Published online Nov 24, 2014. doi: 10.5410/wjcu.v3.i3.376
Revised: June 11, 2014
Accepted: August 27, 2014
Published online: November 24, 2014
Processing time: 230 Days and 18.5 Hours
Phalloplasty is a complex set of procedures used in efforts to improve the anatomical, physiological, and aesthetic deficiencies caused by loss or absence of the penis. Methods have evolved significantly, and the use of free tissue transfer has become common amongst reconstructive surgeons. The inclusion of bone autograft, usually radius or fibula, within the neophallus has caused significant morbidity, and efforts continue to find the optimal solution. We present a novel approach using a pre-fabricated, radial forearm fasciocutaneous free flap containing cadaveric bone graft for phalloplasty following traumatic penis amputation.
Core tip: While there are many options for penile reconstruction, many techniques have associated morbidity when attempting to recreate rigidity in the neophallus by using bone autograft mostly from either the radius or fibula. In our case report, we use a novel approach to addressing rigidity in the neophallus without causing associated morbidity by using a pre-fabricated, radial forearm fasciocutaneous free flap containing cadaveric bone graft for reconstructive phalloplasty.
- Citation: Edens JW, Tran T, Eidelson S, Askari M, Salgado CJ. Pre-fabricated radial forearm phalloplasty with cadaveric bone graft. World J Clin Urol 2014; 3(3): 376-379
- URL: https://www.wjgnet.com/2219-2816/full/v3/i3/376.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v3.i3.376
Phalloplasty is a complex set of procedures that can pose significant anatomical, physiological, and aesthetic challenges, and the surgical techniques implemented have evolved significantly over the past sixty years. In the 1930s, Borgoras used bipedicled abdominal tube flaps to reconstruct the penis[1]. This method was later replaced by the use of fasciocutaneous and extended pedicle island flaps with continued suboptimal results[2,3]. In the 1980s, microsurgical advancements made the use of free flaps a better option, and the use of sensate, radial forearm fasciocutaneous or osteocutaneous flaps and fibular osteocutaneous flaps improved sensation and rigidity of the neophallus, giving a more desirable and functional outcome[4-7]. This method comes with significant donor site morbidity including extensive donor site scarring, bony fracture, and wrist and ankle instability[2,7]. Implant placement in the radial forearm free flap also has risks, including extrusion, costs, infection, and delayed placement (usually 1 year). We report a novel approach using cadaver bone graft to provide penile rigidity without sacrificing donor site morbidity in a patient undergoing phalloplasty following traumatic penis amputation.
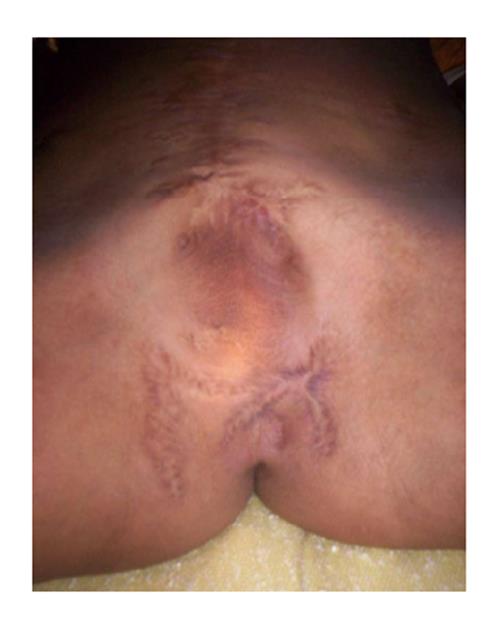
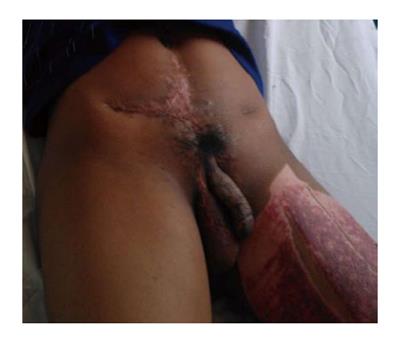
The patient is a 17-year-old male who sustained a gunshot wound to the lower abdomen and perineum at the age of nine. All penile substance and his left testicle were lost. He underwent emergent surgery with creation of a perineal urethrostomy. He has developed normally without other medical problems. The perineal urethrostomy allows him to urinate without incontinence. He has a viable right testicle within his scrotum (Figure 1). Through the work of a charitable organization, he was transferred to our institution for penile reconstruction. This is a retrospective case report and received an exempt status from our institutional review board.
The patient underwent general endotracheal anesthesia and was placed in low lithotomy position. His non-dominant left arm was chosen as the donor site for construction of the neophallus. An Allen’s test was performed on the left wrist which demonstrated adequate flow to the hand via the ulnar artery. Two operative teams were utilized in the operation.
Dissection in the abdomen proceeded through the previous abdominal scar with an extended right Pfannenstiel incision for harvest of the inferior epigastric artery and vein recipient vessels under loupe magnification. These vessels were traced to their origin, and the ilioinguinal nerve was dissected for microscopic nerve anastomosis. The remnant scrotal tissue was elevated to isolate the perineal urethra, and the pudendal nerve and genitofemoral nerve were identified for later nerve anastomosis.
A template was used to design the neophallus on the volar surface of the forearm creating a 5.5 inch long neophallus. Using a tourniquet, the radial forearm flap was elevated in a suprafascial fashion, extending radially to the flexor carpi radialis and ulnarly to the brachioradialis, with identification of the radial artery and its venae comitantes and their inclusion within the flap. The vascular pedicle was dissected to its origin at the brachial artery. The lateral and medial antebrachial cutaneous nerves were also included within the flap.
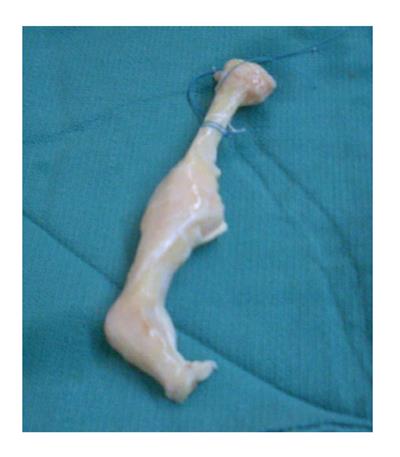
The neophallus was created by first establishing the urethral conduit along the ulnar side of the flap. This area was de-epithelialized for a width of 0.5 cm, giving a 26 mm diameter neourethra. The ulnar aspect of the flap was rolled over an 18-French Foley catheter and secured to the radial aspect of the flap with an inner layer of interrupted 3-0 absorbable suture and an outer layer of running, locking, 3-0 absorbable suture, creating a water-tight closure of the urethra. The remainder of the radial forearm flap was rolled to create a cylindrical penile shape. To give added infrastructure to the neophallus, cadaveric phalangeal and metacarpal bones were placed in its dorsal aspect. These bones were provided by the University of Miami Tissue Bank (Miami, Florida). The cadaveric bones were stripped of periosteum and soft tissue, trimmed into a more narrow shape, and drill holes were created within the substance of the bones to allow for vascular penetration (Figure 2). The bones were anchored to each other with suture, allowing for minimal movement between them. The bone construct was then placed into the flap, dorsal to the neourethra. The metacarpal bone was placed proximal to the proximal phalanx.
The neophallus was then transferred to the perineal area. The neourethra was sutured to the urethral remnant in the perineum in two layers with absorbable suture. The bone construct within the neophallus was secured to the pubic symphysis. Microvascular anastomosis was performed between the radial artery and right inferior epigastric artery, as well as between the radial artery venae comitantes and the inferior epigastric venae comitantes. Adequate flow was established through the vasculature, but due to the small size of the veins, the patient was given a Heparin bolus and started on a drip. Microscopic neurorrhaphy was performed with anastomosis between the lateral antebrachial cutaneous nerve to the ilioinguinal nerve, and between the medial antebrachial cutaneous nerve to the branch of the genitofemoral nerve and pudendal nerve.
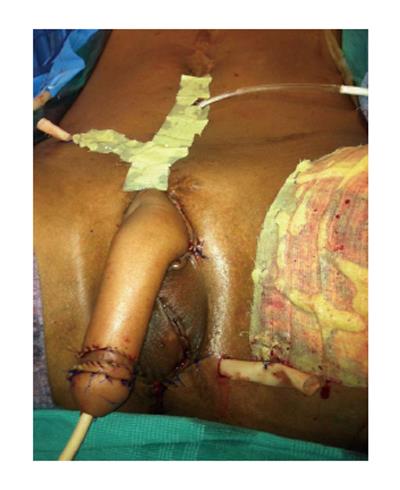
Drains were placed within the abdominal wall dissection and perineum. The perineum was closed with a scrotoplasty to ensure complete soft tissue coverage of the right testicle. A split-thickness skin graft was placed over the donor site on the left forearm. The glans penis was sculptured on the neophallus, using the Norfolk technique to increase its prominence. A skin graft was also placed to further create a transition zone from the shaft to the glans (Figure 3).
The patient was extubated and transferred to the Intensive Care Unit. On post-operative day 2, the patient was taken back to operative room for evacuation of a hematoma that had caused arterial insufficiency to the flap. The microvascular anastomosis was intact, with slight compromise of arterial inflow and venous outflow; however, upon evacuation of the hematoma, there was excellent vascular supply to the flap. The abdominal wall was loosely closed, with a portion of the skin left open due to tension. This area was covered with bovine collagen-dermal bilayer matrix.
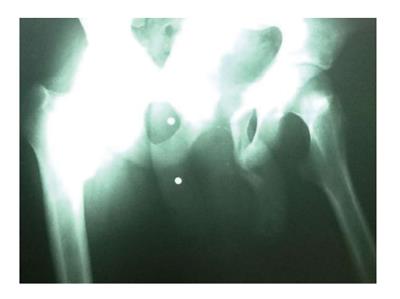
The patient was discharged from the hospital and to his native country on post-operative day 28, and he was satisfied with his overall result. The Foley catheter was removed at 6 wk. The patient developed a urethral-cutaneous fistula three months post-operatively at the penoscrotal junction, but he remained continent of urine. On radiograph, the bone allograft construct is viable without evidence of osteomyelitis (Figure 4). At 6 mo, the patient has a rigid and viable neophallus (Figure 5).
As the technical innovation of phalloplasty continues to evolve, from a fasciocutaneous flap to a radial forearm fasciocutaneous or osteocutaneous flap or fibula osteocutaneous flap to a sensate, prelaminated fibula flap[8], we propose a new approach using cadaveric bone graft reinforced by free radial forearm fasciocutaneous flap for penile reconstruction. We recognize that the radial forearm osteocutaneous flap has morbidity, including donor site functional and aesthetic deformities requiring skin grafting, risk of radius fracture, and aesthetic deformity from autologous bone harvesting[2]. Additionally, the radial osteocutaneous phallus has been reported to develop a “softened phallus” from cortical degeneration. Kim described 12 softened penises out of 58 radial forearm osteocutaneous phalloplasties[9]. Cavadas described a second stage reconstruction using a free fibular osseous flap for rigidity after a radial forearm fasciocutaneous flap[10].
Employing the development and application of over five million musculoskeletal allografts and considering the significant morbidity associated with harvesting of the radius bone autograft, we elected to use a metacarpal and phalangeal allograft from the University of Miami Tissue Bank. Allografts are chemically processed with low dose radiation to prevent the transmission of infection while maintaining biological integrity. Although there is a theoretical risk of disease transmission, it is rare. By using a metacarpal and phalangeal allograft, this eliminates the need for a second operation to provide rigidity with an implant and minimizes donor site morbidity. We were able to provide adequate rigidity and support to the neophallus, as well as place a spacer for a second stage procedure in the event the implant was unsuccessful. This was particularly useful in this humanitarian case, as commonly surgeons only have one opportunity to impact the patient’s care.
Penile prosthesis implantation has also been used in reconstructive phalloplasty, usually as a second stage to the procedure. This form of reconstruction has the disadvantage of significant cost and has not always been covered by insurances in the United States. There are also the added risks of extrusion, infection, and revision surgery[11]. This form of reconstruction was not available for this humanitarian case, as the patient was unable to make multiple trips to our institution for staged operations.
In our case, the patient has good overall success. He has regained his self-esteem as well as the physiological use of his neophallus, although he has not reported sexual intercourse. His complication of urethral fistula is consistent with the reported incidence of 63.6%[2]. This has been managed conservatively without further sequelae. The drawback of this report is that long term rigidity has yet to be determined, and long term photographs have not been able to be obtained due to patient limitations. In spite of this, the use of an allograft bone implant has many advantages, and further study of this technique is warranted to determine its inclusion in the ongoing evolution of phalloplasty.
A 17 year-old male with a traumatic penis amputation.
Absence of penis with the presence of a perineal urethrostomy.
Penile amputation.
Absence of penile substance.
The patient was treated with a pre-fabricated radial forearm phalloplasty with cadaveric bone graft.
Phalloplasty consists of a complex set of procedures, each with its own morbidity, and the issue of creating and maintaining rigidity in penile reconstruction is challenging.
Phalloplasty is the reconstruction or creation of the penis by surgical methods. Pre-fabrication refers to the method of placing a tissue or implant into another vascularized tissue bed and then transferring this construct into a body of tissue that is desired to be reconstructed.
This case report represents a unique method of penile reconstruction in which the authors create rigidity through the use of a cadaveric bone graft rather than utilizing a bone autograft that causes morbidity at the donor site.
This article demonstrates a novel surgical technique of a pre-fabricated radial forearm phalloplasty with cadaveric bone graft in reconstruction of the penis following a traumatic amputation. This type of operation is intended to limit the morbidity of using bone autograft, from either the radius or fibula, as well as complete the operation in one stage, as compared to the multiple stages used with placement of a penile prosthesis implantation.
P- Reviewer: Djordjevic ML, Garaffa G, Gontero P S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Hage JJ. From Peniplastica Totalis to Reassignment Surgery of the External Genitalia in Female-to-Male Transsexuals. Amsterdam: VU University Press 1992; 49-65. |
| 2. | Fang RH, Kao YS, Ma S, Lin JT. Phalloplasty in female-to-male transsexuals using free radial osteocutaneous flap: a series of 22 cases. Br J Plast Surg. 1999;52:217-222. [PubMed] |
| 3. | Perović S. Phalloplasty in children and adolescents using the extended pedicle island groin flap. J Urol. 1995;154:848-853. [PubMed] |
| 4. | Biemer E. Penile construction by the radial arm flap. Clin Plast Surg. 1988;15:425-430. [PubMed] |
| 5. | Koshima I, Tai T, Yamasaki M. One-stage reconstruction of the penis using an innervated radial forearm osteocutaneous flap. J Reconstr Microsurg. 1986;3:19-26. [PubMed] |
| 6. | Sadove RC, Sengezer M, McRoberts JW, Wells MD. One-stage total penile reconstruction with a free sensate osteocutaneous fibula flap. Plast Reconstr Surg. 1993;92:1314-1323; discussion 1324-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 116] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Byun JS, Cho BC, Baik BS. Results of one-stage penile reconstruction using an innervated radial osteocutaneous flap. J Reconstr Microsurg. 1994;10:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Papadopulos NA, Schaff J, Biemer E. The use of free prelaminated and sensate osteofasciocutaneous fibular flap in phalloplasty. Injury. 2008;39 Suppl 3:S62-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Kim SK, Kim TH, Yang JI, Kim MH, Kim MS, Lee KC. The etiology and treatment of the softened phallus after the radial forearm osteocutaneous free flap phalloplasty. Arch Plast Surg. 2012;39:390-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Cavadas PC. Secondary free fibular flap for providing rigidity in a radial forearm phalloplasty. Plast Reconstr Surg. 2008;122:101e-102e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Hoebeke PB, Decaestecker K, Beysens M, Opdenakker Y, Lumen N, Monstrey SM. Erectile implants in female-to-male transsexuals: our experience in 129 patients. Eur Urol. 2010;57:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |