INTRODUCTION
The relationship between exercise and infection has long interested exercise immunologists; however, it is not yet fully understood[1]. Immune reactions to pathogens are generally evaluated by the relative level of plasma antibodies. The production of antibodies is a major protective response against infections. In this review, we have provided the historical background of immune function research, and its relationship to exercise. We then outline the current state of knowledge regarding the effects of exercise on antibody-producing cells and extend antibody half-life. We have also discussed potential mechanisms by which moderate levels of exercise can prevent infections.
EXERCISES ENHANCE SURVIVAL IN ANIMALS INFECTED WITH PATHOGENIC MICROORGANISMS
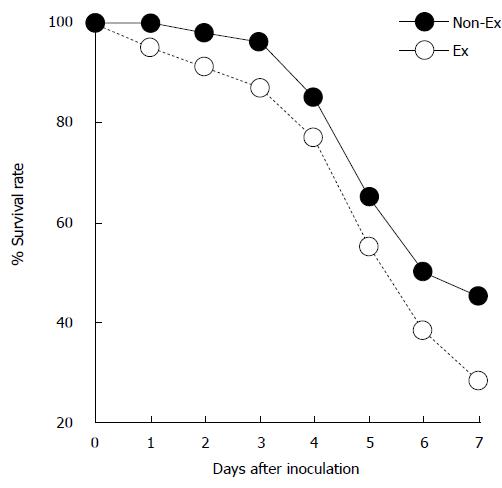
Regular and consistent exercise is known to confer substantial benefits to the immune system, and prevent infection over the long term[2]. Cannon et al[3] were the first to report the effects of exercise on infection. In their study, mice were infected with Salmonella typhimurium; mice that had partaken in some form of exercise had a significantly higher survival rate than sedentary mice. This initial study demonstrated the beneficial effects of exercise on the immune system during an infection (Figure 1). Stressful exercise can exacerbate infections[4,5], while moderate levels of exercise can attenuate disease severity[4,6]. Lowder et al[6] showed that exercise of moderate intensity protected mice from death against the influenza virus. In this study, mice were administered influenza virus A/PR/8/34, a virulent H1N1 strain[7]. Mice were then made to exercise at a moderate intensity (8-12 m/min for 20-30 min over four consecutive days) or over a prolonged period (8-12 m/min for 2.5 h over 4 consecutive days), with some mice used as sedentary controls. Results from a majority of studies support the view that exercise confers a higher survival rate to an infection[6,8]. A limitation of these animal studies is that they have examined primary responses, but not secondary responses to infection. They have been repeatedly confirmed in many studies. First, mice that are made to exercise show significantly higher survival rates than sedentary mice following the injection of an antigen. Second, continuous exercise leads to significantly higher survival rates for active mice than in sedentary mice. Third, antigen-specific antibody responses are more enhanced in active mice than in sedentary mice.
Figure 1 Exercise enhances survival rate in mice infected with Salmonella typhimurium.
Mice voluntarily trained on exercise wheels for 16-18 d (EX) and were infected with Salmonella typhimurium. These EX mice exhibited a small but significant increase in survival rate (34/77) compared with sedentary control (Non-EX) mice (23/79) after 7 d (Modified from Cannon et al[3]). EX: Exercise; Non-EX: Non-exercise.
EXERCISE INDUCES ANTIBODY PRODUCTION
Following exercise and infection studies, exercise immunologists focused on exercise-induced plasma antibody elevation. The antibody response to antigens is a complex process; however, it is important to understand this if the potential for exercise to alter the response is to be investigated. Antibody responses are T cell-dependent and comprise of antigens that are soluble proteins. The first step in the response to an antigen is its uptake and presentation. This is usually performed by specialized antigen presenting cells (APCs), such as macrophages, dendritic cells, or B cells. During T cell-dependent responses, naive B cells recognize the presented antigen, and mature after cognate interaction with T helper (Th) cells. However, for T cell-independent responses, B cells do not need to interact with APCs or T cells. Mature B cells, including plasma cells, are able to secrete antibodies and have a “memory” of encountered antigens.
A significant amount of research has been conducted to investigate whether moderate exercise induces antibody production[1,9-13]. In most of these studies, the administered antigen was not infectious. Liu et al[10] tested the hypothesis that exercise induces the production of plasma antibodies. They examined the plasma antibody levels of sedentary and active (running for 10 min, twice a day) mice following infection with Salmonella typhi. Antibody titers in the active mice were significantly higher (2.76-fold) than those in the sedentary mice over the entire experimental period. The antibody titers in the active mice were 2.76-fold higher than those in sedentary mice. Liu et al[10] observed a primary antibody response after initial exposure to an antigen. Subsequent exposure to the same antigen led to a strong secondary antibody response, resulting in long-lasting immunity. The effects of exercise on secondary antibody responses have been tested in young mice[1,9] and rats[11].
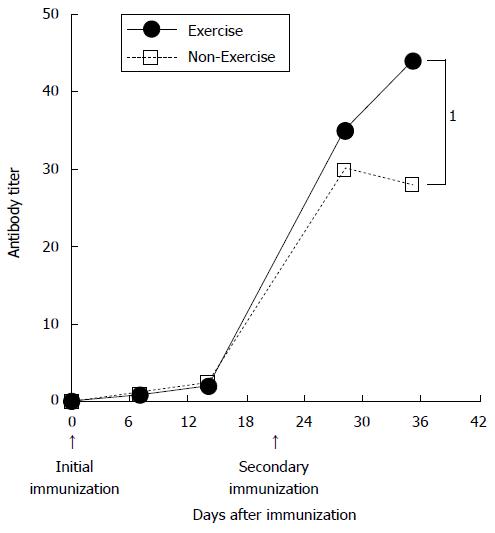
A number of investigations into the influence of exercise on antibody response have been conducted, largely focusing on the secondary antibody response (Figure 2). Exercise immunologists were interested in the dramatic changes to the secondary antibody response in active mice (Figure 2). Moderate exercise, such as voluntary wheel running[12] or treadmill running (8-15 m/min[13] has been shown to have a pronounced effect on the secondary antibody response. These findings indicate that is valuable information encouraged exercise immunologists to investigate other additional components affecting the immune response. Thus, since an early era, investigations of exercise effects on immunity, which reflect the secondary antibody response during exercise. Moderate exercise could be an adjuvant to vaccination, and further research into this is required.
Figure 2 Effects of physical training on the murine immunological response.
Serum IgG levels in active (exercise) and control (non-exercise) mice. Arrows indicate the time of immunization. 1Significant difference between Exercise and Non-Exercise groups (Modified from Douglass[1]).
EXERCISE INDUCES THE SECONDARY ANTIBODY RESPONSE
Exercise is known to affect the secondary antibody response in animal models. In mice, the primary IgG response to tetanus toxoid (TT) was not enhanced by moderate exercise; however, in active mice there was an increased IgG response following a booster dose[12]. Subsequent research findings revealed that booster shots resulted in enhanced antibody production in mice that exercised[10-13]. Antibody responses to TT require recognition of the antigen by Th cells and cooperation with antigen-specific B cells and T lymphocytes. The interaction between Th and B cells involves antigen presentation by B cells to differentiated T cells, activation of Th cells, and the expression of membrane and secreted molecules by the Th cells that bind to and activate B cells. It remains unclear why moderate exercise impacts upon the secondary antibody response, but not the primary response.
Factors involved in the increased secondary antibody response in mice have been investigated in detail by Suzuki et al[12]. They investigated the effects of exercise on the number of cells producing specific IgG in mice by using ELISPOT assays[14]; secondary antibody responses to TT were significantly higher in the active group than in the sedentary group. Exercise induce secondary antibody response to antigen is valuable information and encouraged exercise immunologists. Thus, since an early era, investigations of exercise effects on immunity were beginning.
ROLE OF ENDOGENOUS OPIOIDS IN THE EXERCISE-INDUCED SECONDARY ANTIBODY RESPONSE
The mechanism(s) behind increased specific secondary antibody responses in moderately active animals is unclear[12]. It has been suggested that endogenous opioids are involved in these enhanced secondary antibody response. Enkephalins were first observed in the brain and the endocrine system. Both endorphins and enkephalins are important regulators of pain[15,16]. Recently, inflammatory cells were shown to produce and release these opiates. Endorphins appear to be involved in immune functions[17,18], pain modulation[19], and the exercise pressor response[20-22]. The role(s) of these endogenous opiates with respect to the exercise-related processes they modulate require clarification, in particular at the cellular level.
Kapasi et al[13] immunized mice with an antigen and treated them with a placebo or an opioid antagonist (naltrexone); control mice received no intervention. Mice were further subdivided into sedentary and active groups, with those in the active group made to exercise for 8 wk and following a booster shot. During the secondary response, high antibody titers were seen in the immunized active mice. However, the antibody titer was not increased in naltrexone-treated mice. It has also been concluded that norepinephrine appears to mediate suppression and stimulation of antibody synthesis. Enhancement of the antibody response by endogenous opioids is dependent on the dose of intravenous injections[23]. Antibody production occurs as a result of interactions between the retained antigen on follicular dendritic cells, B lymphocytes, and Th lymphocytes[24].
Exercise induced secondary antibody production mechanism most likely involves opioids binding to specific receptors on B and T cells[25]. Endogenous opioids influence the antibody response through receptors on Th (CD4+) cells and by stimulating proliferation[26]. This is likely a result of IL-4 production being induced, with IL-4 increasing splenic B cell survival[27]. Further research is required to determine whether the effects of exercise on specific enhanced secondary antibody responses are due to such a mechanism.
EFFECTS OF EXERCISE ON IGG HALF-LIFE
The mechanism by which exercise induces elevation of secondary IgG levels is related to IgG half-life[12]. In several species, it was found that the clearance rate of IgG is highly dependent on its concentration in plasma[28]. A physiological concentration of IgG half-life is approximately 10 times longer than at high concentrations[28]. A significant fraction of IgG proteins undergoes endocytosis[29]. IgG does not localize to the lysosome, but is instead redirected to the cell surface and released into plasma or interstitial fluids. The recycling of IgG is mediated by the Brambell receptor, FcRn, which binds to IgG in a pH-dependent manner[30,31]. Within the acidified environment of the early endosome, IgG binds strongly to FcRn. IgG-FcRn complexes are not delivered to the lysosome for catabolism[32]; instead, they migrate to the cell surface for fusion with the cell membrane. The FcRn receptor shows virtually no affinity for IgG at a physiological pH. Upon fusion of the sorting vesicle with the cell membrane, IgG dissociates from the receptor and is rapidly released into the extracellular fluid. The clearance of IgG is increased by approximately 10-fold[31], which is consistent with a recycling efficiency of 90% in wild-type animals expressing FcRn.
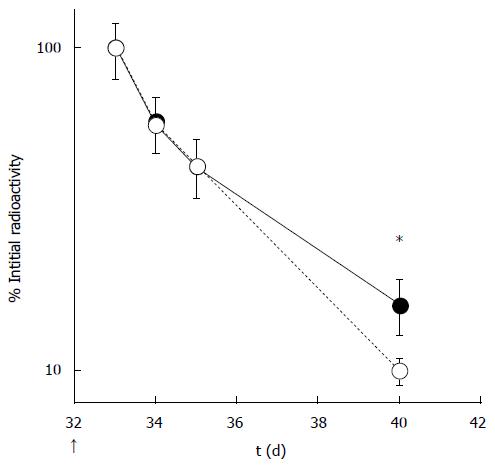
A series of studies examining the effects of exercise on IgG clearance have been published[12,33]. Exercise induced secondary antibody response to antigen was reported by Suzuki et al[33]. They investigated factors that lowered non-specific 125I-IgG clearance from plasma after a booster immunization (Figure 3). A high concentration of circulating antibody can result in lower clearance of antibodies from circulation. The reasons underlying the low clearance of 125I-IgG from circulation in active mice remain to be resolved. The IgG homeostatic mechanism is dependent on the Fc region of IgG[34]. A possible downstream mediator that could explain the lower level clearance is the FcRn receptor. FcRn is normally expressed in the vascular endothelium in the adult rodent, where it is thought to confer a protective function[35]. A functional FcRn molecule is dependent on its dimerization with β2-microglobulin (β2m)[36]. There is strong evidence to indicate that β2m-deficient mice have an abnormally short IgG half-life and reduced homeostatic IgG levels[37]. Suzuki et al[33] reported on the effects of β2m expression in the liver, which were implicated in protecting against IgG catabolism. Mice were intraperitoneally immunized with TT to induce primary and secondary antibody responses. The authors found that the half-lives of TT-specific IgG were prolonged in active mice, with significantly higher blood IgG concentrations in active mice compared with those in sedentary mice. In the active mice, radiolabeled IgG concentrations in the liver were higher than those in sedentary mice, and this was confirmed by immunohistochemical analysis. Expression of the β2m gene was upregulated in the livers of active mice. There was a significant correlation between the accumulation of radiolabeled IgG in the liver and its concentration in the blood. In addition, there was a significant correlation between extracted total hepatic IgG and β2m in the liver.
Figure 3 Clearance of radiolabeled IgGs from active (black circles) and sedentary (white circles) mice (Modified from Suzuki et al[12]).
*Significant difference between active and sedentary mice.
EFFECTS OF EXERCISE ON THE SECONDARY ANTIBODY RESPONSE IN AGED ANIMAL MODEL
Aging is a natural process and is associated with a decline in the normal functioning of the immune system that can be described by the term “immunosenescence”[38]. Accelerated degradation of host immune responses is a key sign of immunosenescence, and can lead to the onset of opportunistic infections[39]. Immunosenescence can also result in poor vaccine responses[40] and the increased incidence of infection that is often seen in the elderly. Restoration of immunological function is expected to have a beneficial effect in reducing pathology and maintaining the health of older individuals. Moderate exercise has been used as an intervention to counteract the aging immune system. Regular exercise has been associated with enhanced vaccination responses[41,42]. Aging is associated with declines in humoral and cellular immunity[43], and therefore reduced immune function. The age-related decline in the function of major cells that take part in the antibody response are reflected by the secondary antibody response[44]. Kapasi et al[45] focused on age-related changes in immune function and the effects of exercise, and their study clearly showed that older mice exhibited a secondary antibody response similar to that seen in young control mice after exercise. Thus, intense exercise exerts positive effects on the secondary antibody response in old animals.
EFFECTS OF EXERCISE ON THE ANTIBODY RESPONSE IN HUMANS
A beneficial effect of exercise on the secondary antibody response to infectious diseases is improved. The use of exercise to augment vaccine responses in humans has been explored, with positive results observed[41,46-49]. Moderate aerobic exercise in older adults[48] and muscle-damaging eccentric contractions in younger adults[50] have been shown to increase immune responses to influenza vaccination. In addition, several cross-sectional studies have found that physically fit[51] or active elderly individuals[52] exhibit elevated antibody responses to recall vaccinations. Shuler et al[46] examined antibody titers in response to influenza vaccination in college students. Measures of physical fitness and physical activity were taken, but neither was found to be associated with the magnitude of the antibody response.
In contrast, several cross-sectional studies of older adult populations have all reported enhanced antibody responses to vaccinations in participants with high levels of physical fitness[51], or who were physically active[46,52,53]. This contrast in effects of chronic exercise on vaccination responses elicited in older adult populations, but not in younger populations, was exemplified by Smith et al[53]. They compared the immune response to a novel antigen, keyhole limpet hemocyanin (KLH). The authors showed that older active men demonstrated stronger antibody and cell-mediated responses to KLH than those in sedentary older men. The responses to KLH in younger men were similar, regardless of their activity habits. Woods et al[54] demonstrated that a 10-mo regimen of cardiovascular exercise (60%-70% maximal oxygen uptake, 45-60 min, three times a week) in previously sedentary older adults resulted in increased seroprotective maintenance, compared with participants who took part in flexibility training over the same period. An increased response to novel antigens has also been observed following chronic exercise. After KLH vaccination, IgG1 and IgM concentrations were greater in participants who had completed a 10-mo cardiovascular training program (11% increase in VO2 max) than in control participants (1% increase in VO2 max)[55]. The majority of results from previous studies supports the hypothesis that regular exercise improves immune function. This is reflected in enhanced antibody or cell-mediated responses to vaccination, especially in older adults. In general, for the majority of papers we reviewed, vaccine response was assessed via antibody titer, the humoral component of the immune response. Previously published findings support the hypothesis that exercise of a moderate intensity appears to enhance immune responses in animals. Therefore, the development of effective exercise regimens that promote antibody responses to vaccination would assist in preventing and reducing the risks of infection.
CONCLUSION
In this review, we have highlighted how moderate exercise interventions can improve immune responses to antigens or pathogens. Currently available evidence shows that exercise has important modulatory effects on antibody responses, and possibly on immune functions. These effects are mediated by a diverse range of factors, including the functions of antibody producing cells, endogenous opioids, aging, and IgG half-life. Most exercise studies have focused on antibody production, with more work required in this area. Almost all studies have investigated the effects of moderate exercise on immune function; however, it remains unknown if exercise is capable of modulating specific antibody-producing cells. While exercise might help boost adaptive immunity by increasing the thymic output of naive T cells[56], and/or by purging senescent T cells[57], further research is required to confirm this. This would provide us with insight into the types of vaccination, which can be ameliorated with exercise. As molecular biological techniques are incorporated into exercise immunology studies, a greater understanding of the pathways of cell activation and regulation should be forthcoming.
P- Reviewer: Jiang YF, Rodrigo L S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK