Published online Apr 28, 2012. doi: 10.5412/wjsp.v2.i2.5
Revised: March 20, 2012
Accepted: March 27, 2012
Published online: April 28, 2012
New technologies are constantly being introduced into the medical and surgical fields. These technologies come in the form of newer medicines, imaging methods and prognostic tools, among others, and allow clinicians to make more rational and informed decisions on the care of their patients. Many of these technologies utilize advanced techniques which are at the forefront of many research fields and represent a transition of bench advances into the clinical realm. This review will highlight four technologies that are at the forefront in the treatment of oncology patients treated by surgeons on a daily basis. Circulating tumor cells, microarray analysis, proteomic studies and rapid sequencing technologies will be highlighted. These technologies will be reviewed and their potential use in the care of surgical patients will be discussed.
- Citation: Kowdley G, Srikantan S, Abdelmohsen K, Gorospe M, Khan J. Molecular biology techniques for the surgeon. World J Surg Proced 2012; 2(2): 5-15
- URL: https://www.wjgnet.com/2219-2832/full/v2/i2/5.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v2.i2.5
The pace of technological advancement in medicine has been substantial. With each advance, not only in the field of biology but in other fields such as information technology, image processing, manufacturing methods and data processing, many aspects of medicine are experiencing great change and our ability to study processes in great detail is expanding vastly. These techniques will become more commonplace and will become a greater part of a surgeon’s daily practice, especially in our cancer patients.
This review will highlight specific techniques in four major areas of study that have been advancing to the forefront of efforts in the treatment of surgical patients with malignancies. Some of these techniques have been known for some time; however, with advancement of related fields, the real-time practical utilization of these techniques in the care of our patients has escalated in recent years.
We will review the techniques associated with the identification and characterization of circulating tumor cells, the genes expressed in cancer cells (microarray analysis, rapid sequencing methods and proteomics). These methods, along with their applications in the field of oncology, will be highlighted in this paper.
Circulating tumor cells (CTC) and disseminated tumor cells (tumor cells in the bone marrow) are becoming more important in the study of patients with cancers as distant metastasis remain a major cause of cancer related deaths[1,2]. The hypothesis that these cells are released into the circulation and may be responsible for the dissemination of cancer cells has been suggested for over a century; however, only recently have we been able to detect them in a commercially viable fashion and have begun to understand their significance in disease[2,3]. The detection of these tumor cells in the clinical treatment of cancer patients is becoming important as many patients thought to have localized disease are actually found to have distant disease on relapse[2,4-7]. As our ability to measure these cells in the circulation from cancer patients increases, the idea of metastatic spread being a late event in tumor development is being questioned.
CTCs exist in the blood and the bone marrow at very low levels (one cell per 105-107 cells)[1,2,5] and an enrichment step is often utilized prior to actual detection of these cells to make the detection of these cells more efficient. Three main methods of enrichment are based on filtration, density gradients and immunomagnetic enrichment[2,5]. Density and filtration methods work on a size basis. Immunomagnetic methods rely on surface markers present on tumor cells that are not present on background cells. Once the samples are enriched, CTCs can be identified and studied.
Several methods utilize size differences between cells to enrich the sample. Isolation by size of epithelial tumor cells is a size-based filtration method. This method utilizes 8 μm pores which allow separation of small leukocytes from larger epithelial cells. This method allows the detection of one tumor cell from a 1 mL sample of blood[8]. Micro electro-mechanical system is another method that has been developed that takes advantage of size differences between CTCs and other blood cells. This method provides a high-efficiency membrane based system that allows for high throughput of samples[9]. The advantages and disadvantages of these methods have been summarized in other reviews[5,10]; briefly, size differences are not absolute for cell lines and thus the sensitivity and specificity of these techniques are low.
CTCs and mononuclear cells have different densities from red blood cells and granulocytes. Density gradient methods using Ficoll-Hypaque make use of this property to enrich CTCs. Often CTCs are lost in the process and the use of these chemicals has been associated with toxicity to cells. This method is also considered to have low specificity and requires a lot of processing time[11,12]. A commercially available method, Oncoquick (Greiner Bio-One, Germany), utilizes a membrane layer in the process to minimize cross contamination and saves some time for processing of samples when compared to the Ficoll method[12]. Other technologies exist as well and utilize a negative selection method[13,14].
Immunomagnetic enrichment is another major technique of enrichment. In this process, magnetic beads are coated with antibodies that are expressed on CTCs. Samples are then mixed with the beads to allow binding of cells with the given markers. The CTCs once bound to the beads are separated from the unbound fraction (background) by use of a magnetic field, thus positively selecting for CTCs. The antibodies employed are usually against epithelial markers or tumor specific antigens [e.g., cytokeratins (CKs), epithelial cell adhesion molecule (EpCAM), carcinoembryonic antigen, the human epidermal growth factor receptor 2]. Negative selection methods in which antibodies against the background cells (leukocytes, megakaryocytes) are used may also be employed and have the advantage of not disturbing the cells of interest[15-17]. These methods require no cell lysis, are less toxic to the cells, and allow for specific identification of CTCs when compared to size methods described previously. Commercially available technologies exist that make use of these immunomagnetic methods and employ semi automated methods for detection of CTCs. Veridex (Johnson and Johnson, Raritan NJ) and the CellSearchTM system are two systems that are available[1,18]. The major drawback to any of these immunological methods is that they rely on markers that are not absolute in distinguishing tumor cells from background. In addition, there is significant variation in expression of markers within tumors themselves and at different stages of tumor growth[5,11,19]. False positive and false negative selection has been identified in both methods, making the detection of all CTCs not possible with current enrichment methods.
Once CTCs are isolated, they are further characterized based on their nucleic acid expression or protein expression. These methods can be broadly characterized into polymerase chain reaction (PCR)-based analysis and cytometric analysis. Each of these techniques, as with all methods, has their pros and cons and is summarized well in another review[5].
PCR-based techniques are very sensitive when compared to immunocytochemistry methods and have been used in the clinical setting[20,21]. These methods achieve their specificity by the design of oligonucleotide primers targeted against genes of interest. Reverse transcription (RT)-PCR is generally the method employed for amplifying mRNAs of interest[21-23]. Multiplex RT-PCR methods have been developed to increase the sensitivity and specificity of this method, also allowing the screening of multiple markers at one time, and are discussed elsewhere in this paper.
Cytometric methods isolate and count cancer cells. This is performed most widely by the use of monoclonal fluorescently labeled antibodies directed against different antigens with the most common ones again being CKs and EpCAM[2,5]. As cells are not lysed, the integrity of the cells is maintained and this allows further characterization of these cells, including their size and their nucleus-to-cytoplasm relationship. The major limitation of this technique is one that is similar to all methods that use antibody-based techniques, specificity of target proteins, heterogeneity of tumors and protein expression. Once cells are coated with the antibody, they are scanned and analyzed using criteria developed for their evaluation[2,24,25].
Fiber-optic array scanning technology is a scanning technology that uses a large field of view for detection of bound antibody. This technology allows the analysis of large volumes of samples without a purification step and allows for the scanning of up to 300 000 cells per second, about 500 times faster than conventional automated digital microscopy[26,27]. This technology is being used in clinical practice at this time[28]. Other automated systems have been developed and are described elsewhere[29-34], including CellSearchTM.
Other technologies make use of chips that are coated with antibodies of interest. Blood is pumped through the chip and cells are captured. This method was shown to have a significantly improved sensitivity when compared to other techniques, with identification of CTCs in 99% of samples from multiple tumors[35,36]. These cells may be further analyzed for function of trapped proteins, as has been shown for telomerase activity recently[37]. More detailed analysis of arrays will be covered in subsequent sections.
In the United States, breast cancer is the most common non-skin cancer and the second leading cause of the cancer-related death in women. Despite advances in screening programs and new chemotherapy agents, 6%-10% of women present with metastatic disease. The 5-year overall survival rate among such patients rarely exceeds 20%[38]. Cristofanilli et al[4] have shown that the presence of high CTC number (≥ 5/7.5 mL of whole blood) is associated with shorter progression-free and overall survival in the metastatic setting and their continued presence following the initiation of a therapy predicts impaired therapeutic responses and overall survival in breast cancer patients. Botteri et al[38] showed that the number of CTCs at baseline and their perpetual changes at different follow-up points is a strong independent predictor of progression-free-survival (PFS) but not overall survival (OS). Currently, CTC assessment has been approved by the Food and Drug Administration (FDA) as a prognostic factor to segregate the patients with metastatic breast cancers into favorable or unfavorable groups and may represent a robust predictive factor in this metastatic population[36,39].
Colorectal cancer (CRC) is the second leading cause of overall cancer death in the United States[40,41]. CTCs can serve as both prognostic and predictive agents for patients with metastatic CRC. In a large prospective multicenter trial of 413 patients with metastatic CRC commencing first, second or third line therapy, patients with baseline CTC number ≥ 3 had significantly worse median PFS (4.4 mo vs 7.8 mo, P = 0.004) and OS (9.4 mo vs 20.6 mo, P < 0.0001) compared with patients with < 3 CTCs[40]. The baseline CTC number was an important predictive factor even within specific patient subgroups defined by treatment or patient characteristics.
In the light of the available evidence, the FDA has approved CTC analysis by Cell SearchTM in metastatic breast, colorectal and prostate cancer patients as an indicator of prognosis[41]. There is scarcity of information regarding CTCs in many other tumors, including melanoma, head/neck cancers, biliary and pancreatic cancers. Preliminary results are encouraging but need further study.
In the nucleus of virtually all cells in the body is a full set of chromosomes, which contain tens of thousands of genes, the DNA genetic code. However, in each cell type, only a fraction of these genes is “expressed”, that is, used for the synthesis of protein. The specific set of proteins produced in each cell confers unique properties to the cell. The term “gene expression” describes a process whereby the information contained in the genes in the form of DNA is used to synthesize (transcribe) a messenger (m) RNA molecule that will be transported to the cytoplasm and used as a temporary template for the synthesis of proteins (translation) that will perform the functions of the cell. Studying the levels and types of mRNAs expressed in the cell provides indirect information about the proteins expressed in that cell. As the cellular needs change to adapt to internal conditions and external stimuli, so do the patterns (type and amount) of expressed mRNAs. The maintenance of adequate gene expression patterns is important for the preservation of cellular homeostasis. Disruptions in the levels or types of expressed mRNAs underlie many disease processes, including cancer.
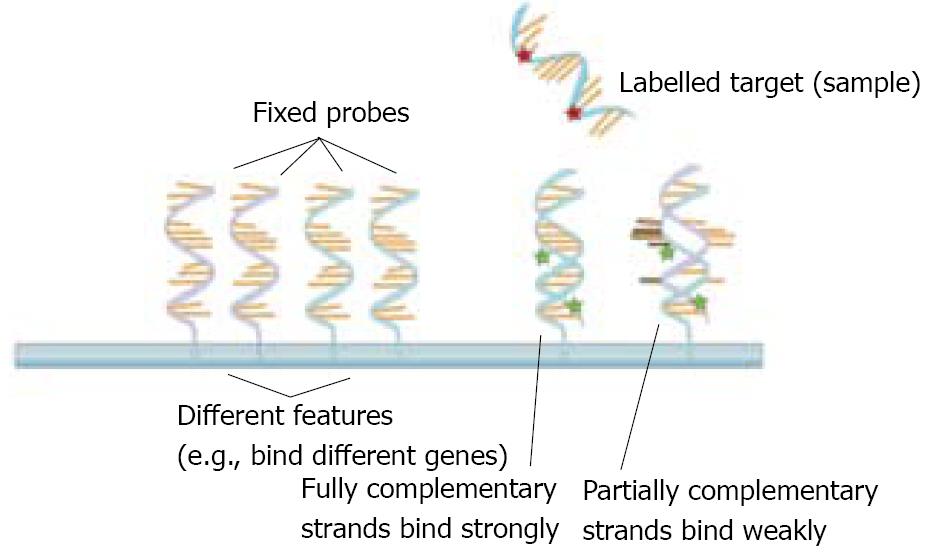
Traditional technologies to study gene expression only allowed scientists to study a handful of expressed mRNAs at a time. In the mid 1990s, the microarray technology emerged as a new tool that began to allow scientists to survey many mRNAs at the same time in a quick, inexpensive and efficient manner. This major methodological advance permitted scientists to explore the patterns of expressed genes during physiological processes (such as development and normal tissue function) and in pathological states, such as cancer and many other diseases[42-44]. The microarray technology is based on the ability of a given mRNA molecule to bind (or “hybridize”) specifically to a DNA template of complementary sequence (Figure 1). Microarrays contain thousands of DNA sequences immobilized at fixed spots on small, solid supports which are typically glass microscope slides, but can also be silicon chips or nylon or nitrocellulose membranes. The DNA sequences themselves are typically oligonucleotides (single-stranded DNA sequences of 5-50 nucleotides) but can also be longer DNA molecules.
There are three basic types of DNA microarrays, each yielding different types of information. Traditional microarrays provide information about the patterns of expressed mRNAs, while new applications of microarrays make available information on changes in the composition of DNA within the samples analyzed.
Microarray analysis of changes in the levels of expressed mRNAs: Also known as “gene expression microarrays”, this technology provides useful information on the types and quantities of mRNAs expressed in a given sample[45]. In these microarrays, the immobilized DNAs are typically oligonucleotides and the samples used in hybridization are prepared from mRNA extracted from cells or tissues. The mRNA is then reverse-transcribed to yield complementary DNA (cDNA) subsets which are labeled with radioactivity or fluorescence. If the samples are derived from healthy tissue and cancer tissue from a patient, for example, after hybridization and signal detection and analysis, the microarray can uncover specific sets of genes differentially expressed between the two tissues. This information can then be used to decide on the appropriate therapeutic regimen for the patient. This information can also be used in the rational design of effective drugs.
Microarray analysis to uncover genomic gains and losses: Comparative genomic hybridization (CGH) microarrays are used to study large genomic gains and losses, as well as to identify changes in the number of copies of a particular gene involved in a disease state[46]. In microarray CGH, each spot of target DNA in the array has a known chromosomal location and the samples tested are large pieces of genomic DNA. For example, the comparison can be made between labeled genomic DNA prepared from a normal tissue with labeled genomic DNA prepared from a cancer tissue. This type of analysis is particularly useful in the study of diseases in which certain genes or groups of genes might be deleted; gross deletions occur frequently in cancer, affecting regions that encode tumor suppressor or DNA repair genes. Regions of selective gene amplification have also been reported in cancers, often affecting oncogenes and genes involved in DNA replication. The microarray CGH analysis can reveal these regions of lost or gained DNA and thereby uncover specific genes increasing or decreasing in the disease[47,48].
Microarray detection of DNA mutations: Although in cancer many more genes are altered in their abundance than are mutated[49], in some cases researchers can use microarrays to detect small mutations or polymorphisms in the sequence of a gene. In this type of analysis, the DNA spots immobilized on the array may differ from one another by only one nucleotide or a few specific nucleotides and the probe used is genomic DNA from the individual. These microarrays are frequently used to identify single nucleotide polymorphisms (SNPs), small variations in gene sequence that occur in the human population. This microarray approach is particularly useful in the detection of specific cancer types and other diseases (or the susceptibility to diseases) associated with a given SNP pattern[50].
Microarrays are particularly useful for studying a large number of genes quickly. They can measure mRNA expression levels for thousands of genes at once and can rapidly identify large or small mutations on a global genomic scale. Microarrays have been extremely valuable in identifying the genes implicated in cancer and other diseases. For example, microarray comparisons of “healthy” (or “control”) tissue from an individual, with cancer tissue from the same individual (for example, from a biopsy or intra-operative sample) have provided valuable information about the specific changes that have occurred in that particular cancer, specifically alterations in the abundance of expressed mRNAs and any gross deletions or amplifications. This information has been useful in guiding specific aspects of cancer therapy and in helping with its prognosis[51-53]. Microarrays have also been used to distinguish between specific types of cancer and to provide clues on the most effective treatment regimens[54,55]. The use of microarrays to compare one individual population to another has been used to identify specific SNPs associated with the likely development of certain diseases and thus have helped with prevention and monitoring plans.
Studies in the clinical setting are beginning to show promise for identifying patients with a poor prognosis based on differential gene expression profiles. In Ntzani et al[51], receiver operating curves for various cancers were reviewed and a sensitivity and specificity for predicting death in patients with breast cancer were 91% and 73%, respectively. In this analysis, only studies that included more than 500 genes were used. In a study of Dukes C colon cancer[52] patients, a total of 236 sequences from 218 genes were noted to be differentially expressed in patients with or without recurrence in 5 years. The expression of K-ras was not predictive of outcome in these patients; however, the expression of RHOA, a ras analogue, was an excellent predictor of recurrence in these patients. In another study of patients with colorectal cancer, the predictive value of outcome using gene array data was significantly better than traditional clinical staging methodologies[53]. In a recent study of breast cancer patients, the over-expression of genes ANLN, KIF2C and the under-expression of MAPT gene strongly correlated with poor outcomes in breast cancer patients[55]. A recent paper comparing gene expression using microarray technology compared profiles between patients that had normal colorectal mucosa with patients with adenomas and adenocarcinomas[56], identified a novel gene locus that is activated very early in the process of neoplasia and may be used in the future as a novel tissue or plasma marker for disease.
At present, microarray analysis is still labor-intensive and requires a minimum of 48 h to collect samples, isolate and process RNA, prepare labeled probes, hybridize microarrays, analyze microarray signals and compare samples to samples and to existing databases. For this reason, microarrays are useful for mid- to long-term planning of health care decisions. For decisions that need to be made in a shorter time-frame (e.g., intraoperatively), the technology will still require a significant amount of automatization and streamlining in order to be practical in such settings. While individualized medicine is still in the early stages, however, based on the concept of microarray analysis, hand-held devices to diagnose disease and to personalize therapy for an individual patient will likely be a reality for physicians in the not too distant future.
As highlighted above, the study of cancer by gene arrays has been widely used to detect changes in gene expression. However, gene arrays do not always reflect changes in protein abundance. Furthermore, posttranslational changes, such as phosphorylation, acetylation, ubiquitinylation, protein interactions and subcellular localization, cannot be resolved by gene arrays. These changes are very important for protein stability and activity and may determine cell fate and thus protein studies (proteomics) are essential to uncover these modifications. There are several proteomic techniques that help scientists to understand and functionally relate protein changes and cell networking. The techniques described below can be used to analyze a wide range of samples, including normal tissue samples as well as solid tumors such as lung, breast, colon and liver. We will cover the techniques first and then point out current applications of the technology in the clinical setting.
This technique determines the mass of charged molecules in tissue samples and is used for protein identification. Mass spectrometry (MS) can be used to study the proteome of mouse solid tumor lysates with similar efficiency as cell culture models and in amounts compatible with biopsies[57]. Cancer prediction, early detection, response to therapy, evaluation and recurrence have been studied by MS among several solid tumors, including lung and gastrointestinal malignancies[58-64]. In addition, MS can be used to determine the abundance of different proteins in cancer tissues compared to normal tissues using two-dimensional gel electrophoresis[65]. This technique of electrophoresis begins with the two-dimensional separation of proteins: the first dimension separates proteins according to their charges and is known as isoelectric focusing, while the second dimension separates proteins according to their molecular weights by sodium dodecyl sulfate polyacrylamide gel electrophoresis. This method is quantitative and can resolve hundreds, possibly thousands, of proteins that are differentially expressed. MS analysis is indeed of high impact in cancer research since it can provide essential information such as protein identity, quantity and its posttranslational modifications such as phosphorylation.
Such techniques are being used to characterize the heterogeneity of tumor cells from a single cancer type. Using these techniques, over 1000 lung tissue samples have been analyzed and the protein PGK1 has been shown to be an excellent predictor of survival in patients with stage I lung cancer[58]. In another study of 174 non small cell lung cancer tumors with normal controls, it was shown that over 25 proteins were differentially expressed between the two lines. Blood from healthy individuals has been studied against patients with a tumor and a protein profile has been identified with a 73% sensitivity and 90% specificity for detection of head and neck cancers[58,64]. Similar protein profiles between healthy and patients with ovarian and prostate cancers have been identified[64]. These observations highlight the importance of these techniques as they apply to prognosis and detection in cancer cell lines and may come into clinical practice in the foreseeable future, necessitating familiarity with such techniques for surgeons.
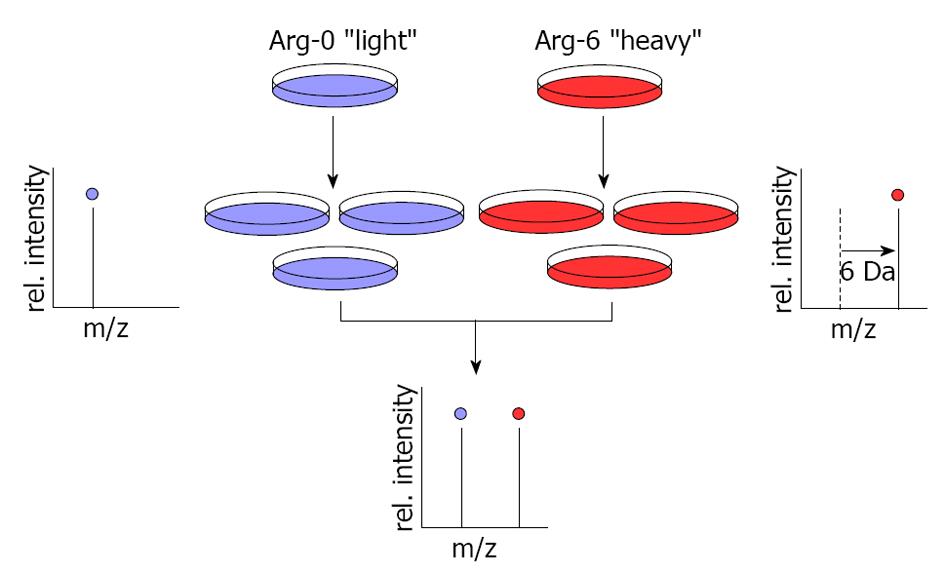
This technology also utilizes MS to globally determine protein abundance in cultured cells. In this method, growing cells are labeled by light medium with normal arginine (Arg-0) or heavy medium with arginine 6 (Arg-6). Arg-0 and Arg-6 metabolically incorporate into proteins, resulting in a shift in the mass of the corresponding peptides that can be detected by MS (Figure 2). Since its development almost ten years ago, stable isotope labeling by/with amino acids in cell culture (SILAC) has been used in cultured cells but not tissues or body fluid analysis; however, scientists are currently expanding this method for use in human cancer tissues and recently developed a technique known as Super-SILAC mix[66]. Although this strategy is still in its infancy, it extends SILAC-based quantitative proteomics to the study of proteins in cancer tissues. It can be used for the early detection of cancers[67] through the identification of potential biomarkers[68], pharmacological targets[69], molecular networks[70] and post-translation modifications[71,72].
Esophageal cancer, often insidious in presentation and unresectable at detection, has been studied with this technique to identify specific protein profiles that differ between normal esophageal epithelial cells and those of esophageal carcinoma cells. In one study, a total of 441 proteins were studied and over 120 proteins were identified as being > 2 fold up regulated in cancer cells. Such techniques potentially allow for the early detection of patients with esophageal cancer, allowing surgical therapy to be of benefit to a larger group of patients[68].
Western blotting (WB) is widely used and based on antibody technology. Protein sequence, molecular weight and post-translational modification can be studied by WB. Lysates from all types of tissue, including solid tumors, can be studied by WB to investigate aspects of protein characteristics, including abundance, stability and posttranslational modification. In cancer research, large numbers of proteins can be investigated using this technology in the form of protein microarrays, discussed elsewhere in this review[73,74].
Immunohistochemistry (IHC) also enables researchers to determine protein abundance, posttranslational modification and, more importantly, direct assessment of its subcellular localization. IHC is the most common way to visualize protein-antibody interaction and is based upon fluorescence technology. This method has clearly advanced our knowledge of cells since tissues can be organized and stained in an array format known as tissue arrays. IHC is becoming widely used since it enables researchers to screen the expression of certain proteins in a large number of tissues (normal and cancer) in a short time and in a cost-effective manner.
The enzyme-linked immunosorbent assay (ELISA) technique is used to detect proteins from samples of different origin, from intact tissue or cell lysates, and from fluids such as blood. ELISA can detect protein abundance and also posttranslational modification to proteins such as phosphorylation. ELISA is quick, simple and amenable to studying large numbers of samples and is used as a diagnostic method to detect tumor markers for earlier detection of cancers[75,76].
The methods summarized above have a great impact on our understanding and ongoing investigation in tumor proteomics. We believe that these techniques will develop further, making them more efficient, accurate, fast and cost-effective.
The completion of sequencing of the human genome has opened a floodgate of opportunities to understand biological processes, variations among individuals and between normal and disease states. Many research groups are now investigating global changes in chromosomal DNA or their transcribed RNA. The need for fast and accurate sequencing methods to carry out these studies has led to the development of many innovative sequencing platforms, collectively known as high-throughput sequencing (HTS) technology. Thanks to this technology, we now have datasets available on various aspects of cellular biology, including genome, transcriptome, epigenome and interactome, leading to a better understanding of biological processes[77]. Here, we discuss some of the applications of HTS approaches and their use in understanding diseases. This will be a fairly detailed look at the techniques and several examples of current clinical use will be highlighted. These methods are in their infancy and direct application to the clinical setting is only beginning to be realized.
Methodology: Distinct chemistry and technology are involved in different HTS platforms. Typically, all of the platforms depend on parallel sequencing on multiple short DNA fragments (typically 50-300 bases) generated from genomic DNA or from cDNA. These sequence reads are then aligned to the available genome databases and processed to get relevant information. The data generated from such experiments are enormous and can serve as a source for unending analysis to get different kinds of information. Upon initial publication, the data is made available in public databases for other scientists to use in their analysis, which ultimately enriches our knowledge of biological processes.
Genome analysis: Scientists studying human genetics ultimately aim to map every genetic variant and link them to phenotype. Many genome-wide association studies reveal that only a modest fraction of heritable risk for common diseases is explained by known genetic variants. This raises the question whether rare variants exist in individuals which could account for unexplained heritability. Recent developments in HTS have helped to address this question by sequencing whole genomes or part of the genomes to identify genetic variations that could lead to disease. For example “exome-sequencing” (sequencing of all protein coding regions) of eight normal individuals and four individuals with a rare dominantly inherited disorder, Freeman-Sheldon syndrome (FSS), led to the identification of many rare and common variants in the coding sequences. Extensive analysis of this data set revealed that MYH3 (identified previously by a candidate gene approach) was a candidate for FSS[78]. This proof of principle analysis has been followed up by other such analysis, leading to identification of novel candidate genes for different diseases[79]. HTS has also been used to detect personalized copy-number variants and gene-fusion events which could explain individual differences in susceptibility to diseases[80]. The use of such information has the potential for identifying individual susceptibilities to various diseases and specifically cancers. This information may allow for potential screening of true at risk individuals based on their sequence specific propensities for cancer development.
Transcriptome analysis: HTS has opened up the doors to study the RNA transcripts generated from the chromosomal DNA in ways which were not possible using traditional sequencing methods or microarrays[81]. For example, using HTS, we not only get the qualitative and quantitative data on expression of all known protein coding RNAs (similar to that of microarray), but also that as yet unknown RNA transcripts. For example, HTS can identify the presence of unknown gene fusions[82], RNA editing and new classes of noncoding RNAs, as well as unknown and less abundant splice variants and splice regions[83]. Combining HTS with the nuclear run-on assay has allowed scientists to monitor transcription rates, as well as to appreciate the prevalence of anti-sense transcription, divergent transcription and transcription extending beyond the pre-messenger RNA 3’ end cleavage site[84]. As an extension of this methodology, one can also monitor the rate of mRNA degradation, as well as understand the degradation processes using HTS. These data not only enrich our knowledge of biological processes but also help us to differentiate normal and diseased states. For example, it can help in the identification of an unknown gene fusion event[82], non-coding RNA[85], splice variant or RNA editing that could be responsible for a given disease.
Epigenome analysis: Epigenetic modifications broadly describe modifications of the basic components of chromatin, i.e., DNA and histone proteins[86], which typically control transcription. It is now increasingly appreciated that epigenetic abnormalities play a key role in cancer and other diseases[87]. HTS coupled to techniques like bisulfate treatment (for DNA methylation status) or chromatin immunoprecipitation of modified histones are employed to understand genome-wide epigenetic differences between normal and abnormal (e.g., disease) phenotypes[88-90]. Many of the protein modifications occurred in genes responsible for pluripotency and differentiation, perhaps indicating their importance in inducing cancer in cell lines. The powerful DNase I hypersensitivity site footprinting coupled to HTS is used to define open chromatin structures which are the potential regulatory sequences[91].
Interactome analysis: To understand how genes bring about a phenotype, it is important to understand the functional and physical interactions of chromosomal genes, as well as their products (RNA and protein). Investigation of these interactions is important to further our knowledge of diseases[92]. Chromatin immunoprecipitation followed by sequencing (ChIP-Seq) is the most sought-after method to identify transcription factor or transcription complex binding to chromosomes. For example, since the sites binding by RNA polymerase II (RNApolII) usually define active transcription, ChIP-Seq of RNApolII has been used to investigate questions such as the transcriptional state, the normal transcription starting sites, and the prevalence of aberrant or alternate transcription starts[89,93]. Combinatorial analysis of different ChIP-Seq experiments can lead to the understanding of the complex nature of chromosome organization and how it is regulated by many cellular factors. For example, Barski et al[89] used ChIP-Seq analysis to generate high-resolution maps for the genome-wide distribution of histones, RNA polymerase II and CCCTC-binding factor (CTCF; the insulator binding protein) across the human genome, which revealed the complex nature of genome organization and how this affects transcription. Similarly, ChIP-Seq analysis of estrogen receptor α (ER-α; key component of estrogen regulated transcription) and RNA polymerase II not only revealed the binding sites of ER-α on human chromosomes but also showed the complexity of transcriptional states of these genes in response to estradiol and/or its antagonists[94,95].
Interaction of a RNA with RNA-binding proteins (RBP) or microRNA-containing RNA-Induced Silencing Complex (RISC) determines the fate of the transcript, including pre-mRNA splicing, and mRNA stability, localization and translation[96-102]. Some of these interactions have been linked to key cellular functions like synaptic plasticity and carcinogenesis[97-99]. Cross-linked immuno-precipitation (CLIP) methods coupled to HTS have helped to elucidate the networks of interaction of RNAs with RNA-binding factors[96-102]. This analysis has led to a better understanding of RNA biology. For example, HTS of RNAs interacting with the RBPs Nova[96] or HuR[100-102] led to the discovery of roles played by these RBPs in the splicing of their target mRNAs. CLIP of Argonaute complexes revealed how the RISC interacts with its target mRNAs and microRNAs and further illuminated microRNA mediated mRNA regulation[98,99]. Similarly, CLIP of ribosomes gives us a global view of different aspects of translation (the decoding of mRNA to synthesize protein) as it happens in the cell, including initiation rates, initiation and termination sites[103]. Coupling microRNA or RBP modulation to ribosome CLIP or nascent RNA/protein translation assays and HTS can determine the role of these factors in the stability or translation of their target mRNAs[104]. Some of the long standing questions of basic biology and medicine are now being tackled with these methodologies[105].
Some examples of current clinical use of these technologies include the use of high-throughput sequencing in colon cancer to study microsatellite instability and its relationship to chemotherapy resistance in colon cancer patients[106]. In this study, colon cancer cell lines were compared to normal colonic mucosa. The expression levels of over 40 proteins involved with chemoresistance, DNA repair, DNA damage and drug metabolism were studied. A significant difference in expression of these proteins was noted between the two cell types. In triple negative breast cancer patients, molecular profiling of breast cancer patients in genome-wide analysis is being used to show heterogeneity among this group of patients. By understanding these differences, it allows us an opportunity to identify novel prognostic and predictive biomarkers in an effort to develop novel therapeutic agents[107] and will better help us tailor our therapeutic options with individual patient differences. These advances will inevitably become a common part of the treatment algorithms available to our patients with cancer in the near future.
This is an exciting time for many technologies and our understanding of molecular events is increasing each day. As this knowledge base is developed, many techniques that appear to be solely in the realm of the laboratory are becoming commonplace in the care of our surgical cancer patients. These technologies will allow us to better match the molecular makeup of individual patients with the diseases that they suffer from. Promising collaborations among bench scientists, computational biologists and clinicians have been forged in the quest to answer these pressing questions.
Peer reviewers: Juan Viñas Salas, Professor of Surgery, Department of Surgery, Arnau de Vilanova University Hospital, Alcalde Rovira Roure 80, 25198 Lleida, Spain; Konstantinos P Economopoulos, MD, Research Fellow in Surgery, Department of General Surgery, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St., Boston, MA 02114, United States
S- Editor Jiang L L- Editor Roemmele A E- Editor Li JY
| 1. | Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 883] [Cited by in F6Publishing: 927] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 2. | Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 488] [Cited by in F6Publishing: 528] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 3. | Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3231] [Cited by in F6Publishing: 3179] [Article Influence: 151.4] [Reference Citation Analysis (0)] |
| 4. | Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420-1430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 820] [Cited by in F6Publishing: 798] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 5. | Alunni-Fabbroni M, Sandri MT. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods. 2010;50:289-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Clare SE, Sener SF, Wilkens W, Goldschmidt R, Merkel D, Winchester DJ. Prognostic significance of occult lymph node metastases in node-negative breast cancer. Ann Surg Oncol. 1997;4:447-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Braun S, Pantel K, Müller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnik A, Dimpfl T, Kindermann G. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000;342:525-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 706] [Cited by in F6Publishing: 729] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 8. | Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M. Isolation by size of epithelial tumor cells : a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol. 2000;156:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 827] [Cited by in F6Publishing: 764] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 9. | Zheng S, Lin H, Liu JQ, Balic M, Datar R, Cote RJ, Tai YC. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A. 2007;1162:154-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 492] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 10. | Zach O, Lutz D. Tumor cell detection in peripheral blood and bone marrow. Curr Opin Oncol. 2006;18:48-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Gertler R, Rosenberg R, Fuehrer K, Dahm M, Nekarda H, Siewert JR. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. Recent Results Cancer Res. 2003;162:149-155. [PubMed] [Cited in This Article: ] |
| 12. | Müller V, Stahmann N, Riethdorf S, Rau T, Zabel T, Goetz A, Jänicke F, Pantel K. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res. 2005;11:3678-3685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 319] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 13. | Naume B, Borgen E, Tøssvik S, Pavlak N, Oates D, Nesland JM. Detection of isolated tumor cells in peripheral blood and in BM: evaluation of a new enrichment method. Cytotherapy. 2004;6:244-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Hayes GM, Busch R, Voogt J, Siah IM, Gee TA, Hellerstein MK, Chiorazzi N, Rai KR, Murphy EJ. Isolation of malignant B cells from patients with chronic lymphocytic leukemia (CLL) for analysis of cell proliferation: validation of a simplified method suitable for multi-center clinical studies. Leuk Res. 2010;34:809-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Neurauter AA, Bonyhadi M, Lien E, Nøkleby L, Ruud E, Camacho S, Aarvak T. Cell isolation and expansion using Dynabeads. Adv Biochem Eng Biotechnol. 2007;106:41-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | McNiece I, Briddell R, Stoney G, Kern B, Zilm K, Recktenwald D, Miltenyi S. Large-scale isolation of CD34+ cells using the Amgen cell selection device results in high levels of purity and recovery. J Hematother. 1997;6:5-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Peters CE, Woodside SM, Eaves AC. Isolation of subsets of immune cells. Methods Mol Biol. 2005;302:95-116. [PubMed] [Cited in This Article: ] |
| 18. | Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897-6904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1810] [Cited by in F6Publishing: 1867] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 19. | Jung R, Petersen K, Krüger W, Wolf M, Wagener C, Zander A, Neumaier M. Detection of micrometastasis by cytokeratin 20 RT-PCR is limited due to stable background transcription in granulocytes. Br J Cancer. 1999;81:870-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Smith BM, Slade MJ, English J, Graham H, Lüchtenborg M, Sinnett HD, Cross NC, Coombes RC. Response of circulating tumor cells to systemic therapy in patients with metastatic breast cancer: comparison of quantitative polymerase chain reaction and immunocytochemical techniques. J Clin Oncol. 2000;18:1432-1439. [PubMed] [Cited in This Article: ] |
| 21. | Xenidis N, Perraki M, Kafousi M, Apostolaki S, Bolonaki I, Stathopoulou A, Kalbakis K, Androulakis N, Kouroussis C, Pallis T. Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol. 2006;24:3756-3762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Stathopoulou A, Ntoulia M, Perraki M, Apostolaki S, Mavroudis D, Malamos N, Georgoulias V, Lianidou ES. A highly specific real-time RT-PCR method for the quantitative determination of CK-19 mRNA positive cells in peripheral blood of patients with operable breast cancer. Int J Cancer. 2006;119:1654-1659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1:1559-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1425] [Cited by in F6Publishing: 1440] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 24. | Fehm T, Braun S, Muller V, Janni W, Gebauer G, Marth C, Schindlbeck C, Wallwiener D, Borgen E, Naume B. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer. 2006;107:885-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Borgen E, Pantel K, Schlimok G, Müller P, Otte M, Renolen A, Ehnle S, Coith C, Nesland JM, Naume B. A European interlaboratory testing of three well-known procedures for immunocytochemical detection of epithelial cells in bone marrow. Results from analysis of normal bone marrow. Cytometry B Clin Cytom. 2006;70:400-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Hsieh HB, Marrinucci D, Bethel K, Curry DN, Humphrey M, Krivacic RT, Kroener J, Kroener L, Ladanyi A, Lazarus N. High speed detection of circulating tumor cells. Biosens Bioelectron. 2006;21:1893-1899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Krivacic RT, Ladanyi A, Curry DN, Hsieh HB, Kuhn P, Bergsrud DE, Kepros JF, Barbera T, Ho MY, Chen LB. A rare-cell detector for cancer. Proc Natl Acad Sci USA. 2004;101:10501-10504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 28. | Somlo G, Lau SK, Frankel P, Hsieh HB, Liu X, Yang L, Krivacic R, Bruce RH. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res Treat. 2011;128:155-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Pachmann K, Dengler R, Lobodasch K, Fröhlich F, Kroll T, Rengsberger M, Schubert R, Pachmann U. An increase in cell number at completion of therapy may develop as an indicator of early relapse: quantification of circulating epithelial tumor cells (CETC) for monitoring of adjuvant therapy in breast cancer. J Cancer Res Clin Oncol. 2008;134:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Pachmann K, Clement JH, Schneider CP, Willen B, Camara O, Pachmann U, Höffken K. Standardized quantification of circulating peripheral tumor cells from lung and breast cancer. Clin Chem Lab Med. 2005;43:617-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Cruz I, Ciudad J, Cruz JJ, Ramos M, Gómez-Alonso A, Adansa JC, Rodríguez C, Orfao A. Evaluation of multiparameter flow cytometry for the detection of breast cancer tumor cells in blood samples. Am J Clin Pathol. 2005;123:66-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Deng G, Herrler M, Burgess D, Manna E, Krag D, Burke JF. Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients. Breast Cancer Res. 2008;10:R69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218-4224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 765] [Cited by in F6Publishing: 752] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 34. | Shaffer DR, Leversha MA, Danila DC, Lin O, Gonzalez-Espinoza R, Gu B, Anand A, Smith K, Maslak P, Doyle GV. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:2023-2029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 35. | Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2735] [Cited by in F6Publishing: 2490] [Article Influence: 155.6] [Reference Citation Analysis (0)] |
| 36. | Pachmann K, Camara O, Kavallaris A, Krauspe S, Malarski N, Gajda M, Kroll T, Jörke C, Hammer U, Altendorf-Hofmann A. Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol. 2008;26:1208-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 37. | Xu T, Lu B, Tai YC, Goldkorn A. A cancer detection platform which measures telomerase activity from live circulating tumor cells captured on a microfilter. Cancer Res. 2010;70:6420-6426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 38. | Botteri E, Sandri MT, Bagnardi V, Munzone E, Zorzino L, Rotmensz N, Casadio C, Cassatella MC, Esposito A, Curigliano G. Modeling the relationship between circulating tumour cells number and prognosis of metastatic breast cancer. Breast Cancer Res Treat. 2010;122:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253:180-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 696] [Cited by in F6Publishing: 672] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 40. | Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213-3221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1354] [Cited by in F6Publishing: 1353] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 41. | Allen JE, El-Deiry WS. Circulating Tumor Cells and Colorectal Cancer. Curr Colorectal Cancer Rep. 2010;6:212-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6477] [Cited by in F6Publishing: 5032] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 43. | DeRisi J, Penland L, Brown PO, Bittner ML, Meltzer PS, Ray M, Chen Y, Su YA, Trent JM. Use of a cDNA microarray to analyse gene expression patterns in human cancer. Nat Genet. 1996;14:457-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1283] [Cited by in F6Publishing: 1367] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 44. | Khan J, Bittner ML, Chen Y, Meltzer PS, Trent JM. DNA microarray technology: the anticipated impact on the study of human disease. Biochim Biophys Acta. 1999;1423:M17-M28. [PubMed] [Cited in This Article: ] |
| 45. | Khan J, Saal LH, Bittner ML, Chen Y, Trent JM, Meltzer PS. Expression profiling in cancer using cDNA microarrays. Electrophoresis. 1999;20:223-229. [PubMed] [Cited in This Article: ] |
| 46. | Albertson DG. Profiling breast cancer by array CGH. Breast Cancer Res Treat. 2003;78:289-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 72] [Reference Citation Analysis (1)] |
| 47. | Ruano Y, Mollejo M, de Lope AR, Hernández-Moneo JL, Martínez P, Meléndez B. Microarray-based comparative genomic hybridization (array-CGH) as a useful tool for identifying genes involved in Glioblastoma (GB). Methods Mol Biol. 2010;653:35-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Al-Mulla F. Microarray-based CGH and copy number analysis of FFPE samples. Methods Mol Biol. 2011;724:131-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Sager R. Expression genetics in cancer: shifting the focus from DNA to RNA. Proc Natl Acad Sci USA. 1997;94:952-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 185] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 50. | Flach J, Dicker F, Schnittger S, Schindela S, Kohlmann A, Haferlach T, Kern W, Haferlach C. An accumulation of cytogenetic and molecular genetic events characterizes the progression from MDS to secondary AML: an analysis of 38 paired samples analyzed by cytogenetics, molecular mutation analysis and SNP microarray profiling. Leukemia. 2011;25:713-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Ntzani EE, Ioannidis JP. Predictive ability of DNA microarrays for cancer outcomes and correlates: an empirical assessment. Lancet. 2003;362:1439-1444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 52. | Arango D, Laiho P, Kokko A, Alhopuro P, Sammalkorpi H, Salovaara R, Nicorici D, Hautaniemi S, Alazzouzi H, Mecklin JP. Gene-expression profiling predicts recurrence in Dukes' C colorectal cancer. Gastroenterology. 2005;129:874-884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Lin YH, Friederichs J, Black MA, Mages J, Rosenberg R, Guilford PJ, Phillips V, Thompson-Fawcett M, Kasabov N, Toro T. Multiple gene expression classifiers from different array platforms predict poor prognosis of colorectal cancer. Clin Cancer Res. 2007;13:498-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 54. | Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7015] [Cited by in F6Publishing: 6109] [Article Influence: 254.5] [Reference Citation Analysis (0)] |
| 55. | Sadi AM, Wang DY, Youngson BJ, Miller N, Boerner S, Done SJ, Leong WL. Clinical relevance of DNA microarray analyses using archival formalin-fixed paraffin-embedded breast cancer specimens. BMC Cancer. 2011;11:253: 1-25313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Graham LD, Pedersen SK, Brown GS, Ho T, Kassir Z, Moynihan AT, Vizgoft EK, Dunne R, Pimlott L, Young GP. Colorectal Neoplasia Differentially Expressed (CRNDE), a Novel Gene with Elevated Expression in Colorectal Adenomas and Adenocarcinomas. Genes Cancer. 2011;2:829-840. [PubMed] [Cited in This Article: ] |
| 57. | Zanivan S, Gnad F, Wickström SA, Geiger T, Macek B, Cox J, Fässler R, Mann M. Solid tumor proteome and phosphoproteome analysis by high resolution mass spectrometry. J Proteome Res. 2008;7:5314-5326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Ocak S, Chaurand P, Massion PP. Mass spectrometry-based proteomic profiling of lung cancer. Proc Am Thorac Soc. 2009;6:159-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Bañez LL, Prasanna P, Sun L, Ali A, Zou Z, Adam BL, McLeod DG, Moul JW, Srivastava S. Diagnostic potential of serum proteomic patterns in prostate cancer. J Urol. 2003;170:442-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Huang YJ, Xuan C, Zhang BB, Liao M, Deng KF, He M, Zhao JM. SELDI-TOF MS profiling of serum for detection of nasopharyngeal carcinoma. J Exp Clin Cancer Res. 2009;28:85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Han KQ, Huang G, Gao CF, Wang XL, Ma B, Sun LQ, Wei ZJ. Identification of lung cancer patients by serum protein profiling using surface-enhanced laser desorption/ionization time-of-flight mass spectrometry. Am J Clin Oncol. 2008;31:133-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Lim JY, Cho JY, Paik YH, Chang YS, Kim HG. Diagnostic application of serum proteomic patterns in gastric cancer patients by ProteinChip surface-enhanced laser desorption/ionization time-of-flight mass spectrometry. Int J Biol Markers. 2007;22:281-286. [PubMed] [Cited in This Article: ] |
| 63. | Wu SP, Lin YW, Lai HC, Chu TY, Kuo YL, Liu HS. SELDI-TOF MS profiling of plasma proteins in ovarian cancer. Taiwan J Obstet Gynecol. 2006;45:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Bons JA, Wodzig WK, van Dieijen-Visser MP. Protein profiling as a diagnostic tool in clinical chemistry: a review. Clin Chem Lab Med. 2005;43:1281-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Zhou G, Li H, DeCamp D, Chen S, Shu H, Gong Y, Flaig M, Gillespie JW, Hu N, Taylor PR. 2D differential in-gel electrophoresis for the identification of esophageal scans cell cancer-specific protein markers. Mol Cell Proteomics. 2002;1:117-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 311] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 66. | Geiger T, Cox J, Ostasiewicz P, Wisniewski JR, Mann M. Super-SILAC mix for quantitative proteomics of human tumor tissue. Nat Methods. 2010;7:383-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 422] [Cited by in F6Publishing: 387] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 67. | Lee KK, Todorova K, Mandinova A. Maximizing early detection of esophageal squamous cell carcinoma via SILAC-proteomics. Cancer Biol Ther. 2010;10:811-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Kashyap MK, Harsha HC, Renuse S, Pawar H, Sahasrabuddhe NA, Kim MS, Marimuthu A, Keerthikumar S, Muthusamy B, Kandasamy K. SILAC-based quantitative proteomic approach to identify potential biomarkers from the esophageal squamous cell carcinoma secretome. Cancer Biol Ther. 2010;10:796-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 69. | Yocum AK, Busch CM, Felix CA, Blair IA. Proteomics-based strategy to identify biomarkers and pharmacological targets in leukemias with t(4; 11) translocations. J Proteome Res. 2006;5:2743-2753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Yang W, Cai Q, Lui VW, Everley PA, Kim J, Bhola N, Quesnelle KM, Zetter BR, Steen H, Freeman MR. Quantitative proteomics analysis reveals molecular networks regulated by epidermal growth factor receptor level in head and neck cancer. J Proteome Res. 2010;9:3073-3082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Imami K, Sugiyama N, Tomita M, Ishihama Y. Quantitative proteome and phosphoproteome analyses of cultured cells based on SILAC labeling without requirement of serum dialysis. Mol Biosyst. 2010;6:594-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Doucet A, Butler GS, Rodríguez D, Prudova A, Overall CM. Metadegradomics: toward in vivo quantitative degradomics of proteolytic post-translational modifications of the cancer proteome. Mol Cell Proteomics. 2008;7:1925-1951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 73. | Haab BB. Antibody arrays in cancer research. Mol Cell Proteomics. 2005;4:377-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 74. | Haab BB. Methods and applications of antibody microarrays in cancer research. Proteomics. 2003;3:2116-2122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 75. | Scholler N, Crawford M, Sato A, Drescher CW, O'Briant KC, Kiviat N, Anderson GL, Urban N. Bead-based ELISA for validation of ovarian cancer early detection markers. Clin Cancer Res. 2006;12:2117-2124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Zangar RC, Varnum SM, Covington CY, Smith RD. A rational approach for discovering and validating cancer markers in very small samples using mass spectrometry and ELISA microarrays. Dis Markers. 2004;20:135-148. [PubMed] [Cited in This Article: ] |
| 77. | Hawkins RD, Hon GC, Ren B. Next-generation genomics: an integrative approach. Nat Rev Genet. 2010;11:476-486. [PubMed] [Cited in This Article: ] |
| 78. | Ng SB, Turner EH, Robertson PD, Flygare SD, Bigham AW, Lee C, Shaffer T, Wong M, Bhattacharjee A, Eichler EE. Targeted capture and massively parallel sequencing of 12 human exomes. Nature. 2009;461:272-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1463] [Cited by in F6Publishing: 1401] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 79. | Bevilacqua L, Doly S, Kaprio J, Yuan Q, Tikkanen R, Paunio T, Zhou Z, Wedenoja J, Maroteaux L, Diaz S. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468:1061-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 80. | Alkan C, Kidd JM, Marques-Bonet T, Aksay G, Antonacci F, Hormozdiari F, Kitzman JO, Baker C, Malig M, Mutlu O. Personalized copy number and segmental duplication maps using next-generation sequencing. Nat Genet. 2009;41:1061-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 534] [Cited by in F6Publishing: 483] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 81. | Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8288] [Cited by in F6Publishing: 7701] [Article Influence: 513.4] [Reference Citation Analysis (0)] |
| 82. | Maher CA, Kumar-Sinha C, Cao X, Kalyana-Sundaram S, Han B, Jing X, Sam L, Barrette T, Palanisamy N, Chinnaiyan AM. Transcriptome sequencing to detect gene fusions in cancer. Nature. 2009;458:97-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 675] [Cited by in F6Publishing: 712] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 83. | Sultan M, Schulz MH, Richard H, Magen A, Klingenhoff A, Scherf M, Seifert M, Borodina T, Soldatov A, Parkhomchuk D. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science. 2008;321:956-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 958] [Cited by in F6Publishing: 917] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 84. | Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845-1848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1489] [Cited by in F6Publishing: 1469] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 85. | Farazi TA, Horlings HM, Ten Hoeve JJ, Mihailovic A, Halfwerk H, Morozov P, Brown M, Hafner M, Reyal F, van Kouwenhove M. MicroRNA sequence and expression analysis in breast tumors by deep sequencing. Cancer Res. 2011;71:4443-4453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 86. | Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1564] [Cited by in F6Publishing: 1459] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 87. | Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3299] [Cited by in F6Publishing: 3283] [Article Influence: 193.1] [Reference Citation Analysis (0)] |
| 88. | Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3346] [Cited by in F6Publishing: 3176] [Article Influence: 211.7] [Reference Citation Analysis (0)] |
| 89. | Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5009] [Cited by in F6Publishing: 4879] [Article Influence: 287.0] [Reference Citation Analysis (0)] |
| 90. | Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1718] [Cited by in F6Publishing: 1669] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 91. | Boyle AP, Davis S, Shulha HP, Meltzer P, Margulies EH, Weng Z, Furey TS, Crawford GE. High-resolution mapping and characterization of open chromatin across the genome. Cell. 2008;132:311-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1023] [Cited by in F6Publishing: 1003] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 92. | Palomero T, Ferrando AA. Genomic tools for dissecting oncogenic transcriptional networks in human leukemia. Leukemia. 2009;23:1236-1242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 93. | Sun H, Wu J, Wickramasinghe P, Pal S, Gupta R, Bhattacharyya A, Agosto-Perez FJ, Showe LC, Huang TH, Davuluri RV. Genome-wide mapping of RNA Pol-II promoter usage in mouse tissues by ChIP-seq. Nucleic Acids Res. 2011;39:190-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Welboren WJ, van Driel MA, Janssen-Megens EM, van Heeringen SJ, Sweep FC, Span PN, Stunnenberg HG. ChIP-Seq of ERalpha and RNA polymerase II defines genes differentially responding to ligands. EMBO J. 2009;28:1418-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 95. | Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462:58-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1306] [Cited by in F6Publishing: 1234] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 96. | Ule J, Jensen KB, Ruggiu M, Mele A, Ule A, Darnell RB. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302:1212-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 833] [Cited by in F6Publishing: 809] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 97. | Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1467] [Cited by in F6Publishing: 1504] [Article Influence: 115.7] [Reference Citation Analysis (0)] |
| 98. | Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479-486. [PubMed] [Cited in This Article: ] |
| 99. | Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jungkamp AC, Munschauer M. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2203] [Cited by in F6Publishing: 2157] [Article Influence: 154.1] [Reference Citation Analysis (0)] |
| 100. | Lebedeva S, Jens M, Theil K, Schwanhäusser B, Selbach M, Landthaler M, Rajewsky N. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol Cell. 2011;43:340-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 512] [Cited by in F6Publishing: 520] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 101. | Mukherjee N, Corcoran DL, Nusbaum JD, Reid DW, Georgiev S, Hafner M, Ascano M, Tuschl T, Ohler U, Keene JD. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol Cell. 2011;43:327-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 500] [Cited by in F6Publishing: 516] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 102. | Srikantan S, Gorospe M. UneCLIPsing HuR nuclear function. Mol Cell. 2011;43:319-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2720] [Cited by in F6Publishing: 2602] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 104. | Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2910] [Cited by in F6Publishing: 3017] [Article Influence: 215.5] [Reference Citation Analysis (0)] |
| 105. | Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10:669-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1327] [Cited by in F6Publishing: 1259] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 106. | Duldulao MP, Lee W, Le M, Chen Z, Li W, Wang J, Gao H, Li H, Kim J, Garcia-Aguilar J. Gene expression variations in microsatellite stable and unstable colon cancer cells. J Surg Res. 2012;174:1-6. [PubMed] [Cited in This Article: ] |
| 107. | Ma CX, Luo J, Ellis MJ. Molecular profiling of triple negative breast cancer. Breast Dis. 2010;32:73-84. [PubMed] [Cited in This Article: ] |