Revised: December 18, 2013
Accepted: February 16, 2014
Published online: March 27, 2014
Processing time: 146 Days and 13.5 Hours
Cigarette smoking (CS) suppresses the immune system, and smoking is a well-known major risk factor for respiratory tract infections, including influenza infection. Both smoking cigarettes and passive smoking alter a wide range of immunological functions, including innate and adaptive immune responses. Past reviews on CS and innate immunity have been focused on the effects of CS on structural changes of the lung, as well as the effects on the function of alveolar macrophages, leukocytes, natural killer cells and dendritic cells. The study of innate immunity has developed rapidly in the last decade with the discovery of new receptors for virus recognition and interferon responses. This review aims to give a brief summary of recent findings on the suppressive effects of CS on the innate response to influenza virus, especially as it pertains to suppression of the function of pattern recognition receptors for influenza virus.
Core tip: Cigarette smoking (CS) alters a wide range of immunological functions, including innate and adaptive immune responses to viral infection. This review aims to give a brief summary of recent findings on the suppressive effects of CS on the innate response to influenza virus, especially as it pertains to suppression of the function of pattern recognition receptors for influenza virus. Studies on CS inhibition to innate response will be important in designing strategies for the development of novel treatments to mitigate the adverse consequences of CS and Flu infection.
- Citation: Wu W, Metcalf JP. Cigarette smoking and innate immune responses to influenza infection. World J Immunol 2014; 4(1): 20-25
- URL: https://www.wjgnet.com/2219-2824/full/v4/i1/20.htm
- DOI: https://dx.doi.org/10.5411/wji.v4.i1.20
Influenza virus is a major cause of infectious morbidity and mortality[1]. Each year in the United States, 5% to 20% of the population are infected, 200000 are hospitalized, and 36000 die due to influenza virus infection, making it the leading infectious cause of death[2,3]. There have been four pandemics (worldwide epidemics) in the last century, including the Spanish flu in 1918, the Asian flu in 1957, the Hong Kong flu in 1968, and the Swine flu in 2009. These were significant outbreaks. For example, the 1918 flu caused more deaths than those due to World War I. Influenza pandemics will continue as a threat to public health. The predisposition of cigarette smokers to have, and to have complications from, influenza infection is well recognized[4]. Epidemiological studies show that influenza infection is seven times more common and is much more severe in smokers than nonsmokers[5]. Influenza infections are more severe, with more cough, acute and chronic sputum production, breathlessness, and wheezing in smokers[6]. Both active and passive cigarette smoke exposure increase the risk of infections[7]. A cohort study of female military recruits showed that smoking was a risk factor for severe influenza-like illness during an outbreak of influenza A (H1N1) subtype infection[8]. Thailand’s National Avian Influenza Surveillance system reported that current or former smoking was among the several risk factors associated with a fatal outcome from human influenza infection[9]. In the spring of 2013, the high mortality of avian influenza H7N9 in humans caused great concern in China and the world. Age along with a history of smoking are the most significant risk factors which predict a fatal outcome in human H7N9 infection[10]. The mechanism of increased susceptibility to influenza infections in smokers is likely multifactorial, but clearly includes alteration of immunologic host defenses. Both smoking cigarettes and second-hand exposure to tobacco smoke alter a wide range of immunological functions, including innate and adaptive immune responses[11].
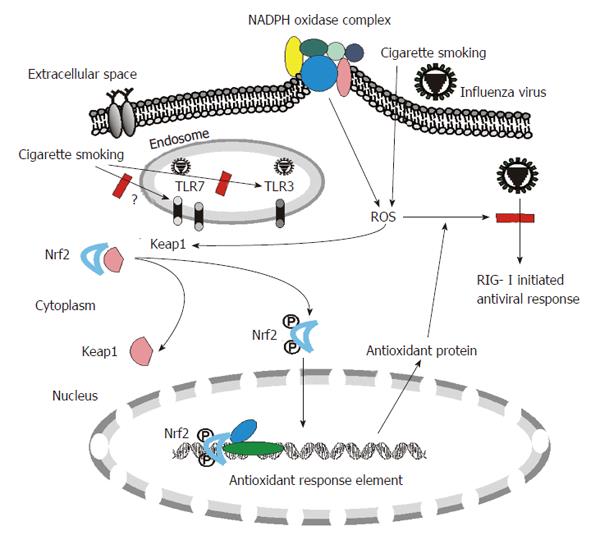
Innate immunity is the first line of host defense against invading microorganisms. Innate immune responses to viruses are triggered by recognition of specific structures of diversified pathogens called pathogen-associated molecular patterns (PAMPs). Host cells have multiple defensive mechanisms including pattern recognition receptors (PRRs) that can eliminate viruses through recognition of various viral PAMPs, such as ssRNA and dsRNA produced in virally infected cells. A recent triumph in research into immunity has been the discovery of three families of PRRs: Toll-like receptors (TLRs), Retinoic acid-inducible gene 1 (RIG-I) like helicases (RLRs) and nucleotide-binding domain and leucine-rich-repeat-containing proteins (NLRs). All three families are involved in influenza virus recognition and responses by the host[12] (Figure 1).
RIG-I is a highly inducible cytoplasmic RNA helicase that activates antiviral responses to influenza virus by cell-signal mediated activation of interferon (IFN) production[13,14]. Stimulation of RIG-I activates specific signaling pathways that lead to activation of nuclear factor-κB (NF-κB) which are crucial for inflammatory cytokine induction, and/or induction of interferon regulatory factor 3/7 (IRF3/7) which is important for the IFN-induced antiviral response. Many studies have confirmed that RIG-I regulation during influenza virus infection is important in the antiviral response and for modulation, either directly or indirectly, of proinflammatory cytokine responses[15,16]. We have shown that RIG-I induction is inhibited by cigarette smoking (CS) in our human organ culture model[17]. We demonstrated that 2%-20% cigarette smoke extract (CSE) inhibited influenza-induced RIG-I mRNA and protein expression as well as expression of the anti-viral cytokines interferon g induced protein 10 and IFN-β in human lung.
Of the 13 mammalian TLRs, TLR3 and 7 are the most important PRRs for influenza virus recognition. The influenza virus ssRNA genome is recognized by TLR7 in plasmacytoid dendritic cells (pDC) in humans[18,19]. Others have shown that CS suppresses key pDC functions upon respiratory syncytial virus (RSV) infection by a mechanism that involves downregulation of TLR7 expression and decreased activation of IRF-7[20]. The effect of CS on TLR7 in influenza virus infected pDC should be similar although it has not been evaluated.
Double-stranded RNA (dsRNA) is produced during viral replication and is recognized by endosomal TLR3[21]. Surprisingly, TLR3-deficient mice appear to be even more resistant to influenza infections than wild type mice, in terms of mortality[22]. Although high viral loads have been detected in the lung, viral load does not appear to underlie disease susceptibility in this model. In in vitro studies, CSE enhances rhinovirus-induced TLR3 expression and interleukin-8 secretion in A549 cells[23]. In human bone marrow mononuclear cells, CSE induces TLR2, TLR3 and TLR4 expression[24]. In vivo, CS augments the expression and responses of TLR3 in human macrophages[25] and in murine lung tissue. However, CS exacerbated poly(I:C)-induced neutrophilia and airway hyperresponsiveness[26]. Recently, Todt et al have reported that smoking decreased the response of human lung macrophages to dsRNA by reducing TLR3 expression. Alveolar macrophage of smokers show reduced C-X-C motif chemokine 10 production in response to poly(I:C) stimulation in vitro[27]. Therefore, CS alone is likely to slightly induce TLR3 expression. However, CS may suppress additional induction of TLR3 by virus. TLR3 is highly expressed in mouse innate immune cells, but shows a low level of expression in human monocytes, macrophages and dendritic cells[28]. This might lead to some conflicting results in studies of TLR3 expression in human and mouse models.
In the NLR family, Sabban et al[29] found that nucleotide-binding oligomerization domain-containing protein 2 (NOD2) confers responsiveness to ssRNA in terms of IRF3 activation and IFN-β production. Furthermore, wild-type cells treated with NOD2-specific small interfering RNA or bone marrow-derived macrophages from NOD2-deficient mice failed to produce an antiviral response after transfection with ssRNA, as is contained in RSV and vesicular stomatitis virus. It has been reported that CSE delays NOD2 expression and affects NOD2/receptor-interacting serine-threonine kinase 2 interactions in intestinal epithelial cells[30]. Thus, CS might interfere with the NLR-initiated innate response to influenza virus although further experiments are needed to examine this possibility.
In addition to inhibition of PRRs, CS could also affect the downstream signaling and transcription factors controlling the expression of IFN. For example, expression of IRF7 is critical for amplification of the type I interferon response. The expression of IRF7 was significantly decreased in influenza-infected nasal epithelium from smokers[31]. Furthermore, the data indicated that DNA methylation of the IRF7 gene and expression of the DNA (cytosine-5-)-methyltransferase1 was enhanced in cells from smokers. Previous studies demonstrated that hypermethylation of IRF7 results in decreased ability of type I IFNs to induce gene expression[32]. In the above report, IRF7 induction after influenza was suppressed both in vitro in long-term differentiated cultures of nasal epithelium, and in freshly biopsied nasal epithelial cells obtained from smokers after inoculation with the live-attenuated influenza virus vaccine. Mechanistically, another group found that cigarette smoke-conditioned medium decreased the expression of IRF-7 transcripts and suppressed the nuclear translocation of the key transcription factors, NF-κB and IRF-3, after poly(I:C) stimulation[33].
CS may affect many physiologic conditions which further alter host defense and virus clearance of lung cells. One of the most important mechanisms of CS-induced alteration is by increasing cellular oxidant stress. CSE contains high concentrations of reactive oxygen species (ROS), nitric oxide, peroxynitrite, and free radicals of organic compounds[34-36]. In addition to these short-lived, highly reactive substances, previous studies have shown that aqueous cigarette tar extracts also contain pro-oxidant substances that increase cellular production of ROS by NADPH oxidases[37-39]. NADPH oxidase-mediated generation of ROS is part of the innate immune defense of phagocytic cells and a variety of non-phagocytic cells against foreign pathogens. Endogenous antioxidant systems cope with the oxidative burden and limit potential toxicity of ROS. However, excess ROS may overwhelm antioxidant capacity and perturb the balance in this reduction-oxidation equilibrium, and damage cells and tissues through oxidative stress. In this regard, ROS are involved in the tissue injury associated with a number of inflammatory diseases, including rheumatoid arthritis[40], ischemia-reperfusion injury[41] and the adult respiratory distress syndrome[42]. Most importantly, mice lacking a functional NADPH oxidase exhibit increased viral clearance, reduced lung damage and improved lung function during influenza virus infection[43]. Human and animal studies show that CS produces generalized endothelial dysfunction[44-46], which is usually an indicator of increased oxidative stress which can be mediated by NADPH oxidases. Thus the increased NADPH oxidase activity induced by CS might play a major role in oxidative stress in human lung and inhibit the innate response to influenza virus (Figure 1).
CS increases the level of oxidants in the lungs, resulting in depletion of antioxidants. In response to CS, pulmonary epithelial cells counteract increased levels of oxidants by activating Nrf2-dependent pathways to augment the expression of detoxification and antioxidant enzymes. Nrf2 is a transcription factor and the Nrf2 antioxidant response pathway is the primary cellular defense against the cytotoxic effects of oxidative stress. Among other effects, Nrf2 increases the expression of numerous antioxidant and pollutant-detoxifying genes and is essential to protect the lungs from oxidative injury and inflammation. Yageta et al[47] have examined the role of Nrf2 in protection against influenza virus-induced pulmonary inflammation after CS exposure with both in vitro and in vivo approaches. Their data indicate that the antioxidant pathway controlled by Nrf2 is pivotal for protection against the development of influenza virus-induced pulmonary inflammation and injury under oxidative conditions[47]. The results further proved that oxidant stress contributes to CS-mediated susceptibility to influenza infections.
Blake et al[48] have developed a novel mouse model in which the cytosolic inhibitor of Nrf2, Keap1, is genetically deleted in Clara cells, which predominate in the upper airways in mice. Deletion of Keap1 in Clara cells resulted in increased expression of Nrf2-dependent genes. Deletion of Keap1 in airway epithelium also protected Clara cells against oxidative stress ex vivo and attenuated oxidative stress and CS-induced inflammation in vivo[48]. Therefore, current reports suggest that reducing oxidative stress in cells has a potential therapeutic effect, not only restoring virus recognition by PRRs suppressed by CS, but also by decreasing oxidant-mediated inflammation and cellular injury.
Recent data from our laboratory also demonstrated that CS-mediated cellular oxidant stress is the major mechanism of suppression of viral-mediated induction of the major RNA virus sentinel, RIG-I, in human lung[17]. We found that CSE treatment inhibited influenza-induced anti-viral cytokine expression in our human lung organ culture model. This is associated with CSE-inhibited mRNA and protein expression of RIG-I, which is important in the antiviral host response. However, inhibition of viral-mediated RIG-I induction by CSE was prevented and antiviral cytokine responses were restored by the antioxidant, N-acetyl cysteine (NAC). These findings show that CSE suppresses anti-viral responses in influenza virus infected human lung through oxidative inhibition of RIG-I. CS is the major cause of chronic obstructive pulmonary disease (COPD) and exacerbates the susceptibility of the host to respiratory infectious diseases and the attendant pathology[49]. Restoration of these responses by NAC may be an important mechanism for the recent finding that treatment of COPD patients with high-dose NAC resulted in decreased exacerbations[50].
In summary, epidemiological studies suggest that CS is a major risk factor for influenza caused morbidity and mortality. The innate immune system senses influenza virus invasion through recognition of specific ligands by TLR3/7, NLR and cytosolic RIG-I. CS inhibits RIG-I, TLR3 and possibly TLR7 recognition of influenza virus. ROS induced by CS are involved in the interference of PRR function. Reducing oxidative stress in cells, either by using antioxidants or by manipulating Nrf2 overexpression, has a potential therapeutic effect of restoring virus recognition by PRRs suppressed by CS.
More studies will be required to enhance our understanding of the mechanism whereby CS suppresses the human immune system and also of the process that controls influenza virus infection. This will be important in designing strategies for the development of novel treatments to mitigate the adverse consequences of CS and flu infection.
P- Reviewers: Fujita J, Julio RL, Ryffel B, Yokota S S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Davis MM, Taubert K, Benin AL, Brown DW, Mensah GA, Baddour LM, Dunbar S, Krumholz HM. Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. J Am Coll Cardiol. 2006;48:1498-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1620] [Cited by in RCA: 1635] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 3. | Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, Black S, Shinefield H, Fukuda K. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 722] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 4. | Finklea JF, Sandifer SH, Smith DD. Cigarette smoking and epidemic influenza. Am J Epidemiol. 1969;90:390-399. [PubMed] |
| 5. | Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 674] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 6. | Kark JD, Lebiush M, Rannon L. Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N Engl J Med. 1982;307:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Peat JK, Keena V, Harakeh Z, Marks G. Parental smoking and respiratory tract infections in children. Paediatr Respir Rev. 2001;2:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Kark JD, Lebiush M. Smoking and epidemic influenza-like illness in female military recruits: a brief survey. Am J Public Health. 1981;71:530-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Hanshaoworakul W, Simmerman JM, Narueponjirakul U, Sanasuttipun W, Shinde V, Kaewchana S, Areechokechai D, Levy J, Ungchusak K. Severe human influenza infections in Thailand: oseltamivir treatment and risk factors for fatal outcome. PLoS One. 2009;4:e6051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Liu S, Sun J, Cai J, Miao Z, Lu M, Qin S, Wang X, Lv H, Yu Z, Amer S. Epidemiological, clinical and viral characteristics of fatal cases of human avian influenza A (H7N9) virus in Zhejiang Province, China. J Infect. 2013;67:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 854] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 12. | Wu S, Metcalf JP, Wu W. Innate immune response to influenza virus. Curr Opin Infect Dis. 2011;24:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1059] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 14. | Sumpter R, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 724] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 15. | Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 726] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 16. | Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2934] [Cited by in RCA: 3121] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 17. | Wu W, Patel KB, Booth JL, Zhang W, Metcalf JP. Cigarette smoke extract suppresses the RIG-I-initiated innate immune response to influenza virus in the human lung. Am J Physiol Lung Cell Mol Physiol. 2011;300:L821-L830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2469] [Cited by in RCA: 2553] [Article Influence: 121.6] [Reference Citation Analysis (0)] |
| 19. | Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598-5603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1343] [Cited by in RCA: 1404] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 20. | Castro SM, Chakraborty K, Guerrero-Plata A. Cigarette smoke suppresses TLR-7 stimulation in response to virus infection in plasmacytoid dendritic cells. Toxicol In Vitro. 2011;25:1106-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571-5580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 532] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 22. | Le Goffic R, Balloy V, Lagranderie M, Alexopoulou L, Escriou N, Flavell R, Chignard M, Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 414] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 23. | Wang JH, Kim H, Jang YJ. Cigarette smoke extract enhances rhinovirus-induced toll-like receptor 3 expression and interleukin-8 secretion in A549 cells. Am J Rhinol Allergy. 2009;23:e5-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Zhou J, Eksioglu EA, Fortenbery NR, Chen X, Wang H, Epling-Burnette PK, Djeu JY, Wei S. Bone marrow mononuclear cells up-regulate toll-like receptor expression and produce inflammatory mediators in response to cigarette smoke extract. PLoS One. 2011;6:e21173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Koarai A, Yanagisawa S, Sugiura H, Ichikawa T, Akamatsu K, Hirano T, Nakanishi M, Matsunaga K, Minakata Y, Ichinose M. Cigarette smoke augments the expression and responses of toll-like receptor 3 in human macrophages. Respirology. 2012;17:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Kimura G, Ueda K, Eto S, Watanabe Y, Masuko T, Kusama T, Barnes PJ, Ito K, Kizawa Y. Toll-like receptor 3 stimulation causes corticosteroid-refractory airway neutrophilia and hyperresponsiveness in mice. Chest. 2013;144:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Todt JC, Freeman CM, Brown JP, Sonstein J, Ames TM, McCubbrey AL, Martinez FJ, Chensue SW, Beck JM, Curtis JL. Smoking decreases the response of human lung macrophages to double-stranded RNA by reducing TLR3 expression. Respir Res. 2013;14:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Heinz S, Haehnel V, Karaghiosoff M, Schwarzfischer L, Müller M, Krause SW, Rehli M. Species-specific regulation of Toll-like receptor 3 genes in men and mice. J Biol Chem. 2003;278:21502-21509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 595] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 30. | Aldhous MC, Soo K, Stark LA, Ulanicka AA, Easterbrook JE, Dunlop MG, Satsangi J. Cigarette smoke extract (CSE) delays NOD2 expression and affects NOD2/RIPK2 interactions in intestinal epithelial cells. PLoS One. 2011;6:e24715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Jaspers I, Horvath KM, Zhang W, Brighton LE, Carson JL, Noah TL. Reduced expression of IRF7 in nasal epithelial cells from smokers after infection with influenza. Am J Respir Cell Mol Biol. 2010;43:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Li Q, Tang L, Roberts PC, Kraniak JM, Fridman AL, Kulaeva OI, Tehrani OS, Tainsky MA. Interferon regulatory factors IRF5 and IRF7 inhibit growth and induce senescence in immortal Li-Fraumeni fibroblasts. Mol Cancer Res. 2008;6:770-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Bauer CM, Dewitte-Orr SJ, Hornby KR, Zavitz CC, Lichty BD, Stämpfli MR, Mossman KL. Cigarette smoke suppresses type I interferon-mediated antiviral immunity in lung fibroblast and epithelial cells. J Interferon Cytokine Res. 2008;28:167-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Nakayama T, Church DF, Pryor WA. Quantitative analysis of the hydrogen peroxide formed in aqueous cigarette tar extracts. Free Radic Biol Med. 1989;7:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 112] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Pryor WA, Prier DG, Church DF. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environ Health Perspect. 1983;47:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 234] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Zang LY, Stone K, Pryor WA. Detection of free radicals in aqueous extracts of cigarette tar by electron spin resonance. Free Radic Biol Med. 1995;19:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 152] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Pryor WA, Stone K, Zang LY, Bermúdez E. Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem Res Toxicol. 1998;11:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 188] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Squadrito GL, Cueto R, Dellinger B, Pryor WA. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radic Biol Med. 2001;31:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 280] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 39. | Bock FG, Swain AP, Stedman RL. Carcinogenesis assay of subfractions of cigarette smoke condensate prepared by solvent-solvent separation of the neutral fraction. J Natl Cancer Inst. 1972;49:477-483. [PubMed] |
| 40. | Kitsis E, Weissmann G. The role of the neutrophil in rheumatoid arthritis. Clin Orthop Relat Res. 1991;63-72. [PubMed] |
| 41. | Zimmerman BJ, Granger DN. Mechanisms of reperfusion injury. Am J Med Sci. 1994;307:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 168] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Boxer LA, Axtell R, Suchard S. The role of the neutrophil in inflammatory diseases of the lung. Blood Cells. 1990;16:25-40; discussion 41-2. [PubMed] |
| 43. | Snelgrove RJ, Edwards L, Rae AJ, Hussell T. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur J Immunol. 2006;36:1364-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Adams MR, Jessup W, Celermajer DS. Cigarette smoking is associated with increased human monocyte adhesion to endothelial cells: reversibility with oral L-arginine but not vitamin C. J Am Coll Cardiol. 1997;29:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Czernin J, Waldherr C. Cigarette smoking and coronary blood flow. Prog Cardiovasc Dis. 2003;45:395-404. [PubMed] |
| 46. | Raij L, DeMaster EG, Jaimes EA. Cigarette smoke-induced endothelium dysfunction: role of superoxide anion. J Hypertens. 2001;19:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Yageta Y, Ishii Y, Morishima Y, Masuko H, Ano S, Yamadori T, Itoh K, Takeuchi K, Yamamoto M, Hizawa N. Role of Nrf2 in host defense against influenza virus in cigarette smoke-exposed mice. J Virol. 2011;85:4679-4690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | Blake DJ, Singh A, Kombairaju P, Malhotra D, Mariani TJ, Tuder RM, Gabrielson E, Biswal S. Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. Am J Respir Cell Mol Biol. 2010;42:524-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 49. | Sutherland ER, Cherniack RM. Management of chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2689-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 50. | Tse HN, Raiteri L, Wong KY, Yee KS, Ng LY, Wai KY, Loo CK, Chan MH. High-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest. 2013;144:106-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |