Published online Nov 27, 2014. doi: 10.5411/wji.v4.i3.158
Revised: June 3, 2014
Accepted: October 14, 2014
Published online: November 27, 2014
Processing time: 236 Days and 23.3 Hours
Reactive oxygen species (ROS) take part in diverse biological processes like cell growth, programmed cell death, cell senescence, and maintenance of the transformed state through regulation of signal transduction. Cancer cells adapt to new higher ROS circumstance. Sometimes, ROS induce cancer cell proliferation. Meanwhile, elevated ROS render cancer cells vulnerable to oxidative stress-induced cell death. However, this prominent character of cancer cells allows acquiring a resistance to oxidative stress conditions relative to normal cells. Activated signaling pathways that increase the level of intracellular ROS in cancer cells not only render up-regulation of several genes involved in cellular proliferation and evasion of apoptosis but also cause cancer cells and cancer stem cells to develop a high metabolic rate. In over the past several decades, many studies have indicated that ROS play a critical role as the secondary messenger of tumorigenesis and metastasis in cancer from both in vitro and in vivo. Here we summarize the role of ROS and anti-oxidants in contributing to or preventing cancer. In addition, we review the activated signaling pathways that make cancer cells susceptible to death.
Core tip: Reactive oxygen species originally used to induce injurious cellular effects are now recognized as key physiological molecules for the induction of host defense genes, activation of transcription factors, and regulation of signal transduction. Tumorigenic cells can induce a new redox balance, resulting in cellular adaptation and proliferation. Here, we review the role of oxidative stress in cancer cells using a pathophysiological view.
- Citation: Kim D, Park GB, Hur DY. Apoptotic signaling through reactive oxygen species in cancer cells. World J Immunol 2014; 4(3): 158-173
- URL: https://www.wjgnet.com/2219-2824/full/v4/i3/158.htm
- DOI: https://dx.doi.org/10.5411/wji.v4.i3.158
The high intracellular ROS levels are related to various human diseases, including neurodegenerative disease[1-7], inflammatory disease[8,9], cardiovascular disease[10,11], immune system dysfunctions[12], obesity[13], and diabetes[14,15]. Survival of tumor cells is greatly dependent on their capacity to control expression of endogenous antioxidants to maintain the upper standard level of ROS below the threshold that will induce tumor cell death[16,17]. ROS could contribute to the initiation of cancer by accelerating tumorigenic signaling pathways, increasing DNA mutations and changing the activity of the tyrosine phosphatases superfamily[18-21]. For example, cancer inactivates the tumor suppressor phosphatase and tensin homolog (PTEN) by oxidation[22,23] and inhibits the mitogen-activated protein kinase (MAPK) phosphatase by ROS, which in turn induces activation of extracellular signal-regulated kinases (ERK). Although greater oxidative stresses activate nuclear factor-kappa B (NF-κB) for growth or survival, high intracellular ROS levels also lead to activation of c-Jun N-terminal kinase (JNK) and p38 kinases, and their activities often facilitate cell apoptosis[24,25]. Normally, the inhibition of PTEN by ROS activates the phosphoinositide-3 kinase (PI3K)/Akt signaling pathway and blocks cell apoptosis[26,27]. In contrast to the apoptotic death, necrosis induces via mitochondrial production of ROS after signaling from tumor necrosis factor-α (TNF-α) or death receptor[28,29]. Interestingly, apoptotic cells inhibit ERK1/2 but induce p38 and JNK inside macrophage, while necrotic cells induce macrophage ERK1/2[30-32]. ROS-mediated signaling has received more attention in oncological studies than ROS-mediated cellular stress and damage of cancer cells. In this article, we present and summarize the interaction between redox status or redox signaling systems and apoptosis in tumor cell death and anti-cancer treatments.
ROS are mainly generated from mitochondrial electron transfer complex (ETC) during the reduction of oxygen. Superoxide anion (·O2-) generated by O2 from the mitochondrial electron transport chain, which is usually changed into hydrogen peroxide (H2O2) by several cytoprotective enzymes, including superoxide dismutase (SOD)[33,34]. Although scientists are now considering the consequences of different levels of oxidative stress, ROS formation in cells can inflict serious hazards and was originally known for their ability to induce injurious cellular effects.
Reactive oxygen species (ROS) are the most abundantly produced oxygen species in mitochondria. Reactive nitrogen species (RNS) are also produced during intracellular metabolic processes in mitochondrial ETC. Extracellular ROS can be also found in a variety of natural or acquired environment. NAD(P)H oxidase (NOX) can be found in cell membrane phagosomes in neutrophil. The NOX complex is composed of seven members, NOX1-5, and two dual oxidases (Duox), Duox1 and Duox2[35]. Although activation mechanisms and tissue distribution are significantly different, all these enzymes, including cytochrome c oxidase and cyclo-oxygenase (COX) are able to generate superoxide anion[36,37]. Nitric oxide (NO·) is produced from arginine catalyzed by a nitric oxide synthase (NOS). Fast reaction between ·O2- and NO· gives rise to peroxynitrite (ONOO-) and ONOO- is oxidizing molecule that connected to cancer. NO· is finally converted into a hydroxyl radical and nitrite anion (NO2-)[38,39]. Numerous agents, including anti-cancer drugs, have been shown to induce proliferation or apoptosis through ROS production in various cancer types. Low sodium arsenite induces MCF-7 epithelial breast cancer cell proliferation by ROS production, activation of NF-κB, and increases in c-Myc and heme oxygenase-1 (HO-1)[40]. ROS-enhancing compound, such as piperlongumine, is insufficient to induce death of cancer cell lines including osteosarcoma cells, breast, and glioblastoma cancer cells, but not in normal cells[41-43].
Although the ROS levels modestly increases in tumorigenic cells, intracellular ROS is maintained below a toxic level in normal cells by various scavengers and anti-oxidative enzymes. Besides mitochondrial superoxide dismutases (SODs), catalase, glutathione (GSH), peroxidase (GPx), and peroxiredoxin also modulate oxidative status[44]. SODs are metalloenzymes which catalyze the dismutation of ·O2- to O2 and H2O2. They ubiquitously exist in eukaryotes and prokaryotes. SODs also play a critical role in inhibiting oxidative inactivation of NO, thereby preventing ONOO- formation and mitochondrial dysfunction[45]. SODs utilize metal ions such as copper (Cu2+), zinc (Zn2+), manganese (Mn2+) or iron (Fe2+) as cofactors. Ferric ions catalyze hydrogen peroxide, which is the Fenton reaction[46]. Catalase, which is located in peroxisomes, facilitates the decomposition of H2O2 to water and oxygen and protects cells from H2O2 produced within the cell[46]. GPx catalyzes the reduction of hydrogen peroxide using cellular GSH as the reducing reagent. GPx converts H2O2 to H2O + O2[47]. Peroxiredoxins are thioredoxin peroxidases that catalyze the reduction of hydrogen peroxide, organic hydroperoxides and peroxynitrite[48]. In neutrophil, many species of bacteria are killed readily by a myeloperoxidase/hydrogen peroxide/chloride system. HOCl, oxidizing chloride ions, is the most bactericidal oxidant produced by myeloperoxidase[49].
Besides, genetic factors have important roles in the transforming events that lead to carcinogenesis. The enhanced oxidative stress is generally associated with cancer promotion and progression. Meanwhile, high levels of ROS are less harmful in cancer cells than they would be in normal cells because cancer cells have developed mechanisms to keep themselves from intrinsic oxidative stress through regulation of antioxidant functions and pro-survival molecules[16,17]; however, oxidative stress still has a negative impact on various types of cancer cells as well[50,51]. The identification of specific alterations in critical cellular components by ROS can provide evidences for early detection, prevention of cancer.
Over the several years’ studies about the cytokine, inflammatory cells and cytokines found in neoplastic tissues seems to contribute to tumor growth, progression, and immunosuppression. ROS induced by these cells and cytokines facilitate cancer growth, invasion, and metastasis through DNA damage or inhibition of DNA repair. Chronic inflammation predisposes cells for an oncogenic transformation through overproduction of ROS, increased COX-2, and aberrant NF-κB expression[52,53]. Defective mitochondria have also been characterized by excessive ROS production in several chronic human diseases associated with inflammation. ROS derived from mitochondria (mtROS) enhance signaling pathways to produce pro-inflammatory cytokine subsets. mtROS activates NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome-dependent pro-inflammatory cytokine production[18,54].
Oxidative stress can activate various transcription factors including NF-κB, activating protein-1 (AP-1), p53, and hypoxia inducible factor-1α (HIF-1α). Activation of these transcription factors can result in the expression of numerous different genes, including cytokines and chemokines[55,56]. Nuclear factor erythroid-related factor 2 (Nrf2) is one of the master transcription regulators in controlling antioxidant responses. Nrf2 controls the expression of hundreds of genes, including NAD(P)H:quinone oxidoreductase 1 (NQO1), Glutathione S-transferase (GST), GPx and oxidoreductases for inflammatory responses, tissue remodeling and fibrosis, carcinogenesis, and metastasis[57-59]. Kelch-like protein 1 (Keap1), a suppressor protein anchored in the cytoplasm that physically binds Nrf2, controls the access of Nrf2 to promoters of Antioxidant Response Element (ARE)-regulated antioxidant enzymes[60,61]. MAPK, PI3K, atypical protein kinase C (PKC), and other pathways are also found as alternative pathways for Nrf2 activation[62,63]. Importantly, somatic mutations that disrupt the Nrf2-Keap1 interaction are identified in cancer patients. In non-small-cell lung cancer (NSCLC) cells with Keap1 gene mutations, Nrf2 is constitutively activated and cells proliferate independently of epidermal growth factor receptor (EGFR) signaling[64-66]. Although the above-mentioned studies show the effects of ROS-mediated inflammation in carcinogenesis, It is contrast with blockade of NF-κB predisposes murine skin to squamous cell carcinoma. RelA subunit of NF-κB has tumor suppressing activity under some circumstances[67-71]. Because NF-κB is modulated by ROS, the effects of ROS on carcinogenesis may be unfavorable to certain type of cells and conditions.
Although the significance of ROS and antioxidant systems in carcinogenesis is still controversial, substantial evidence suggests that an increase of intracellular ROS might contribute to carcinogenesis[72-74]. ROS also might stimulate the expansion of initiated cell clones through stimulation of cell proliferation and suppression of apoptosis[72]. The involvement of mitochondria in disease has been largely recognized to their essential role in production of ROS and to the damaging effect of chemical agents or pathological conditions on these organelles[33,52]. Recently, several studies have reported that tumorigenic mitochondrial DNA (mtDNA) mutations affect respiratory chain complexes. Decreased mitochondrial activity is considered to be tumorigenic, mainly because of the enhanced ROS production. H2O2 exported to the nucleus enhances the transcription of selected genes that favor tumor progression[75,76]. Depletion of mtDNA, especially encoded OXPHOS genes, plays a key role in transformation of breast epithelial cells. Breast epithelial cells results in in vitro tumorigenic phenotype as well as breast tumorigenesis in a xenograft model[77]. Claudin-1 and 7 in p53 network of breast epithelial cells are down-regulated in tumorigenesis[77]. In humans, mtDNA mutations coding (ND1, ND4, ND5, and cytochrome b genes) or noncoding regions are frequently detected in breast cancer tissue[78,79]. However, the pathological relevance of mtDNA mutations in cancer cells is still controversial[80]. Nonetheless, a clear-cut correlation between the occurrence of pathogenic mtDNA mutations and mitochondrial energetic impairment is a well-demonstrated feature of oncocytomas, characterized by disruptive mutations of mtDNA, especially in complex I subunits[81]. Initial enhanced ROS generation may induce supercomplex disorganization, eventually leading to a possible decrease of complex I assembly[82]. Dissociation of the supercomplex might further induce ROS generation and have harmful consequences, such as disassembly of complex I and III[83,84]. However, ROS measurements in tumor biopsies are not practicable currently in oncocytoma. Further studies are needed to understand whether ROS may influence the proliferative potential and accumulation of mutations in oncocytoma. Because elevated ROS have been proposed to induce apoptosis, additional studies are required to determine the role of apoptosis in regulating the survival and proliferation of oncocytic cells. It has been reported that the cell line carrying the heteroplasmic ND5 mtDNA mutation showed progressively decline of respiratory function and significantly enhancement of dependence on glucose in tumor growth, while cells with homoplasmic ND5 mutation inhibited tumor formation[85,86].
ROS-related pathways are considerably activated in many types of cancers. In particular, transient formation of H2O2, as second messengers, participates in growth, proliferation, and metabolism[87]. The level of intracellular ROS has a considerable influence on various signal pathways, including MAPK signaling cascades[88,89], PI3K/Akt signaling cascades[90,91], and IκB kinase/NF-κB signaling pathway[40,92]. Oxidative stress-mediated signaling involves all characters of cancer cell behaviors, such as cell survival, apoptosis, energy metabolism, angiogenesis, metastasis, and cancer stem cell generation. Researchers have noticed that some cancer cells show death or arrested growth when exposed to increased ROS, whereas others are able to eliminate even high levels of ROS for survival[93,94]. Emerging research indicates that modest increases in ROS are oncogenic, whereas dramatic increases in ROS seem to suppress tumors[95]. GTPase Rac1 in the cytoplasm activates NF-κB and markedly blocks the activity of caspase-3 and TNF-induced apoptosis, whereas mitochondria-derived ROS promote TNF-induced apoptosis[96]. ROS generated by the newly described NOX5 are essential for prostate cancer growth[97]. NOX4-mediated ROS generation in extracellular matrix of cancer partially transfers cell survival signals through the Akt/apoptosis signal-regulating kinase 1(ASK1) pathway in pancreatic cancer cells[98]. Inhibiting ROS with the antioxidants, NOX4 antisense, or MnSOD overexpression efficiently stimulates apoptosis in pancreatic cancer cells[99]. ROS produced by NADPH oxidase also inhibit protein tyrosine phosphatases (PTPs) and sustain the activation of Janus kinase 2 (Jak2)[91,100].
The high intracellular ROS levels in cancer cells are largely the byproducts of the highly metabolic nature of these cells. These ROS levels could be protumorigenic, but also increase the susceptibility of cancer cells to cell death. High levels of ROS in cancer cells indicate hyperactive PI3K/Akt signaling generated by increased mitochondrial metabolism and by the suppression of antioxidant gene expression, through the inhibition of forkhead box O (FOXO) transcription factor[101]. In addition to elevating SOD2 and catalase, FOXO induces the expression of Sestrin3[102,103]. Sestrin3 is a member of a family of proteins that includes Sestrin1 and Sestrin2, which were originally identified as antioxidants induced by the tumor suppressor p53[104,105]. Thus, the suppression of Sestrins expression in cancer cells could increase intracellular ROS and activate mammalian target of rapamycin complex 1 (mTORC1). ROS produced by reactive oxygen species modulator 1 (Romo1), a mitochondria-localized protein[106-108], are necessary to the ERK-dependent proliferation of lung cancer cells[108]. Similarly, high intracellular ROS levels generated by inactivation of antioxidant mechanisms has been connected with increased proliferation of breast[109] and ovarian[110] cancer cells.
Methionine sulfoxide reductase A (MsrA), a ROS scavenger, is down-regulated in a number of breast cancers. Moreover, reduction of MsrA levels results in increased ROS levels, which reduces the PTEN activity and activates PI3K pathway and leads to increased cell proliferation and a more aggressive cellular phenotype consequently[109]. Cancer cells adopt alternative mechanisms of antioxidation in order to maintain the intracellular level of ROS below a toxic threshold level[109-111]. Forkhead box M1 (FOXM1) is expressed at low levels in normal cells, but its expression is markedly elevated in cancer cells[111]. FOXM1 controls multiple pro-tumorigenic activities, but also reduces ROS levels through the transcriptional induction of SOD2, catalase, and mitochondrial-dependent peroxide reductase[112]. In addition, the expression of detoxifying enzymes such as GST and NQO1 are elevated in cancer cells[113,114]. The high intracellular ROS levels generated by exogenous administration of ROS enhance the proliferation of several cancer types. Akt activity and cell growth are significantly stimulated by treating hepatoma cells with low concentration of ROS, which could be abolished by adding antioxidants. PI3K inhibitor, wortmannin, inhibits Akt phosphorylation induced by ROS[115]. In another study, monomethylarsonous acid (MMAIII), ROS inducer, induces proliferation and activation of MAPK pathway as well as up-regulation of COX-2 and EGFR in human urothelium cells[116]. Generally, COX-2 expression is induced by NF-κB, not CCAAT/enhancer binding protein (C/EBP) in chronic gastritis and gastric cancer. Sphingosine kinase 1 and Sphingosine-1-phosphate are required for TNF-induced COX-2 induction in lung cancer[117,118].
Elevated intracellular ROS levels in cancer cells render these cells more vulnerable to oxidative stress-induced cell death. Therefore, cancer cells can be selectively killed without harming normal cells. Intracellular ROS levels in tumor cells are more likely to reach a threshold that triggers death after exposing to exogenous ROS-producing or -stimulating agents[119-121]. Apoptosis is prompted through extrinsic and intrinsic pathways[122]. In the extrinsic pathway, ROS are generated by ligation of cell surface death receptors [CD95(Fas), TNFR1, death receptor 3 (DR3), and DR5]. In turn, death receptor-ligand interaction leads to the subsequently activation of Fas-associated protein with death domain (FADD) and caspase-8[123-126]. In the intrinsic pathway, apoptosis is induced by mitochondria membrane disruption without involving death receptors. Subsequently, ROS activated by Bcl-2 family members, which are located in the outer mitochondrial membrane make pore. That results in cytochrome c release, apoptosome formation, and activation of caspases-3 and -7[127]. Exogenous administration of H2O2 induces apoptosis in various cancer cells, including lymphoma cells[128], leukemia cells[129,130], hepatoma cells[42,131], and bladder cancer cells[132] through the activation of MAPK signaling pathways.
ROS have been paid little attention in adaptive immunity because ROS production by the transformed and primary human B cells is very low compared to the levels of ROS are released by phagocytes[133,134]. However, later studies showed T cell receptor (TCR) or B cell receptor (BCR) engagement elicits ROS production transiently and superoxide controls pro-apoptotic and proliferative signal transduction, respectively[135,136]. In previous reports, we have shown that ROS might regulate apoptosis of lymphocytes directly or indirectly using EBV-transformed B cells as lymphoma or using an activated B cell model. Engagement of B7-H4, a negative regulator of T-cell mediated responses, induces the high levels of intracellular ROS and the expression of FasL. B7-H4 ligation induces Fas/FasL-mediated apoptosis through activation of caspase. Subsequently, cytochrome c, apoptosis-inducing factor (AIF), and endonuclease G (EndoG) are released from the mitochondria on EBV-transformed B cells after stimulation of B7-H4[137]. B7-H1 stimulation in EBV-transformed B cells also induces both transcription and translation of FasL. B7-H1 stimulation activated the phosphorylation of JNK and down-regulated ERK1/2 and p-Akt. N-acetylcysteine (NAC), ROS scavenger, and SP600125 completely blocked the induction of FasL and activation of JNK. B7-H1-mediated apoptosis on EBV-transformed B cells may be involved in the induction of FasL, which is evoked by ROS generation and JNK activation after cross-linking of B7-H1[138]. Ligation of CD70, the ligand for CD27, expressed on EBV-transformed B cells induced production of ROS and triggered ER stress-mediated apoptosis via ROS generation and MAPK pathway activation. These reports suggest that ROS-mediated alternate signaling pathways induce apoptosis and provide information supporting ROS as a target against EBV-related tumors[139,140]. The present paradigm of cell death is caspase-dependent apoptosis, whereas necroptosis is a regulated through caspase-independent manner[141-143]. TNFα, FasL, and Trail, the same ligands that can initiate apoptosis also trigger the necroptosis. Receptor-interacting protein-1 (RIP-1) and RIP-3 play a critical role in TNF-induced necroptosis[144]. TNF-mediated ROS generation and their lethal action are confined to the inner mitochondrial membrane in L929 cells[145,146]. ROS scavenger butylated hydroxyanisole (BHA) efficiently blocks TNF-induced necroptosis. Interestingly, inhibitors of NF-κB facilitate TNF-induced necrotic cell death, suggesting that NF-κB suppresses the necrotic cell death pathway[147]. However, antioxidant treatment against ROS is unable to protect all cell lines from necroptosis[141,148]. Recent work revealed the critical role of RIP-3 kinase activity in linking TNFR1/RIP-1-associated events. RIP-3 binds RIP-1 through this unique C-terminal segment to inhibit RIP- and TNF receptor-1-mediated NF-κB activation and necrostatin-1, RIP-1 kinase inhibitor, prevents RIP-1/RIP-3 interaction from necroptosis[149,150].
ROS mediate induction of matrix metalloproteinases (MMPs) involved in cancer invasion and metastasis. ROS have been reported to cause a significant increase in the production and expression of MMP-7. MMP-7 expression after H2O2 exposure is mediated by AP-1-dependent MAPKs in colorectal cancer cells[151]. ROS also up-regulate Akt and CXCR4 expression as well as inactivating PTEN. ROS mediate CXCR4-dependent cell migration cancer progression in prostate cancer cells[152]. Meanwhile, Hydrogen peroxide and hydroxyl radical prevent migration of non-small cell lung cancer cell from inhibiting Caveolin-1 down-regulation[153]. TNF or ROS can induce p38 MAPK- and MMP-9-dependent angiogenesis of endothelial cells[154,155]. Furthermore, oxidative stress induced by H2O2 stimulates angiogenesis and tumor progression by altering the gene expression of CXC chemokine ligand 14 (CXCL14) and IL-8 through the EGFR/MAPK signaling pathway[156]. EGF treatment induces H2O2 production, leading to activation of the Akt and vascular endothelial growth factor (VEGF) expression for angiogenesis in ovarian cancer cells[90]. Stimulation of tumor angiogenesis is connected with intracellular level of ROS. ROS regulate HIF-1α and VEGF expression[157]. Antioxidant N-acetyl-l-aspartate (NAC) decreased vessels number via suppressing HIF-1α expression in colon[158], liver[159] cancer cells. Conditions of energetic stress could lead to oxidative stress. Cancer cells that consume high levels of glucose create energetic stress during the formation of solid tumors. Higher levels of stress may occur when cells detach from the matrix and translocate to the lumen or during metastasis[160]. Decreased glucose uptake during these processes suppresses ATP production and activates AMP-activated protein kinase (AMPK), but also inhibits the generation of NADPH via the pentose phosphate pathway. Reduced levels of NADPH result in increased intracellular ROS, which could eventually cause cell death[161,162]. However, the concomitant activation of AMPK elicits alternative mechanisms that maintain intracellular NADPH levels.
Several studies have reported that cancer-associated fibroblasts (CAFs) play a critical role in the metastatic spread of cancer cells[163,164]. ROS-controlled signaling mechanisms involved in myofibroblast differentiation have diverse cellular effects. Recent data from human breast cancers and animal models established that myofibroblasts are derived from bone marrow derived cells such as fibrocytes or mesenchymal stem cells[165,166]. Moreover, various mesenchymal cell types including endothelial cells, pericytes, or pre-adipocytes can also be converted into myofibroblasts in breast carcinomas as well as local resident fibroblasts[167-169]. Mitochondrial ROS generation results in expression of NOX4, an enzyme that is required for TGF-β-driven conversion of fibroblasts into myofibroblasts[170]. In addition, fibroblasts suffering from chronic oxidative stress exhibit properties normally found in myofibroblasts[171]. Indeed, fibroblasts derived from mouse models exhibiting chronic oxidative stress (such as JunD−/− or NRF-2−/− mice, depleted for key anti-oxidant transcription factors) are converted into myofibroblasts[172]. Cancer cells themselves produce H2O2, which is a highly diffusible species. NOX enzymes, located at the plasma membrane in various carcinomas, might contribute to the production of H2O2 and the conversion of surrounding fibroblasts into myofibroblasts[173]. The increase of ROS in the stromal fibroblasts results in the promotion of tumor cell motility and neo-angiogenesis, further increasing metastatic dissemination[174,175]. CAFs, similar to fibroblasts exposed to chronic oxidative stress, express genes that encode for proteases involved in extracellular matrix (ECM) remodeling, including collagens, cell adhesion molecules, and MMPs[171,173]. ROS remodel the ECM and create tracks for collective migration of tumor cells through the activation of Rho-dependent pathways[176]. Improved understanding of ROS functions in cancer progression or metastasis could therefore help pave the way for new concepts in therapy.
As a cancerous tumor grows, the cancer cells repeatedly face limited oxygen supply due to the imbalance between growth rate and neovascularization. Cancer cells are able to adapt to oxidative stress and switch to glycolysis. Tumors utilize the Warburg effect, relying on glycolysis to supply energy for cancer cell survival[177]. The ROS released from mitochondria during the hypoxia act as signaling molecules that initiate diverse functional responses[178]. The increased ROS under low oxygen conditions, HIF-1α becomes transcriptionally active and accumulates at low levels of manganese (Mn)-containing SOD (MnSOD) activity. However, at moderate levels of MnSOD activity, hypoxic induction of VEGF and HIF-1α protein are suppressed in human breast carcinoma cells. This suggests that superoxide may contribute to accumulation of HIF-1α[179].
HIF-1α expression has been correlated with poor prognosis and increased cancer cell invasiveness and recent studies have also shown that the antitumorigenic effect of antioxidants is HIF-dependent[180]. HIF-1α regulates glycolysis-related genes in response to hypoxia and leads to glycolytic ATP generation[181,182]. However, it is not yet clear why tumor cells rely on glycolysis in the presence of oxygen and whether cellular ROS involved in regulation of glucose metabolism. ROS Accumulation and HIF-1 stabilization in CAFs results from the down-regulation of sirtuin-3 (SIRT3), a mitochondrial NAD-dependent deacetylase. HIF-1α can work with SIRT3 to regulate CAF metabolism, driving to metabolic reprogramming towards glycolysis[183]. In addition, hydrogen peroxide stabilizes HIF-1α thus leading to transcription of genes that code for signaling stromal cells such as macrophages and fibroblasts to support an invasive tumor cell phenotype. ROS-mediated HIF-1α helps the tumor cell convert energy production from OXPHOS to glycolysis, a metabolic switch that has been associated with increased metastatic potential[184].
Stem cells can be difficult to obtain, which makes it challenging to directly evaluate the role of ROS and the regulatory mechanism in stem cells[185]. However, interesting research has been conducted using Hematopoietic stem cells (HSCs) in the bone marrow. HSCs are principally located in a low-oxygen environment, which allows long-term protection from ROS-related oxidative stress. The ROSlow population has a higher self-renewal potential. In contrast, ROShigh population expresses high levels of the activated p38 MAPK and mammalian target of rapamycin (mTOR)[186]. ROS production and NF-κB activation triggered by GTPase Rac 1 are critical events for facilitating tumorigenesis after APC loss[187]. In comparison with cancer cells, cancer stem cells (CSCs) also have a lower intracellular ROS content than non-CSCs, which is similar to HSCs and may be caused by the increased expression of antioxidant systems[38,188].
ROSlow breast cancer cells are predominantly in quiescent phase of the cell cycle compared with ROShigh cells. The expression of ESR1 or MYC, which are both necessary for MCF7 proliferation in ROSlow breast cancer cells do not change[189]. Since ROS are critical mediators of ionizing radiation induced-therapy and chemotherapy, the expression of antioxidants in CSCs prevented DNA damage and protected cells from irradiation- or drug-induced cell death[38]. Due to high levels of antioxidant signaling, cancer stem cells also may not be responsive to other (chemotherapeutic) treatments that target cancer cells by increasing intracellular ROS levels[52]. Niclosamide, antihelminthic agent, increases induces apoptosis through up-regulation of ROS in progenitor/stem cells from acute myelogenous leukemia (AML) patients as well as Niclosamide inhibits the transcription and DNA binding of NF-κB[190]. Niclosamide is synergistic with the frontline chemotherapeutic agents, such as cytarabine, etoposide, and daunorubicin[190]. The peroxisome proliferator-activated receptor γ (PPARγ) is a ligand-dependent transcription factor belonging to the nuclear hormone receptor superfamily[191]. PPARγ agonists inhibit the stem cell-like features and repress tumor growth of human hepatocellular carcinoma (HCC) cells through NOX2-mediated ROS generation[192]. To date, it is unclear how ROS kill CSCs, although it does appear that ROS levels in normal or cancer environment may influence development and differentiation of stem cells[193].
Autophagy is activated under stress conditions as protective process for the cell and in various pathological conditions, including cancer and neurodegenerative diseases[194-196]. One major breakthrough in both the understanding of autophagy regulation and its implication in cancer was the discovery of Beclin-1. Mutation of Beclin-1 is detected in human breast, ovarian, and prostate cancer[197]. Beclin-1(-/-) mutant mice die in early embryonic stage and Beclin-1(+/-) mutant mice have shown the decreased autophagy formation and suffer from a high incidence of spontaneous tumors[198]. Beclin-1 is the first identified tumor suppressor protein that functions in the lysosomal degradation pathway of autophagy. Bcl-2, a specific inhibitor for apoptosis, inhibits starvation-induced autophagy in cancer cells and mice through interacting with Beclin-1[199,200]. In addition, oncogenic signaling molecules, including class I PI3K, Akt, and mTOR suppress the macroautophagic pathway. However, PTEN and p53 stimulate autophagy[201-203]. Autophagy-related genes (Atg) 4, a direct target for oxidation by Intracellular H2O2 generated during starvation, is regulated by conjugating Atg8 at the site of autophagosome formation via the lipidation of Atg8[204]. Low levels of ROS modify Atg4 and HMGB1 proteins, which activate AMPK and ASK1/JNK pathways or transactivate various proteins that could up-regulate autophagy, leading to reductions in apoptosis[205]. MAPKs, such as JNK and p38 MAPK, play a critical role in ROS-mediated autophagy events[206,207]. Meanwhile, activated ERK and JNK are also upstream effectors controlling both autophagy and apoptosis in response to elevated intracellular ROS[208]. Antioxidant, N-acetyl-l-cysteine (NAC), clearly reduces K-Ras-induced Atg5 and Atg7 induction, autophagy, and malignant cell transformation[209]. Starvation-induced production of ·O2- induces autophagy and cell death. Exogenous H2O2 is effectively converted to intracellular ·O2- leading to autophagy-induced cell death. Overexpression of SOD2 and 3-methyladenine, autophagy inhibitor, attenuate starvation-induced autophagy[210]. 2-Methoxyestradiol inhibits SODs and induces autophagy-mediated cell death in the transformed or cancer cell lines, but not in astrocytes[210]. From these results, understanding the mechanisms that regulate the crosstalk between ROS and autophagy regulation is important for various disorders, including neurodegenerative diseases and cancer.
Many chemotherapeutic drugs are designed to increase cellular ROS levels with the goal of inducing irreversible damage, consequently resulting in tumor cell apoptosis. For example, intracellular ROS levels increase in a dose-dependent manner in A549 human lung cancer cells after treatment with paclitaxel. Addition of NAC or GSH, two H2O2 scavengers, induces a four-times increase in paclitaxel IC50[211]. Combined treatment with trichostatin A and gemcitabine synergistically inhibits growth of pancreatic adenocarcinoma cell lines and induces apoptosis through the induction of ROS by gemcitabine[212]. Wogonin, a flavonoid isolated from Scutellaria baicalensis, synergistically sensitizes cancer cells to TNF-induced apoptosis through inhibition of catalase activity and an increase of cellular H2O2. Wogonin-induced ROS inhibit TNF-induced phosphorylation on the NF-κB p65 subunit[213]. Mutations in mitochondrial genes (mtDNA), such as the gene encoding cytochrome c oxidase II, are associated with increased ROS generation and involved in cancer initiation and progression[74-76]. However, the susceptibility of mitochondrial DNA to ROS-induced mutation may also be utilized for therapy[214]. Low levels of exogenous H2O2 or H2O2 produced by mitochondria induce a modest drop in ATP level, delayed toxicity, and G2/M arrest without affecting cell viability. Concomitant inhibition of glycolysis was found to markedly sensitize cells to death in the presence of nontoxic concentrations of H2O2[215]. Another approach to regulating intracellular ROS levels is the use of antioxidants to prevent tumor cells from entering the ROS-mediated survival signaling pathway. ROS, as second messengers in signaling pathways, regulate not only kinase phosphorylation (MAPK, Rho kinase) but also transcription factors (NF-κB, AP-1, and HIF-1). ROS also up-regulate proto-oncogene and pro-inflammatory gene expression and activity[216,217]. The protective effects of antioxidants have generated significant interest in developing synthetic and natural antioxidants as therapeutic agents to prevent and/or treat patients with cancer.
Several clinical trials have been published regarding the effects of antioxidant vitamins on the risk of cardiovascular disease. However, clinical trial data has been inconsistent and inconclusive, until now[218]. Although oxidative stress against cancer cells induces apoptosis, several exogenous antioxidants also produce favorable effects in various cancer patients[219]. The major antioxidant vitamin systems include vitamin E, vitamin C, and GSH[220]. Vitamin C (ascorbic acid) is a water-soluble antioxidant involved in the reduction of radicals by recycling radicals produced by oxidation of vitamin E. A 20 μmol/L rise in plasma ascorbic acid concentration is associated with an approximately 20% reduction in the risk of all-cause mortality. Ascorbic acid was inversely related to cancer mortality in men but not women[221]. In a study conducted in Japan, Vitamin C was found to reduce oxidative stress among subjects with atrophic gastritis[222]. However, Vitamins E and C, at high concentrations, also function as pro-oxidants causing cell damage[223]. Unfortunately, one report showed Vitamin E supplement significantly increased the risk of prostate cancer compared with placebo group and selenium combination group[224]. There is possibility that the bioavailability of anti-oxidants may be insufficient after oral administration, or that they may be inaccessible to the source of free radicals, particularly if ROS are generated in specific cellular compartments and organelles[225]. In addition, antioxidants do not inhibit the production of ROS; rather, they scavenge ROS after ROS has been generated. Selenium supplementation has been shown to reduce total and prostate cancer incidence but was not significantly associated with lung and colorectal cancer incidence[226]. In addition, selenium shows the most prominent protective effect on former male smokers[226]. However, the benefits of selenium were only observed in those patients with the lowest baseline blood selenium levels[226]. A phase II clinical trial about the effect of pomegranate for men reveals that prostate-specific antigen (PSA) doubling time is significantly extended after treatment with pomegranate juice in patients received surgery or radiotherapy. Further, a decrease in cell proliferation and an increase in apoptosis is observed in patients who consumed pomegranate[227]. Green tea is popular because it contains epigallocatechin gallate, a polyphenolic compound that provides potential benefits for prostate cancer control[228,229].
Lycopene in tomato decreases serum prostate-specific antigen levels and oxidative DNA damage and increases apoptotic cells in carcinomas[230]. In another study, tomato sauce consumption suppresses the progression of prostate carcinoma[231]. Curcumin is also demonstrated the inhibitory effects in colon carcinogenesis[232].
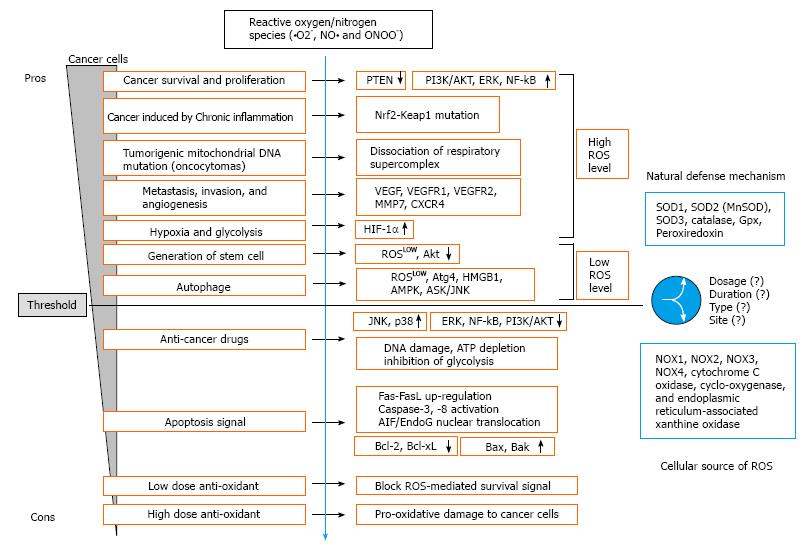
Daily fruits and vegetables intake has been inversely correlated to the risk of the development of chronic diseases, including cancer. Cancer is caused by both internal factors and environmental factors[233]. The link between diet and cancer indicates that cancer is a disease which can be prevented largely by lifestyle changes. In addition, ROS have plenty roles in carcinogenesis or anti-tumor effects though numerous pathways (Figure 1). Since ROS are involved in the transformation of nonmalignant cells to malignant cells, regulation of ROS can be a critical approach to prevent cancer development. ROS act as the second messenger for generating further intracellular events. ROS play a crucial role in tumorigenesis and cancer cell survival as well as apoptotic signaling in cancer cells. In biological systems, enzymatic and nonenzymatic systems have evolved to protect against oxidative damage. The potential of pro-oxidants or antioxidants in treating cancer associated with oxidative stress is reinforced by experimental researches, several clinical studies, and epidemiological data. Therefore, future studies should continue to clarify the different roles of ROS in cancer cells.
P- Reviewer: Hann HW, Hou WH, Koch TR, Morales-Gonzalez JA, Sanchez-Alcazar JA S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Bruce-Keller AJ, Gupta S, Knight AG, Beckett TL, McMullen JM, Davis PR, Murphy MP, Van Eldik LJ, St Clair D, Keller JN. Cognitive impairment in humanized APP×PS1 mice is linked to Aβ(1-42) and NOX activation. Neurobiol Dis. 2011;44:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G. Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem Biophys Res Commun. 2000;273:5-9. [PubMed] |
| 3. | Murakami K, Murata N, Noda Y, Tahara S, Kaneko T, Kinoshita N, Hatsuta H, Murayama S, Barnham KJ, Irie K. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid β protein oligomerization and memory loss in mouse model of Alzheimer disease. J Biol Chem. 2011;286:44557-44568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | Umeda-Kameyama Y, Tsuda M, Ohkura C, Matsuo T, Namba Y, Ohuchi Y, Aigaki T. Thioredoxin suppresses Parkin-associated endothelin receptor-like receptor-induced neurotoxicity and extends longevity in Drosophila. J Biol Chem. 2007;282:11180-11187. [PubMed] |
| 5. | Hu X, Weng Z, Chu CT, Zhang L, Cao G, Gao Y, Signore A, Zhu J, Hastings T, Greenamyre JT. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J Neurosci. 2011;31:247-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756-10764. [PubMed] |
| 7. | Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59-62. [PubMed] |
| 8. | Blake DR, Hall ND, Treby DA, Halliwell B, Gutteridge JM. Protection against superoxide and hydrogen peroxide in synovial fluid from rheumatoid patients. Clin Sci (Lond). 1981;61:483-486. [PubMed] |
| 9. | Mazzetti I, Grigolo B, Borzì RM, Meliconi R, Facchini A. Serum copper/zinc superoxide dismutase levels in patients with rheumatoid arthritis. Int J Clin Lab Res. 1996;26:245-249. [PubMed] |
| 10. | Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci USA. 1991;88:10045-10048. [PubMed] |
| 11. | Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916-1923. [PubMed] |
| 12. | Krause KH, Bedard K. NOX enzymes in immuno-inflammatory pathologies. Semin Immunopathol. 2008;30:193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Krieger-Brauer HI, Medda PK, Kather H. Insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Galphai2. J Biol Chem. 1997;272:10135-10143. [PubMed] |
| 14. | Aggeli IK, Theofilatos D, Beis I, Gaitanaki C. Insulin-induced oxidative stress up-regulates heme oxygenase-1 via diverse signaling cascades in the C2 skeletal myoblast cell line. Endocrinology. 2011;152:1274-1283. [PubMed] |
| 15. | Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752-1761. [PubMed] |
| 16. | Farber E, Rubin H. Cellular adaptation in the origin and development of cancer. Cancer Res. 1991;51:2751-2761. [PubMed] |
| 17. | Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390-395. [PubMed] |
| 18. | Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603-1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4137] [Cited by in RCA: 3708] [Article Influence: 247.2] [Reference Citation Analysis (0)] |
| 19. | Visconti R, Grieco D. New insights on oxidative stress in cancer. Curr Opin Drug Discov Devel. 2009;12:240-245. [PubMed] |
| 20. | Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. [PubMed] |
| 21. | Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833-846. [PubMed] |
| 22. | Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, Manevich Y, Beeson C, Neumann CA. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, Nowill AE, Leslie NR, Cardoso AA, Barata JT. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118:3762-3774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 358] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 24. | Marshall HE, Hess DT, Stamler JS. S-nitrosylation: physiological regulation of NF-kappaB. Proc Natl Acad Sci USA. 2004;101:8841-8842. [PubMed] |
| 26. | Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179-183. [PubMed] |
| 27. | Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261-1274. [PubMed] |
| 28. | Li M, Carpio DF, Zheng Y, Bruzzo P, Singh V, Ouaaz F, Medzhitov RM, Beg AA. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166:7128-7135. [PubMed] |
| 30. | Sen P, Wallet MA, Yi Z, Huang Y, Henderson M, Mathews CE, Earp HS, Matsushima G, Baldwin AS, Tisch RM. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood. 2007;109:653-660. [PubMed] |
| 31. | Ren G, Su J, Zhao X, Zhang L, Zhang J, Roberts AI, Zhang H, Das G, Shi Y. Apoptotic cells induce immunosuppression through dendritic cells: critical roles of IFN-gamma and nitric oxide. J Immunol. 2008;181:3277-3284. [PubMed] |
| 32. | Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561-574. [PubMed] |
| 33. | Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 2008;477:183-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Pervaiz S, Taneja R, Ghaffari S. Oxidative stress regulation of stem and progenitor cells. Antioxid Redox Signal. 2009;11:2777-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 35. | Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245-313. [PubMed] |
| 36. | del Río LA, Sandalio LM, Palma JM, Bueno P, Corpas FJ. Metabolism of oxygen radicals in peroxisomes and cellular implications. Free Radic Biol Med. 1992;13:557-580. [PubMed] |
| 37. | Inoue M, Sato EF, Nishikawa M, Park AM, Kira Y, Imada I, Utsumi K. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem. 2003;10:2495-2505. [PubMed] |
| 38. | Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2102] [Cited by in RCA: 1935] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 39. | Chen W, Druhan LJ, Chen CA, Hemann C, Chen YR, Berka V, Tsai AL, Zweier JL. Peroxynitrite induces destruction of the tetrahydrobiopterin and heme in endothelial nitric oxide synthase: transition from reversible to irreversible enzyme inhibition. Biochemistry. 2010;49:3129-3137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Ruiz-Ramos R, Lopez-Carrillo L, Rios-Perez AD, De Vizcaya-Ruíz A, Cebrian ME. Sodium arsenite induces ROS generation, DNA oxidative damage, HO-1 and c-Myc proteins, NF-kappaB activation and cell proliferation in human breast cancer MCF-7 cells. Mutat Res. 2009;674:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Adams DJ, Boskovic ZV, Theriault JR, Wang AJ, Stern AM, Wagner BK, Shamji AF, Schreiber SL. Discovery of small-molecule enhancers of reactive oxygen species that are nontoxic or cause genotype-selective cell death. ACS Chem Biol. 2013;8:923-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 886] [Cited by in RCA: 849] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 43. | Liu JM, Pan F, Li L, Liu QR, Chen Y, Xiong XX, Cheng K, Yu SB, Shi Z, Yu AC. Piperlongumine selectively kills glioblastoma multiforme cells via reactive oxygen species accumulation dependent JNK and p38 activation. Biochem Biophys Res Commun. 2013;437:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Fridovich I. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515-18517. [PubMed] |
| 45. | Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1411] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 46. | Goyal MM, Basak A. Human catalase: looking for complete identity. Protein Cell. 2010;1:888-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 48. | Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3513] [Cited by in RCA: 4194] [Article Influence: 262.1] [Reference Citation Analysis (0)] |
| 49. | Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007-3017. [PubMed] |
| 50. | Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881-1896. [PubMed] |
| 51. | Gupta A, Rosenberger SF, Bowden GT. Increased ROS levels contribute to elevated transcription factor and MAP kinase activities in malignantly progressed mouse keratinocyte cell lines. Carcinogenesis. 1999;20:2063-2073. [PubMed] |
| 52. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [PubMed] |
| 53. | Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 493] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 54. | Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 601] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 55. | Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. [PubMed] |
| 56. | Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 2667] [Article Influence: 177.8] [Reference Citation Analysis (0)] |
| 57. | Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Banning A, Deubel S, Kluth D, Zhou Z, Brigelius-Flohé R. The GI-GPx gene is a target for Nrf2. Mol Cell Biol. 2005;25:4914-4923. [PubMed] |
| 59. | Köhle C, Bock KW. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem Pharmacol. 2007;73:1853-1862. [PubMed] |
| 60. | Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76-86. [PubMed] |
| 61. | Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908-11913. [PubMed] |
| 62. | Yu R, Lei W, Mandlekar S, Weber MJ, Der CJ, Wu J, Kong AN. Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J Biol Chem. 1999;274:27545-27552. [PubMed] |
| 63. | Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol Pharmacol. 2002;62:1001-1010. [PubMed] |
| 64. | Yamadori T, Ishii Y, Homma S, Morishima Y, Kurishima K, Itoh K, Yamamoto M, Minami Y, Noguchi M, Hizawa N. Molecular mechanisms for the regulation of Nrf2-mediated cell proliferation in non-small-cell lung cancers. Oncogene. 2012;31:4768-4777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 65. | Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, Eom HS, Yoo NJ, Lee SH. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 304] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 66. | Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. [PubMed] |
| 67. | Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639-643. [PubMed] |
| 68. | Gapuzan ME, Yufit PV, Gilmore TD. Immortalized embryonic mouse fibroblasts lacking the RelA subunit of transcription factor NF-kappaB have a malignantly transformed phenotype. Oncogene. 2002;21:2484-2492. [PubMed] |
| 69. | Ricca A, Biroccio A, Trisciuoglio D, Cippitelli M, Zupi G, Del Bufalo D. relA over-expression reduces tumorigenicity and activates apoptosis in human cancer cells. Br J Cancer. 2001;85:1914-1921. [PubMed] |
| 70. | Seitz CS, Lin Q, Deng H, Khavari PA. Alterations in NF-kappaB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-kappaB. Proc Natl Acad Sci USA. 1998;95:2307-2312. [PubMed] |
| 71. | van Hogerlinden M, Rozell BL, Ahrlund-Richter L, Toftgård R. Squamous cell carcinomas and increased apoptosis in skin with inhibited Rel/nuclear factor-kappaB signaling. Cancer Res. 1999;59:3299-3303. [PubMed] |
| 72. | Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1-11. [PubMed] |
| 73. | Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239-267. [PubMed] |
| 74. | Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. APMIS. 2007;115:81-103. [PubMed] |
| 75. | Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359-407. [PubMed] |
| 76. | Wallace DC. Mitochondrial DNA mutations in disease and aging. Environ Mol Mutagen. 2010;51:440-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 283] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 77. | Kulawiec M, Safina A, Desouki MM, Still I, Matsui S, Bakin A, Singh KK. Tumorigenic transformation of human breast epithelial cells induced by mitochondrial DNA depletion. Cancer Biol Ther. 2008;7:1732-1743. [PubMed] |
| 78. | Parrella P, Xiao Y, Fliss M, Sanchez-Cespedes M, Mazzarelli P, Rinaldi M, Nicol T, Gabrielson E, Cuomo C, Cohen D. Detection of mitochondrial DNA mutations in primary breast cancer and fine-needle aspirates. Cancer Res. 2001;61:7623-7626. [PubMed] |
| 79. | Wang CY, Wang HW, Yao YG, Kong QP, Zhang YP. Somatic mutations of mitochondrial genome in early stage breast cancer. Int J Cancer. 2007;121:1253-1256. [PubMed] |
| 80. | Frezza C, Gottlieb E. Mitochondria in cancer: not just innocent bystanders. Semin Cancer Biol. 2009;19:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 81. | Bonora E, Porcelli AM, Gasparre G, Biondi A, Ghelli A, Carelli V, Baracca A, Tallini G, Martinuzzi A, Lenaz G. Defective oxidative phosphorylation in thyroid oncocytic carcinoma is associated with pathogenic mitochondrial DNA mutations affecting complexes I and III. Cancer Res. 2006;66:6087-6096. [PubMed] |
| 82. | Porcelli AM, Angelin A, Ghelli A, Mariani E, Martinuzzi A, Carelli V, Petronilli V, Bernardi P, Rugolo M. Respiratory complex I dysfunction due to mitochondrial DNA mutations shifts the voltage threshold for opening of the permeability transition pore toward resting levels. J Biol Chem. 2009;284:2045-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Lenaz G, Genova ML. Kinetics of integrated electron transfer in the mitochondrial respiratory chain: random collisions vs. solid state electron channeling. Am J Physiol Cell Physiol. 2007;292:C1221-C1239. [PubMed] |
| 84. | Zimmermann FA, Mayr JA, Neureiter D, Feichtinger R, Alinger B, Jones ND, Eder W, Sperl W, Kofler B. Lack of complex I is associated with oncocytic thyroid tumours. Br J Cancer. 2009;100:1434-1437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Shidara Y, Yamagata K, Kanamori T, Nakano K, Kwong JQ, Manfredi G, Oda H, Ohta S. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res. 2005;65:1655-1663. [PubMed] |
| 86. | Park JS, Sharma LK, Li H, Xiang R, Holstein D, Wu J, Lechleiter J, Naylor SL, Deng JJ, Lu J. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum Mol Genet. 2009;18:1578-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 87. | Chiarugi P, Fiaschi T. Redox signalling in anchorage-dependent cell growth. Cell Signal. 2007;19:672-682. [PubMed] |
| 88. | Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1989] [Cited by in RCA: 2278] [Article Influence: 151.9] [Reference Citation Analysis (0)] |
| 89. | Cosentino C, Grieco D, Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30:546-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 336] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 90. | Liu LZ, Hu XW, Xia C, He J, Zhou Q, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1alpha expression through activation of AKT and P70S6K1 in human ovarian cancer cells. Free Radic Biol Med. 2006;41:1521-1533. [PubMed] |
| 91. | Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699-3707. [PubMed] |
| 92. | Manna SK, Zhang HJ, Yan T, Oberley LW, Aggarwal BB. Overexpression of manganese superoxide dismutase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-kappaB and activated protein-1. J Biol Chem. 1998;273:13245-13254. [PubMed] |
| 93. | Kong Q, Beel JA, Lillehei KO. A threshold concept for cancer therapy. Med Hypotheses. 2000;55:29-35. [PubMed] |
| 94. | Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191-1212. [PubMed] |
| 95. | Ramsey MR, Sharpless NE. ROS as a tumour suppressor? Nat Cell Biol. 2006;8:1213-1215. [PubMed] |
| 96. | Deshpande SS, Angkeow P, Huang J, Ozaki M, Irani K. Rac1 inhibits TNF-alpha-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J. 2000;14:1705-1714. [PubMed] |
| 97. | Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353-C369. [PubMed] |
| 98. | Edderkaoui M, Hong P, Vaquero EC, Lee JK, Fischer L, Friess H, Buchler MW, Lerch MM, Pandol SJ, Gukovskaya AS. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1137-G1147. [PubMed] |
| 99. | Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643-34654. [PubMed] |
| 100. | Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, Pandol SJ, Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637-1648. [PubMed] |
| 101. | Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 633] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 102. | Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 285] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 103. | Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1089] [Cited by in RCA: 1081] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 104. | Kopnin PB, Agapova LS, Kopnin BP, Chumakov PM. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 2007;67:4671-4678. [PubMed] |
| 105. | Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306-1313. [PubMed] |
| 106. | Chung YM, Kim JS, Yoo YD. A novel protein, Romo1, induces ROS production in the mitochondria. Biochem Biophys Res Commun. 2006;347:649-655. [PubMed] |
| 107. | Hwang IT, Chung YM, Kim JJ, Chung JS, Kim BS, Kim HJ, Kim JS, Yoo YD. Drug resistance to 5-FU linked to reactive oxygen species modulator 1. Biochem Biophys Res Commun. 2007;359:304-310. [PubMed] |
| 108. | Na AR, Chung YM, Lee SB, Park SH, Lee MS, Yoo YD. A critical role for Romo1-derived ROS in cell proliferation. Biochem Biophys Res Commun. 2008;369:672-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 109. | De Luca A, Sanna F, Sallese M, Ruggiero C, Grossi M, Sacchetta P, Rossi C, De Laurenzi V, Di Ilio C, Favaloro B. Methionine sulfoxide reductase A down-regulation in human breast cancer cells results in a more aggressive phenotype. Proc Natl Acad Sci USA. 2010;107:18628-18633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 110. | Hu Y, Rosen DG, Zhou Y, Feng L, Yang G, Liu J, Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J Biol Chem. 2005;280:39485-39492. [PubMed] |
| 111. | Pilarsky C, Wenzig M, Specht T, Saeger HD, Grützmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6:744-750. [PubMed] |
| 112. | Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, Lau LF, Costa RH, Raychaudhuri P. FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009;28:2908-2918. [PubMed] |
| 113. | Belinsky M, Jaiswal AK. NAD(P)H: quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 1993;12:103-117. [PubMed] |
| 114. | Di Pietro G, Magno LA, Rios-Santos F. Glutathione S-transferases: an overview in cancer research. Expert Opin Drug Metab Toxicol. 2010;6:153-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 115. | Dong-Yun S, Yu-Ru D, Shan-Lin L, Ya-Dong Z, Lian W. Redox stress regulates cell proliferation and apoptosis of human hepatoma through Akt protein phosphorylation. FEBS Lett. 2003;542:60-64. [PubMed] |
| 116. | Eblin KE, Jensen TJ, Wnek SM, Buffington SE, Futscher BW, Gandolfi AJ. Reactive oxygen species regulate properties of transformation in UROtsa cells exposed to monomethylarsonous acid by modulating MAPK signaling. Toxicology. 2009;255:107-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 117. | Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411-1421. [PubMed] |
| 118. | Seo JH, Kim H, Kim KH. Cyclooxygenase-2 expression by transcription factors in Helicobacter pylori-infected gastric epithelial cells: comparison between HP 99 and NCTC 11637. Ann N Y Acad Sci. 2002;973:477-480. [PubMed] |
| 119. | Yi J, Yang J, He R, Gao F, Sang H, Tang X, Ye RD. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108-116. [PubMed] |
| 120. | Jing Y, Yang J, Wang Y, Li H, Chen Y, Hu Q, Shi G, Tang X, Yi J. Alteration of subcellular redox equilibrium and the consequent oxidative modification of nuclear factor kappaB are critical for anticancer cytotoxicity by emodin, a reactive oxygen species-producing agent. Free Radic Biol Med. 2006;40:2183-2197. [PubMed] |
| 121. | Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241-252. [PubMed] |
| 122. | Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96:2181-2196. [PubMed] |
| 123. | Denning TL, Takaishi H, Crowe SE, Boldogh I, Jevnikar A, Ernst PB. Oxidative stress induces the expression of Fas and Fas ligand and apoptosis in murine intestinal epithelial cells. Free Radic Biol Med. 2002;33:1641-1650. [PubMed] |
| 124. | Medan D, Wang L, Toledo D, Lu B, Stehlik C, Jiang BH, Shi X, Rojanasakul Y. Regulation of Fas (CD95)-induced apoptotic and necrotic cell death by reactive oxygen species in macrophages. J Cell Physiol. 2005;203:78-84. [PubMed] |
| 125. | Reinehr R, Becker S, Eberle A, Grether-Beck S, Häussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J Biol Chem. 2005;280:27179-27194. [PubMed] |
| 126. | Uchikura K, Wada T, Hoshino S, Nagakawa Y, Aiko T, Bulkley GB, Klein AS, Sun Z. Lipopolysaccharides induced increases in Fas ligand expression by Kupffer cells via mechanisms dependent on reactive oxygen species. Am J Physiol Gastrointest Liver Physiol. 2004;287:G620-G626. [PubMed] |
| 127. | Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1-15. [PubMed] |
| 128. | Hampton MB, Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 1997;414:552-556. [PubMed] |
| 129. | Bejarano I, Espino J, Marchena AM, Barriga C, Paredes SD, Rodríguez AB, Pariente JA. Melatonin enhances hydrogen peroxide-induced apoptosis in human promyelocytic leukaemia HL-60 cells. Mol Cell Biochem. 2011;353:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 130. | Price M, Terlecky SR, Kessel D. A role for hydrogen peroxide in the pro-apoptotic effects of photodynamic therapy. Photochem Photobiol. 2009;85:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 131. | Valdameri G, Trombetta-Lima M, Worfel PR, Pires AR, Martinez GR, Noleto GR, Cadena SM, Sogayar MC, Winnischofer SM, Rocha ME. Involvement of catalase in the apoptotic mechanism induced by apigenin in HepG2 human hepatoma cells. Chem Biol Interact. 2011;193:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 132. | Choudhary S, Wang KK, Wang HC. Oncogenic H-Ras, FK228, and exogenous H2O2 cooperatively activated the ERK pathway in selective induction of human urinary bladder cancer J82 cell death. Mol Carcinog. 2011;50:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 133. | Maly FE, Nakamura M, Gauchat JF, Urwyler A, Walker C, Dahinden CA, Cross AR, Jones OT, de Weck AL. Superoxide-dependent nitroblue tetrazolium reduction and expression of cytochrome b-245 components by human tonsillar B lymphocytes and B cell lines. J Immunol. 1989;142:1260-1267. [PubMed] |
| 134. | Maly FE, Cross AR, Jones OT, Wolf-Vorbeck G, Walker C, Dahinden CA, De Weck AL. The superoxide generating system of B cell lines. Structural homology with the phagocytic oxidase and triggering via surface Ig. J Immunol. 1988;140:2334-2339. [PubMed] |
| 135. | Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J Exp Med. 2002;195:59-70. [PubMed] |
| 136. | Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281-293. [PubMed] |
| 137. | Song H, Park G, Kim YS, Hur I, Kim H, Ryu JW, Lee HK, Cho DH, Choi IH, Lee WJ. B7-H4 reverse signaling induces the apoptosis of EBV-transformed B cells through Fas ligand up-regulation. Cancer Lett. 2008;266:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 138. | Kim YS, Park GB, Lee HK, Song H, Choi IH, Lee WJ, Hur DY. Cross-linking of B7-H1 on EBV-transformed B cells induces apoptosis through reactive oxygen species production, JNK signaling activation, and fasL expression. J Immunol. 2008;181:6158-6169. [PubMed] |
| 139. | Hintzen RQ, Lens SM, Koopman G, Pals ST, Spits H, van Lier RA. CD70 represents the human ligand for CD27. Int Immunol. 1994;6:477-480. [PubMed] |
| 140. | Park GB, Kim YS, Lee HK, Song H, Cho DH, Lee WJ, Hur DY. Endoplasmic reticulum stress-mediated apoptosis of EBV-transformed B cells by cross-linking of CD70 is dependent upon generation of reactive oxygen species and activation of p38 MAPK and JNK pathway. J Immunol. 2010;185:7274-7284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 141. | Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112-119. [PubMed] |
| 142. | Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 824] [Cited by in RCA: 887] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 143. | Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, Vandenabeele P. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998;188:919-930. [PubMed] |
| 144. | Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489-495. [PubMed] |
| 145. | Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400-410. [PubMed] |
| 146. | Goossens V, De Vos K, Vercammen D, Steemans M, Vancompernolle K, Fiers W, Vandenabeele P, Grooten J. Redox regulation of TNF signaling. Biofactors. 1999;10:145-156. [PubMed] |
| 147. | Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, Tran JH, Nedospasov SA, Liu ZG. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822-10828. [PubMed] |
| 148. | He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1882] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 149. | Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277:9505-9511. [PubMed] |
| 150. | Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1651] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 151. | Ho BY, Wu YM, Chang KJ, Pan TM. Dimerumic acid inhibits SW620 cell invasion by attenuating H₂O₂-mediated MMP-7 expression via JNK/C-Jun and ERK/C-Fos activation in an AP-1-dependent manner. Int J Biol Sci. 2011;7:869-880. [PubMed] |
| 152. | Chetram MA, Don-Salu-Hewage AS, Hinton CV. ROS enhances CXCR4-mediated functions through inactivation of PTEN in prostate cancer cells. Biochem Biophys Res Commun. 2011;410:195-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 153. | Rungtabnapa P, Nimmannit U, Halim H, Rojanasakul Y, Chanvorachote P. Hydrogen peroxide inhibits non-small cell lung cancer cell anoikis through the inhibition of caveolin-1 degradation. Am J Physiol Cell Physiol. 2011;300:C235-C245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 154. | Rajashekhar G, Kamocka M, Marin A, Suckow MA, Wolter WR, Badve S, Sanjeevaiah AR, Pumiglia K, Rosen E, Clauss M. Pro-inflammatory angiogenesis is mediated by p38 MAP kinase. J Cell Physiol. 2011;226:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 155. | Yasuda M, Ohzeki Y, Shimizu S, Naito S, Ohtsuru A, Yamamoto T, Kuroiwa Y. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 1999;64:249-258. [PubMed] |
| 156. | Maehata Y, Ozawa S, Kobayashi K, Kato Y, Yoshino F, Miyamoto C, Izukuri K, Kubota E, Hata R, Lee MC. Reactive oxygen species (ROS) reduce the expression of BRAK/CXCL14 in human head and neck squamous cell carcinoma cells. Free Radic Res. 2010;44:913-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 157. | Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823-10830. [PubMed] |
| 158. | Khromova NV, Kopnin PB, Stepanova EV, Agapova LS, Kopnin BP. p53 hot-spot mutants increase tumor vascularization via ROS-mediated activation of the HIF1/VEGF-A pathway. Cancer Lett. 2009;276:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 159. | Lin CC, Huang CY, Mong MC, Chan CY, Yin MC. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J Agric Food Chem. 2011;59:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 160. | Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 833] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 161. | Nagalingam A, Arbiser JL, Bonner MY, Saxena NK, Sharma D. Honokiol activates AMP-activated protein kinase in breast cancer cells via an LKB1-dependent pathway and inhibits breast carcinogenesis. Breast Cancer Res. 2012;14:R35. [PubMed] |
| 162. | Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 928] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 163. | Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2949] [Cited by in RCA: 2767] [Article Influence: 172.9] [Reference Citation Analysis (0)] |
| 164. | Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557-563. [PubMed] |
| 165. | Paunescu V, Bojin FM, Tatu CA, Gavriliuc OI, Rosca A, Gruia AT, Tanasie G, Bunu C, Crisnic D, Gherghiceanu M. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med. 2011;15:635-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 166. | Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 857] [Cited by in RCA: 869] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 167. | Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 810] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 168. | Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, Andreeff M, Marini FC. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One. 2012;7:e30563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 169. | Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA. 2010;107:20009-20014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 687] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 170. | Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J Biol Chem. 2013;288:770-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 313] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 171. | Artaud-Macari E, Goven D, Brayer S, Hamimi A, Besnard V, Marchal-Somme J, Ali ZE, Crestani B, Kerdine-Römer S, Boutten A. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2013;18:66-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 172. | Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029-12038. [PubMed] |
| 173. | Toullec A, Gerald D, Despouy G, Bourachot B, Cardon M, Lefort S, Richardson M, Rigaill G, Parrini MC, Lucchesi C. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2:211-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 174. | Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335-348. [PubMed] |
| 175. | Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848-851. [PubMed] |
| 176. | Sanz-Moreno V, Gaggioli C, Yeo M, Albrengues J, Wallberg F, Viros A, Hooper S, Mitter R, Féral CC, Cook M. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell. 2011;20:229-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 360] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 177. | Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68-72. [PubMed] |
| 178. | Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807-819. [PubMed] |
| 179. | Wang M, Kirk JS, Venkataraman S, Domann FE, Zhang HJ, Schafer FQ, Flanagan SW, Weydert CJ, Spitz DR, Buettner GR. Manganese superoxide dismutase suppresses hypoxic induction of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Oncogene. 2005;24:8154-8166. [PubMed] |
| 180. | Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230-238. [PubMed] |
| 181. | Tacchini L, Dansi P, Matteucci E, Desiderio MA. Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis. 2001;22:1363-1371. [PubMed] |
| 182. | Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437-443. [PubMed] |
| 183. | Fiaschi T, Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol. 2012;2012:762825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 184. | Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, Salim A, Wang Y, Kristensen GB, Helland A, Børresen-Dale AL. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3:e47. [PubMed] |
| 185. | Abbott A. Stem cells: The cell division. Nature. 2011;480:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 186. | Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056-3063. [PubMed] |
| 187. | Myant KB, Cammareri P, McGhee EJ, Ridgway RA, Huels DJ, Cordero JB, Schwitalla S, Kalna G, Ogg EL, Athineos D. ROS production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 319] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 188. | Ye XQ, Li Q, Wang GH, Sun FF, Huang GJ, Bian XW, Yu SC, Qian GS. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int J Cancer. 2011;129:820-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 189. | Dey-Guha I, Wolfer A, Yeh AC, G Albeck J, Darp R, Leon E, Wulfkuhle J, Petricoin EF, Wittner BS, Ramaswamy S. Asymmetric cancer cell division regulated by AKT. Proc Natl Acad Sci USA. 2011;108:12845-12850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 190. | Jin Y, Lu Z, Ding K, Li J, Du X, Chen C, Sun X, Wu Y, Zhou J, Pan J. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010;70:2516-2527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 191. | Wang T, Xu J, Yu X, Yang R, Han ZC. Peroxisome proliferator-activated receptor gamma in malignant diseases. Crit Rev Oncol Hematol. 2006;58:1-14. [PubMed] |
| 192. | Liu L, Yang Z, Xu Y, Li J, Xu D, Zhang L, Sun J, Xia S, Zou F, Liu Y. Inhibition of oxidative stress-elicited AKT activation facilitates PPARγ agonist-mediated inhibition of stem cell character and tumor growth of liver cancer cells. PLoS One. 2013;8:e73038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 193. | Abdel-Wahab O, Levine RL. Metabolism and the leukemic stem cell. J Exp Med. 2010;207:677-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 194. | Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell. 2008;15:344-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 422] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 195. | Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5421] [Cited by in RCA: 5293] [Article Influence: 311.4] [Reference Citation Analysis (0)] |
| 196. | Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol. 2008;3:427-455. [PubMed] |
| 197. | Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809-1820. [PubMed] |
| 198. | Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077-15082. [PubMed] |
| 199. | Pattingre S, Levine B. Bcl-2 inhibition of autophagy: a new route to cancer? Cancer Res. 2006;66:2885-2888. [PubMed] |
| 200. | Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927-939. [PubMed] |
| 201. | Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3’-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275:992-998. [PubMed] |
| 202. | Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243-35246. [PubMed] |
| 203. | Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA. 2005;102:8204-8209. [PubMed] |
| 204. | Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749-1760. [PubMed] |
| 205. | Kaminskyy VO, Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid Redox Signal. 2014;21:86-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 325] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 206. | Baregamian N, Song J, Bailey CE, Papaconstantinou J, Evers BM, Chung DH. Tumor necrosis factor-alpha and apoptosis signal-regulating kinase 1 control reactive oxygen species release, mitochondrial autophagy, and c-Jun N-terminal kinase/p38 phosphorylation during necrotizing enterocolitis. Oxid Med Cell Longev. 2009;2:297-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 207. | Wu H, Wang MC, Bohmann D. JNK protects Drosophila from oxidative stress by trancriptionally activating autophagy. Mech Dev. 2007;126:624-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 208. | Wong CH, Iskandar KB, Yadav SK, Hirpara JL, Loh T, Pervaiz S. Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PLoS One. 2010;5:e9996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 209. | Kim MJ, Woo SJ, Yoon CH, Lee JS, An S, Choi YH, Hwang SG, Yoon G, Lee SJ. Involvement of autophagy in oncogenic K-Ras-induced malignant cell transformation. J Biol Chem. 2011;286:12924-12932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 210. | Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 615] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 211. | Alexandre J, Batteux F, Nicco C, Chéreau C, Laurent A, Guillevin L, Weill B, Goldwasser F. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 2006;119:41-48. [PubMed] |
| 212. | Donadelli M, Costanzo C, Beghelli S, Scupoli MT, Dandrea M, Bonora A, Piacentini P, Budillon A, Caraglia M, Scarpa A. Synergistic inhibition of pancreatic adenocarcinoma cell growth by trichostatin A and gemcitabine. Biochim Biophys Acta. 2007;1773:1095-1106. [PubMed] |
| 213. | Yang L, Zheng XL, Sun H, Zhong YJ, Wang Q, He HN, Shi XW, Zhou B, Li JK, Lin Y. Catalase suppression-mediated H(2)O(2) accumulation in cancer cells by wogonin effectively blocks tumor necrosis factor-induced NF-κB activation and sensitizes apoptosis. Cancer Sci. 2011;102:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 214. | Carew JS, Zhou Y, Albitar M, Carew JD, Keating MJ, Huang P. Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia. 2003;17:1437-1447. [PubMed] |
| 215. | Panieri E, Gogvadze V, Norberg E, Venkatesh R, Orrenius S, Zhivotovsky B. Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radic Biol Med. 2013;57:176-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 216. | Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51:1271-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 217. | Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol. 2011;301:H647-H653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 218. | Kizhakekuttu TJ, Widlansky ME. Natural antioxidants and hypertension: promise and challenges. Cardiovasc Ther. 2010;28:e20-e32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 219. | Lamson DW, Brignall MS. Antioxidants in cancer therapy; their actions and interactions with oncologic therapies. Altern Med Rev. 1999;4:304-329. [PubMed] |
| 220. | Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 757] [Cited by in RCA: 596] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 221. | Khaw KT, Bingham S, Welch A, Luben R, Wareham N, Oakes S, Day N. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet. 2001;357:657-663. [PubMed] |
| 222. | Sasazuki S, Hayashi T, Nakachi K, Sasaki S, Tsubono Y, Okubo S, Hayashi M, Tsugane S. Protective effect of vitamin C on oxidative stress: a randomized controlled trial. Int J Vitam Nutr Res. 2008;78:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 223. | Gori T, Münzel T. Oxidative stress and endothelial dysfunction: therapeutic implications. Ann Med. 2011;43:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 224. | Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1223] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 225. | Farbstein D, Kozak-Blickstein A, Levy AP. Antioxidant vitamins and their use in preventing cardiovascular disease. Molecules. 2010;15:8098-8110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 226. | Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Slate EH, Fischbach LA, Marshall JR, Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630-639. [PubMed] |
| 227. | Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ, Seeram N, Liker H, Wang H, Elashoff R. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12:4018-4026. [PubMed] |
| 228. | Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475-510. [PubMed] |
| 229. | Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108:130-135. [PubMed] |
| 230. | Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, van Breemen R, Ashton D, Bowen PE. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J Natl Cancer Inst. 2001;93:1872-1879. [PubMed] |
| 231. | Kim HS, Bowen P, Chen L, Duncan C, Ghosh L, Sharifi R, Christov K. Effects of tomato sauce consumption on apoptotic cell death in prostate benign hyperplasia and carcinoma. Nutr Cancer. 2003;47:40-47. [PubMed] |
| 232. | Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597-601. [PubMed] |
| 233. | Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1115] [Cited by in RCA: 1202] [Article Influence: 70.7] [Reference Citation Analysis (0)] |