
Since 1993, parathyroidectomy has been guided by IPM for patients with pHPT at the University of Miami. BNE is no longer the initial approach in these patients with pHPT unless preoperative localization studies are negative or when preoperative imaging has identified the wrong side of the neck. At the authors’ institution, operative success is defined as calcium levels within normal limits for > 6 mo following successful parathyroidectomy. The definition of operative failure is persistent elevated PTH and elevated calcium measurements occurring < 6 mo following focused parathyroidectomy. Disease recurrence is defined as elevated PTH and elevated calcium measurements occurring > 6 mo following succes sful parathyroidectomy. The definition of MGD is two or more hypersecreting parathyroid glands identified intraoperatively during parathyroidectomy as demonstrated by IPM or if excision of one gland results in operative failure.
While criteria for IPM may vary among surgeons, the principle remains the same. By obtaining PTH levels in real time and achieving a desired reduction, the surgeon may have greater confidence intraoperatively that the offending hyperfunctioning parathyroid gland has been excised. While IPM has become common practice in most experienced centers, the Miami criterion has been compared to other stricter protocols in predicting post-operative eucalcemia. Stricter criteria proposed include a larger > 65%-70% PTH drop and/or return of absolute PTH level to within normal limits, or a PTH decrease at 5 min after gland removal[26-28]. In comparison to other criteria, the > 50% PTH drop was found to accurately predict operative success in > 95% of patients who had IPM guided parathyroidectomy for pHPT. In fact, the Miami criterion demonstrated the highest accuracy in predicting operative success when compared to other protocols, which included the Vienna, Rome, and Halle criteria[27]. In a study, which applied stricter protocols, the false positive rate would be reduced; however, at the expense of a lower sensitivity and an increased false negative rate. This false negative rate would then result in performance of BNE not necessary for the patient[29].
An additional protocol from the Mayo clinic was compared to different criteria in a study of 1882 patients with pHPT who had parathyroidectomy with IPM[30]. The Mayo criterion defined a successful parathyroidectomy as > 50% from baseline in addition to a normal or near-normal intraoperative PTH measurement at 10 min following removal of the abnormal gland. The Mayo criterion was compared with the following criteria for monitoring: A > 50% PTH drop at 10 min, > 50% PTH drop at 5 min, and intraoperative PTH within normal range at 10 min. The authors described an operative success of 97% equivalent to that of the Miami criterion. Results were similar when comparing Mayo criterion which had a sensitivity of 96%, PPV of 99%, and an accuracy of 95%, whereas the Miami criterion had had a sensitivity of 96%, PPV of 97%, and an accuracy of 94%. The criterion, however, differed with respect to MGD. Authors reported that MGD was found in 271 patients (14.5%). A total of 134 of 1858 patients (7.2%) were not able to meet criteria predictive of cure, which indicated the presence of MGD. The authors reported that using the > 50% PTH criterion alone would have theoretically resulted in a failed parathyroidectomy in 22.4% of patients affected with MGD[30].
Critics of the focused parathyroidectomy predicted that the combination of both preoperative localization imaging studies and IPM would miss abnormal parathyroid glands, resulting in greater recurrence rates in patients undergoing parathyroidectomy. In a study of simulated focused parathyroidectomy, both preoperative sestamibi and ultrasound for localization and IPM were performed in all 916 patients with pHPT[31]. All patients underwent BNE, 16% of which had additional enlarged parathyroid glands. The researchers determined that the long term failure or recurrence rate of the focused approach may be greater than initially described in previous studies[31]. Other studies, however, demonstrated that focused parathyroidectomy had long-term surgical success that was similar to BNE. In another study of the 181 patients who underwent image-guided parathyroidectomy, no patients developed recurrent disease with a mean follow-up of approximately 5 years[32]. In a randomized clinical trial which had a five year follow-up, recurrence rates for targeted parathyroidectomy and traditional approach were 5% and 3%, respectively[30]. A study of 164 patients with an average follow-up of close to seven years demonstrated a 3% disease recurrence rate following successful focused parathyroidectomy guided by IPM[33]. Additionally, other studies found that parathyroid gland size or pathology do not show a correlation with PTH secretion reliably, as a result they may not be useful indicators for identifying hyperfunctioning parathyroid glands[4,5,34]. Together, such findings demonstrate that the focused parathyroidectomy has a durable operative success rate and does not miss MGD as a cause of disease recurrence. These postoperative outcomes indicate that IPM guided parathyroidectomy may allow for minimal dissection for patients with single gland disease in pHPT with durable long-term eucalcemia.
The implementation of IPM in patients with pHPT has shifted the surgical approach to parathyroidectomy from BNE to less invasive operations. Many studies have confirmed that the success of focused parathyroidectomy guided by IPM demonstrate operative success rates comparable to conventional BNE[6-10]. One study of 718 patients over thirty-four years demonstrated rates of operative success for focused parathyroidectomy and traditional approach to be 97% and 94%, respectively[6]. A review of 656 patients with 255 undergoing focused parathyroidectomy and 401 undergoing BNE demonstrated success rates of 99% and 97%, respectively[8]. The overall rates of complications for focused parathyroidectomy and BNE within this same study were 1.2% and 3%, respectively[8]. Patients who underwent focused parathyroidectomy experienced reduced operating room times of 1.3 h in contrast to patients undergoing BNE with operating times of 2.4 h[8]. There were shorter hospitalizations of 0.24 d for focused parathryoidectomy in comparison to 1.64 d for BNE[8]. Focused parathyroidectomy demonstrated equivalent long-term results when compared to conventional BNE for patients with pHPT in one randomized controlled trial with a 5-year follow-up[35].
CONCLUSION
Over the past 25 years, IPM has been an effective surgical adjunct that can be of help during parathyroidectomy in patients with pHPT. IPM has been shown to effectively confirm operative success with a focused or targeted approach that allows for minimal dissection and selected parathyroid gland excision. Using the Miami or “> 50% PTH drop” criterion, the surgeon excises only the hyperfunctioning parathyroid gland(s) without identifying the remaining normal parathyroid glands. Instead of identifying abnormal parathyroid glands by size, color, and/or pathology, IPM allows for quantitative recognition of parathyroid gland hyperfunction based on PTH secretion during parathyroidectomy where pHPT is recognized as a disease of function rather than form. IPM guided parathyroidectomy has become the preferred initial approach over traditional BNE, and there has been a shift of treatment paradigm from comprehensive to limited parathyroidectomy for pHPT over the last few decades. Parathyroidectomy guided by IPM has evolved into a highly successful and rapid operation, usually requiring minimal dissection that can be performed in an ambulatory setting. IPM has proven to be a vital adjunct to focused parathyroidectomy demonstrated by its high postoperative success rate and long term outcomes, and its efficacy ensures that this important tool will continue to benefit surgeons in the future.
P- Reviewer: Musetti C, Papavramidis TS S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK