Revised: May 24, 2014
Accepted: June 14, 2014
Published online: July 27, 2014
Processing time: 134 Days and 12.5 Hours
Reactive arthritis (ReA), also known as sterile postinfectious arthritis, belongs to the group of related arthropathies known as spondyloarthritis (SpA). ReA can arise 1-4 wk after a gastrointestinal or genitourinary infection, but once arthritis develops, the microorganism is not found in the joint. The classical microbes associated with ReA development include Gram-negative aerobic or microaerophilic bacteria containing LPS in their outer membrane. The immunopathogenic mechanisms involved in ReA development are still unknown. A hypothesis suggested that the bacteria probably persist outside the joint, at sites such as gut mucosa or lymph nodes, and bacterial antigens might then be transported to the joints. On the other hand, an altered immune response and the unbalanced production of cytokines have been reported in subjects with ReA. Currently, there is increased evidence to suggest that both mechanisms would operate in the immunopathogenesis of ReA. In this review we highlight recent advances on the role of cytokines in the ReA. Particularly, we discuss the roles of some pro- and anti-inflammatory cytokines involved in the immunopathogenesis of ReA.
Core tip: The immunopathogenic mechanisms involved in reactive arthritis (ReA) development are still unknown. However, in the last years, increased evidence suggests that the immune response in particular certain cytokines could be involved in the pathogenesis of ReA. Currently, the use of biological agents that block the action of certain cytokines has contributed to improving the treatment of some rheumatic pathology. Understanding the role of cytokines in the pathogenesis of ReA could contribute to the development of future treatments. In this review, we highlight recent advances on the role of certain cytokines in the pathogenesis of ReA.
- Citation: Eliçabe RJ, Genaro MSD. Immunopathogenesis of reactive arthritis: Role of the cytokines. World J Immunol 2014; 4(2): 78-87
- URL: https://www.wjgnet.com/2219-2824/full/v4/i2/78.htm
- DOI: https://dx.doi.org/10.5411/wji.v4.i2.78
Reactive arthritis (ReA), also known as sterile postinfectious arthritis, belongs to the group of related arthropathies known as spondyloarthritis (SpA)[1]. This group also includes undifferentiated SpA, psoriatic arthritis (PsA), arthritis associated with inflammatory bowel disease and ankylosing spondylitis (AS). The SpA arthropathies have common several epidemiological, pathological, clinical and radiological features. ReA, as with other SpA, exhibits an absence of rheumatoid factor and has a genetic association with the molecule HLA-B27[1-3]. ReA can arise 1-4 wk after a gastrointestinal or genitourinary infection, but once arthritis develops, the microorganism is not found in the joint[2]. The ReA symptoms were recognized and studied in 1942 by Bauer and Engelmann, who associated these symptoms with those described in 1916 by the German physician Hans Reiter. At that time, Reiter described the clinical triad: arthritis, non-gonococcal urethritis and conjunctivitis in a German soldier after an episode of bloody diarrhea. So, Bauer and Engelmann coined the term Reiter’s syndrome to describe this new pathology[2]. However, most patients do not have the complete triad of symptoms. These observations drove Ahvonen to propose the name of ReA as the term most adapted to describe the “arthritis that happens during or after an infection in another site of the body without evidence of microorganisms in the joint”[4]. Yet, this operational definition of ReA has led to uncertain diagnosis in different clinical settings. Thus, several attempts have been made to create classification criteria; however, lack of consensus has led to a failure to achieve any universally validated diagnostic criteria. Based on discussions at the 4th International Workshop on ReA, this term should be used only in patients with clinical features of ReA and in cases where a pathogen known to cause ReA is implicated[5].
ReA most commonly affects young adults aged 20 to 40 years old and is rare in children[6-8]. Both sexes are equally affected by ReA after a gastrointestinal infection, while ReA is more frequent in men when triggered by a urogenital tract infection[3]. The presence of the HLA-B27 allele does not seem to be related to the onset of ReA; however, HLA-B27 positive patients have more severe arthritis with a tendency to progress to a chronic stage and they also have a greater chance of developing extra-articular symptoms. One hypothesis suggests that this molecule favors the cross-reaction between antigen and host, or it might be itself a target of the immune response[9].
The symptoms of ReA typically start between 1 to 4 wk after the gastrointestinal infection. However, the triggering infection could be asymptomatic, such as Chlamydia-induced ReA, resulting in underdiagnosis[2]. Clinical features of ReA are characterized by asymmetrical oligoarthritis, often in large joints of the lower extremities or in the upper extremities. A mild polyarticular form, particularly in the small joints, can also occur. Patients can have dactylitis. The typical extra-articular manifestations are enthesitis, tendinitis and bursitis. ReA share these clinical characteristics and inflammatory back pain with other members of SpA, such as AS and PsA[1]. Other extra-articular features include eye disease, where conjunctivitis is most prevalent, followed by acute anterior uveitis, and skin changes, such as erythema nodosum, keratodermia blennorrhagica and circinate balanitis[3].
The clinical diagnosis is made based on the clinical symptoms. Evidence for infection triggering the arthropathy is most convincing when microbe isolation or antigen detection is successful. In this respect, fecal culture of enteric pathogens associated with ReA or the finding of Chlamydia trachomatis nucleic acids in urine, cervical or urethral swabs are secondary criteria used to confirm the diagnosis.
Animal models of ReA have complemented studies in human materials. However, these animal models are limited since even when they are developed after bacterial infection as in human ReA, in some of them the route of infection was intravenous instead of oral. Table 1 shows animal models of ReA similar to the human form of the disease[10-17]. We have described an experimental model useful for studying the pathogenesis of Yersinia enterocolitica (Y. enterocolitica) ReA. In our model, TNFRp55 deficient mice develop ReA after oral infection with Y. enterocolitica O: 3, the most common serotype associated with human ReA. TNFRp55-/- mice exhibited macroscopic signs of severe and progressive arthritis with significantly higher clinical score compared with wild-type mice from d 14 to 56 after infection[14]. Extensively, increased scores for inflammation and bone/cartilage degradation resulted when histopathological changes were analyzed in the joints. In these animals, we observed luminal disorganization of the synovial membrane, which was densely infiltrated with various types of leucocytes, sometimes concomitant with follicle formation. The articular cartilage and bone were degraded. Proliferation of synovial lining cells was also detected[14,15]. This evidence and the data presented in Table 1 indicate ReA development in animal models that resemble this disease in humans. Nevertheless, the convergence of these models with human studies will contribute to understand the pathogenic mechanisms of ReA.
| Animal | Bacteria | Route of infection | Arthritis onset/remission | Clinical symptoms | Cytokineinvolved | Ref. |
| Lewis rats | Y. enterocolitica O:81 | iv1 | 1 wk/6 wk | Polyarticular arthritis, erythema | ND | Hill et al[10] |
| DBA/2 and BDF1 mice | Y. enterocolitica O:8 plasmid cured1 | iv1 | Day 31/3 wk | Polyarticular arthritis | ND | Yong et al[11] |
| SHR rats | Y. enterocolitica O:81 | iv1 | 1-4 wk/7-25 wk | Polyarticular arthritis, erythema, swelling and impaired movement of the joint | ND | Merilahti-Palo et al[12] |
| Swiss, BALB/c and C3H/HeJ mice | Y. enterocolitica O:3 | iv1/Oral | 1-3 wk/2-8 mo | Monoarticular arthritis, swelling redness, deformations and conjunctivitis | ND | de los Toyos et al[13] |
| C57BL/6 TNFRp55-/- mice | Y. enterocolitica O:3 | ig | 2 wk/chronic until 8 wk | Polyarticular arthritis, swelling, erythema | IL-17 IFN-γ | Di Genaro et al[14] |
| IL-6 IL-1β | Eliçabe et al[15] | |||||
| BALB/c mice | S. enteritides | ig | 1 wk/ND | Synovial inflammation | TNF-α IL-17 | Noto Llana et al[16] |
| Noto Llana et al[17] |
The classical bacteria associated with gastrointestinal ReA are Yersinia, Salmonella, Shigella and Campylobacter, while C. trachomatis is by far the most common cause of ReA associated with genital infection[3,18]. All these pathogens are Gram-negative aerobic or microaerophilic bacteria containing LPS in their outer membrane.
The immunopathogenic mechanisms involved in ReA development are still unknown. Even when bacterial cultures of synovial fluids are negative in ReA, bacterial antigens have been found in the joints of patients. In Chlamydia-induced ReA, bacterial DNA and RNA have been detected in the joint, suggesting that live Chlamydia are present[19-21]. Positive reaction of antibodies specific to Salmonella and Yersinia antigens in synovial fluid cells of ReA patients suggests the presence of bacterial antigen in the joint[22,23]. Based on these findings, some authors have suggested that the bacteria probably persist outside the joint at sites such as gut mucosa or lymph nodes, and bacterial antigens might then be transported by monocytes to the joints[24,25]. On the other hand, an altered immune response and the unbalanced production of cytokines have been reported in subjects with ReA[26,27]. This altered immune response benefits the bacterial persistence and disfavors the elimination of the antigen by the host.
In this review, we highlight recent advances on the role of cytokines in ReA. Particularly, we discuss the roles of pro- and anti-inflammatory cytokines, especially interleukin (IL)-17, IL-12, IL-23, IL-6, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) as well as IL-10 in the pathophysiology of the ReA. Finally, we discuss the latest advances in the treatment of ReA based on the use of biological agents that neutralize the functions of certain cytokines, such as TNF-α or IL-6.
Conflicting data have been reported on the production of cytokines in ReA patients. CD4+ T cells mediate immunity as a balance between different lineages of T helper (Th)-1, Th2, Th3 and Th17 which secrete IFN-γ, IL-4, TGF-γ and IL-17, respectively, as the main cytokine for each profile. Some studies revealed low levels of Th1 cytokines in ReA, especially of TNF-α but also of IFN-γ in peripheral blood and synovium[28-34]. Since Th1 cells secreting IFN-γ and TNF-α have been proposed for bacterial clearance, defective Th1 response may contribute to bacterial persistence. Other data suggest that a Th2 cytokine profile and Th3 response with expression of TGF-β is common in ReA[32]. Temporal relationships of these different Th1 and Th2 cytokines or blunting of initial cytokine response might also be important in the disease manifestations and its maintenance. On the other hand, the discovery of Th17 cells and their importance in the pathogenesis of chronic inflammatory diseases suggested that these cells may have a pathogenic role in ReA. However, the available studies are not large enough to support the role of certain cytokines in the pathogenesis of ReA.
IL-17 is a 15-20-kDa glycoprotein produced by a novel subset of Th cells, termed Th17 cells, and to a lesser extent by innate lymphoid cells, including T-cells, innate-like lymphoid cells, mast cells and neutrophils[35]. Th17 cells are critical in the pathogenesis of the arthritis, as demonstrated in several animal models[36-38]. Th17 differentiation, survival and expansion depend on a variety of cytokines and transcription factors that work in concert to drive the induction of increased Th17 numbers. TGF-β in synergy with IL-6 has been described as the central factor involved in generating Th17 cells in mice. It has been shown in humans that TGF-β, IL-1β and IL-6, combined with IL-21 or IL-23, can induce Th17 differentiation[39]. IL-17 binds to IL-17RA/IL-17RC, which is expressed by a variety of cells, such as monocytes, lymphocytes, lymphoid tissue inducer cells, epithelial cells, synoviocytes, fibroblasts and keratinocytes[35].
Th17 cell responses and IL-17 expression provide protection against bacterial and fungal pathogens through production and induction of inflammatory cytokines and granulopoiesis, or by the recruitment of neutrophils. However, Th17 cells producing IL-17 have been suggested as the central effector lineage involved in the pathogenicity of ReA[40]. Thus, it has been shown that ReA patients have elevated levels of IL-17 in synovial fluid and that this cytokine contributes to the development of joint inflammation[40,41]. Furthermore, high expression of IL-17 was found in the synovial fluid of patients with SpA and an increased number of circulating memory Th17 cells has been recently reported in these patients[42,43]. Moreover, in patients with C. trachomatis-induced ReA, increased percentages of IL-17-positive CD4+ T cells[44] and higher IL-17 concentrations were detected in synovial fluid[45].
Recent works suggest that Salmonella-induced ReA in mice dependent on CD4+ T cells secreting IL-17[17]. Interestingly, these authors observed that the expression of IL-17 in the large intestine and in mesenteric lymph nodes (MLN) resembles that of popliteal and inguinal lymph nodes (ILN)[17]. Accordingly, previous results from our laboratory demonstrated that IL-17 plays a major role in Yersinia-induced ReA[15]. Furthermore, we detected a strong correlation among IL-17 levels in MLNs, ILNs and joints from TNFRp55-/- mice with arthritis, supporting a link between the intestinal mucosa and the articular immune response. In addition, we observed that neutralization of IL-17 resulted in the abrogation of synovitis[15]. In line with these results, other authors have reported recently that modulating intestinal IL-23/IL-17 expression by consumption of Lactobacillus casei prior to Salmonella infection in mice abolishes intestinal and joint inflammation[46].
These data in animal models and patients support the hypothesis that Th17 cells may be involved in ReA pathogenesis. However, there are few reports for understanding and elucidating the true role of IL-17 in the pathogenesis of ReA.
IL-12 and IL-23 are heterodimeric cytokines that share subunits and have important roles in autoimmunity. These IL-12 family cytokines share some biological characteristics but have functional differences. IL-12 is composed of two covalently linked subunits, IL-12p35 and IL-12p40, while IL-23 is composed of two covalently linked subunits, IL-23p19, which is distantly related to IL-12p35, and the IL-12p40 subunit[47,48]. Furthermore, the receptors of IL-23 and IL-12 are also heterodimers that share the receptor 1 chain and have unique 2 chains[49]. IL-12 is released by antigen presenting cells such as dendritic cells (DCs) and monocytes/macrophages in response to bacterial products and immune signals. Furthermore, IL-12 is the main stimulator of IFN-γ production by inducing development of Th1 responses[49,50]. In addition, IL-23 is produced by macrophages and activated DCs and plays a crucial role in the generation of the Th17 cells. Since IL-12 has the ability to orchestrate the Th1 response, this cytokine plays a crucial role in the protective immunity against many pathogens associated with ReA. Thus, the low concentrations of IL-12 have been linked to the bacterial persistence hypothesis and then to the pathogenesis of ReA[28]. On the other hand, data on IL-23 concentrations in synovial fluid or serum of patients with ReA are limited, but high levels of IL-17 found in synovial fluids and sera of patients with ReA may reflect IL-23 activity. Moreover, abnormality of IL-12p40 gene expression in humans has been reported and IL-12 deficiency has been detected in patients with ReA[51,52]. Yin et al[28] found that the balance of anti-inflammatory cytokines (IL-10) and IL-12 in the synovial fluid is also important. This may contribute to the decreased clearance of the bacteria or their components from the joint and lead to ReA[28]. In relation to these findings, a recent study has shown that monocyte-derived macrophages from subjects with a history of ReA show low IL-12 and IL-23 production[53]. Conversely, some authors have reported that IL-12/23p40 levels in synovial fluids of patients with ReA and other SpA are higher compared to synovial fluids of patients with osteoarthritis (OA) used as control[41,54].
Interestingly, we demonstrated that the p40-deficient mice develop acute ReA after oral infection with Y. enterocolitica, suggesting that IL-12 or IL-23 could exert a protective effect on the development of ReA[55]. However, we have observed elevated levels of p40 in regional lymph nodes to joints of TNFRp55-/- mice with Yersinia-induced ReA. This effect has been accompanied by high levels of IFN-γ and IL-17 in affected joints[15]. These results are in accordance with the concept that the IL-12/IL-23 pathway plays a dual role protecting from infection and eliciting tissue damage, and support future study to determine whether IL-12/23p40 could be a possible target for ReA treatment.
IL-6 is a pleiotropic cytokine that is involved in numerous biological processes. The pleiotropy and redundancy of IL-6 functions have been identified by characterizing a unique receptor system comprising two functional proteins: a receptor specific for IL-6 (IL-6R)[56] and gp130, the common signal transducer of cytokines related to IL-6, including the IL-12 family cytokines IL-27 and IL-35[57,58]. In the early phase of infectious inflammation, IL-6 is produced by monocytes and macrophages immediately after the stimulation with distinct pathogen-associated molecular patterns. In noninfectious inflammation, damage-associated molecular patterns from damaged or dying cells stimulate monocytes and macrophages to produce IL-6. The pathogenic role of IL-6 in rheumatic diseases like rheumatoid arthritis (RA) has been well established. The critical role for IL-6 in the pathogenesis of RA is provided by clinical trials, in which tocilizumab, a humanized mAb specific for IL-6R, has been shown to suppress disease activity and erosive progression in patients with RA[59]. In ReA, elevated IL-6 concentrations in the plasma and sera of the patients has been reported[60,61]. Moreover, synovial fluid concentrations of IL-6 were higher in patients with ReA[41]. Interestingly, we found that mice TNFRp55-deficient macrophages are hyperactivated to secrete common pro-inflammatory mediators such as NO and IL-6 following stimulation with Yersinia antigens. The higher concentrations of IL-6 production detected in stimulated TNFRp55-/- macrophages may be associated with our previous in vivo results demonstrating the increased susceptibility of TNFRp55-/- mice to Yersinia-induced ReA[14]. Furthermore, higher concentrations of IL-6 were detected in the joints of these mice which showed a severe chronic synovitis[15]. This data suggests that over-synthesis of IL-6 may be related to the development of ReA.
TNF-α is a cytokine prototype of a large family of over 40, known as TNF superfamily, and TNF receptor (TNFR) proteins. TNF-α is a cytokine with pleiotropic functions produced by a large number of cells, but are monocytic lineage cells (macrophages, astroglia, microglia, Kupffer cells and alveolar macrophages) major sources. Initially, this cytokine is produced as a pro-TNF and is expressed on the cell surface. Subsequently it is cleaved by the action of a metalloproteinase (TACE) and released into the extracellular medium as a soluble protein[62]. Often, TNF-α is not detected in high concentrations in serum or tissues, but increases intensively on various inflammatory and infectious conditions. Two receptors, TNF-R1 (TNF receptor type 1; CD120a; p55/60) and TNF-R2 (TNF receptor type 2; CD120b; p75/80) bind to membrane-integrated TNF (memTNF) as well as soluble TNF-α (sTNF-α). In the vast majority of cells, TNF-R1 appears to be the key mediator of TNF-α signaling, whereas in the lymphoid system, TNF-R2 seems to play a major role. Low TNF-α secretion by blood mononuclear cells may be related to ReA development since TNF-α deficiency may interfere with eradication of bacterial infection in its early stages[34,63-66]. However, other studies suggest that TNF-α could have a pathogenic role during the chronic stage of ReA in line with the role of this cytokine in RA. In this regard, some studies have revealed significant increase of TNF-α production in chronic ReA compared with acute ReA[66]. These data support the possibility that anti-TNF-α treatment in ReA during the chronic phase of the disease could be beneficial. However, considering that TNF-α may be required for the elimination of ReA-associated bacteria, anti-TNF-α biologics might favor bacteria growth. Results obtained in our laboratory showed that TNFRp55 deficiency favors the development of ReA after infection with Y. enterocolitica[14]. These data support the idea that the relative lack of TNF-α may play a protective role in ReA at acute phase of disease. On the other hand, we have demonstrated an in vivo regulatory role for TNFRp55 signaling in fine-tuning of Th17 and Th1 programs during bacterial-induced ReA through modulation of the common p40 subunit of IL-23 and IL-12[15]. This evidence suggests that TNF-α might have a dual role in ReA, playing a protective role first and during the initial stage. However, during the chronic stage of the disease, TNF-α would act as a pro-inflammatory cytokine.
IFN-γ is produced mainly by natural killer (NK) cells and a particular subset of T cells, namely Th1 cells[67]. As previously mentioned, IL-12 is the main stimulator of IFN-γ production[47,50]. Thus, IL-12 and IFN-γ coordinate the link between pathogen recognition by innate immune cells and the induction of specific immunity by mediating a positive feedback loop to amplify the Th1 response. The functional IFN-receptor (IFN-R) consists of 2 ligand-binding IFNGR1 chains and 2 signal-transducing IFNGR2 chains[68]. Mice deficient in IFN-γ or its receptor are susceptible to an array of intracellular pathogens[69-71]. It was thought that Th1 cells cause damage in the joints mainly through IFN-γ driven inflammatory mechanisms. However, similar to TNF-α, conflicting data have been reported about the role of IFN-γ in ReA. As previously mentioned, some authors have reported an aberrant lower production of IFN-γ in patients with ReA[28-34,52]. In contrast, in patients with C. trachomatis-induced ReA, the synovial fluid concentrations of IFN-γ were significantly higher than in OA patients but no significant differences were found between ReA and RA patients[45]. Similar results were reported by Singh et al[41]. Other studies have shown that the percentages of IFN-γ positive CD3+ cells were significantly higher in peripheral blood and synovial fluid of chronic ReA patients[66]. These data support the idea that, as with TNF-α, IFN-γ may play a significant protective role in ReA in the acute phase of disease. However, in the chronic phase, this cytokine, as in RA, could play a pathogenic role in ReA.
IL-10 is an anti-inflammatory cytokine with a major role in preventing inflammatory and autoimmune pathologies[72]. Based on a large body of evidence, T cells are thought to be the main source of IL-10 in vivo. Regulatory T (Treg) subsets are also a key source of IL-10 in vivo and play a central role in mediating the inflammation control. However, it is now accepted that IL-10 is expressed by subsets of all CD4+ T helper populations, including Th1, Th2 and Th17[73]. Nevertheless, this cytokine is also expressed by B cells and cells of the innate immune system (DCs, stimulated macrophages, mast cells, NK cells, eosinophils and neutrophils)[74]. This cytokine binds to IL-10 receptor (IL-10R), which consists of two subunits. They are members of the interferon receptor family and belong to JAK/STAT3 class of receptors[74]. Extensive studies have demonstrated that IL-10 inhibits the production of pro-inflammatory cytokines and chemokines in activated monocytes/macrophages and inhibits proliferation of CD4+ T cells[75]. However, the role of IL-10 in ReA is less clear. Appel et al[32] reported that the amount of IL-10 and TGF-β secreting cells was higher in ReA than in RA patients. This result was accompanied by a lower level of TNF-α secretion in ReA patients. Interestingly, all ReA patients had a disease course of less than 6 mo. These authors suggest that this cytokine milieu might contribute to the lack of elimination of the triggering agent. Similar results were reported by Yin et al[28]. These authors found that synovial fluid mononuclear cells secreted low amounts of IFN-γ and TNF-α, but high amounts of IL-10 upon stimulation with specific bacteria, which was responsible for the suppression of IFN-γ and TNF-α[28]. There is also evidence indicating association of the IL-10 promoter region with the development of ReA. This raises the possibility that high levels of IL-10 in the joints of patients with ReA may be genetically determined, making these individuals more prone to the persistence of arthritogenic bacteria[76].
Despite these clinical findings suggesting a pathogenic role of IL-10 in human ReA, IL-10 depletion and IL-10 treatment in other types of arthritis models have demonstrated the anti-inflammatory properties of IL-10 in arthritis[77-80]. Results obtained in our laboratory showed that the number of Treg cells as well as the FoxP3 mRNA expression and IL-10 levels were significantly decreased in joint regional lymph nodes of TNFRp55-/- mice at the arthritis onset[81]. These results would indicate that IL-10 plays a protective role during the acute phase of arthritis. However, the clinical evidence suggests that high levels of IL-10 could promote bacterial persistence, favoring the development of ReA.
Published data on the effects of IL-6 blockade in patients with SpA are very scarce. Thus, in 1996 a report describes a patient with ReA who received a murine anti-IL-6 antibody[82] and, in 2009, tocilizumab was reported to be successful in another patient with ReA[83]. Only two injections of tocilizumab led to complete clinical remission from symptoms caused by ReA[83]. Recently, Kwan et al[84] reported successful results of tocilizumab in the treatment of a case of ReA precipitated by intravesical bacillus Galmette-Guèrin (BCG) which did not respond completely to disease modifying antirheumatic drugs (DMARDs). As previously mentioned, IL-6 is one of the cytokines that favor the differentiation of nave T cells into Th17 cells[39]. Therefore, it is possible that the inhibitory action of tocilizumab is exerted indirectly interfering with the differentiation of Th17 cells. These data indicate that IL-6 may play a pivotal role directly or indirectly in the pathogenesis of ReA and tocilizumab treatment can be an option for an alternative treatment.
The pathogenic role during the critical stage of the disease supports the idea that TNF-α blocking agents could be an effective treatment for patients with ReA who develop severe arthritis that does not respond to conventional lines of treatment. Thus, Kaipiainen-Seppnen et al[85] reported two cases of ReA post Y. enterocolitica treated early with infliximab (an anti-TNF-α antibody). One patient that received this treatment within 2 mo after the disease onset exhibited an improvement after the third infusion. The second patient that was treated after one month of evolution showed an immediate clinical improvement with almost complete regression after 15 d[85]. Recently, Thomas-Pohl et al[86] obtained the same result in one patient with ReA triggered by a gastrointestinal infection. Similar results were reported by Edrees in a patient with a severe case of C. trachomatis-related ReA that was successfully treated with etanercept (a fusion protein of TNFRp75)[87]. Thus, anti-TNF-α therapy has proved efficacious in some cases. However, sufficient data are lacking and theoretical concerns with their use remain. Large controlled trials are needed to evaluate the role of TNF-α blocking agents in ReA.
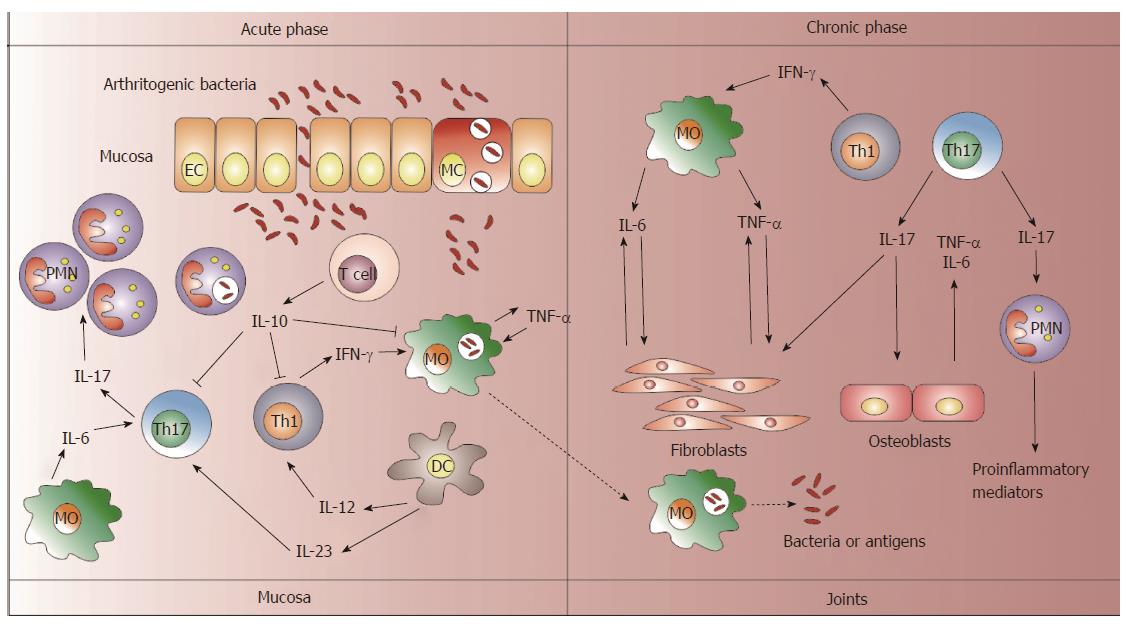
The network of cytokines is complex with feedback regulatory circuits that make it difficult to elucidate the role of a particular cytokine in ReA. In addition, the clinical reports of cytokine levels in patients with ReA have included patients in different stages of the disease or they are not large enough to support the role of different cytokines in ReA development. However, the current evidence in patients with anti-cytokine treatments suggests that IL-6 and TNF-α may play central roles in ReA pathogenesis. Furthermore, the IL-17/23 axis should be considered in the picture of ReA development, although further investigations are necessary for these cytokines. According to the presented evidence in this review, Figure 1 shows the different functions of the cytokines in ReA depending on the disease phases. Moreover, animal models may contribute to provide insight into the immunopathogenic mechanisms mediated by a particular cytokine in ReA and to support anti-cytokine treatments.
P- Reviewer: Gazouli M, Konttinen Yrj, Pixley J S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Wang CH
| 1. | Townes JM. Reactive arthritis after enteric infections in the United States: the problem of definition. Clin Infect Dis. 2010;50:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Carter JD, Hudson AP. Reactive arthritis: clinical aspects and medical management. Rheum Dis Clin North Am. 2009;35:21-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Hannu T. Reactive arthritis. Best Pract Res Clin Rheumatol. 2011;25:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Leirisalo-Repo M. Reactive arthritis. Scand J Rheumatol. 2005;34:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Braun J, Kingsley G, van der Heijde D, Sieper J. On the difficulties of establishing a consensus on the definition of and diagnostic investigations for reactive arthritis. Results and discussion of a questionnaire prepared for the 4th International Workshop on Reactive Arthritis, Berlin, Germany, July 3-6, 1999. J Rheumatol. 2000;27:2185-2192. [PubMed] |
| 6. | Rudwaleit M, Richter S, Braun J, Sieper J. Low incidence of reactive arthritis in children following a salmonella outbreak. Ann Rheum Dis. 2001;60:1055-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Mattila L, Leirisalo-Repo M, Koskimies S, Granfors K, Siitonen A. Reactive arthritis following an outbreak of Salmonella infection in Finland. Br J Rheumatol. 1994;33:1136-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Mattila L, Leirisalo-Repo M, Pelkonen P, Koskimies S, Granfors K, Siitonen A. Reactive arthritis following an outbreak of Salmonella Bovismorbificans infection. J Infect. 1998;36:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Colmegna I, Cuchacovich R, Espinoza LR. HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin Microbiol Rev. 2004;17:348-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Hill JL, Yu DT. Development of an experimental animal model for reactive arthritis induced by Yersinia enterocolitica infection. Infect Immun. 1987;55:721-726. [PubMed] |
| 11. | Yong Z, Hill JL, Hirofuji T, Mander M, Yu DT. An experimental mouse model of Yersinia-induced reactive arthritis. Microb Pathog. 1988;4:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Merilahti-Palo R, Gripenberg-Lerche C, Söderström KO, Toivanen P. Long term follow up of SHR rats with experimental yersinia associated arthritis. Ann Rheum Dis. 1992;51:91-96. [PubMed] |
| 13. | de los Toyos JR, Vázquez J, Sampedro A, Hardisson C. Yersinia enterocolitica serotype O: 3 is arthritogenic for mice. Microb Pathog. 1990;8:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Di Genaro MS, Cargnelutti DE, Eliçabe JR, Lacoste MG, Valdez S, Gómez N, de Guzmán AM. Role of TNFRp55 in Yersinia enterocolitica O: 3-induced arthritis: triggering bacterial antigens and articular immune response. Rheumatology (Oxford). 2007;46:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Eliçabe RJ, Cargnelutti E, Serer MI, Stege PW, Valdez SR, Toscano MA, Rabinovich GA, Di Genaro MS. Lack of TNFR p55 results in heightened expression of IFN-γ and IL-17 during the development of reactive arthritis. J Immunol. 2010;185:4485-4495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Noto Llana M, Sarnacki SH, Giacomodonato MN, Caccuri RL, Blanco GA, Cerquetti MC. Sublethal infection with Salmonella Enteritidis by the natural route induces intestinal and joint inflammation in mice. Microbes Infect. 2009;11:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Noto Llana M, Sarnacki SH, Vázquez MV, Gartner AS, Giacomodonato MN, Cerquetti MC. Salmonella enterica induces joint inflammation and expression of interleukin-17 in draining lymph nodes early after onset of enterocolitis in mice. Infect Immun. 2012;80:2231-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Rich E, Hook EW, Alarcón GS, Moreland LW. Reactive arthritis in patients attending an urban sexually transmitted diseases clinic. Arthritis Rheum. 1996;39:1172-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Schumacher HR, Gérard HC, Arayssi TK, Pando JA, Branigan PJ, Saaibi DL, Hudson AP. Lower prevalence of Chlamydia pneumoniae DNA compared with Chlamydia trachomatis DNA in synovial tissue of arthritis patients. Arthritis Rheum. 1999;42:1889-1893. [PubMed] |
| 20. | Bas S, Griffais R, Kvien TK, Glennås A, Melby K, Vischer TL. Amplification of plasmid and chromosome Chlamydia DNA in synovial fluid of patients with reactive arthritis and undifferentiated seronegative oligoarthropathies. Arthritis Rheum. 1995;38:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Gérard HC, Carter JD, Hudson AP. Chlamydia trachomatis is present and metabolically active during the remitting phase in synovial tissues from patients with chronic Chlamydia-induced reactive arthritis. Am J Med Sci. 2013;346:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Granfors K, Jalkanen S, von Essen R, Lahesmaa-Rantala R, Isomäki O, Pekkola-Heino K, Merilahti-Palo R, Saario R, Isomäki H, Toivanen A. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N Engl J Med. 1989;320:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 316] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Granfors K, Jalkanen S, Lindberg AA, Mäki-Ikola O, von Essen R, Lahesmaa-Rantala R, Isomäki H, Saario R, Arnold WJ, Toivanen A. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet. 1990;335:685-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 216] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Gripenberg-Lerche C, Söderström KO, Toivanen A, Toivanen P. Antibiotic prophylaxis and treatment of reactive arthritis. Lessons from an animal model. Arthritis Rheum. 1996;39:1238-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Granfors K, Merilahti-Palo R, Luukkainen R, Möttönen T, Lahesmaa R, Probst P, Märker-Hermann E, Toivanen P. Persistence of Yersinia antigens in peripheral blood cells from patients with Yersinia enterocolitica O: 3 infection with or without reactive arthritis. Arthritis Rheum. 1998;41:855-862. [PubMed] |
| 26. | Gracey E, Inman RD. Chlamydia-induced ReA: immune imbalances and persistent pathogens. Nat Rev Rheumatol. 2012;8:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Anttonen K, Orpana A, Leirisalo-Repo M, Repo H. Aberrant TNF secretion by whole blood in healthy subjects with a history of reactive arthritis: time course in adherent and non-adherent cultures. Ann Rheum Dis. 2006;65:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Yin Z, Braun J, Neure L, Wu P, Liu L, Eggens U, Sieper J. Crucial role of interleukin-10/interleukin-12 balance in the regulation of the type 2 T helper cytokine response in reactive arthritis. Arthritis Rheum. 1997;40:1788-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Simon AK, Seipelt E, Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci USA. 1994;91:8562-8566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 268] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Smeets TJ, Dolhain RJ, Breedveld FC, Tak PP. Analysis of the cellular infiltrates and expression of cytokines in synovial tissue from patients with rheumatoid arthritis and reactive arthritis. J Pathol. 1998;186:75-81. [PubMed] |
| 31. | Kotake S, Schumacher HR, Arayssi TK, Gérard HC, Branigan PJ, Hudson AP, Yarboro CH, Klippel JH, Wilder RL. Gamma interferon and interleukin-10 gene expression in synovial tissues from patients with early stages of Chlamydia-associated arthritis and undifferentiated oligoarthritis and from healthy volunteers. Infect Immun. 1999;67:2682-2686. [PubMed] |
| 32. | Appel H, Neure L, Kuhne M, Braun J, Rudwaleit M, Sieper J. An elevated level of IL-10- and TGFbeta-secreting T cells, B cells and macrophages in the synovial membrane of patients with reactive arthritis compared to rheumatoid arthritis. Clin Rheumatol. 2004;23:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | van Holten J, Smeets TJ, Blankert P, Tak PP. Expression of interferon beta in synovial tissue from patients with rheumatoid arthritis: comparison with patients with osteoarthritis and reactive arthritis. Ann Rheum Dis. 2005;64:1780-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Braun J, Yin Z, Spiller I, Siegert S, Rudwaleit M, Liu L, Radbruch A, Sieper J. Low secretion of tumor necrosis factor alpha, but no other Th1 or Th2 cytokines, by peripheral blood mononuclear cells correlates with chronicity in reactive arthritis. Arthritis Rheum. 1999;42:2039-2044. [PubMed] |
| 35. | Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 36. | Hickman-Brecks CL, Racz JL, Meyer DM, LaBranche TP, Allen PM. Th17 cells can provide B cell help in autoantibody induced arthritis. J Autoimmun. 2011;36:65-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Di Padova FE, Boots AM, Gram H, Joosten LA, van den Berg WB. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 38. | Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986-5990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 391] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 39. | Bedoya SK, Lam B, Lau K, Larkin J. Th17 cells in immunity and autoimmunity. Clin Dev Immunol. 2013;2013:986789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 40. | Singh AK, Misra R, Aggarwal A. Th-17 associated cytokines in patients with reactive arthritis/undifferentiated spondyloarthropathy. Clin Rheumatol. 2011;30:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Singh R, Aggarwal A, Misra R. Th1/Th17 cytokine profiles in patients with reactive arthritis/undifferentiated spondyloarthropathy. J Rheumatol. 2007;34:2285-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 43. | Wendling D, Cedoz JP, Racadot E, Dumoulin G. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine. 2007;74:304-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Shen H, Goodall JC, Gaston JS. Frequency and phenotype of T helper 17 cells in peripheral blood and synovial fluid of patients with reactive arthritis. J Rheumatol. 2010;37:2096-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Bas S, Neff L, Viatte S, Vuillet M, Spenato U, Guerne PA, Michel M, Tiercy JM, Butrimiene I, Gabay C. Relationship between gamma-interferon and interleukin-17 in Chlamydia trachomatis reactive arthritis. Clin Exp Rheumatol. 2009;27:885-886. [PubMed] |
| 46. | Noto Llana M, Sarnacki SH, Aya Castañeda Mdel R, Bernal MI, Giacomodonato MN, Cerquetti MC. Consumption of Lactobacillus casei fermented milk prevents Salmonella reactive arthritis by modulating IL-23/IL-17 expression. PLoS One. 2013;8:e82588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2687] [Cited by in RCA: 2881] [Article Influence: 131.0] [Reference Citation Analysis (0)] |
| 48. | Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2046] [Cited by in RCA: 2110] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 49. | Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699-5708. [PubMed] |
| 50. | Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospísil M, Young HA, Wolf SF, Young D, Clark SC, Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869-879. [PubMed] |
| 51. | Haraguchi S, Day NK, Nelson RP, Emmanuel P, Duplantier JE, Christodoulou CS, Good RA. Interleukin 12 deficiency associated with recurrent infections. Proc Natl Acad Sci USA. 1998;95:13125-13129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Bas S, Kvien TK, Buchs N, Fulpius T, Gabay C. Lower level of synovial fluid interferon-gamma in HLA-B27-positive than in HLA-B27-negative patients with Chlamydia trachomatis reactive arthritis. Rheumatology (Oxford). 2003;42:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Välimäki E, Aittomäki S, Karenko L, Kantonen J, Pettersson T, Turunen U, Matikainen S, Leirisalo-Repo M, Repo H. Normal inflammasome activation and low production of IL-23 by monocyte-derived macrophages from subjects with a history of reactive arthritis. Scand J Rheumatol. 2013;42:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Wendling D, Cedoz JP, Racadot E. Serum and synovial fluid levels of p40 IL12/23 in spondyloarthropathy patients. Clin Rheumatol. 2009;28:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Di Genaro MS, Cargnelutti DE, Castro DO, Eliçabe RJ, Gutiérrez JV, Correa SG, de Guzmán AM. Yersinia-triggered arthritis in IL-12p40-deficient mice: relevant antigens and local expression of Toll-like receptor mRNA. Scand J Rheumatol. 2007;36:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Yamasaki K, Taga T, Hirata Y, Yawata H, Kawanishi Y, Seed B, Taniguchi T, Hirano T, Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 825] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 57. | Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1019] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 58. | Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1008] [Cited by in RCA: 1075] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 59. | Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, Woodworth T, Alten R. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1047] [Cited by in RCA: 1080] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 60. | Metsärinne KP, Nordström DC, Konttinen YT, Teppo AM, Fyhrquist FY. Plasma interleukin-6 and renin substrate in reactive arthritis, rheumatoid arthritis, and systemic lupus erythematosus. Rheumatol Int. 1992;12:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Straub RH, Paimela L, Peltomaa R, Schölmerich J, Leirisalo-Repo M. Inadequately low serum levels of steroid hormones in relation to interleukin-6 and tumor necrosis factor in untreated patients with early rheumatoid arthritis and reactive arthritis. Arthritis Rheum. 2002;46:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 62. | Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1713] [Cited by in RCA: 1764] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 63. | Westendorp RG, Langermans JA, de Bel CE, Meinders AE, Vandenbroucke JP, van Furth R, van Dissel JT. Release of tumor necrosis factor: an innate host characteristic that may contribute to the outcome of meningococcal disease. J Infect Dis. 1995;171:1057-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Netea MG, van der Meer JW, van Deuren M, Kullberg BJ. Proinflammatory cytokines and sepsis syndrome: not enough, or too much of a good thing? Trends Immunol. 2003;24:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 65. | Saleh M, Vaillancourt JP, Graham RK, Huyck M, Srinivasula SM, Alnemri ES, Steinberg MH, Nolan V, Baldwin CT, Hotchkiss RS. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature. 2004;429:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 66. | Butrimiene I, Jarmalaite S, Ranceva J, Venalis A, Jasiuleviciute L, Zvirbliene A. Different cytokine profiles in patients with chronic and acute reactive arthritis. Rheumatology (Oxford). 2004;43:1300-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Frucht DM, Fukao T, Bogdan C, Schindler H, O’Shea JJ, Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 339] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 68. | Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2690] [Cited by in RCA: 3031] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 69. | Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249-2254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1855] [Cited by in RCA: 1921] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 70. | Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1257] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 71. | Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 338] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 72. | O’Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 2008;223:114-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 73. | Hedrich CM, Bream JH. Cell type-specific regulation of IL-10 expression in inflammation and disease. Immunol Res. 2010;47:185-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | Yao Y, Simard AR, Shi FD, Hao J. IL-10-producing lymphocytes in inflammatory disease. Int Rev Immunol. 2013;32:324-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 75. | Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4725] [Cited by in RCA: 4965] [Article Influence: 206.9] [Reference Citation Analysis (0)] |
| 76. | Kaluza W, Leirisalo-Repo M, Märker-Hermann E, Westman P, Reuss E, Hug R, Mastrovic K, Stradmann-Bellinghausen B, Granfors K, Galle PR. IL10.G microsatellites mark promoter haplotypes associated with protection against the development of reactive arthritis in Finnish patients. Arthritis Rheum. 2001;44:1209-1214. [PubMed] |
| 77. | Finnegan A, Kaplan CD, Cao Y, Eibel H, Glant TT, Zhang J. Collagen-induced arthritis is exacerbated in IL-10-deficient mice. Arthritis Res Ther. 2003;5:R18-R24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Finnegan A, Mikecz K, Tao P, Glant TT. Proteoglycan (aggrecan)-induced arthritis in BALB/c mice is a Th1-type disease regulated by Th2 cytokines. J Immunol. 1999;163:5383-5390. [PubMed] |
| 79. | Fellowes R, Etheridge CJ, Coade S, Cooper RG, Stewart L, Miller AD, Woo P. Amelioration of established collagen induced arthritis by systemic IL-10 gene delivery. Gene Ther. 2000;7:967-977. [PubMed] |
| 80. | Kasama T, Strieter RM, Lukacs NW, Lincoln PM, Burdick MD, Kunkel SL. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J Clin Invest. 1995;95:2868-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 195] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Cargnelutti E, Di Genaro MS. Reactive Arthritis: from clinical features to pathogenesis. Int J Clin Med. 2013;4:20-30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 82. | Wendling D, Racadot E, Toussirot E, Wijdenes J. Combination therapy of anti-CD4 and anti-IL6 monoclonal antibodies in a case of severe spondylarthropathy. Br J Rheumatol. 1996;35:1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Tanaka T, Kuwahara Y, Shima Y, Hirano T, Kawai M, Ogawa M, Arimitsu J, Hagihara K, Narazaki M, Ogata A. Successful treatment of reactive arthritis with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Arthritis Rheum. 2009;61:1762-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Kwan K, Bharadwaj S, Inderjeeth C. Response to treatment with tocilizumab of reactive arthritis induced by intravesical bacillus Galmette-Guérin unresponsive to DMARDs. Int J Rheum Dis. 2012;15:e73-e75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Kaipiainen-Seppönen O, Niinisalo H, Korpilähde T, Virolainen J. Treatment of reactive arthritis with infliximab. Scand J Rheumatol. 2003;32:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Thomas-Pohl M, Tissot A, Banal F, Lechevalier D. Spectacular evolution of reactive arthritis after early treatment with infliximab. Joint Bone Spine. 2012;79:524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 87. | Edrees A. Successful use of etanercept for the treatment of Reiter’s syndrome: a case report and review of the literature. Rheumatol Int. 2012;32:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |