Peer-review started: September 11, 2016
First decision: November 14, 2016
Revised: November 18, 2016
Accepted: December 7, 2016
Article in press: December 9, 2016
Published online: March 27, 2017
Processing time: 193 Days and 18.9 Hours
Macrophages are key players in various immune responses. In addition to functions in innate immunity such as antigen phagocytosis and cytokine production, antigen presentation by macrophage represents a link between innate and acquired immunity. During inflammatory processes, naïve monocytes differentiate into pro-inflammatory M1 and anti-inflammatory M2 macrophages. Resident monocytes/macrophages contribute to immune response that maintains tissue-specific homeostasis. In the target organs of autoimmune diseases, macrophages have dual functions in both the induction and suppression of autoimmune responses, which are mediated by production of various cytokines and chemokines, or by interaction with other immune cells. This review focuses on selected autoimmune diseases, such as systemic lupus erythematosus, multiple sclerosis, rheumatoid arthritis, and Sjögren’s syndrome, to illustrate the key roles of macrophages in the cellular or molecular pathogenesis of autoimmunity. In addition, the contribution of macrophages to each autoimmune disease is compared.
Core tip: Macrophages are well known as phagocytic cells and the source of cytokines and other immunomodulators of the innate immune system. There are many reviews of macrophage function, but not many that focus on their role in autoimmunity and autoimmune disease. This review focuses on the role of tissue resident macrophages in autoimmunity both in general and several selected autoimmune diseases, develops a novel context for evaluation and a slightly different way of thinking of the complex interactions involved in “mistaken self-identity”.
- Citation: Ushio A, Arakaki R, Yamada A, Saito M, Tsunematsu T, Kudo Y, Ishimaru N. Crucial roles of macrophages in the pathogenesis of autoimmune disease. World J Immunol 2017; 7(1): 1-8
- URL: https://www.wjgnet.com/2219-2824/full/v7/i1/1.htm
- DOI: https://dx.doi.org/10.5411/wji.v7.i1.1
Autoimmunity proceeds via a complex interaction of immune responses by a variety of immune cells in both lymphoid and target organs[1]. In some autoimmune diseases, T-cell-mediated autoimmune responses are involved in the onset or development of disease. Autoreactive T cells are generated in the thymus and other peripheral lymphoid organs in an environment that includes other immune cells, stromal cells, and various epithelial cells[2,3]. Because the activities of autoreactive T cells are regulated by interactions with regulatory T (Treg) cells, dendritic cells, macrophages, and B cells, the pathogenesis of autoimmune diseases cannot be considered simply as a T-cell-mediated immune response[4]. The interactions of T cells with other immune cells in the pathogenesis of autoimmune disease have been described in many research reports[5].
Macrophages differentiate from bone marrow-derived monocytes or tissue resident cells that are derived from yolk sac or fetal liver, such as histiocytes, Kupffer cells, microglia, alveolar, peritoneal, and synovial macrophages[6,7]. In the innate immunity system, macrophages function as phagocytic cells that engulf and digest cellular debris, foreign substances, microbes, and pathogens[8]. They also secrete cytokines and chemokines that modulate the activities of other immune cells in the inflammatory lesions[8]. The third macrophage function in innate immune system is antigen presentation to T cells which represents a link between innate and acquired immunity as well as dendritic cell[8]. Macrophages also contribute to the recovery of injured tissue by promoting angiogenesis or fibrosis[9]. The functions of tissue-resident macrophages have been the topic of recent reviews.
Classically activated (M1) macrophages produce pro-inflammatory cytokines, such as interleukin (IL)-1β, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α that promote various inflammatory responses[6,10]. Alternatively activated (M2) macrophages produce anti-inflammatory cytokines, such as IL-10 and IL-4. There are three macrophage subsets (M2a, b, and c) with characteristic stimuli or cytokine profiles[6,10].
Although macrophages are involved in inflammatory stimuli, including autoimmunity, the lesions accompanying such responses are not induced by macrophages only. In addition, these cells also support tissue repair and immune homeostasis restoration. Therefore, the complex pathogenesis of autoimmune diseases can be seen as reflecting macrophage dysfunction. Further, the comparison regarding the contribution of macrophages to the pathogenesis between representative autoimmune diseases would be important for understanding the cellular mechanisms of the onset or development of autoimmune diseases. We review recent studies elucidating the role of macrophages in cellular and molecular mechanisms of autoimmune disease; moreover, we discuss potential novel clinical approaches to treat autoimmune diseases by targeting macrophages.
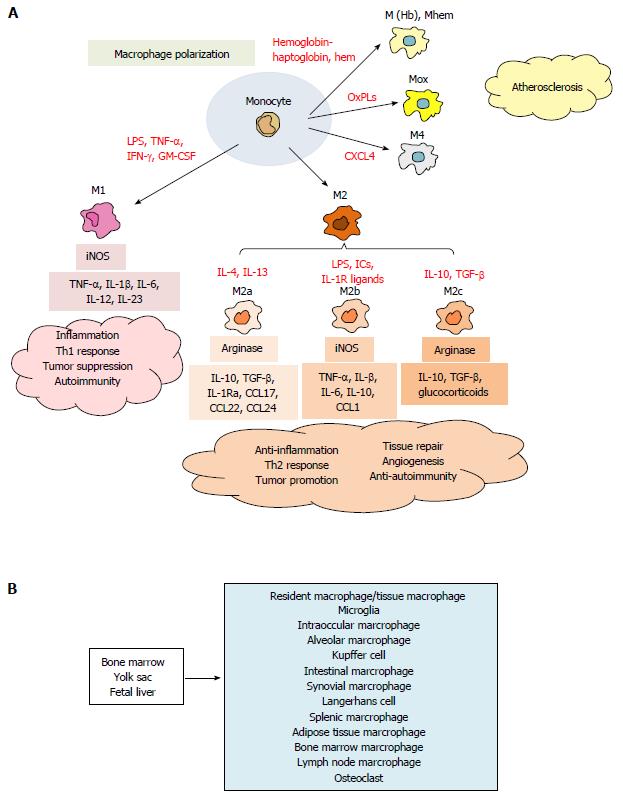
Monocytes differentiate into classically activated (M1) or alternatively activated (M2) macrophages following exposure to polarization signals such as cytokeines, chemokines, hormones, bacterial products, and lipids (Figure 1A). Exposure of naïve monocytes to IFN-γ, TNF-α, or lipopolysaccharide (LPS) induces M1 development. M1 macrophages produce pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-12 and IL-23, which in turn promote development and responses of Th1 cells[6,9]. By contrast, M2 macrophages are further classified into three subpopulations, depending on the response to various stimuli[6,10]. Exposure of naïve monocytes to IL-4 and IL-13 promotes M2a macrophages which express arginase I and produce IL-10, TGF-β, IL-1Ra, CCL17, CCL22 and CCL24 to promote Th2 cells, eosinophils and basophils (Figure 1A)[6-10]. M2b macrophage development is induced by LPS, immune complexes (ICs), or apoptotic cells, In turn, M2b macrophages express inducible nitric oxide synthase and produce high level of IL-10, TNF-α, IL-1β, IL-6 and CCL1, which then promote recruitment of eosinophils and Treg cells (Figure 1A)[6-10]. M2c macrophages are elicited by IL-10, TGF-β, or glucocorticoids and express arginase I. M2c macrophages exert an immunosuppressive function by promoting the development of Th2 cells and Treg cells (Figure 1A)[6-10].
In addition to the functional macrophage subtypes, there are subpopulations of tissue resident macrophages. These include microglia in the brain, intraocular macrophage in the eye, Langerhans cells in the skin (dermal DCs), salivary macrophage in the salivary glands, alveolar macrophage in the lung, splenic macrophages, Kupffer cells in the liver, intestinal macrophages, subcapsular sinusoidal macrophages and medullary macrophage in the lymph nodes, bone marrow macrophages, and osteoclasts in bone, all of which contribute to various tissue-specific immune surveillance (Figure 1B)[9]. For example, alveolar macrophages remove foreign antigens or allergens in the lung: Kupffer cells contribute to clearance of pathogens and toxin in the liver[8,9]. Recent studies demonstrate that resident macrophages derives from yolk sac and fetal liver in addition to bone marrow (Figure 1B)[7]. Adipose tissue macrophage (ATM) are involved in the pathogenesis of metabolic diseases such as obesity and type 2 diabetes, in which accumulation of M1 ATMs promote inflammation and insulin resistance[11,12]. Resident macrophages are located in the all tissues, and play important roles in immune surveillance function to maintain the homeostasis.
Macrophage accumulation within the vascular wall is a hallmark of atherosclerosis. The lesional macrophages are derived from both blood monocytes and smooth muscle cells, and accumulate lipoproteins by macropinocytosis, phagocytosis, and binding to scavenger receptors, such as SR-A, SR-BI, CD36, and LOX1[13]. The vascular subsets include M(Hb) and Mhem macrophages that are resistant to lipid loading and are induced by hemoglobin-haptoglobin complexes and hem[13-15]. The Mox macrophage subset is induced by exposure to oxidized phospholipids and is characterized by expression of high levels of heme oxygenase-1[13,16,17]. In addition, M4 macrophage is induced by CXCL4, and associated with the pathogenesis of atherosclerosis (Figure 1A)[13,16-18].
Macrophages are also a functional link between inflammation and cancer. There is strong evidence that tumor-associated macrophages (TAMs) can promote tumor progression[19,20]. Cytotoxic killing by M1 TAMs, has an antitumor effect, but angiogenesis stimulated by vascular endothelial growth factor produced by M2 macrophages promotes tumor growth[21,22]. A number of diverse macrophage-associated phenotypes contribute to tumorigenesis in the complex tumor microenvironment.
Impaired engulfment of dead cells by macrophage results in activation of autoimmune responses leading, severe autoimmune anemia, and chronic arthritis[23-26]. Apoptotic cells release “find me” signals, such as lysophosphatidylcholine, to attract phagocytes and expose “eat me” signals on the cell surface that stimulate engulfment[23,27]. One of the “eat me” signals is phosphatidylserine, which is exposed on the cell surface during apoptosis. Macrophages recognize phosphatidylserine, engulf the apoptotic cells, which are transferred to the lysosomes and degraded by lysosomal enzymes. Bridging molecules such as milk fat globule EGF factor 8 (MFG-E8) and growth arrest-specific 6 (Gas6) protein mediate binding between phosphatidylserine on the apoptotic cells, and integrin and tyrosine-kinase receptors on macrophages[23,26]. The clearance of apoptotic cells in the body is controlled by complex molecular and cellular interaction with macrophages.
If apoptotic cells are not engulfed, then they undergo secondary necrosis, in which the plasma membrane disintegrates with release of the cellular contents, which then bind to immunoglobulins and complement proteins, and activate macrophages and B cells. In addition to Fc receptors and B cell receptors, also Toll-like receptors are able to recognize the necrotic cell components and activate macrophages and B cells. The activated macrophages secrete cytokines that stimulate B cells to produce autoantibodies able to cause pathological conditions such as systemic erythematosus (Table 1)[23,26]. In addition, if lysosomal digestion is defective, the dead cell components accumulate in the lysosomes, leading to intracellular activation of pro-inflammatory cytokines such as IFN-β and TNF-α production by the innate immune system[23,26]. Thus, both extracellular activation of immune responses by apoptotic cells that are not phagocytized and intracellular activation of macrophages by impaired processing of apoptotic cells contribute to the onset or development of autoimmunity.
| Autoimmune disease | Remarks of macrophage |
| SLE | Impaired engulfment, IFN-β, TNF-α, B cell activation |
| MS | M1 (CCR4, TNF-α, IL-1β, IL-12, NO), M2 (IL-4, IL-10, IL-13, TGF-β) |
| RA | M1 (TNF-α, IL-1β, IRF5), M2 (IL-10, IL-4, IL-13, IRF3, IRF4, NF-κB) |
| SS | M1 (IL-1β, IL-6, TNF-α, MCP-1) |
Multiple sclerosis (MS) is a debilitating neurological disorder of the central nervous system (CNS). It is a T-cell-mediated autoimmune disease with clinical signs and symptoms characterized by weakness and progressive paralysis[28,29]. Studies of experimental autoimmune encephalomyelitis (EAE), an animal model of MS, have demonstrated that autoreactive T cells against myelin proteins play a key role in disease development[30,31]. Th1 and Th17 cells trigger autoreactive responses within the CNS through pro-inflammatory cytokines including IFN-γ, IL-17, IL-12, and IL-23. At later phases of MS, Th2 and Treg cells contribute to controlling inflammation.
Macrophages participate in the pathogenesis of EAE[32,33]. Indeed, there are few infiltrating macrophages in the CNS under physiological conditions. However, during induction and exacerbations of EAE, macrophages infiltrate the meninges surrounding the CNS, the perivascular space, and the choroid plexus[34,35]. The number of infiltrating macrophages in the CNS decreased during remissions in parallel with the decrease in lymphocyte infiltrates[34,35]. The expression of chemokines and chemokine receptors by CNS macrophages contributes to the induction and progression of EAE[36]. EAE studies have demonstrated that up-regulation of CCR2, CCL2, CCL3, CCL4 and CCL22 induces macrophage accumulation and effector function in the CNS[37,38]. Moreover, CCR4, a receptor for CCL17 and CCL22, is up-regulated in macrophages present in the CNS lesions[39]. CCR4 gene knockout mice exhibit lower macrophage infiltrates in the CNS, and exhibit milder EAE symptoms than those seen in wild type mice[40].
Both M1 and M2 macrophages play important roles in enhancing and regulating the pathogenesis of EAE. TNF-α, IL-1β, IL-12 and nitric oxide, expressed by activated M1 macrophages, induce inflammation and tissue damage in the CNS[41-43]. Fewer M2 than M1 macrophages are present in the CNS during exacerbations in EAE mice. An increase in the M2 macrophage population contributes to anti-inflammatory effect associated with increased production of IL-4, IL-10, IL-13 and TGF-β[44]. Moreover, M2 macrophages are thought to have more regulatory function in the pathogenesis of EAE[45]. In addition to tissue repair, the anti-inflammatory cytokines produced by M2 macrophages drive differentiation and recruitment of Th2 and Treg cells to suppress the autoimmune response in EAE[45]. Adoptive transfer of M2 macrophages into EAE mice significantly inhibits disease development[41,44]. Thus, macrophages play key roles in the pathogenesis of MS (Table 1).
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by inflammation of the synovial lining of the joint capsule. Pathological characteristics of RA lesions include immune cell infiltration, synovial cell hyperproliferation, fibrosis (pannus formation), and destruction of cartilage and bone[46]. Various immune cells are observed in RA lesions, including CD4+ T cells, CD8+ T cells, B cells, natural killer (NK) cells, γδT cells, mast cells, dendritic cells, and macrophages[47,48]. Monocyte/macrophage-derived cytokines, such as TNF-α, IL-1β, IL-12, IL-6, IL-15, IL-18 and IL23, trigger and enhance the activation and recruitment of Th1 and Th17 T cells in the synovial tissues of RA patients and animal models[49]. In addition, activated macrophages also play an important role in controlling Treg cells in the pathogenesis of RA[49,50].
Synovial macrophages are resident cells in the synovial tissues of healthy joints[49]. The macrophages become activated and polarized to form M1 or M2 phenotype within RA lesions. However, inflammatory synovial macrophages have not yet been classified into a phenotype. Many M1 macrophages that produce TNF-α and IL-1β are observed in the synovial tissues of RA patients along with M2 macrophages that produce IL-10. The ratio of M1 to M2 macrophages present in synovial lesions varies in relation to disease stage. IFN-γ and TNFα promote polarization to M1 macrophages during synovial inflammation in early stage disease[51,52]. IFN regulatory factor (IRF) 5 is thought to be a key transcription factor for M1 macrophage differentiation[53]. IL-10, IL-4, IL-13 and ICs promote polarization to M2 macrophages and suppression of synovial inflammation at later stage[54]. M2 macrophage differentiation is controlled by a lot of number of transcription factors, including IRF3, IRF4, and nuclear factor (NF)-κB (Table 1)[55-57].
Therapies targeting monocyte/macrophage have been used to treat RA. Inhibition of TNF-α produced by synovial inflammatory macrophages promotes IL-10 expression by CD4+ T cells, enhances Treg cell function, promotes monocyte apoptosis via transmembrane (tm)TNF-α, and is associated with an antiosteoclast effect[58-63]. Inhibition of IL-6 signaling enhances the frequency of Treg cell, and monocyte apoptosis[64-69]. Abatacept [cytotoxic T-lymphocyte antigen 4-Ig (CTLA4-Ig)] inhibits both the interaction between monocytes/macrophages and T cells, and monocyte differentiation into osteoclasts[70]. Therefore, macrophages contribute to the pathogenesis of RA directly or indirectly. Clinical use of agents that target macrophage function would likely be effective for treating RA.
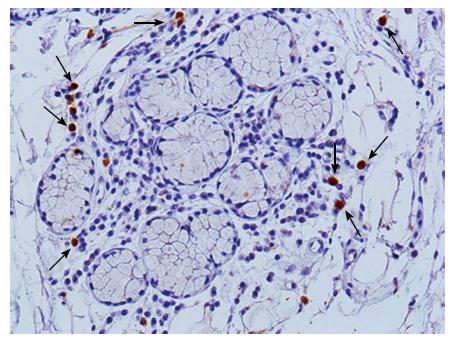
Sjögren’s syndrome (SS) is a chronic autoimmune disease that targets exocrine glands, such as salivary and lacrimal glands, and also causes systemic autoimmune lesions[71]. The mononuclear cell populations infiltrating the salivary gland tissues of SS patients include CD4+ T cells, CD8+ T cells, Treg cells, B cells, NK cells, DCs and macrophages[72]. Among them, infiltration of CD4+ T cells, Treg cells, B cells, DCs and macrophages is correlated to lesion severity[73]. SS is triggered by T-cell-mediated autoimmune responses; however, also other immune cells contribute to the onset or development of SS, including macrophages. Macrophages are observed in the autoimmune lesions of the salivary gland tissues from SS patients (Figure 2). Indeed, an elevated expression of macrophage-derived molecules, such as chitinase-3-like protein 1 and chitinase 1, is associated with increased severity of SS lesions, suggesting that macrophages are involved in the pathogenesis of SS[74]. In addition, pro-inflammatory cytokines produced by macrophages, such as TNF-α, IL-1β, IL-6 and IL-12, have been associated with the induction of autoimmune lesions in the target glands of MRLlpr/lpr mice, a murine model of SS[75]. In a SS model using autoimmune regulator (AIRE) gene knockout (KO) mice, many macrophages in addition to CD4+ T cells infiltrated the corneal stroma, limbus, and lacrimal glands[76]. Adoptive transfer of CD4+ T cells from AIRE KO mice into immunodeficient recipient mice resulted in local infiltration of macrophages in the target tissue[76]. Moreover, in vivo depletion of macrophages by injection of clodronate liposome into AIRE KO mice attenuated dry eye symptoms[76]. Therefore, autoreactive T cells may elicit macrophage infiltration into the target organs of macrophage-associated autoimmune lesions (Table 1).
Aromatase is an enzyme that converts androgens to estrogens. Aromatase gene knockout (ArKO) mice develop marked abdominal adiposity, suggesting that aromatase regulates lipid metabolism[77,78]. ArKO mice also spontaneously develop an autoimmune disease in exocrine glands, such as salivary and lacrimal glands that resembles SS[79]. We reported significantly increased expression of mRNA encoding pro-inflammatory cytokines, IL-1β, IL-6, IFN-γ, TNF-α, and monocyte chemotactic protein-1 (MCP-1) in white adipose tissue of ArKO mice[80]. We also found an increased number of inflammatory M1 macrophages in white adipose tissue of ArKO mice, and significant enhancement of MCP-1 mRNA expression in the salivary gland tissue[80]. The severity of autoimmune lesions in a murine SS model exacerbated by administration of an aromatase inhibitor, and the percentage of macrophages in the spleen of SS model mice treated with aromatase inhibitor was significantly higher than that in control mice[80]. Collectively, the data indicates that aromatase may be involved in the pathogenesis of SS-like lesions by controlling the target organ- and adipose tissue-associated M1 macrophages.
Macrophages have dual functions in promotion and regulation of inflammation. The differentiation and distribution of macrophages influence the onset or development of systemic and organ-specific autoimmune diseases. Macrophages serve as a bridge between innate and adoptive immunity to maintain immunological homeostasis, and macrophage dysfunction or impairment leads to the induction of severe immune disorder. Clinical interventions targeting macrophages may result in discovery of novel treatments of immune disorders, including autoimmune diseases.
Manuscript source: Invited manuscript
Specialty type: Immunology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: La Cava A, Martinez-Costa OH, Yankee T, Zhou S S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Cho JH, Feldman M. Heterogeneity of autoimmune diseases: pathophysiologic insights from genetics and implications for new therapies. Nat Med. 2015;21:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 2. | Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 3. | Waldmann H. Tolerance: an overview and perspectives. Nat Rev Nephrol. 2010;6:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 5. | Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat Immunol. 2014;15:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1178] [Cited by in RCA: 1565] [Article Influence: 142.3] [Reference Citation Analysis (0)] |
| 7. | Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3452] [Article Influence: 287.7] [Reference Citation Analysis (0)] |
| 8. | Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1742] [Cited by in RCA: 2248] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 9. | Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4053] [Cited by in RCA: 3860] [Article Influence: 275.7] [Reference Citation Analysis (0)] |
| 10. | Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2914] [Article Influence: 194.3] [Reference Citation Analysis (0)] |
| 11. | Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5334] [Cited by in RCA: 5420] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 12. | Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 1030] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 13. | Tabas I, Bornfeldt KE. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ Res. 2016;118:653-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 805] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 14. | Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174:1097-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 15. | Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 16. | Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 449] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 17. | Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 505] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 18. | Erbel C, Akhavanpoor M, Okuyucu D, Wangler S, Dietz A, Zhao L, Stellos K, Little KM, Lasitschka F, Doesch A. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J Immunol. 2014;193:4344-4355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8312] [Article Influence: 488.9] [Reference Citation Analysis (0)] |
| 20. | Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3122] [Cited by in RCA: 3918] [Article Influence: 261.2] [Reference Citation Analysis (0)] |
| 21. | Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1660] [Article Influence: 87.4] [Reference Citation Analysis (2)] |
| 22. | Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2416] [Cited by in RCA: 2564] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 23. | Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 668] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 24. | Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y, Nagata S. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. 2001;292:1546-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 25. | Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 26. | Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 759] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 27. | Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207-2216. [PubMed] |
| 28. | Hickey WF. The pathology of multiple sclerosis: a historical perspective. J Neuroimmunol. 1999;98:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Banwell B, Bar-Or A, Arnold DL, Sadovnick D, Narayanan S, McGowan M, O’Mahony J, Magalhaes S, Hanwell H, Vieth R. Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: a prospective national cohort study. Lancet Neurol. 2011;10:436-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 30. | Almolda B, Gonzalez B, Castellano B. Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front Biosci (Landmark Ed). 2011;16:1157-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Jiang Z, Li H, Fitzgerald DC, Zhang GX, Rostami A. MOG(35-55) i.v suppresses experimental autoimmune encephalomyelitis partially through modulation of Th17 and JAK/STAT pathways. Eur J Immunol. 2009;39:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Rawji KS, Yong VW. The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clin Dev Immunol. 2013;2013:948976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 33. | Abourbeh G, Thézé B, Maroy R, Dubois A, Brulon V, Fontyn Y, Dollé F, Tavitian B, Boisgard R. Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis rats by positron emission tomography using the mitochondrial 18 kDa translocator protein radioligand [18F]DPA-714. J Neurosci. 2012;32:5728-5736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 656] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 35. | Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nat Neurosci. 2012;15:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 36. | Dogan RN, Long N, Forde E, Dennis K, Kohm AP, Miller SD, Karpus WJ. CCL22 regulates experimental autoimmune encephalomyelitis by controlling inflammatory macrophage accumulation and effector function. J Leukoc Biol. 2011;89:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 476] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 38. | Jiang Z, Jiang JX, Zhang GX. Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis. Immunol Lett. 2014;160:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 39. | Forde EA, Dogan RN, Karpus WJ. CCR4 contributes to the pathogenesis of experimental autoimmune encephalomyelitis by regulating inflammatory macrophage function. J Neuroimmunol. 2011;236:17-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Columba-Cabezas S, Serafini B, Ambrosini E, Sanchez M, Penna G, Adorini L, Aloisi F. Induction of macrophage-derived chemokine/CCL22 expression in experimental autoimmune encephalomyelitis and cultured microglia: implications for disease regulation. J Neuroimmunol. 2002;130:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Jiang HR, Milovanović M, Allan D, Niedbala W, Besnard AG, Fukada SY, Alves-Filho JC, Togbe D, Goodyear CS, Linington C. IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages. Eur J Immunol. 2012;42:1804-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 42. | Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1365] [Cited by in RCA: 1828] [Article Influence: 152.3] [Reference Citation Analysis (0)] |
| 43. | Porcheray F, Viaud S, Rimaniol AC, Léone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 557] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 44. | Denney L, Kok WL, Cole SL, Sanderson S, McMichael AJ, Ho LP. Activation of invariant NKT cells in early phase of experimental autoimmune encephalomyelitis results in differentiation of Ly6Chi inflammatory monocyte to M2 macrophages and improved outcome. J Immunol. 2012;189:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 45. | Liu C, Li Y, Yu J, Feng L, Hou S, Liu Y, Guo M, Xie Y, Meng J, Zhang H. Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PLoS One. 2013;8:e54841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 46. | Klareskog L, Padyukov L, Rönnelid J, Alfredsson L. Genes, environment and immunity in the development of rheumatoid arthritis. Curr Opin Immunol. 2006;18:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 47. | McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1662] [Cited by in RCA: 1866] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 48. | McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3395] [Cited by in RCA: 3856] [Article Influence: 275.4] [Reference Citation Analysis (0)] |
| 49. | Kennedy A, Fearon U, Veale DJ, Godson C. Macrophages in synovial inflammation. Front Immunol. 2011;2:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 50. | Afzali B, Mitchell P, Lechler RI, John S, Lombardi G. Translational mini-review series on Th17 cells: induction of interleukin-17 production by regulatory T cells. Clin Exp Immunol. 2010;159:120-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 51. | Erwig LP, Kluth DC, Walsh GM, Rees AJ. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol. 1998;161:1983-1988. [PubMed] |
| 52. | Wallet MA, Wallet SM, Guiulfo G, Sleasman JW, Goodenow MM. IFNgamma primes macrophages for inflammatory activation by high molecular weight hyaluronan. Cell Immunol. 2010;262:84-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 1010] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 54. | Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4154] [Cited by in RCA: 4832] [Article Influence: 241.6] [Reference Citation Analysis (1)] |
| 55. | Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood. 2006;107:2112-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 528] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 56. | Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 917] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 57. | Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, Dieli F, Ghisletti S, Natoli G, De Baetselier P. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci USA. 2009;106:14978-14983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 501] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 58. | Evans HG, Roostalu U, Walter GJ, Gullick NJ, Frederiksen KS, Roberts CA, Sumner J, Baeten DL, Gerwien JG, Cope AP. TNF-α blockade induces IL-10 expression in human CD4+ T cells. Nat Commun. 2014;5:3199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 59. | Boks MA, Kager-Groenland JR, Mousset CM, van Ham SM, ten Brinke A. Inhibition of TNF receptor signaling by anti-TNFα biologicals primes naïve CD4(+) T cells towards IL-10(+) T cells with a regulatory phenotype and function. Clin Immunol. 2014;151:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 894] [Cited by in RCA: 962] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 61. | Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 628] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 62. | Meusch U, Krasselt M, Rossol M, Baerwald C, Klingner M, Wagner U. In vitro response pattern of monocytes after tmTNF reverse signaling predicts response to anti-TNF therapy in rheumatoid arthritis. J Transl Med. 2015;13:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Smolen JS, Han C, Bala M, Maini RN, Kalden JR, van der Heijde D, Breedveld FC, Furst DE, Lipsky PE. Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: a detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum. 2005;52:1020-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 347] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 64. | Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J, Ornetti P, Maillefert JF, Miossec P, Bonnotte B. Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:2499-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 284] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 65. | Pesce B, Soto L, Sabugo F, Wurmann P, Cuchacovich M, López MN, Sotelo PH, Molina MC, Aguillón JC, Catalán D. Effect of interleukin-6 receptor blockade on the balance between regulatory T cells and T helper type 17 cells in rheumatoid arthritis patients. Clin Exp Immunol. 2013;171:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 66. | Thiolat A, Semerano L, Pers YM, Biton J, Lemeiter D, Portales P, Quentin J, Jorgensen C, Decker P, Boissier MC. Interleukin-6 receptor blockade enhances CD39+ regulatory T cell development in rheumatoid arthritis and in experimental arthritis. Arthritis Rheumatol. 2014;66:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 67. | Kikuchi J, Hashizume M, Kaneko Y, Yoshimoto K, Nishina N, Takeuchi T. Peripheral blood CD4(+)CD25(+)CD127(low) regulatory T cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: increase in regulatory T cells correlates with clinical response. Arthritis Res Ther. 2015;17:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 68. | Sarantopoulos A, Tselios K, Gkougkourelas I, Pantoura M, Georgiadou AM, Boura P. Tocilizumab treatment leads to a rapid and sustained increase in Treg cell levels in rheumatoid arthritis patients: comment on the article by Thiolat et al. Arthritis Rheumatol. 2014;66:2638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Tono T, Aihara S, Hoshiyama T, Arinuma Y, Nagai T, Hirohata S. Effects of anti-IL-6 receptor antibody on human monocytes. Mod Rheumatol. 2015;25:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Bozec A, Zaiss MM, Kagwiria R, Voll R, Rauh M, Chen Z, Mueller-Schmucker S, Kroczek RA, Heinzerling L, Moser M. T cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6:235ra60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 71. | Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1086] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 72. | Fox RI, Robinson CA, Curd JG, Kozin F, Howell FV. Sjögren’s syndrome. Proposed criteria for classification. Arthritis Rheum. 1986;29:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 454] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 73. | Katsifis GE, Moutsopoulos NM, Wahl SM. T lymphocytes in Sjögren’s syndrome: contributors to and regulators of pathophysiology. Clin Rev Allergy Immunol. 2007;32:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Greenwell-Wild T, Moutsopoulos NM, Gliozzi M, Kapsogeorgou E, Rangel Z, Munson PJ, Moutsopoulos HM, Wahl SM. Chitinases in the salivary glands and circulation of patients with Sjögren’s syndrome: macrophage harbingers of disease severity. Arthritis Rheum. 2011;63:3103-3115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Mustafa W, Zhu J, Deng G, Diab A, Link H, Frithiof L, Klinge B. Augmented levels of macrophage and Th1 cell-related cytokine mRNA in submandibular glands of MRL/lpr mice with autoimmune sialoadenitis. Clin Exp Immunol. 1998;112:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Zhou D, Chen YT, Chen F, Gallup M, Vijmasi T, Bahrami AF, Noble LB, van Rooijen N, McNamara NA. Critical involvement of macrophage infiltration in the development of Sjögren’s syndrome-associated dry eye. Am J Pathol. 2012;181:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735-12740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 553] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 78. | Misso ML, Murata Y, Boon WC, Jones ME, Britt KL, Simpson ER. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology. 2003;144:1474-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 79. | Shim GJ, Warner M, Kim HJ, Andersson S, Liu L, Ekman J, Imamov O, Jones ME, Simpson ER, Gustafsson JA. Aromatase-deficient mice spontaneously develop a lymphoproliferative autoimmune disease resembling Sjogren’s syndrome. Proc Natl Acad Sci USA. 2004;101:12628-12633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 80. | Iwasa A, Arakaki R, Honma N, Ushio A, Yamada A, Kondo T, Kurosawa E, Kujiraoka S, Tsunematsu T, Kudo Y. Aromatase controls Sjögren syndrome-like lesions through monocyte chemotactic protein-1 in target organ and adipose tissue-associated macrophages. Am J Pathol. 2015;185:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |