Published online Nov 24, 2014. doi: 10.5410/wjcu.v3.i3.330
Revised: June 21, 2014
Accepted: July 25, 2014
Published online: November 24, 2014
Processing time: 175 Days and 22.8 Hours
Recently, clinical and epidemiologic data indicating the involvement of metabolic syndrome (MetS) in the pathogenesis and progression of lower urinary tract symptom (LUTS)/benign prostatic hyperplasia (BPH) are reported. This review evaluates the reports on the influence of MetS in the development and progression of LUTS/BPH, and discusses possible clinical implications for the management and treatment of this disease. Recent studies on the epidemiological relationship between MetS and LUTS hypothesize that MetS may be associated with an overactivity of the autonomic nervous system for which hyperinsulinemia, a key element of the MetS, might be responsible. An alternative explanation is that LUTS are associated with chronic ischemia of pelvis resulting from atherosclerotic changes in blood vessels, which leads the production of reactive oxygen species, which can damage the bladder detrusor. Control of autonomic nervous system overactivity and control of chronic bladder ischemia have potential as new targets for LUTS treatment. Studies suggest an association of MetS with LUTS/BPH, although further research is needed to understand how MetS influences LUTS/BPH. MetS should be considered a new domain in basic and clinical research in patients with LUTS/BPH and as a target for treatment.
Core tip: Associations between metabolic syndrome (MetS) and lower urinary tract symptom (LUTS) are noteworthy. MetS treatment may have the potential to prevent LUTS from worsening as well as to prevent the occurrence of new LUTS. Medication for LUTS patients with MetS should not only target the lower urinary tract, but should also be recognized as systemic treatment. Medications which are essentially thought to be unrelated to LUTS treatment may have potential for development as a specific medical treatment for LUTS with MetS.
- Citation: Ito H, Yokoyama O. Metabolic syndrome and lower urinary tract symptoms. World J Clin Urol 2014; 3(3): 330-335
- URL: https://www.wjgnet.com/2219-2816/full/v3/i3/330.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v3.i3.330
Lower urinary tract symptoms (LUTS) are a symptom complex which decreases quality of life in both the males and females. It is known that the number of LUTS patients increases in an age-dependent manner. It has been reported that bladder outlet obstruction (BOO), which occurs in patients with benign prostatic hyperplasia (BPH), is the main factor that triggers LUTS and efforts have focused on BOO. However, recent reports have shown that various etiologies are also associated with LUTS. Metabolic syndrome (MetS) is a cluster of several metabolic abnormalities or risk factors, including visceral obesity, dyslipidemia, hypertension, insulin resistance, and glucose intolerance[1]. Recently, it has been suggested that MetS may play an integral role in LUTS/BPH etiologies. In order to improve the quality of LUTS treatment, it will be useful to understand how MetS influences LUTS. The present review highlights current knowledge about the influence of MetS on LUTS/BPH and about the expected effect of MetS treatment on LUTS improvement. The application of this knowledge in clinical use should contribute to improvements in the treatment quality of LUTS patients with MetS.
Associations between LUTS/BPH and various components of MetS (obesity, hypertension, and fasting blood glucose) have been previously reported[2-4]. Moreover, cases of LUTS/BPH have also been positively associated with the number of MetS components[5].
It is known that hyperinsulinemia caused by insulin resistance, the core pathophysiology of MetS, may increase prostate smooth muscle tone[6], resulting in the increased prostate volume[7,8]. The insulinlike growth factor (IGF) pathway may contribute to the relation between insulin resistance and prostate enlargement. Insulin presents a structural similarity to IGF-1 and can bind its receptor, result in activating of a complex pathway influencing proliferation of prostatic cells. Another relevant study has demonstrated that the chronic inflammation induced by MetS may lead to the prostate enlargement[9,10]. It has been proven that insulin resistance and inflammation increase as the number of MetS components increases[11]. T-cell activity in prostate with inflammation infiltrates may result in increased stromal and epithelial cell proliferation that is sustained by an autoimmune mechanism. Repetitive tissue damage and the subsequent wound healing induced by inflammation may result in prostate enlargement[7].
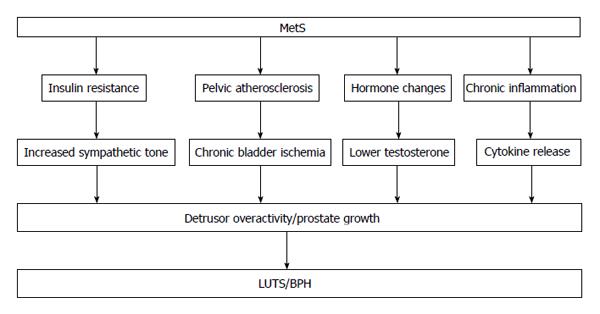
Alternative hypotheses include overactivity of the sympathetic nervous system by hyperinsulinemia, which may lead to more sever LUTS independent of prostate enlargement, and pelvic atherosclerosis causes chronic ischemia of the bladder, which may lead to functional impairments clinically manifested as LUTS (Figure 1).
Insulin resistance is thought to be the central underlying mechanism of MetS and is known to cause sympathetic overactivity[12]. Recent studies trying to show the epidemiological link between MetS and LUTS hypothesize that MetS is related with increased autonomic nervous system activity for which hyperinsulinemia, a key element of MetS, might be responsible[13,14]. Briefly, hyperinsulinemia that induces increased autonomic nervous system activity results in prostate enlargement and detrusor overactivity. McVary et al[14] revealed that LUTS are related with changes in blood pressure. The important role of α1-adrenoceptor (α1-AR) in the sympathetic nerve terminals of the prostate has been indicated. It has been also indicated that dynamic obstruction is mediated by stimulation of α1-AR. Previous studies indicate a relationship between overactivity of autonomic nervous system and LUTS. Spontaneously hypertensive rats (SHRs) have been reported to void more often than normotensive control rats[15], and have also been reported to have an overabundance of sympathetic fibers innervating the bladder. Urodynamic studies on SHRs have indicated spontaneous bladder contractions at low volumes, an effect that is largely ameliorated by α1-AR blocker and appears to be mediated by increased nerve factor. Thus, sympathetic overactivity has been suggested to have relation with bladder storage function. Autonomous nervous system overactivity because of MetS might contribute to detrusor overactivity. Hammarsten et al[16] represented an association between a more rapid enlargement of prostate and diseases related with sympathetic activation, including obesity, non-insulin-dependent diabetes mellitus and treated hypertension (HT). Furthermore, it has been proven that the over input of autonomic neural stimulation to the prostate may lead to the prostatic enlargement[17].
An association among smoking, vascular risk factors and LUTS has been reported[18]. Furthermore, previous study has shown decreased bladder compliance, reduced contractile force in the bladders and increased micturition frequency in pelvic ischemic model of rabbits[19]. Various researchers have shown the influence of chronic ischemia of the bladder detrusor resulting from atherosclerotic changes in blood vessels on LUTS; specifically atherosclerosis narrows the bladder vessels. Bladder filling in atherosclerotic models results in a significant decrease in blood flow of the bladder. Repeated ischemia and reperfusion produces reactive oxygen species, which damages the tissues[20,21]. Shimizu et al[22] found that an angiotensin receptor blocker (ARB), olmesartan, protects bladder function in a chronic bladder ischemic model by recovering blood flow and decreasing oxidative stress. Protective effects of coenzyme Q10[23] and melatonin[24] on bladder hyperactivity in a chronic bladder ischemia model due to their action as antioxidants have been indicated. Treatment targeting the bladder blood flow and protection from oxidative stress caused by chronic ischemia can be a new strategy for LUTS treatment.
Medical treatment of voiding and/or storage symptoms is now the initial choice of therapy and α1-AR blockers remain the most widely used pharmacological agents aimed at the dynamic component of prostatic obstruction[25]. α1-ARs predominate in prostatic stroma at the mRNA and protein levels, and it is reasonable to think that they may play a role in combatting the dynamic component of obstruction[26]. Increased activity of the autonomic nervous system may result in an acceleration of the sympathetic tone of the lower urinary tract, lead to C-fibers stimulation, which in turn causes storage symptoms. By bringing these findings together, it is suggested that α1-AR-blocker exerts an suppressive effect on C-fiber urethral afferent nerves by inhibiting the muscle tone of prostate activated by HT, which may lead to improve storage symptoms. We previously showed that the effect of tamsulosin on storage symptoms was more prominent in patients with HT than in patients without it[27]. An α1-ARs subtype analysis showed that the expression levels of α1-AR mRNAs in the detrusor were equally at low densities[26], indicating that α1-AR blockers have little effect on contractility of detrusor, which in turn may have little effect on voiding symptoms. Although α1-AR-blockers improve functional obstruction in BPH, detrusor contractility may not be improved in elderly patients.
There is no special treatment for LUTS patients with MetS at the present time. Strict control of MetS may be needed.
Increased physical activity have been associated with a decreased risk of LUTS[28], although the mechanism of this effect is not completely understood. MetS patients may receive medications for HT, hyperlipidemia and diabetes. These medications may possibly improve LUTS as well as control MetS. As the populations of LUTS and HT both increase with aging, a great number of LUTS patients receive medications for HT. Ang-II is a potent vasoconstrictor and plays an important role in the systemic renin-angiotensin system (RAS), which controls blood pressure by regulation of vascular tone and sodium homeostasis. Medications which inhibit the RAS, such as ARBs, are mainstays in HT treatment. In addition to its systemic effects, Ang-II may affect cellular proliferation and function in several organ systems, including bladder and prostate, through local RAS. It has been well established that Ang-II receptors exists in the bladder wall and Ang-II on the detrusor muscle has contractile effects[29,30]. Because Ang-II is the principal mediator of the RAS and strongly stimulates cell growth[31], researchers have also reported its possible role as a mediator in collagen production and smooth muscle growth in BOO[32,33]. Previous studies report that Ang-II induces bladder muscle contractions[29,34]. Ang-II in the bladder is known to regulate smooth muscle growth and connective tissue production[35], and has been partly associated with the pathogenesis of urinary dysfunction with subsequent outlet obstruction[36] and stress urinary incontinence in rats[37].
It has been proven that morphological changes are remarkable in the bladder smooth muscle cells, connective tissues, and innervation in the detrusor muscles in patients with chronic BOO[38]. It has been shown that repeated captopril administration, the angiotensin-converting enzyme inhibitor, in neonatal BOO rabbits significantly reduced the expression level in total DNA and decreased the amount of total collagen in the bladder[39]. It may hypothesized that these pathological variations in the BOO bladder may be caused by the activation of Ang-II-mediated changes. It has been indicated that urethral resistance in a rat model of stress incontinence may be reduced by the blockade of Ang-II receptors[37]. Taken together, it can be proposed that the development of BOO results in a significant change of Ang-II receptors in the lower urinary tract. It has also been indicated that the Ang-II antagonist telmisartan suppresses both the suppression of Ang-II receptors in the bladder and the bladder hypertrophy in BOO rats[36]. Consequently, telmisartan is effective in attenuating the increase in bladder weight in BOO. We previously showed that ARB administration in hypertensive patients improves LUTS[40]. One possible mechanism indicating the improvement of storage symptoms by ARB administration may be the effect of stabilization of the smooth muscle activity by down regulation of the local RAS in the bladder. It is also discussed that Ang-II may contribute in the prostate. It has been reported that RAS has been overactivated in BPH[41]. The elevated local levels of Ang-II in the prostate in BPH patients has also been reported[42]. Ang-II may have effect on cell proliferation and smooth muscle tone in the prostate via Ang-II receptors. Nevertheless, the functional role of elevated Ang-II in the prostate is not clearly understood. It is expected that ARB may improve LUTS by down regulating noradrenaline release in the prostate. Recent reports indicated that the effect of apoptosis induced by ARB in prostatic tissue of SHR[43]. ARB may improve voiding symptoms by minimizing the prostatic microenvironment, result in lowering urethral resistance. As ARB can also improve blood flow, as mentioned above, the possibility exists that ARB may improve LUTS.
The role of MetS in the occurrence of LUTS remains controversial. Some reports, in particular in Asian populations, found no association between MetS and LUTS[44-46]. Moreover, a report from Korea showed favorable effects of MetS on LUTS[47], that is, the opposite result. The differences in these results could stem from several factors. First, the ages of the study participants are different among studies. Second, the criteria used to define the presence of MetS or LUTS vary among studies. And third, the prevalence of MetS and BPH are lower in Asian populations.
It is important to emphasize that most studies indicating the possible association between MetS and LUTS are derived from observational studies, which are often limited to a specific area or population. Future clinical trials are expected.
Associations between MetS and LUTS are noteworthy. MetS treatment may have the potential to prevent LUTS from worsening as well as to prevent the occurrence of new LUTS. Medication for LUTS patients with MetS should not only target the lower urinary tract, but should also be recognized as systemic treatment. Statin and ARB, which are essentially thought to be unrelated to LUTS treatment, may have potential for development as a specific medical treatment for LUTS with MetS.
P- Reviewer: Das UN, Faienza MF S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3852] [Cited by in RCA: 4235] [Article Influence: 222.9] [Reference Citation Analysis (0)] |
| 2. | Rohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Int J Obes (Lond). 2005;29:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Kupelian V, McVary KT, Kaplan SA, Hall SA, Link CL, Aiyer LP, Mollon P, Tamimi N, Rosen RC, McKinlay JB. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston area community health survey. J Urol. 2013;189:S107-S114; discussion S115-S116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Lee RK, Chung D, Chughtai B, Te AE, Kaplan SA. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int. 2012;110:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Park YW, Kim SB, Kwon H, Kang HC, Cho K, Lee KI, Kim YJ, Lee JH. The relationship between lower urinary tract symptoms/benign prostatic hyperplasia and the number of components of metabolic syndrome. Urology. 2013;82:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Sarma AV, Parsons JK, McVary K, Wei JT. Diabetes and benign prostatic hyperplasia/lower urinary tract symptoms--what do we know? J Urol. 2009;182:S32-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schröder F, Sciarra A, Tubaro A. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011;60:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 318] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 8. | Zhang X, Zeng X, Liu Y, Dong L, Zhao X, Qu X. Impact of Metabolic Syndrome on Benign Prostatic Hyperplasia in Elderly Chinese Men. Urol Int. 2014;93:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Alcaraz A, Hammerer P, Tubaro A, Schröder FH, Castro R. Is there evidence of a relationship between benign prostatic hyperplasia and prostate cancer? Findings of a literature review. Eur Urol. 2009;55:864-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Gacci M, Vignozzi L, Sebastianelli A, Salvi M, Giannessi C, De Nunzio C, Tubaro A, Corona G, Rastrelli G, Santi R. Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate Cancer Prostatic Dis. 2013;16:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Belfki H, Ben Ali S, Bougatef S, Ben Ahmed D, Haddad N, Jmal A, Abdennebi M, Ben Romdhane H. Relationship of C-reactive protein with components of the metabolic syndrome in a Tunisian population. Eur J Intern Med. 2012;23:e5-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Landsberg L. Role of the sympathetic adrenal system in the pathogenesis of the insulin resistance syndrome. Ann N Y Acad Sci. 1999;892:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83:1853-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 208] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | McVary KT, Rademaker A, Lloyd GL, Gann P. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2005;174:1327-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Steers WD, Clemow DB, Persson K, Sherer TB, Andersson KE, Tuttle JB. The spontaneously hypertensive rat: insight into the pathogenesis of irritative symptoms in benign prostatic hyperplasia and young anxious males. Exp Physiol. 1999;84:137-147. [PubMed] |
| 16. | Hammarsten J, Högstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001;39:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 222] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Golomb E, Rosenzweig N, Eilam R, Abramovici A. Spontaneous hyperplasia of the ventral lobe of the prostate in aging genetically hypertensive rats. J Androl. 2000;21:58-64. [PubMed] |
| 18. | Koskimäki J, Hakama M, Huhtala H, Tammela TL. Association of smoking with lower urinary tract symptoms. J Urol. 1998;159:1580-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Gill HS, Monson FC, Wein AJ, Ruggieri MR, Levin RM. The effects of short-term in-vivo ischemia on the contractile function of the rabbit urinary bladder. J Urol. 1988;139:1350-1354. [PubMed] |
| 20. | Nomiya M, Sagawa K, Yazaki J, Takahashi N, Kushida N, Haga N, Aikawa K, Matsui T, Oka M, Fukui T. Increased bladder activity is associated with elevated oxidative stress markers and proinflammatory cytokines in a rat model of atherosclerosis-induced chronic bladder ischemia. Neurourol Urodyn. 2012;31:185-189. [RCA] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Erdem E, Leggett R, Dicks B, Kogan BA, Levin RM. Effect of bladder ischaemia/reperfusion on superoxide dismutase activity and contraction. BJU Int. 2005;96:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Shimizu S, Saito M, Oiwa H, Ohmasa F, Tsounapi P, Oikawa R, Dimitriadis F, Martin DT, Satoh I, Kinoshita Y. Olmesartan ameliorates urinary dysfunction in the spontaneously hypertensive rat via recovering bladder blood flow and decreasing oxidative stress. Neurourol Urodyn. 2014;33:350-357. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Kim JW, Jang HA, Bae JH, Lee JG. Effects of coenzyme Q10 on bladder dysfunction induced by chronic bladder ischemia in a rat model. J Urol. 2013;189:2371-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Nomiya M, Burmeister DM, Sawada N, Campeau L, Zarifpour M, Yamaguchi O, Andersson KE. Effect of melatonin on chronic bladder-ischaemia-associated changes in rat bladder function. BJU Int. 2013;112:E221-E230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Stoevelaar HJ, Van de Beek C, Casparie AF, McDonnell J, Nijs HG. Treatment choice for benign prostatic hyperplasia: a matter of urologist preference? J Urol. 1999;161:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Sigala S, Peroni A, Mirabella G, Fornari S, Palazzolo F, Pezzotti G, Simeone C, Cunico SC, Spano P. Alpha1 adrenoceptor subtypes in human urinary bladder: sex and regional comparison. Life Sci. 2004;76:417-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Ito H, Yoshiyasu T, Yamaguchi O, Yokoyama O. Male LUTS: hypertension as a risk factor for storage symptoms, but not voiding symptoms. LUTS. 2012;4:68-72. |
| 28. | Platz EA, Kawachi I, Rimm EB, Colditz GA, Stampfer MJ, Willett WC, Giovannucci E. Physical activity and benign prostatic hyperplasia. Arch Intern Med. 1998;158:2349-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Tanabe N, Ueno A, Tsujimoto G. Angiotensin II receptors in the rat urinary bladder smooth muscle: type 1 subtype receptors mediate contractile responses. J Urol. 1993;150:1056-1059. [PubMed] |
| 30. | Lam DS, Dias LS, Moore KH, Burcher E. Angiotensin II in child urinary bladder: functional and autoradiographic studies. BJU Int. 2000;86:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am J Cardiol. 2002;89:3A-9A; discussion 10A. [PubMed] |
| 32. | Cheng EY, Grammatopoulos T, Lee C, Sensibar J, Decker R, Kaplan WE, Maizels M, Firlit CF. Angiotensin II and basic fibroblast growth factor induce neonatal bladder stromal cell mitogenesis. J Urol. 1996;156:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 33. | Kato H, Suzuki H, Tajima S, Ogata Y, Tominaga T, Sato A, Saruta T. Angiotensin II stimulates collagen synthesis in cultured vascular smooth muscle cells. J Hypertens. 1991;9:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Saito M, Kondo A, Kato T, Miyake K. Response of the human urinary bladder to angiotensins: a comparison between neurogenic and control bladders. J Urol. 1993;149:408-411. [PubMed] |
| 35. | Cheng EY, Decker RS, Lee C. Role of angiotensin II in bladder smooth muscle growth and function. Adv Exp Med Biol. 1999;462:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Yamada S, Takeuchi C, Oyunzul L, Ito Y. Bladder angiotensin-II receptors: characterization and alteration in bladder outlet obstruction. Eur Urol. 2009;55:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Phull H, Salkini M, Escobar C, Purves T, Comiter CV. The role of angiotensin II in stress urinary incontinence: A rat model. Neurourol Urodyn. 2007;26:81-88; discussion 89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Gosling JA, Gilpin SA, Dixon JS, Gilpin CJ. Decrease in the autonomic innervation of human detrusor muscle in outflow obstruction. J Urol. 1986;136:501-504. [PubMed] |
| 39. | Cheng EY, Lee C, Decker RS, Sensibar JA, Lang S, Kaplan WE, Maizels M, Firlit CF. Captopril (an inhibitor of angiotensin converting enzyme) inhibits obstructive changes in the neonatal rabbit bladder. Urology. 1997;50:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Ito H, Taga M, Tsuchiyama K, Akino H, Yokoyama O. IPSS is lower in hypertensive patients treated with angiotensin-II receptor blocker: posthoc analyses of a lower urinary tract symptoms population. Neurourol Urodyn. 2013;32:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Dinh DT, Frauman AG, Sourial M, Casley DJ, Johnston CI, Fabiani ME. Identification, distribution, and expression of angiotensin II receptors in the normal human prostate and benign prostatic hyperplasia. Endocrinology. 2001;142:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Dinh DT, Frauman AG, Somers GR, Ohishi M, Zhou J, Casley DJ, Johnston CI, Fabiani ME. Evidence for activation of the renin-angiotensin system in the human prostate: increased angiotensin II and reduced AT(1) receptor expression in benign prostatic hyperplasia. J Pathol. 2002;196:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Yu W, Zhao YY, Zhang ZW, Guo YL, Jin J. Angiotension II receptor 1 blocker modifies the expression of apoptosis-related proteins and transforming growth factor-beta1 in prostate tissue of spontaneously hypertensive rats. BJU Int. 2007;100:1161-1165. [PubMed] |
| 44. | Yang TK, Hsieh JT, Chen SC, Chang HC, Yang HJ, Huang KH. Metabolic syndrome associated with reduced lower urinary tract symptoms in middle-aged men receiving health checkup. Urology. 2012;80:1093-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Ohgaki K, Hikima N, Horiuchi K, Kondo Y. Association between metabolic syndrome and male lower urinary tract symptoms in Japanese subjects using three sets of criteria for metabolic syndrome and International Prostate Symptom Score. Urology. 2011;77:1432-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Gao Y, Wang M, Zhang H, Tan A, Yang X, Qin X, Hu Y, Zhang Y, Liao M, Mo Z. Are metabolic syndrome and its components associated with lower urinary tract symptoms? Results from a Chinese male population survey. Urology. 2012;79:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Eom CS, Park JH, Cho BL, Choi HC, Oh MJ, Kwon HT. Metabolic syndrome and accompanying hyperinsulinemia have favorable effects on lower urinary tract symptoms in a generally healthy screened population. J Urol. 2011;186:175-179. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |