Peer-review started: August 5, 2015
First decision: October 13, 2015
Revised: November 24, 2015
Accepted: December 17, 2015
Article in press: December 18, 2015
Published online: March 27, 2016
Processing time: 249 Days and 9.6 Hours
The classical view of signaling between cells of immune system includes two major routes of intercellular communication: Through the release of extracellular molecules or a direct interaction between membrane bound receptor and its membrane bound ligand, which initiate a cascade of signaling in target cell. However, recent studies indicate that besides these canonical modes of signaling there are also noncanonical routs of intercellular communications through membrane stripping/membrane exchange/trogocytosis, extracellular traps, exosomes and ectososmes/microparticles. In this review we discuss what are the components of noncanonical pathways of signaling and what role they play in immune cells interactions.
Core tip: Noncanonical routes of intercellular communications through membrane stripping, trogocytosis, extracellular traps, microparticles and exosomes and their function in immune response are highlighted.
- Citation: Kloc M, Kubiak JZ, Li XC, Ghobrial RM. Noncanonical intercellular communication in immune response. World J Immunol 2016; 6(1): 67-74
- URL: https://www.wjgnet.com/2219-2824/full/v6/i1/67.htm
- DOI: https://dx.doi.org/10.5411/wji.v6.i1.67
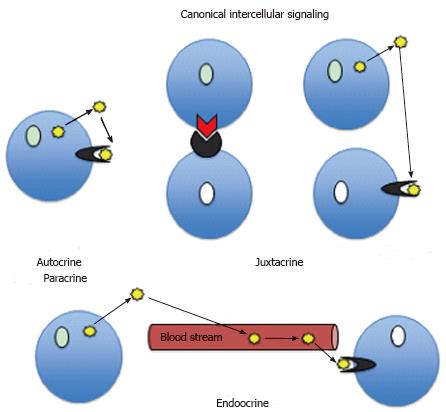
For many decades general belief has been that during an immune response the immune cells communicate either by a direct contact between molecules anchored at the plasma membrane of adjacent cells (juxtracrine signaling) or via short (autocrine and paracrine) or long (endocrine) distance signaling using various cytokines or hormones and their cognate receptors systems (Figure 1). However, recent years studies, amounting to hundreds of publications, indicate that besides these well-known (canonical) signaling pathways there is a cornucopia of nonclassical (noncanonical) signaling mechanisms, which modify behavior of immune cells and shape the immune response. Below we give a brief overview of features and functions of these noncanonical signaling pathways.
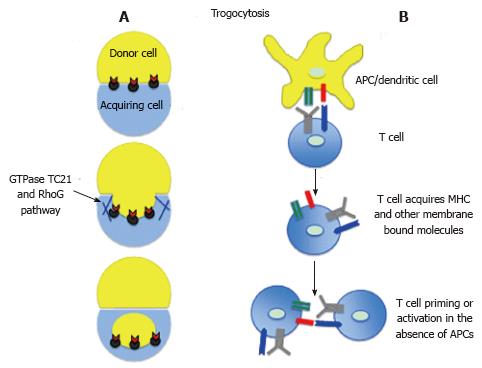
The trogocytosis - the nibbling (gnawing) off the fragments of live cells is probably an ancient mechanism applied by feeding amoebas[1,2]. However, in contrast to amoebic trogocytosis, which ultimate goal is devouring, and death of cellular pray, the immune cells developed mechanisms of vital trogocytosis or membrane stripping or exchange (Figure 2). During such a process, upon close contact between cells, the recipient cells acquire (borrow) foreign molecules, which they normally do not produce, and the donor cells either completely lose given molecules or the level of these molecules become, at least temporarily, reduced[3,4]. Recent studies indicate that the membrane exchange between various cell types, including immune cells, is a much more common and frequent phenomenon than previously thought. The in vitro and in vivo studies in different model systems showed that upon disengagement from immunological synapse with antigen presenting cells (APCs) or from a direct contact with other target cells, the activated CD4+, CD8+ T cells, regulatory T cells, helper T cells, macrophages, B cells, monocytes, granulocytes and natural killer (NK) cells, are able to internalize fragments of APCs/target cell membrane together with monoclonal antibodies, antigens, ligands, major histocompatibility complex (MHC), adhesion or co-stimulatory molecules[5-18]. For example, studies of Baba et al[19] showed that OX40 ligand (OX40L) expressed by COS-1 cells is transferred to CD4+ T (OX40L-, OX40+) cells, and that the acquired OX40L is functionally active. Other studies showed that the trogocytic acquisition of m157 (the murine cytomegalovirus-encoded ligand for the Ly49H-activating receptor) from target cells regulates NK cells function making them hypo-responsive both in vivo and in vitro[8]. In contrast, acquisition of anti-CD19 chimeric antigen receptors by NK cells enhances their cytotoxicity against the B-cell acute lymphoblastic leukemia cells[7]. Trogocytosis can also lead to acquisition of the MHC complexes by the non-APCs, which in turn may reinforce and/or propagate immune response, and activate or regulate T cells[4]. There are indications that trogocytosis/membrane internalization depends on GTPase TC21 and RhoG-dependent phagocytosis pathway (Figure 2)[16,20]. Membrane internalization not only leads to the acquisition of novel qualities by recipient cells but may also down-regulates the MHC/antigen/co-stimulatory molecules level in bestower APCs[11,16]. There are also instances of multicellular exchange and serial trogocytosis when immune cells acquire novel molecules from multiple sources and then transfer them to other recipient cells. For example the membrane bound molecules from multiple cancer cells can be acquired by CD4+ and CD8+ T cells and monocytes through multiple trogocytosis[21]. It has been shown that monocytes are able to transfer these molecules to other T cells[21]. Thus, trogocytosis/membrane exchange/stripping leads to acquisition/depletion of molecules and their cognate functions in recipient/donor cell, and ultimately modify or modulate an immune response (Figure 2)[3,4,6,18,22,23]. Trogocytosis and its outcomes can be either beneficial or harmful for the organism. Depending on circumstances and cell partners involved the trogocytosis may either promote or prevent development of various pathological conditions or diseases. For example trogocytosis is involved in the ablation of red blood cells in autoimmune hemolytic anemia[24] but when it removes antibodies binding to self-antigens it can prevent autoimmune diseases[25]. Another example of harmful trogocytosis is “oncologic trogocytosis” occurring between ovarian epithelial cancer cells and stromal cells allows cancer cells to acquire multiple drug resistance protein and thus chemoresitance[26].
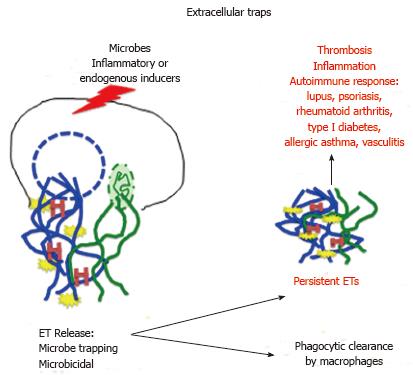
Extracellular traps (ETs) were discovered in 2004 in neutrophils and thus have been named Neutrophil Extracellular Traps (NETs)[27]. The process of ETs and NETs formation is called ETosis and NETosis, respectively. ETs consist of filamentous network of chromosomal and/or mitochondrial DNA, which is released from the cell after the break of nuclear/mitochondrial membrane (Figure 3). Because the process of ETosis involves nuclear/mitochondrial/plasma membrane breakage it usually leads to a suicidal, distinct from apoptosis or necrosis, death of ETs’ producing cells[28]. However, there are instances of non-suicidal (vital) NETosis, when nucleus-deprived neutrophils retain motility and chemotactic and phagocytic functions[29,30]. Another example is the vital mitochondrial NETosis when neutrophils primed, for example, with granulocyte/macrophage colony-stimulating factorand stimulated with short-term toll-like receptor 4 or complement factor 5a receptor retain intact nucleus and produce NETs containing exclusively mitochondrial DNA[31]. Besides DNA, the ETs contain various histones (which by themselves have antimicrobial properties) and a plethora of antimicrobial enzymes (Figure 3)[32-35]. The main role of ETs is the immobilization of microbes, which prevents dissemination, and exposing them to a high concentration of antimicrobial agents. Interestingly, many microbes developed defense mechanisms allowing them to escape from or neutralize ETs or ETs producing cells. For example Staphylococcus and Vibrio cholera produce endonucleases, which digest NETs, or/and convert NETs’ DNA into toxic metabolite (deoxyadenosine), which induces apoptosis and promotes death of immune cells[35-37]. The fact that ETosis occurs in many different cell types, and not only in vertebrates but also in invertebrates and plants, suggests that ETs are one of the primordial and evolutionary ancient mechanism of host defense. Studies of molecular pathways involved in initiation and execution of NETosis indicate that stimulation with microbes, inflammatory molecules or endogenous inducers leads, via protein kinase C and NADPH oxidase, to the production of reactive oxygen species and nitrix oxide[35]. These, in turn, induce nuclear/mitochondrial/granule membrane rapture, followed by proteolytic cleavage, deamination (citrullination) of histones, chromatin decondensation and eventual release of NETs[38]. It has been shown that besides consistent presence of DNA the other components of ETs vary, as they are stimulatory signal-specific and cell type-specific[32]. Although ETs play beneficial role in host defense, the presence of DNA and various enzymes makes ETs harmful, especially if they persist for long period of time; they become a very potent inducer of autoimmune response and various pathological conditions, such as lupus, psoriasis, vasculitis, rheumatoid arthritis, type I diabetes, allergic asthma and deep-vein thrombosis (Figure 3)[33,35,39]. It has been also shown that, at least in vitro, ETs influence the behavior of immune cells. NETs are able to down regulate lipopolysaccharide-induced activation of monocyte-derived dendritic cells, inhibit their capacity to activate proliferation of CD4+ T lymphocytes and to polarize naïve CD4+ T cells toward Th1/Th17 phenotypes, promoting Th2 response instead[40]. In addition, prolonged exposure to NETs can induce macrophage and dendritic cells death, which may limit ongoing inflammation[41]. However, it is still unknown, which components of NETs are responsible for these effects. The fact that persisting ETs can modify molecular and cellular components of immune system indicates that fast clearing of ETs is extremely important for proper functioning of immune response[42]. Recent studies indicate that macrophages serve as such clearing agents. Thus, macrophages seem to have a dual role; they can produce ETs and also remove them through phagocytosis[35]. Macrophage ETs, named METs, were discovered in 2010 in murine RAW 264. 7 (Abelson murine leukemia virus transformed macrophages) cell line and since then have been described in many different macrophage types[35,43]. In contrast to the neutrophils where the NETs formation is their main strategy (neutrophils are short-lived “by design”) the formations of METs in macrophages, which are long-lived cells, is an auxiliary strategy and is (regardless of stimulation) self-limited to less than 25% of total macrophage population[35]. Recently, the eosinophil extracellular traps (EETs) and their role in allergic diseases such as human eosinophilic chronic rhinosinusitis and eosinophilic otitis and eosinophilic esophagitis have been described[44,45]. Eosinophil traps released during local cytolysis contain DNA/histone H1 complex, which form globular fibers ticker than those present in neutrophil-derived traps. The EETs can trap fungi and bacteria and at least in eosinophilic esophagitis (characterized by esophageal epithelial barrier defects) can guard against pathogens infiltration through the impaired esophageal wall[45].
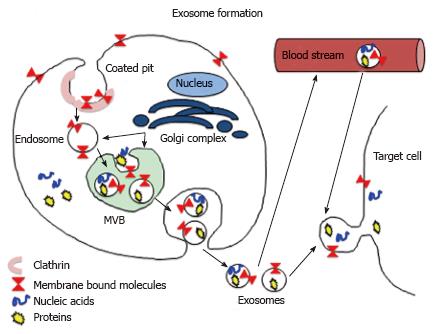
Exosomes are small (30-150 nm) endocytic membranous vesicles, which are produced by various cell types including immune cells[46]. They were discovered over 30 years ago and for many years they were believed to be the non-functional cell debris or debris disposal vehicles. However, over the last several years numerous studies have proven that exosomes are an important component of intercellular communication circuit and as such play a crucial role in initiation and/or modulation of immune response[46-54]. Exosomes form inside the cell through endosome/mulitvesicular body (MVB) pathway in which they acquire various cytoplasmic, membrane bound and/or nuclear components (Figure 4). Fully matured exosomes released (via exocytosis) from a founder cells deliver, sometimes over long distances, various molecules to their targets. Exososmes may contain a variety of molecules such as: (1) genetic material: retrotransposomal DNA, mitochondrial DNA, mRNAs, miRNAs, rRNA, tRNA; (2) lipids; and (3) proteins: Cytoskeletal proteins, heat shock proteins, channels and transporters, adhesion proteins, tetraspanis and various receptors (Table 1), listed in Exocarta database http://www.exocarta.org[49,55-61]. There are many studies showing how exososmal signaling can influence and modify immune cells and immune response. For example the exosomes released by B cells and dendritic cells contain functional MHC - antigenic peptide complexes, which induce adaptive immune responses in vitro and in vivo[62,63]. Andreola et al[64], showed that death-inducing Fas ligand - bearing exosomes secreted by tumor cells induce lymphocyte apoptosis, which in turn suppress anti-tumor response. In addition, other studies showed that exosomes derived from B lymphocytes expressing FasL can kill T helper (TH) lymphocytes[65], and that antigen-specific suppression of immune response is exerted by microRNA-150 (miRNA-150)-containing exosomes derived from T CD8+ suppressor (Ts) cells[66]. There are also studies showing that exosomes participate in the signaling between pathogens and immune cells. For example exosomes derived from Schistosoma japonicum worm induce macrophage differentiation into M1 subtype[67] and Leishmania-derived exosomes deliver Leishmania specific molecules into the host macrophages and induce secretion of IL-8[68]. Bhatnagar et al[69] showed that pathogen-associated molecular patterns (PAMPs)-rich exosomes secreted from macrophages infected with various mycobacteria are able to stimulate proinflammatory response in naïve macrophages, and when transferred into mice they stimulate synthesis of IL-12 and TNF-α and promote infiltration of lungs with neutrophils and macrophages. Authors suggest that PAMPs-containing exosomes play a major role in immune surveillance[69]. Because exosomes’ content is cell/pathogen specific and they are able to carry and deliver biologically active molecules to the target cells there is also tremendous interest in application of exosomes as biomarkers and the custom engineered exosomes as an immune and anti-cancer therapeutics[70-75].
| Nucleic acids | Exosome lumen proteins | Exosome membrane proteins | Lipids |
| mRNA miRNA tRNA rRNA mitochondrial DNA retrotransposons | Actin Cofillin GAPDH Hsp70 Rab Tubulin | Annexins Channels EGFR FasL I-CAM1 Integrins LBPA/CD63 LAMP1/2 MHC PD-1L Tetraspanins Tsg 101 | Cholesterol Diglycerides Eicosanoids fatty acids Gangliosides Lyso-phosphatidylcholine Lyso-bis phosphatidic acid Phosphatidylcholine phosp hatidylethanolamine Phosphatidylserine phosphatidylinositol Sphingomyelin |
Besides exosomes, various cells are able to release another type of membranous vesicles called the ectosomes/microparticles (MPs). The MPs are 0.2-2 mm in diameter and unlike exosomes they bud off the plasma membrane without the involvement of endosome/MVB pathway. The MPs contain a variety of bioactive molecules such as procoagulation compounds (for example P-selectin glycoprotein ligand-1 and tissue factor TF) and/or oncogenic proteins, mRNAs and micro RNAs[76-78]. Similar to exososmes, the circulating MPs may promote/inhibit inflammation, immune response, resistance to chemotherapeutics or activate oncogenic pathways[76-78].
One of the most fascinating aspects of noncanonical signaling is the fact that its cellular processes such as trogocytosis/membrane exchange, ETs, exosomes and microparticles are evolutionary ancient (amoebic trogocytsosis, ETs in acoelomate) and conserved in plants, invertebrates and vertebrates[79]. This indicates that noncanonical processes served as the primordial defense mechanisms and canonical signaling had developed later in evolution adding a new and more sophisticated quality to the ancient safeguards. Ironically, the existence of these ancient noncanonical pathways has been discovered much latter than canonical pathways, and only in recent decade they have been recognized as an extremely important regulators of innate and adaptive immunity and inflammatory responses.
P- Reviewer: Li W, Ramirez GA, Sakkas L, Vermi W S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Ralston KS. Chew on this: amoebic trogocytosis and host cell killing by Entamoeba histolytica. Trends Parasitol. 2015;31:442-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Ralston KS, Solga MD, Mackey-Lawrence NM, Somlata A, Petri WA. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature. 2014;508:526-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4:815. [PubMed] |
| 4. | Nakayama M. Antigen Presentation by MHC-Dressed Cells. Front Immunol. 2014;5:672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Pham T, Mero P, Booth JW. Dynamics of macrophage trogocytosis of rituximab-coated B cells. PLoS One. 2011;6:e14498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Taylor RP, Lindorfer MA. Fcγ-receptor-mediated trogocytosis impacts mAb-based therapies: historical precedence and recent developments. Blood. 2015;125:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Cho FN, Chang TH, Shu CW, Ko MC, Liao SK, Wu KH, Yu MS, Lin SJ, Hong YC, Chen CH. Enhanced cytotoxicity of natural killer cells following the acquisition of chimeric antigen receptors through trogocytosis. PLoS One. 2014;9:e109352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Miner CA, Giri TK, Meyer CE, Shabsovich M, Tripathy SK. Acquisition of activation receptor ligand by trogocytosis renders NK cells hyporesponsive. J Immunol. 2015;194:1945-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952-954. [PubMed] |
| 10. | Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, Sprent J. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137-1148. [PubMed] |
| 11. | Walker MR, Mannie MD. Acquisition of functional MHC class II/peptide complexes by T cells during thymic development and CNS-directed pathogenesis. Cell Immunol. 2002;218:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Kedl RM, Schaefer BC, Kappler JW, Marrack P. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat Immunol. 2002;3:27-32. [PubMed] |
| 13. | Wetzel SA, McKeithan TW, Parker DC. Peptide-specific intercellular transfer of MHC class II to CD4+ T cells directly from the immunological synapse upon cellular dissociation. J Immunol. 2005;174:80-89. [PubMed] |
| 14. | Zhou G, Ding ZC, Fu J, Levitsky HI. Presentation of acquired peptide-MHC class II ligands by CD4+ regulatory T cells or helper cells differentially regulates antigen-specific CD4+ T cell response. J Immunol. 2011;186:2148-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Romagnoli PA, Premenko-Lanier MF, Loria GD, Altman JD. CD8 T cell memory recall is enhanced by novel direct interactions with CD4 T cells enabled by MHC class II transferred from APCs. PLoS One. 2013;8:e56999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Dhainaut M, Moser M. Regulation of immune reactivity by intercellular transfer. Front Immunol. 2014;5:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Rossi EA, Goldenberg DM, Michel R, Rossi DL, Wallace DJ, Chang CH. Trogocytosis of multiple B-cell surface markers by CD22 targeting with epratuzumab. Blood. 2013;122:3020-3029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Zhu X, Niedermann G. Rapid and efficient transfer of the T cell aging marker CD57 from glioblastoma stem cells to CAR T cells. Oncoscience. 2015;2:476-482. [PubMed] |
| 19. | Baba E, Takahashi Y, Lichtenfeld J, Tanaka R, Yoshida A, Sugamura K, Yamamoto N, Tanaka Y. Functional CD4 T cells after intercellular molecular transfer of 0X40 ligand. J Immunol. 2001;167:875-883. [PubMed] |
| 20. | Martínez-Martín N, Fernández-Arenas E, Cemerski S, Delgado P, Turner M, Heuser J, Irvine DJ, Huang B, Bustelo XR, Shaw A. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35:208-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Alegre E, Howangyin KY, Favier B, Baudhuin J, Lesport E, Daouya M, Gonzalez A, Carosella ED, Lemaoult J. Membrane redistributions through multi-intercellular exchanges and serial trogocytosis. Cell Res. 2010;20:1239-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | LeMaoult J, Caumartin J, Daouya M, Switala M, Rebmann V, Arnulf B, Carosella ED. Trogocytic intercellular membrane exchanges among hematological tumors. J Hematol Oncol. 2015;8:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Uzana R, Eisenberg G, Merims S, Frankenburg S, Pato A, Yefenof E, Engelstein R, Peretz T, Machlenkin A, Lotem M. Human T cell crosstalk is induced by tumor membrane transfer. PLoS One. 2015;10:e0118244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Chadebech P, Michel M, Janvier D, Yamada K, Copie-Bergman C, Bodivit G, Bensussan A, Fournie JJ, Godeau B, Bierling P. IgA-mediated human autoimmune hemolytic anemia as a result of hemagglutination in the spleen, but independent of complement activation and FcαRI. Blood. 2010;116:4141-4147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Masuda S, Iwasaki S, Tomaru U, Baba T, Katsumata K, Ishizu A. Possible implication of Fc γ receptor-mediated trogocytosis in susceptibility to systemic autoimmune disease. Clin Dev Immunol. 2013;2013:345745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Rafii A, Mirshahi P, Poupot M, Faussat AM, Simon A, Ducros E, Mery E, Couderc B, Lis R, Capdet J. Oncologic trogocytosis of an original stromal cells induces chemoresistance of ovarian tumours. PLoS One. 2008;3:e3894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532-1535. [PubMed] |
| 28. | Wartha F, Henriques-Normark B. ETosis: a novel cell death pathway. Sci Signal. 2008;1:pe25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 718] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 30. | Zhao W, Fogg DK, Kaplan MJ. A novel image-based quantitative method for the characterization of NETosis. J Immunol Methods. 2015;423:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 31. | Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 739] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 32. | Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318-1321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 987] [Cited by in RCA: 1189] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 33. | Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 883] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 34. | Badimon L, Vilahur G. Neutrophil extracellular traps: a new source of tissue factor in atherothrombosis. Eur Heart J. 2015;36:1364-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Boe DM, Curtis BJ, Chen MM, Ippolito JA, Kovacs EJ. Extracellular traps and macrophages: new roles for the versatile phagocyte. J Leukoc Biol. 2015;97:1023-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Seper A, Hosseinzadeh A, Gorkiewicz G, Lichtenegger S, Roier S, Leitner DR, Röhm M, Grutsch A, Reidl J, Urban CF. Vibrio cholerae evades neutrophil extracellular traps by the activity of two extracellular nucleases. PLoS Pathog. 2013;9:e1003614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342:863-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 38. | Stoiber W, Obermayer A, Steinbacher P, Krautgartner WD. The Role of Reactive Oxygen Species (ROS) in the Formation of Extracellular Traps (ETs) in Humans. Biomolecules. 2015;5:702-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 39. | Pinegin B, Vorobjeva N, Pinegin V. Neutrophil extracellular traps and their role in the development of chronic inflammation and autoimmunity. Autoimmun Rev. 2015;14:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 40. | Barrientos L, Bignon A, Gueguen C, de Chaisemartin L, Gorges R, Sandré C, Mascarell L, Balabanian K, Kerdine-Römer S, Pallardy M. Neutrophil extracellular traps downregulate lipopolysaccharide-induced activation of monocyte-derived dendritic cells. J Immunol. 2014;193:5689-5698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Donis-Maturano L, Sánchez-Torres LE, Cerbulo-Vázquez A, Chacón-Salinas R, García-Romo GS, Orozco-Uribe MC, Yam-Puc JC, González-Jiménez MA, Paredes-Vivas YL, Calderón-Amador J. Prolonged exposure to neutrophil extracellular traps can induce mitochondrial damage in macrophages and dendritic cells. Springerplus. 2015;4:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Anjos PM, Fagundes-Netto FS, Volpe CM, Nogueira-Machado JA. Impaired clearance of neutrophils extracellular trap (NET) may induce detrimental tissular effect. Recent Pat Endocr Metab Immune Drug Discov. 2014;8:186-190. [PubMed] |
| 43. | Chow OA, von Köckritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 44. | Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, Honda K, Ohta N, Yamamoto S, Takagi Y. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J Allergy Clin Immunol. 2016;137:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 45. | Simon D, Radonjic-Hösli S, Straumann A, Yousefi S, Simon HU. Active eosinophilic esophagitis is characterized by epithelial barrier defects and eosinophil extracellular trap formation. Allergy. 2015;70:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 46. | Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 738] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 47. | Hegmans JP, Gerber PJ, Lambrecht BN. Exosomes. Methods Mol Biol. 2008;484:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Bu N, Wu H, Zhang G, Zhan S, Zhang R, Sun H, Du Y, Yao L, Wang H. Exosomes from Dendritic Cells Loaded with Chaperone-Rich Cell Lysates Elicit a Potent T Cell Immune Response Against Intracranial Glioma in Mice. J Mol Neurosci. 2015;56:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 483] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 50. | Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 836] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 51. | Lai FW, Lichty BD, Bowdish DM. Microvesicles: ubiquitous contributors to infection and immunity. J Leukoc Biol. 2015;97:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Agarwal A, Fanelli G, Letizia M, Tung SL, Boardman D, Lechler R, Lombardi G, Smyth LA. Regulatory T cell-derived exosomes: possible therapeutic and diagnostic tools in transplantation. Front Immunol. 2014;5:555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | De Jong OG, Van Balkom BW, Schiffelers RM, Bouten CV, Verhaar MC. Extracellular vesicles: potential roles in regenerative medicine. Front Immunol. 2014;5:608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 54. | Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16:24-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 554] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 55. | Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241-D1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 836] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 56. | Benito-Martin A, Di Giannatale A, Ceder S, Peinado H. The new deal: a potential role for secreted vesicles in innate immunity and tumor progression. Front Immunol. 2015;6:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 57. | Yoon YJ, Kim OY, Gho YS. Extracellular vesicles as emerging intercellular communicasomes. BMB Rep. 2014;47:531-539. [PubMed] |
| 58. | Salido-Guadarrama I, Romero-Cordoba S, Peralta-Zaragoza O, Hidalgo-Miranda A, Rodríguez-Dorantes M. MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. Onco Targets Ther. 2014;7:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 59. | Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-659. [PubMed] |
| 60. | Yao Y, Wei W, Sun J, Chen L, Deng X, Ma L, Hao S. Proteomic analysis of exosomes derived from human lymphoma cells. Eur J Med Res. 2015;20:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 604] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 62. | Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161-1172. [PubMed] |
| 63. | Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594-600. [PubMed] |
| 64. | Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303-1316. [PubMed] |
| 65. | Lundy SK, Klinker MW, Fox DA. Killer B lymphocytes and their fas ligand positive exosomes as inducers of immune tolerance. Front Immunol. 2015;6:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Nazimek K, Ptak W, Nowak B, Ptak M, Askenase PW, Bryniarski K. Macrophages play an essential role in antigen-specific immune suppression mediated by T CD8⁺ cell-derived exosomes. Immunology. 2015;146:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Wang L, Li Z, Shen J, Liu Z, Liang J, Wu X, Sun X, Wu Z. Exosome-like vesicles derived by Schistosoma japonicum adult worms mediates M1 type immune- activity of macrophage. Parasitol Res. 2015;114:1865-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 68. | Silverman JM, Clos J, de’Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci. 2010;123:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 69. | Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234-3244. [PubMed] |
| 70. | Kalani A, Tyagi A, Tyagi N. Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol. 2014;49:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 71. | De Toro J, Herschlik L, Waldner C, Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 462] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 72. | Zhang B, Yin Y, Lai RC, Lim SK. Immunotherapeutic potential of extracellular vesicles. Front Immunol. 2014;5:518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 73. | Lässer C. Exosomes in diagnostic and therapeutic applications: biomarker, vaccine and RNA interference delivery vehicle. Expert Opin Biol Ther. 2015;15:103-117. [PubMed] |
| 74. | Waldenström A, Ronquist G. Role of exosomes in myocardial remodeling. Circ Res. 2014;114:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 75. | Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205-212. [PubMed] |
| 76. | Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 1080] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 77. | de Souza PS, Faccion RS, Bernardo PS, Maia RC. Membrane microparticles: shedding new light into cancer cell communication. J Cancer Res Clin Oncol. 2015;Aug 19; Epub ahead of print. [PubMed] |
| 78. | Morel O, Morel N, Jesel L, Freyssinet JM, Toti F. Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis. Semin Immunopathol. 2011;33:469-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 79. | Robb CT, Dyrynda EA, Gray RD, Rossi AG, Smith VJ. Invertebrate extracellular phagocyte traps show that chromatin is an ancient defence weapon. Nat Commun. 2014;5:4627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |