Published online Mar 24, 2017. doi: 10.5410/wjcu.v6.i1.18
Peer-review started: August 16, 2016
First decision: September 28, 2016
Revised: October 27, 2016
Accepted: December 13, 2016
Article in press: December 15, 2016
Published online: March 24, 2017
Processing time: 202 Days and 14.5 Hours
To investigate the association of urinary chemokines with the treatment response in chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients.
Between 2007-2011, 18 out of 21 male CP/CPPS patients met the exclusion/inclusion criteria of the 16 wk longitudinal study on twice daily oral treatment with Phosphodiesterase 4 inhibitor called Apremilast for 12 wk. Symptom scores and urine specimen were collected at baseline and every visit at 4 wk interval from CP/CPPS patients who completed at least 8 wk of drug treatment. Urine collected at each visit was frozen and then analyzed together after thawing for chemokines and growth factors using MILLIPLEX™ MAP immunoassay. Cross sectional association of Chronic Prostatitis Symptom Index (CPSI) and visual analog scale (VAS) with chemokine levels in urine collected at baseline was assessed in 18 CP/CPPS patients relative to 10 asymptomatic male subjects. Longitudinal association between urine chemokine levels and symptom scores was assessed in 8 treatment-adherent CP/CPPS patients at baseline and at 4, 8, 12 and 16 wk.
Urine chemokines levels of CXCL-1 (GRO-a), CXCL-8 (IL-8), CXCL-10 (IP-10) and CCL5 (RANTES) in CP/CPPS patients at baseline were significantly elevated relative to asymptomatic subjects, whereas levels of sIL-1RA in CP/CPPS were significantly lower compared to controls (P < 0.05). Quantitatively, urine levels of CXCL-10 were higher than other chemokines in CP/CPPS, but its 5 fold change relative to controls was lower than the 20 fold change noted for CXCL-8. The mean age of enrolled patients who completed at least 8 wk of treatment (n = 8) was 46.5 ± 9.4 years and analysis found that elevation of CXCL-8 and CCL5 increased the odds for higher score of CPSI by 54% and 25%, respectively (F test, P = 0.00007). Urine levels of CCL2 (MCP-1) and CXCL-10 together explained approximately 85% of variance in longitudinal data on multivariate analysis. Bivariate analysis of 5 patients who fully complied and completed the assigned dose regimen, showed strong linear correlation of reduced urine levels of CXCL-10, CXCL-8, CCL5, CCL2 and PDGF with improvement in clinical activity as measured by pain VAS and CPSI (Pearson r = 0.83-0.97; P < 0.05).
Urine levels of CXCL-10, CCL2 and PDGF can be sensitive, objective and non-invasive markers of response to new therapeutic intervention in CP/CPPS patients.
Core tip: Chronic prostatitis or chronic pelvic pain syndrome is a poorly understood and prevalent male condition, which is generally described by pelvic pain in the absence of demonstrable urinary or genital tract infection. Inflammation is considered to play a critical role in the prostatitis and so we carried out a small physician initiated clinical study to investigate the potential efficacy of a new phosphodiesterase 4 inhibitor drug, Apremilast. Urine was collected from the study participants at the baseline and at each visit to assay the chemokine levels and then determine their association with the treatment response. We are the first to report that chemokine levels in urine instead of the semen or prostatic secretions have the potential to serve as non-invasive biomarkers of severity and treatment response of the prostatitis patients.
- Citation: Tyagi P, Killinger K, McLennan G, Jayabalan N, Chancellor M, Peters KM. Urine chemokine levels correlate with treatment response to phosphodiesterase 4 inhibitor in prostatitis. World J Clin Urol 2017; 6(1): 18-26
- URL: https://www.wjgnet.com/2219-2816/full/v6/i1/18.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v6.i1.18
Chronic prostatitis (CP) or chronic pelvic pain syndrome (CPPS) is a poorly understood and prevalent male condition that affects 10% to 14% of men of all ages and ethnic origins[1]. According to the National Institutes of Health (NIH) consensus definition, CPPS is regarded as category III prostatitis described by genital or pelvic pain in the absence of demonstrable urinary or genital tract infection[2].
Unlike NIH category I and II prostatitis, the pathophysiology of category III (CP/CPPS) is an enigma and may not be as organ (prostate) centric as categories I and II[2]. With progression of symptom severity, CP/CPPS may evolve into regional pain syndrome with symptoms resembling interstitial cystitis[3]. Considering the significant inflammatory component in CP/CPPS, most prior therapies have focused on targeting inflammation[4]. Leukocyte infiltration is a primary event in inflammation and because expression of chemokines temporally precede infiltration[5] it makes the chemokines well suited to serve as biomarkers for pelvic pain disorders[6].
Interestingly, leukocytes and immune cells predominantly express a cyclic adenosine monophosphate cAMP metabolizing isoenzyme called phosphodiesterase 4 (PDE4)[7]. Consequently, selective pharmacological inhibition of PDE4 by Apremilast, a novel, orally available small molecule, can cause buildup of cAMP which affects production of cytokinesand chemokines, including CXCL- 10 (IP-10), CCL2/monocyte chemoattractant protein-1 (MCP-1), CCL5/Regulated on Activation, Normal T cell Expressed and Secreted (RANTES) and vascular endothelial growth factor (VEGF)[8]. Since mechanism of action for Apremilast is different from traditional anti-inflammatory drugs (NSA IDS), it could have potential efficacy in CP/CPPS patients refractory NSAIDS[9], which work by inhibiting cyclooxygenase (COX) instead of PDE4.
Previous studies have reported correlations between increased cytokines, chemokines and growth factors in the semen and prostatic secretions with symptom scores of CP/CPPS[10,11]. Here of seminal plasma, we aimed to correlate here, in a longitudinal study, instead the disease severity and treatment response to Apremilast by non-invasive measurement of chemokines in urine of CP/CPPS patients.
Adult male subjects aged ≥ 18 years at time of informed consent were recruited in a prospective open-label, one arm, physician initiated study (NCT00701311)[9] that was approved by the institutional review board (HIC-2007-135). The inclusion and exclusion criteria for CP/CPPS patients enrolled in a 12 wk oral therapy of PDE4 inhibitor, Apremilast (20 mg BID; provided by Celgene), can also be found at http://clinicaltrials.gov/ct2/show/NCT00701311. The inclusion criteria were patients with clinical symptoms of CP/CPPS (pain in the pelvic area, penis, scrotum, or perineum) who were refractory to other therapies (e.g., NSAIDS) for at least 3 of the 6 mo immediately before the first visit. Enrolled patients also fulfilled the NIH definition of CP/CPPS (category III prostatitis) and had a NIH-CPSI total score of 15 or higher. Exclusion criteria were subjects with genital infection, clinical epididymitis, documented positive urine culture (> 100000 CFU/mL) within the past three months, pelvic mass, or known active or prior genitourinary malignancies (including renal, ureteral, bladder or prostate). Urine samples from 10 age-matched males without CP/CPPS diagnosis or symptoms were also included as controls in the analysis for comparison.
Clinical and laboratory assessments were performed at baseline and then every 4 wk till the end of treatment (at 12 wk) with a final follow up at week 16. Clinical assessment for improvement in symptoms after treatment included change between week 0 and week 12 in the chronic prostatitis symptom index (CPSI), which is a validated urinary and pain measurement tool from the NIH. Pain was measured using a visual analog scale (VAS) for pain (0 = no pain and 10 = worst pain imaginable). Laboratory assays included urine culture at baseline, complete blood count, metabolic blood chemistry, urinalysis, liver and kidney function tests, and PSA.
Urine specimens were collected from consented patients at baseline and at 4, 8, 12 and 16 wk after starting treatment. Urine dipstick was done after each urine collection. After collection, all the spot-urine samples were frozen at -80 °C until analysis.
On the day of analysis, all the frozen urine samples were thawed quickly and nine different chemokines/cytokines in urine were measured by MILLIPLEX human cytokine/chemokine immunoassay following the procedure as previously described[6]. An automated immunoassay analyzer Bioplex Luminex 200 IS System, (Luminex, Austin, TX) purchased through Bio-Rad, was used to assay chemokines using commercially available microspheres (Millipore, Billerica, MA) following manufacturer’s instructions. Microspheres of defined spectral properties conjugated to antibody directed against CXCL-1, CXCL-8, CXCL-10, IL-6, soluble interleukin-1 receptor antagonist (sIL-1RA), MCP-1 (CCL2), RANTES (CCL5), VEGF, platelet derived growth factor (PDGF) were pipetted into 96-well plate. The median fluorescence intensity of microspheres specific for each chemokine was recorded for each well to calculate chemokine concentration.
The differences in urine levels of chemokines between controls and CP/CPPS patients were measured by Mann-Whitney U test. The test assesses significant differences between groups without making assumptions of normality. Both bivariate and multivariate models using principal component analysis (PCA) were examined to assess the degree to which each baseline predictor was associated with the clinical outcome measures independently or in conjunction with the other variables using Unscrambler® X 10.1 software (CAMO Software Inc, NJ, United States). Assumptions of linearity were verified for each model and age, urine chemokines, and duration of CP/CPPS symptoms were included as covariates in all models. Pearson (r) and Spearman (rs) correlation test were used to assess the correlations among reductions in urinary chemokines and clinical outcomes. All statistical tests were evaluated for statistical significance at α < 0.05 2-tailed, and trends at 0.05 < α > 0.10. Continuous variables are expressed as mean ± standard error of mean.
Of the 21 patients recruited for the study only 18 met the exclusion/inclusion criteria and provided urine at baseline, 7 provided a urine specimen at baseline but did not complete at least 8 wk of treatment, and 3 others withdrew before completing the study. The mean age of enrolled patients who completed at least 8 wk of treatment (n = 8) was 46.5 ± 9.4 years. Among 8 CP/CPPS patients, those who fully complied and completed the assigned dose regimen are referred to as treatment adherent (n = 5) and those not completely adherent to regimen are referred to as treatment non-adherent CP/CPPS patients (n = 3).
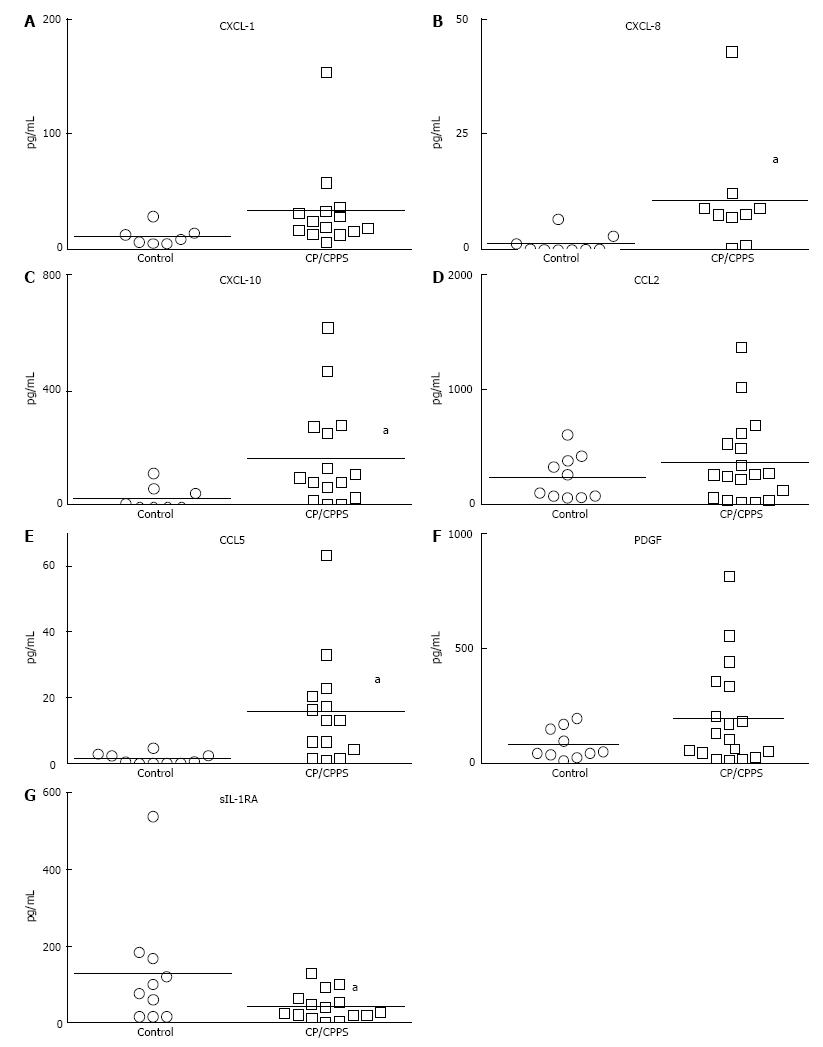
Urine dipstick done after each collection ruled out presence of urinary infection. At baseline, levels of CX CL-1 (15 fold), CXCL-8 (20 fold) and CXCL-10 (5 fold) were significantly elevated in CP/CPPS (P < 0.05) (Figure 1A-C). Higher levels of CXCL-10 (169.8 ± 46.38 pg/mL vs 31.45 ± 16.83 pg/mL) relative to controls is in agreement with the intra-acinar infiltration of T-lymphocytes previously noted in prostate biopsy of CP/CPPS patients[12]. Levels of neutrophil attracting CXC chemokines CXCL-1 (32.46 ± 9.9 pg/mL) and CXCL-8 (5.3 ± 1.8 pg/mL) were comparatively lower in CP/CPPS[12] (notice scale on Y-axis Figure 1).
CCL5, which is also known to drive trafficking of lymphocytes[13] (Figure 1E), was also significantly elevated in CP/CPPS relative to controls (19.26 ± 6.8 pg/mL vs 0.14 ± 0.02 pg/mL). CCL2 and PDGF were detected in substantial amount in all the CP/CPPS patients, but due to high variability in the control group, results were not significant (Figure 1D and F, respectively). Levels of CXCL-1, CXCL-8 and CCL5 in the control group were uniformly below or very near the detection limit of our assay method.
Urine levels of CXCL-8 and CCL5 levels were not only quantitatively lower in CP/CPPS patients compared to other chemokines (Figure 1), but were also detected only in 7 and 8 patients, respectively, of the 18 enrolled CP/CPPS patients at baseline. The urine levels of CXCL-1, CXCL-8 and CCL5 in the control group were uniformly below or very near the detection limit of our assay method. Significantly higher levels of sIL-1RA in controls relative to CP/CPPS (Figure 1G) are consistent with its anti-inflammatory role (P < 0.05).
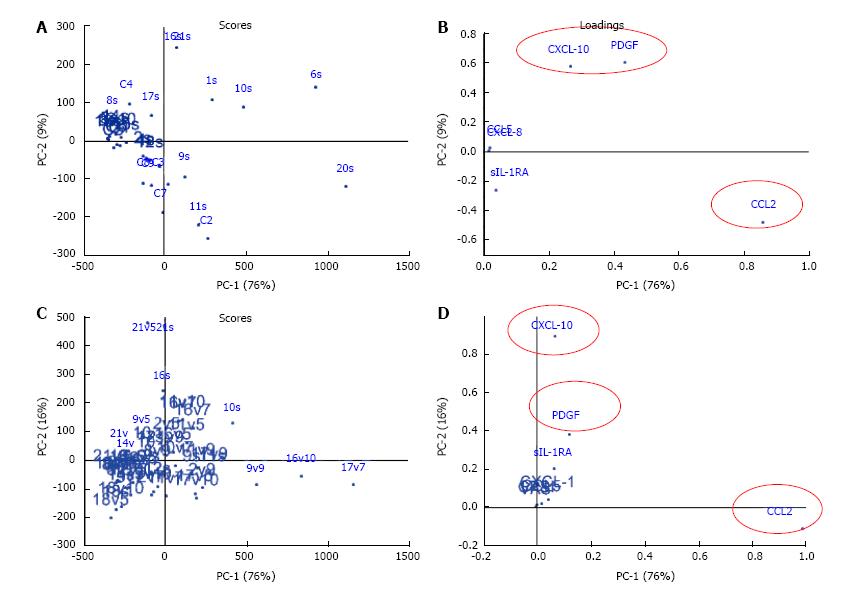
PCA was used to reduce the dimensionality by extracting two principal components (PCs), PC1 and PC2 from the cross-sectional data of 9 cytokines from 18 CP/CPPS patients and 10 controls at baseline (Figure 2A and B). PC1 and PC2 together explain most of the variation, 76% and 9% respectively, and are defined by a weighted sum of the individual cytokine values referred to as loadings (Figure 2B). The majority of variance at baseline was explained by CCL2, CXCL-10 and PDGF. The value of PC1 and PC2 for each patient and controls were calculated and these values are referred to as “scores”. The scores are represented graphically to summarize the variation (Figure 2A). The majority of CP/CPPS mapped separately from controls, but a close cluster of CP/CPPS patients like most controls was absent which can be explained by disease heterogeneity as revealed by the variation in the levels of different chemokines in CP/CPPS at baseline (Figure 1).
Significant improvement in clinical outcome measures (VAS and CPSI) was noted post treatment in adherent CP/CPPS patients (Figure 3). Multivariate modeling of data from treatment adherent and non-adherent patients together (n = 8) revealed that age and duration of CP/CPPS symptoms had no effect on the CPSI or VAS scores or on the response to treatment. The longitudinal effect of treatment was visualized by PCA (Figure 2C) after excluding the data of Patient 6 due to high variation relative to other treated CP/CPPS patients. PCA allowed grouping of patients with overall similar cytokine expression profiles at individual time points. With regard to cross-sectional data (Figure 2A and B), the contribution of CCL2 was slightly increased whereas contribution of CXCL-10 was slightly reduced in PC1. CXCL-1, CCL5 and CPSI scores together contributed < 1% to both PC1 and PC2 scores.
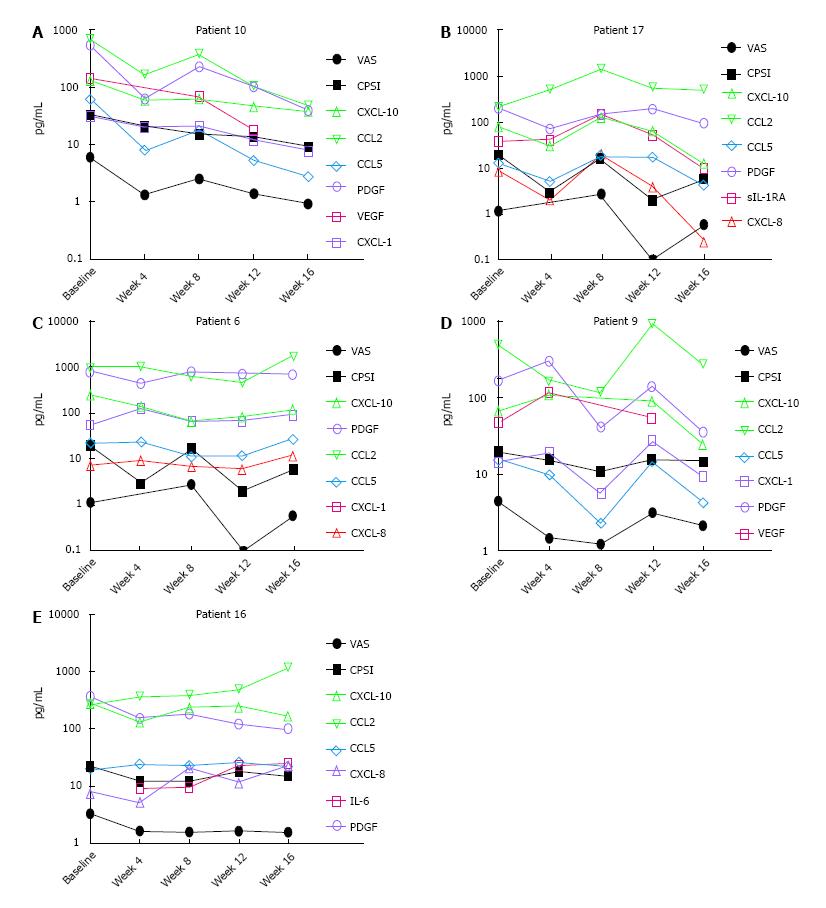
Considering the variability of treatment response, we used both parametric and non-parametric bivariate correlation analysis for clinical outcome scores measured at each visit with the respective urine levels of chemokines individually for each of the 5 treatment adherent patients (Figure 3). Analysis of longitudinal data from treatment adherent Patient 10 (Figure 3A) demonstrated that lower VAS strongly correlated with reduced levels of CXCL-10 (Pearson r = 0.97; P = 0.003), CCL2 (r = 0.97; P = 0.005), CCL5 (r = 0.99; P < 0.0001), VEGF (r = 0.98; P = 0.003) and PDGF (r = 0.99; P < 0.0001). Lower CPSI also linearly correlated with reduced levels of CXCL-10 (r = 0.96; P = 0.009), CCL2 (r = 0.86; P = 0.05), CCL5 (r = 0.91; P = 0.03), VEGF (r = 0.80; P = 0.1), PDGF (r = 0.86; P = 0.06) and non-linearly correlated with CXCL-1 (rs = 0.9; P = 0.08). In contrast, only the VAS of Patient 17 (Figure 3B) strongly correlated with CXCL-10 (r = 0.84; P = 0.07), CXCL-8 (r = 0.94; P = 0.01) and sIL-1RA (r = 82; P = 0.08) and CPSI showed rs coefficient of 0.4-0.5 for PDGF, CXCL-10 and CXCL-8 without achieving statistical significance.
In Patient 6 (Figure 3C), reduced CXCL-10 was linearly correlated with VAS (r = 0.89; P = 0.04) and CPSI (r = 0.82; P = 0.08) whereas PDGF was non-linearly correlated with VAS (rs = 0.89; P = 0.08) and CPSI (rs = 0.9; P = 0.08). For Patient 9 (Figure 3D), lower CCL5 linearly correlated with VAS (r = 0.80; P = 0.09) and CPSI (r = 0.86; P = 0.05), and CCL2 show non-linear with VAS (rs = 0.9; P = 0.08). In Patient 16 (Figure 3E) PDGF was linearly correlated with VAS (r = 0.95; P = 0.01) and CPSI was non-linearly correlated with CXCL-10 (rs = 0.82; P = 0.13).
In our study, we focused on chemokines, which are mainly subdivided into 2 families (CXC and CC) based on the position and number of conserved cysteine as well as the presence of intervening amino acid(s) between the first two conserved cysteine residues[14]. The CXC chemokines (CXCL-1, CXCL-8, CXCL-10) mainly act on neutrophils and lymphocytes, while the CC chemokines (CCL2/MCP-1 and CCL5/RANTES) act on monocytes and lymphocytes without affecting neutrophils[14]. Chemokines not only induce chemotaxis but also actiavte of these target cells in affected tissue[15] through their influence on the expression and/or affinity of leukocyte integrins to cause inflammatory cell adhesion and infiltration.
A major finding of our clinical study is the successful measurement of chemokines in urine of CP/CPPS patients, which have been previously detected only in seminal plasma (CXCL-8 and CCL2)[16,17]. Previous longitudinal studies on CP/CPPS only reported subjective symptom scores[18], but we were able to correlate the subjective scores with objective urine biomarker data. CP/CPPS, being a chronic disease with substantial variation in symptoms across patients[18], probably contributes to the variable levels of different chemokines at baseline (Figure 1) and may explain why different chemokines correlate with treatment induced reduction in VAS and CPSI scores in different patients (Figure 3). The disease heterogeneity and variability in treatment response of CP/CPPS patients justify the need for personalized treatment guided by urinary biomarkers.
CCL2 previously detected in seminal plasma[17] can also be non-invasively measured in urine (Figure 1D). Loading plots of cross-sectional and longitudinal data identified CCL2 as the key variable explaining the variance in data (Figure 2). CCL2, together with significant elevation of another CC chemokine (CCL5) in CP/CPPS (Figure 1E), agrees with previous suggestions of a dominant T-helper Th1-mediated inflammation in CP/CPPS[19]. Previously observed activated T-lymphocytes in prostate biopsy of CP/CPPS patients[12] supported the role of Th1-mediated inflammation and biopsy findings concurs with the results of our study that showed higher urine levels of CXCL-10 (Figure 1C) that chemoattracts activated T-lymphocytes[14] relative to CXCL-1 and CXCL-8 (Figure 1A and B) that attract neutrophils[20]. CXCL-10 is induced by pro-inflammatory interferfon-γ (IFN-γ) or TNF-α directly or through activation of nuclear factor-κB (NF-κB)[20] and it chemoattracts T cells, which inhibit the proliferation of endothelial cells and inflammatory angiogenesis[21]. Since sIL-1RA is known to block T lymphocyte activation[22], the significantly lower levels of sIL-1RA (Figure 1G) in urine of CP/CPPS relative to controls (P < 0.05) also supports the predominance of T-lymphocytes in the pathophysiology of CP/CPPS.
Interestingly, the same CCR5 receptor acted on by CCL2 and CCL5 for recruitment of macrophages, monocytes and lymphocytes to inflammatory foci highlight the inbuilt redundancy in chemokines signaling[23]. Because chemokines operate in integrated networks[24], a more complete understanding of their role can be gained by measuring multiple chemokines for examining patterns associated with symptoms and prognosis of CP/CPPS. The results of our study and the statistical logic lend impetus to the assertion that a multiple panel of chemokines instead of single protein like PSA[25] will be able to better serve as a “proteomic signature” to predict treatment response and stratify CP/CPPS patients according to disease severity.
The predictive association of CXCL-8 and CCL5 with CPSI scores is corroborated by previous studies with regard to CXCL-8 (IL-8)[16] where CXCL-8 is known to act as a secretagogue for histamine from basophils[26]. Reduced levels of CXCL-8, CXCL-10, CCL2, CCL5, and PDGF measured individually in treatment adherent patients strongly correlated with coincident lower VAS and CPSI scores (Figure 3). PCA analysis of longitudinal data agree (Figure 2D) with regard to CXCL-10, and CCL2 is in agreement with the bivariate correlation of lower CXCL-10 and CCL2 with the lower VAS in Patients 6, 9, 10 and 17 (Figure 3).
The negative association of lower CXCL-10 and CCL2 with pain scores of CP/CPPS is presumably linked to the concentration dependent suppression of opioid receptors[27] and augmentation of TRPV1 receptor responses[28,29] known to be caused by these elevated chemokines. Drug related lowering of chemokines will unmask the endogenous analgesic effect of opioid receptors and block the algesic effect of afferents sensitization to explain the lower pain scores. The lower VAS and CPSI scores in Patients 6, 9, 10, 16, 17 may also be related to reduced inflammatory angiogenesis in prostate as a consequence of reduced expression of PDGF[30], CXCL-10[21] and CCL5[31] caused by Apremilast.
The dose of Apremilast 20 mg BID was selected based on pre-clinical studies to maintain the serum level above the IC50 of 77 nM for TNF-α inhibition in serum for the duration of therapy. As predicted by pre-clinical studies[8], steady PDE4 inhibition by Apremilast reduced the chemokine levels in urine collected without any prostatic massage from treatment adherent CP/CPPS patients (Figure 3). Considering that CP/CPPS may not be as organ (prostate) centric as categories I and II[2], the ability to track disease severity and treatment response by non-invasive measurement of chemokines in urine is a significant improvement. The urine levels probably reflect the inflammation not only localized in prostate but in pelvic floor muscle as well.
These results demonstrate that chemokines are better biomarker candidates than the cytokines TNF-α or IL-1β that have been previously measured only in seminal plasma[10]. Chemokines are the downstream member of the inflammation cascade[20,24] and are therefore produced in copious amounts[6] which explains their substantial presence in urine of CP/CPPS patients (Figure 1). Very small amounts of TNF-α have been detected previously in urine[22] and very low levels of IL-6 and VEGF (Figure 3) were detected in one or two patients of our study. Possibly the secretion of IL-6 and VEGF is just enough from the inflamed site to bind with receptors in nearby tissue and not available for release into urine of other patients. Unlike PDGF, VEGF was only detected in urine of 3 patients at various time points, which is probably related to the differences in expression of cognate receptors of two growth factors at inflamed site leading to trapping of secreted VEGF within the tissue.
These findings show the promise of urinary chemokines for monitoring disease activity of CP/CPPS and objectively evaluate the response to therapeutic intervention. The present study was limited by small size of the cohort and incomplete adherence to treatment in some patients; nevertheless these results can build the framework for a larger prospective longitudinal study to investigate the causal relationship of these chemokines with patient symptoms. Perhaps, urine may be able to serve as a non-surgical diagnostic tool for prostate disease in the future.
In conclusion, a major finding of our study is the successful longitudinal measurement of chemokines in urine of CP/CPPS patients instead of seminal plasma and objective urine chemokine data strongly correlated with subjective symptom scores in treatment adherent patients. Urine levels of chemokines such as CXCL-10, CCL2 and PDGF can be sensitive, objective and non-invasive biomarkers for monitoring patient response to novel therapy in CP/CPPS. The study also offers insight into the mechanisms underlying the benefit from Apremilast in CP/CPPS.
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a poorly understood and prevalent male condition that is described by genital or pelvic pain in the absence of demonstrable urinary or genital tract infection.
Unlike NIH category I and II prostatitis, the pathophysiology of category III (CP/CPPS) is an enigma and may not be as organ (prostate) centric as categories I and II. With progression of symptom severity, CP/CPPS may evolve into regional pain syndrome with symptoms resembling interstitial cystitis, which necessitates the search for objective tools to assist in differential diagnosis and treatment.
The significant inflammatory component in CP/CPPS can be leveraged as a target for symptomatic drug treatment and paracrine messengers mediating inflammation can be tracked in non-invasive bio-fluids as objective surrogate markers.
Data described here can be the basis of a large scale clinical study to assess the potential of identified chemokines as treatment surrogates and for basic research to understand the biological significance of identified chemokines.
CP/CCPS is chronic prostatitis or chronic pelvic pain syndrome also called NIH category III prostatitis.
Available papers on urine analysis of CP/CPPS patients without prostate massage are scarce. Authors are first analyze the urine from a longitudinal study uncover their potential as non-invasive biomarkers for CP/CPPS.
Manuscript source: Invited manuscript
Specialty Type: Urology and Nephrology
Country of Origin: United States
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cai T, Schatz F, Slobedman B S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Nickel JC, Tripp DA, Chuai S, Litwin MS, McNaughton-Collins M, Landis JR, Alexander RB, Schaeffer AJ, O’Leary MP, Pontari MA. Psychosocial variables affect the quality of life of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome. BJU Int. 2008;101:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Nickel JC, Alexander RB, Anderson R, Berger R, Comiter CV, Datta NS, Fowler JE, Krieger JN, Landis JR, Litwin MS. Category III chronic prostatitis/chronic pelvic pain syndrome: insights from the National Institutes of Health Chronic Prostatitis Collaborative Research Network studies. Curr Urol Rep. 2008;9:320-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Pontari MA. Chronic prostatitis/chronic pelvic pain syndrome and interstitial cystitis: are they related? Curr Urol Rep. 2006;7:329-334. [PubMed] |
| 4. | Anothaisintawee T, Attia J, Nickel JC, Thammakraisorn S, Numthavaj P, McEvoy M, Thakkinstian A. Management of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and network meta-analysis. JAMA. 2011;305:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Pérez de Lema G, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, Schlöndorff D. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001;12:1369-1382. [PubMed] |
| 6. | Tyagi P, Killinger K, Tyagi V, Nirmal J, Chancellor M, Peters K. Urinary chemokines as Non-Invasive predictors of Ulcerative interstitial cystitis. J Urol. 2012;187:2. |
| 7. | Lagente V, Martin-Chouly C, Boichot E, Martins MA, Silva PM. Selective PDE4 inhibitors as potent anti-inflammatory drugs for the treatment of airway diseases. Mem Inst Oswaldo Cruz. 2005;100 Suppl 1:131-136. [PubMed] |
| 8. | Buenestado A, Grassin-Delyle S, Guitard F, Naline E, Faisy C, Israël-Biet D, Sage E, Bellamy JF, Tenor H, Devillier P. Roflumilast inhibits the release of chemokines and TNF-α from human lung macrophages stimulated with lipopolysaccharide. Br J Pharmacol. 2012;165:1877-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | McLennan GP, Khourdaji I, Killinger KA, Boura JA, Peters KM. Apremilast in the Treatment of Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A Pilot Study. Low Urin Tract Symptoms. 2012;4:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Miller LJ, Fischer KA, Goralnick SJ, Litt M, Burleson JA, Albertsen P, Kreutzer DL. Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology. 2002;59:603-608. [PubMed] |
| 11. | Watanabe T, Inoue M, Sasaki K, Araki M, Uehara S, Monden K, Saika T, Nasu Y, Kumon H, Chancellor MB. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU Int. 2011;108:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | John H, Barghorn A, Funke G, Sulser T, Hailemariam S, Hauri D, Joller-Jemelka H. Noninflammatory chronic pelvic pain syndrome: immunological study in blood, ejaculate and prostate tissue. Eur Urol. 2001;39:72-78. [PubMed] |
| 13. | Kabelitz D, Wesch D. Features and functions of gamma delta T lymphocytes: focus on chemokines and their receptors. Crit Rev Immunol. 2003;23:339-370. [PubMed] |
| 14. | Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2697] [Cited by in RCA: 2671] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 15. | Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1081] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 16. | Penna G, Mondaini N, Amuchastegui S, Degli Innocenti S, Carini M, Giubilei G, Fibbi B, Colli E, Maggi M, Adorini L. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51:524-533; discussion 533. [PubMed] |
| 17. | Thumbikat P, Shahrara S, Sobkoviak R, Done J, Pope RM, Schaeffer AJ. Prostate secretions from men with chronic pelvic pain syndrome inhibit proinflammatory mediators. J Urol. 2010;184:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Propert KJ, McNaughton-Collins M, Leiby BE, O’Leary MP, Kusek JW, Litwin MS. A prospective study of symptoms and quality of life in men with chronic prostatitis/chronic pelvic pain syndrome: the National Institutes of Health Chronic Prostatitis Cohort study. J Urol. 2006;175:619-623; discussion 623. [PubMed] |
| 19. | Motrich RD, Maccioni M, Riera CM, Rivero VE. Autoimmune prostatitis: state of the art. Scand J Immunol. 2007;66:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Antoniou KM, Tzanakis N, Tzortzaki EG, Malagari K, Koutsopoulos AV, Alexandrakis M, Wells AU, Siafakas NM. Different angiogenic CXC chemokine levels in bronchoalveolar lavage fluid after interferon gamma-1b therapy in idiopathic pulmonary fibrosis patients. Pulm Pharmacol Ther. 2008;21:840-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Luster AD, Cardiff RD, MacLean JA, Crowe K, Granstein RD. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Am Physicians. 1998;110:183-196. [PubMed] |
| 22. | Sadeghi M, Daniel V, Naujokat C, Weimer R, Opelz G. Strikingly higher interleukin (IL)-1alpha, IL-1beta and soluble interleukin-1 receptor antagonist (sIL-1RA) but similar IL-2, sIL-2R, IL-3, IL-4, IL-6, sIL-6R, IL-10, tumour necrosis factor (TNF)-alpha, transforming growth factor (TGF)-beta and interferon IFN-gamma urine levels in healthy females compared to healthy males: protection against urinary tract injury? Clin Exp Immunol. 2005;142:312-317. [PubMed] |
| 23. | Burke SM, Issekutz TB, Mohan K, Lee PW, Shmulevitz M, Marshall JS. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood. 2008;111:5467-5476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595-3602. [PubMed] |
| 25. | Nadler RB, Collins MM, Propert KJ, Mikolajczyk SD, Knauss JS, Landis JR, Fowler JE, Schaeffer AJ, Alexander RB. Prostate-specific antigen test in diagnostic evaluation of chronic prostatitis/chronic pelvic pain syndrome. Urology. 2006;67:337-342. [PubMed] |
| 26. | Ley K. Arrest chemokines. Microcirculation. 2003;10:289-295. [PubMed] |
| 27. | Zhang N, Rogers TJ, Caterina M, Oppenheim JJ. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. J Immunol. 2004;173:594-599. [PubMed] |
| 28. | Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007;3:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Krzystek-Korpacka M, Neubauer K, Matusiewicz M. Platelet-derived growth factor-BB reflects clinical, inflammatory and angiogenic disease activity and oxidative stress in inflammatory bowel disease. Clin Biochem. 2009;42:1602-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Barcelos LS, Coelho AM, Russo RC, Guabiraba R, Souza AL, Bruno-Lima G, Proudfoot AE, Andrade SP, Teixeira MM. Role of the chemokines CCL3/MIP-1 alpha and CCL5/RANTES in sponge-induced inflammatory angiogenesis in mice. Microvasc Res. 2009;78:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |