Published online Nov 24, 2014. doi: 10.5410/wjcu.v3.i3.310
Revised: July 3, 2014
Accepted: September 6, 2014
Published online: November 24, 2014
Processing time: 210 Days and 12 Hours
Secondary lymphedema of male external genital organs, characterized by increase in genital organs volume, tissue fibrosis, erysipelas, and objective difficulties in the normal use of lower limbs and the penis, is a very common and impairing consequence of invasive surgery, radical lymphadenectomy and radiotherapy of the pelvic-inguinal area. Standard surgical approach to lymphedema are either very invasive and/or at high risk of lymphedema recurrence and do not guarantee an efficient long-term treatment. Alternatively, we developed a microsurgical technique to perform direct anastomoses between the lymphatic collectors of the spermatic funiculum afferent to the external iliac chains and the vessels tributary to the spermatic vein. This innovative approach, although surgically demanding, provided a long term successful treatment of external genitals with no clinical complications, low invasivity, rapid post-surgical recovery, minor tissue demolition and satisfactory post-surgical functional and esthetic results. In addition, lympho-venous microsurgery seems to trigger the local development of new lymphatic vessels that not only canalize along new collecting channels, but also form complex meshes in proximity to the anastomosis area, thus improving lymphedema also in adjacent tissues like lower limbs, supplied by lymphatics emptying into common developed lymphatic shunt.
Core tip: Treatment of secondary lymphedema of male external genital organs through invasive standard surgical techniques may be complicated by impairing consequences for the patient and often do not guarantee an efficient long-term outcome. Alternatively, microsurgical suture of lympho-venous anastomoses between lymphatic collectors of the spermatic funiculum efferent to the external iliac chains and the pampiniform plexus tributary to the spermatic vein provides a successful long term treatment of genitals lymphedema and triggers the development of new lymphatic meshes in proximity to the anastomosis area, thus improving lymphedema also in tissues, like lower limbs, supplied by lymphatics emptying into common developed shunts.
- Citation: Mukenge SM, Negrini D, Catena M, Ferla G. Innovative microsurgical treatment of male external genitals lymphedema. World J Clin Urol 2014; 3(3): 310-319
- URL: https://www.wjgnet.com/2219-2816/full/v3/i3/310.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v3.i3.310
The lymphatic system is a closed vascular network, whose smallest vessels (initial lymphatics) originate as a dead-end tubes directly from the extracellular tissue space[1] to subsequently confluence in progressive larger collecting lymphatics that paralleling main artery and veins, propel the lymph unidirectionally. At variance with what observed in initial lymphatics, the wall of collecting lymphatics is supplied with one or more layers of smooth muscle cells arranged in the media externally to the endothelial cells layer and interspersed with collagen and elastic fibres. Fluid flux along the lymphatic conduits is maintained unidirectional by the presence of primary valves in the wall of initial lymphatics[2,3] and secondary or intraluminar valves all along the lymphatic network. Intraluminar valves are formed by two endothelial leaflet attached at opposite sites to the lymph channel wall, connected with zonulae and sustained by a basement membrane composed of collagen, fibrillar material and scanty fibroblasts[4]. Two consecutive valves, arranged at a distance ranging between approximately 1 μm and approximately 15-20 μm delimit the “lymphangion”[1] a sort of primitive heart often considered the “functional unit” of the lymphatic vascular system.
In its flow along the main lymphatic ducts, the lymph is conveyed through secondary lymphoid organs, the lymph nodes. The lymph enters the node through afferent lymphatic vessels, percolates through the node’s inner structure dense of lymphocytes and emerge through efferent vessels to be eventually returned into the venous blood stream.
The upper and lower extremities are drained by a superficial and a deep lymphatic network, both equipped with intraluminar valves and smooth muscle cells. Lymph from the skin and superficial connective tissues are carried by superficial ducts, while the muscles and the periosteum drain into the deeper network which accompanies the main vascular fascia. The superficial and deep networks connect only at the level of the popliteal nodes, the adductor canal of the lower leg and the supratrocheal node of the arm. Collecting ducts from the upper limbs drain into the axillary lymph nodes while those of the lower extremities drain into inguinal lymph nodes. The latter also receive lymph from the gluteal area, the uterus, the anterior abdominal wall, the external genital organs and the lower anal canal.
Lymphatic drainage of the external male genitals occurs through two main pathways. A superficial diffuse lymphatic mesh which supplies the scrotal tissues, empties into collecting ducts running along the scrotal raphe and reaches the inguinal lymph nodal chains. A deeper lymphatic network which supplies the gonads is basically organized in two plexuses: (1) one from the testis parenchyma which run proximally along the testicular artery and discharges into the para- and pre-aortic lymph nodal stations; and (2) the other, from the vaginal tunica, which runs in the spermatic funiculum and empties into the external iliac lymphnodes.
The lymphatic vessels efferent from the external, internal iliac and obturator nodes merge into the common iliac lymphatic duct and travel through the corresponding lymph nodes which also receive lymph from the perineum, the buttock, thigh and the pelvic viscera. Efferent lymphatics from iliac nodes drain into para-aortic lymph nodes plexus; the latter, which also receive from all abdominal organs, discharge into the cysterna chyli, a dilated saccular structure located along the abdominal aorta. The larger and main lymphatic collector, the thoracic duct, departs from the cysterna chyli, runs dorsally to the aorta and eventually empties into the venous stream at the junction between the left internal jugular and subclavian veins. The right lymphatic duct collects instead the lymph from the right arm, the right and most of the left lung, the heart, the right side of head, neck and the right anterior chest to empty into the right brachiocephalic vein at the confluence of the right internal jugular and subclavian veins.
The connection between the large collecting ducts and the venous system is usually controlled by unidirectional valves that prevent backflow of blood into the lymphatic vessel. Additional communications between smaller collecting lymphatic and veins may be found along the iliac, renal, subclavian, azygos, and portal veins. However, functional study have revealed that these lymphatico-venous communications are not recruited under normal conditions and may become functional only in pathological conditions[5,6]. No direct lympho-venous anastomosis have instead been detected in lymph nodes.
Because of its peculiar anatomical arrangement the lymphatic system fulfils several important functions in the body: (1) by removing fluid from the interstitial tissue and returning it to the blood stream, it maintains the normal tissue hydration and plasma volume; (2) it returns proteins and high molecular weight soluble molecules from the interstitial tissue to the blood; (3) it returns leukocytes, cell debris and tumoral cells to the blood stream; and (4) the dissemination of lymph nodes along the lymphatic network provides an important contribution to host immune defence by presenting antigens and antigen-presenting cells to the B and T lymphocytes in the lymph node hylum.
Transport of fluid and solute from the interstitial space to the lymphatic lumen occurs in initial lymphatics by bulk flow through the highly permeable interendothelial junctions[7] that allow free passage of fluid, interstitial hydrophilic molecules of any size, cells, viruses and bacteria. Since the initial lymphatic endothelium offers no effective sieving to large macromolecules, the protein concentration and the colloid osmotic pressure of lymph in initial lymphatics equals that of the surrounding interstitium.
Therefore, the requirement necessary for the formation of the lymph is that the hydraulic pressure in the interstitium be higher than that existing in the initial lymphatics, while propulsion of the lymph along the lymphatic conduits is sustained by intraluminar pressure gradients developing between adjacent lymphangions. The maintenance of the pressure gradients which sustain lymph formation and propulsion is supported by both local tissue movements such as cardiogenic oscillations, heart activity[8] and respiratory muscle contraction[9], as well as by the action of synchronous contraction of the smooth muscle wall of the collecting lymphatic vessels[1]. Through these mechanisms, lymph flow may increase up to 20-30 folds its physiological value, to counteract an increased interstitial fluid volume depending, for example, upon an increased fluid filtration across the blood microvasculature wall. Such a modulation allows an efficient control of tissue fluid volume only until attainment of a saturation threshold, beyond which the filtration rate through the wall of blood capillaries overwhelms the maximal flow capability of the normally performing lymphatic network, leading to a progressive development of tissue edema. Expansion of tissue fluid is instead defined lymphedema when it depends not only from an increased capillary filtration rate, but rather to an absent or greatly impaired lymphatic drainage. Depending on the underlying cause lymphedema can be distinguished in: (1) primary lymphedema, caused by absence or deficient development of the lymphatic vasculature and/or lymph nodes. In its more severe forms, the primary lymphedema appears at birth (congenital primary lymphedema), and is accompanied by functional impairment of most organs, peritoneal and pleural effusions, pulmonary edema, etc. Congenital lymphedema is likely due to genetic faults or abnormalities in the formation of lymph nodes and/or lymphatic vessels due to a defect of the lymphangiogenic vascular endothelial growth factor receptor (VEGFR-3)-gene or of the FOXC-2 (fork head transcription factor for the formation of collecting vessels) gene, both involved in the development and control of lymphangiogenesis. The primary idiopathic lymphedema occurs instead in adolescence or within the 35th year of age and typically affects the upper and lower limbs and/or the external genitalia[10]. The causes of this pathology are still unclear although phenomena like abnormalities of lymph nodes and/or lymphatic vessels, hypoplasia of the lymphatic structures or extracellular matrix degradation might all interplay to determine the primary lymphatic drainage impairment. Due to its complexity, congenital and idiopatic lymphedema represents a challenging, often unsolvable clinical and, more so, surgical problem which, at the moment, goes beyond the focus of the present Review.
Instead, we will focus on a recent application of microsurgery in the treatment of secondary lymphedema, a more localized lymphatic insufficiency caused by excision, lesion or radiation of the lymphatic vasculature and of the lymph nodes secondary, as an example, to major surgery and/or oncological therapies. Impairment of the lymphatic fluid and solute drainage, such as that observed in secondary lymphedema, causes uncontrolled increase of tissue fluid volume, as well changes in tissue matrix composition, thus deeply compromising tissue function.
Secondary lymphedema of the external genital organs is a very common consequence of invasive surgery of the pelvic[11] and inguinal area[12] with associated radical lymphadenectomy and radiotherapy[13]. In particular, lymphedema of the male genital organs and of the hypogastric area is a very impairing condition characterized by an important increase in the volume of the external genital organs, tissue fibrosis, erysipelas, and objective difficulties in the normal use of lower limbs and the penis. Hence, although pelvic lymph node dissection (PLND) is an essential staging approach to cancer patients, the secondary lymphedema that can develop as a complication of this procedure is very impairing to the patient’s normal quality of life, even from a psychological standpoint.
In the upper and lower limbs, the first treatment of secondary lymphedema consists in local physical therapy, massage, bandage and external compression, which improves the lymphatic drainage by slowly reducing tissue fluid imbibition, increasing local tissue pressure and/or muscular tone and, eventually, shunts development. However, when the lymphatic drainage pathways have been excised and/or interrupted by fibrous scars developed after surgery and/or radiotherapy, medical treatment of lymphedema very seldom provides satisfactory results[14]. This is particularly true in patients affected by secondary lymphedema of the external male and female genital organs in which effective physical therapy cannot be completely performed and that can therefore be treated only by surgery. Various surgical approaches has been utilized: (1) lymphangiectomy associated to adipectomy, the excision of involved subcutaneous tissue, remains the most common approach to the treatment of chronic male external genital organs lymphedema[15]; and (2) more recently, lymphangenctomy/adipectomy has been improved by a plastic surgery procedure consisting in resection of scrotal and penile lymphedematous tissue, reconstruction with a posterior fascio-cutaneous flap from the scrotum itself and penile reconstruction with a skin graft[16,17].
These classical surgical approaches are usually very invasive and imply important demolition of a large part of the scrotal and penile tissues with high risk of lymphedema recurrence. Therefore, in the last decade, with the development of more sophisticated microsurgical techniques and aiming at favoring new lymphangiogenesis in the lymphedematous tissues, the homologous lymph nodes contained in vascularized tissue flap excised from the inguinal, crural or axillary districts of the same patient have been transplanted in lymphedematous external male genitals or limbs[18]. An alternative microsurgical derivative procedure utilizes only the subcutaneous scrotal lymphatic vessels, ignoring the alternative draining pathways[19]. In spite of the great technical improvement offered by these pioneering microsurgical approaches, they do not seem yet to offer enough guarantees to ensure an efficient long-term treatment of the extended, severe, secondary lymphedema of the external genitals organs.
The development of microsurgical techniques has allowed a minimally invasive approach to the treatment of secondary lymphedema of lower or upper extremities and more rarely of other body districts. The present Review describes the application of a new microsurgical lymphovenous derivation technique for the treatment of male external genital organs secondary lymphedema. Indeed, following a careful autoptic observation of the lymph node-chain tributaries of the collecting lymphatic ducts draining the testis during previous external iliac-chain lymphadenectomy, we developed a technique that allowed us to perform direct anastomosis between the lymphatic collectors running within the spermatic cord afferent to the external iliac chains and the vessels tributary to the spermatic veins[14,20]. The idea of developing new drainage pathways of testicular lymphatic collectors in spermatic cord originated from the consideration that excision of lymph node-chain tributaries of lymphatic ducts draining the testis could determine lymphedema of external genitals as a consequence of the insufficient drainage through the superficial external genitals lymphatics.
On the other hand, locoregional PLND with excision of the pelvic fibrous-fatty tissue using as margins the external iliac vein and the posterolateral aspect of obturator fossa, is required in prostate cancer to attain an accurate diagnosis of occult micrometastases and to stratify patients who might benefit from adjuvant therapeutic measures.
From January 2006 to November 2013, 18 patients suffering from lymphedema of male external genitals or male genital and/ or lower limbs lymphedema were screened for lymphedema treatment. Out of these patients, 10 were candidate to microsurgery according to the following inclusion criteria: (1) patients from 25 to 75 years old; (2) at least 3rd degree lymphedema, with no satisfactory and/or durable results after physical and/or pharmacological therapy; (3) free of tumor for at least two years; (4) at least one year since the last post-surgical adjuvant oncological treatment; and (5) less than 15 years since the primary oncological surgery lymphedema development. Patients with co-morbidity as cardiopathy, respiratory diseases, renal failure or severe bilateral lower lymphedema were oriented towards medical and physical therapy.
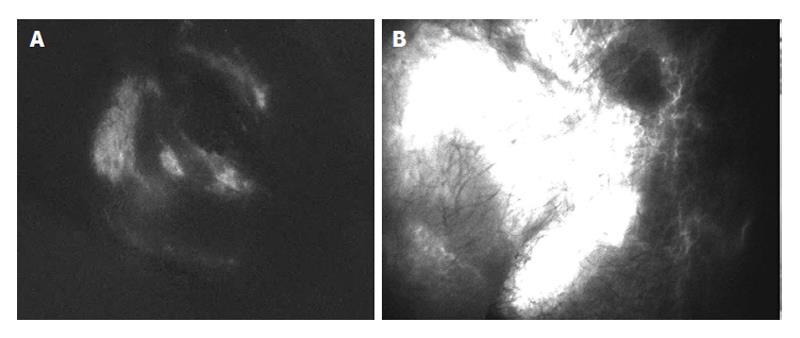
The pre-operative screening included: (1) lower abdomen and genitals echography, lymphoscintigraphy of lower limbs and inguinal area. Indeed, since there are currently no studies documenting the use of lymphoscintigraphy for the identification of genital organs pathologies, echography was the only diagnostic instrument available for this purpose. Subsequently, the green indocyanine lymphography, Photodynamic eye (PDE, Hamamatsu Photonic K.K., Tokyo, Japan) imaging[21] was utilized to investigate the patency of the genitalia lymphatic network (Figure 1). Briefly, 0.2 to 1 mL green indocyanine were injected bilaterally at the base of the scrotum through an insulin needle. After few minutes of massage, the fluorescent dye in subcutaneous lymphatic vessels at a maximum depth of 2-3 cm were visualized through a portable near-infrared camera system. Images were conveyed on a PC monitor and stored. The Echo-color doppler of lower limbs was also performed to exclude vascular disease such as deep veins thrombosis.
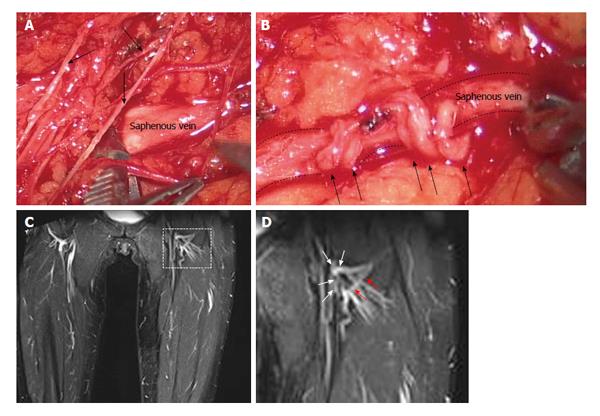
In the first two candidate patients, lymphedema affected both the external genitals and the lower limb: therefore, a preliminary lympho-venous shunt of lower limb was performed followed, after six months, by the planned lympho-venous shunt of spermatic cord. Under general anaesthesia, bilateral funiculum lymphatic collectors (diameter approximately 500 μm) were visualized under stereomicroscopic magnification (40 ×) after cutaneous inguino-scrotal, cremaster and vaginal tunica incision. Identified lymphatic collectors were used to perform (with interrupted stitches, Vicryl 10-0 or 9-0) the lympho-venous termino-lateral or termino-terminal anastomosis, depending upon the proximity and the pathway followed by either the veins of the pampiniforms plexus or the spermatic vein previously prepared through adventixectomy. The number of anastomosis (up to four) depended upon the number of lymphatic collectors found, the higher the anastomosis number the higher the chances of a more effective drainage.
The microsurgical procedure lasted between five and seven hours, depending upon the extent of tissue fibrosis and surgery complexity: indeed, the microsurgical approach is highly demanding in terms of intra-operative technical skill, surgical and laboratory training and on-site imaging equipment. On the other hand, the described microsurgical effort is worth, as it offers several major advantages with respect to the standard approach, such as: (1) absence of clinical complications, (2) low invasivity and more rapid post-surgical recovery; and (3) minor tissue demolition and more satisfactory post-surgical functional and esthetic results.
Since low-weight molecular heparin has been used during the surgery, incidence of thrombosis into the lymphatic channels or in the anastomosis was minimized. Antibiotic was maintained for seven days, while heparin therapy continued for at least 30 d. Neither warfarin nor thrombin inhibitors were necessary.
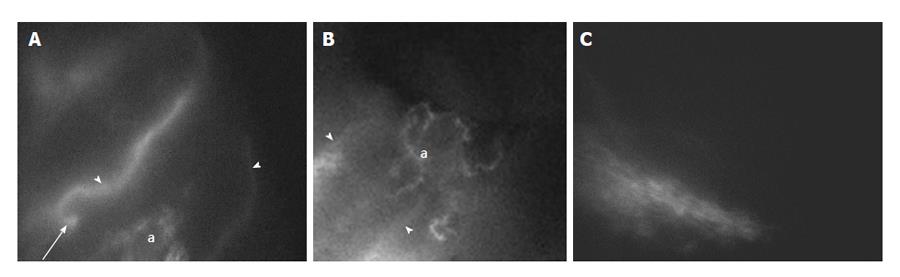
At three months after surgery, the patency of lympho-venous anastomosis was assessed by PDE: of the 10 patients who underwent microsurgery of spermatic cord, 8 showed early improvement, while an almost complete (95%-100% of scrotal swelling) remission was observed at six months after surgery. The clearest observation was the correspondence between the clinical outcome, the restored motility of lymphatic collectors observable during PDE lymphography[22], and the anastomosis patency. Indeed in responding patients, the distribution and canalization of the injected fluorescent dye improved over time with clearly visible patent anastomosis (Figure 2A and B). Clear identification of patent scrotal subcutaneous and spermatic lymphatic vessels in responding patients is accompanied by a significant reduction of tissue fluid volume and of the hypogastric abdominal wall thickness. Viceversa, only two patients (not-responders) showed much slower improvement, with only partial recovery (approximately 60%) at six months. In these non-responding patients, PDE images obtained at three months after microsurgery (Figure 2C) highlight the disperse distribution of fluorescent dye in the subcutaneous with difficult localization of the upstream lymphatic collectors and anastomosis.
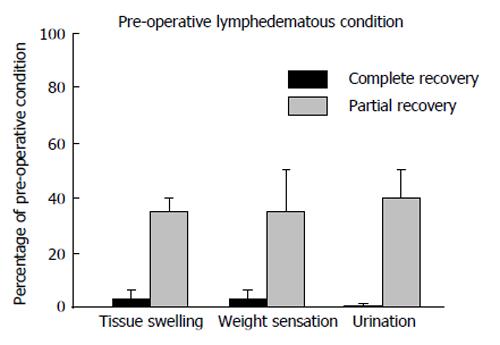
The post-surgical patient self-evaluation demonstrated (Figure 3): (1) improvement of scrotal tissue softness and scrotal skin normochromic aspect; (2) pain absence, (3) disappearance of the edematous volume with evident reduction of scrotal and penile dimensions with normal palpability of the testis and decreased weight sensation, (4) decreased urination and (5) the disappearance of episodic erysipelas with respect to the pre-operative condition. The postsurgical improvement assessed at three months after surgery by PDE and self evaluation was maintained over several years in all responding patients. The restoration of the preoperative scrotal size may sometime require an additional plastic surgery to remove the excessive scrotal skin.
The lower limb is supplied by: (1) a superficial lymphatic network, merging into lymphatic collectors directed to the inguinal lymph nodes from which the lymph is carried through the iliac collectors into the cisterna chili and thereafter to the thoracic duct and, eventually, into the subclavian veins; and (2) a deeper lymphatic network, whose collectors follow the sub-fascial limb neuro-vascular plexus to reach the peri-aortic lymph node chains and the thoracic duct. As a consequence, patients undergoing to inguinal and/or pelvic lymphadenectomy may develop a secondary lymphedema of the lower limb. We chose to micro-surgically treat the secondary lymphedema of the lower limb by performing a lympho-venous termino-terminal anastomosis between inguinal pre-nodal lymphatic collectors of the limb and collateral branches of the saphena or, when collaterals were not available, a termino-lateral anastomosis between inguinal pre-nodal lymphatic collectors and the saphena itself[14,20]. In the lower limb, the lympho-venous anastomosis is preferable compared to the lymphatic grafting technique usually performed in the treatment of upper and/or lower limb lymphedema[23]: (1) to more efficiently exploit the strong muscular contraction of the thigh musculature; and (2) to avoid the risk of inducing lymphedema in the previously healthy controlateral leg.
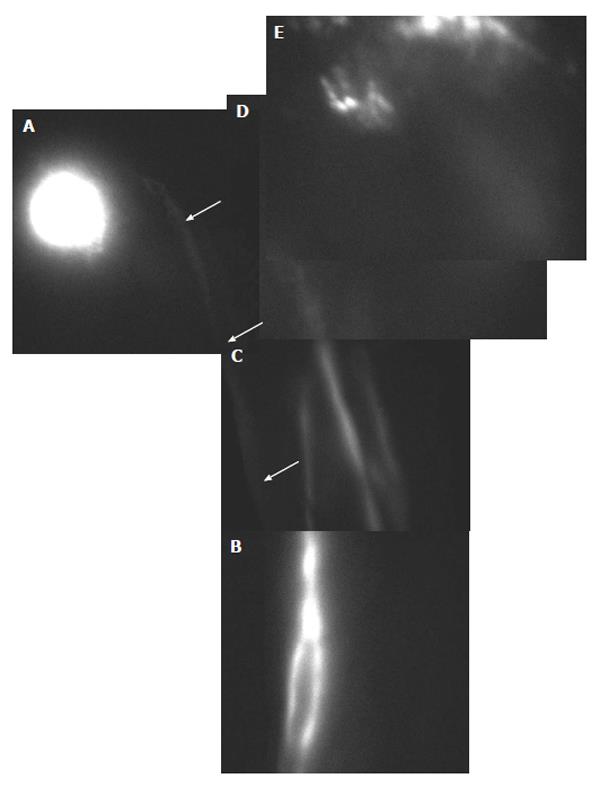
A common clinical observation is that lymphedema of the external genitals is often combined with either unilateral or bilateral chronic swelling of the lower limbs. In the first two cases coming to our observation, a two-stage treatment was performed: first, the microsurgical lympho-venous shunt of the lower limb and then, after six months, the derivative lympho-venous anastomosis of genital organs. The results of this approach clearly indicated that surgical treatment lymphatic collectors of the thigh draining lower limb edema may result in an improvement of the genitalia edema (Figure 4); in turn, shunting of the spermatic cord in treating of genital organ edema often results in recovery from lower limb edema so that lower limb surgery was deemed unnecessary. This could be explained by the fact that, after excision of inguinal lymph nodes, lymph from lower limbs is shunted through the scrotal and testicular pathways. Similarly, when testicular drainage is restored, edema of the scrotum and penis is also significantly improved, possibly because of lymphatic connections between the testicular and scrotal lymphatics networks. These results point to the existence of complex shunt network connecting the local lymphatic drainage pathways. It is worth noting that the target of physical therapy is to improve lymphedematous tissue drainage bypassing the obstruction by recruiting shunted pre-existing lymphatic vessels usually supplying adjacent tissue districts.
In addition to recruitment of patent superficial and spermatic cord lymphatic vessels, the development of peri-anastomotic lymphatic meshes in different tissues such as the spermatic cord, the inguino-crural and brachial regions was observed and documented for the first time in several patients who positively responded to microsurgery. Meshes were instead never encountered by PDE in non-responding patients or in normal tissues. Lymphatic meshes (Figure 2) consist of several lymphatic vessels sprouting from the anastomosis and/or between afferent lymphatic collectors and merging into well-canalized and complex networks. Subcutaneous meshes could be detected as early as three months after surgery and their complexity and extension seemed to improve over time, as shown by PDE images at 6 mo.
Lymphatic meshes were observed not only in patients who underwent spermatic funicular microsurgery, but also in patients who positively responded to the treatment of the lower limb swelling (Figure 5). On the contrary, no canalized lymphatics were evidenced at the same post-surgical follow-up in non-responding patients, who showed no significant improvement when compared to the pre-surgical PDE.
The development of this network might well explain the successful outcome of the microsurgical procedure in responding vs non-responding patients with little or no improvement. Indeed, it seems difficult to attribute the positive results of the surgical treatment to improvement of only one single lymphatic draining district. It is at present not known whether lymphatic meshes of the type observed in this study after microsurgical procedure may develop also in conservative physical therapy.
The post-surgical development of peri-anastomotic lymphatic meshes might serve as: (1) capacitance reservoirs to improve the draining capability of the existing lymphatics and maximize the drainage capacity of the postsurgically-canalized lymphatic vessels; and (2) intermediate compartments placed in series between the smallest initial lymphatics taking place from the edematous tissue and the larger collecting vessels transporting the lymph out of the tissue. At present, we are unable to precisely establish whether these lymphatic meshes reflect recruitment of previously existing but obliterated networks or, rather, new lymphangiogenesis. The fact that meshes were never observed in PDE lymphography of normal healthy tissues, but only during the post-surgical follow up in previously oedematous tissues of patients which positively respond to microsurgery might support the hypothesis of local lymphangiogenesis triggered by the minimally invasive microsurgical procedure within a process of extracellular matrix and lymphatic repair. In particular, the visual observation that the sprouting was particular intense in proximity of the patent anastomosis, is in line with the experimental observation[24] that, in the rat tail in presence of interstitial fluid flow, such as that induced by the new patent lympho-venous anastomosis, lymphatics may bypass a tissue lesion, reconstructing the original lymphatic pathway.
Although the lymphatic system still represents the least known compartment, particularly in humans, of the cardiovascular-extracellular tissue system, a growing amount of information is available on lymphatic cell biology and on the mechanical and molecular factors promoting functional lymphangiogenesis[25-32]. During embryogenesis the endothelial cells of the cardinal vein express the lymphatic endothelium-specific hyaluronan receptor (LYVE-1) and the VEGFR-3, which serves as a signalling receptor for the lymphatic-specific growth factors C (VEGF-C) and VEGF-D. Activation of VEGFR-3 signal is sufficient to induce lymphangiogenensis[30]. Later in adult life both LYVE-1 and VEGFR-3 are expressed by the lymphatic, but not by the blood endothelial cells. Subsequently, a still undefined mesenchymal factor induces, in specific endothelial cells of the cardinal veins the expression of the protein Prox1[31,32] which in the adult, is expressed by the lymphatic endothelium and by several non endothelial cells like lens, retina, central nervous system, heart, liver and pancreas[33-35]. Prox1-positive cells emerge from the original vessels to form new lymphatic sprouts. Prox1 is necessary to the development and differentiation of lymphatic endothelial phenotype[31,32], a phenomenon which implies induction of lymphatic specific genes such as VEGFR-3, podoplanin and desmoplakin-1[25,28] and simultaneous repression of blood vascular specific genes like collagens, laminin, VEGF-C, E-selectin, neuropilin-1 and interleukin-8. The transmembrane mucin-type glycoprotein podoplanin, expressed during embryogenesis by all cardinal vein and Prox1-sensitive cells seems to play an important role in lymphatic endothelial cells migration, adhesion and vessel formation[29].
At present, no information are available concerning the expression of pro-lymphangiogenetic endothelial and tissue molecules in human lymphedematous compared to normal tissue in murine models of primary lymphedema[33], dermal tissue swelling, interstitial fluid and solute transport critically depend on collagen and lipid dermal composition, suggesting that lymphatic function may be modulated by extracellular structure and macromolecular content. However, the behavior of normal lymphatic vessels exposed to either radiation or trauma, as in secondary lymphedema patients, may be quite different from those induced by primary genetic mutation.
The choice of the microsurgical lympho-venous derivation in the spermatic cord is highly recommended in the treatment of secondary severe chronic lymphedema of the external male genitals. Indeed, in addition to its direct significant advantages with respect to the standard approaches, such as: (1) minor tissue demolition and more satisfactory post-surgical functional and esthetic results; (2) absence of clinical complications; and (3) low invasivity and more rapid post-surgical recovery, the microsurgical lympho-venous derivation offers the unique possibility of improving lymphedema in adjacent tissues which are supplied by lymphatic collectors emptying into common lymphatic shunt.
Last but not least, lympho-venous microsurgery triggers the development of a complex network of new lymphatic vessels in proximity to the anastomosis area. This phenomenon, that we documented for the first time in human patients, seems to play a crucial favorable role in guaranteeing the long term recovery from chronic lymphedema after microsurgical treatment.
P- Reviewer: Coban Y, Negosanti L S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1-78. [PubMed] |
| 2. | Grimaldi A, Moriondo A, Sciacca L, Guidali ML, Tettamanti G, Negrini D. Functional arrangement of rat diaphragmatic initial lymphatic network. Am J Physiol Heart Circ Physiol. 2006;291:H876-H885. [PubMed] |
| 3. | Trzewik J, Mallipattu SK, Artmann GM, Delano FA, Schmid-Schönbein GW. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J. 2001;15:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Takada M. The ultrastructure of lymphatic valves in rabbits and mice. Am J Anat. 1971;132:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Edwards JM, Kinmonth JB. Lymphovenous shunts in man. Br Med J. 1969;4:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Ruszynak I, Foldi M, Szabo G. Lymphatics and lymph circulation. Physiology and pathology. London: Pergamon Press 1967; . |
| 7. | Schmid-Schönbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987-1028. [PubMed] |
| 8. | Negrini D, Moriondo A, Mukenge S. Transmural pressure during cardiogenic oscillations in rodent diaphragmatic lymphatic vessels. Lymphat Res Biol. 2004;2:69-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Moriondo A, Mukenge S, Negrini D. Transmural pressure in rat initial subpleural lymphatics during spontaneous or mechanical ventilation. Am J Physiol Heart Circ Physiol. 2005;289:H263-H269. [PubMed] |
| 10. | Burnard K, Mortimer P, Partsch H. Diagnosis and investigation of lymphedema. Diseases of the lymphatics. London: Arnold Eds 2003; 115-136. |
| 11. | Loughlin KR. Re: radical open inguinal lymphadenectomy for penile carcinoma: surgical technique, early complications and late outcomes: L. Koifman, D. Hampl, N. Koifman, A. J. Vides and a. A. Ornellas J Urol 2013; 190: 2086-2092. J Urol. 2014;191:1474-1475. [PubMed] |
| 12. | Nelson BA, Cookson MS, Smith JA, Chang SS. Complications of inguinal and pelvic lymphadenectomy for squamous cell carcinoma of the penis: a contemporary series. J Urol. 2004;172:494-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Franks KN, Kancherla K, Sethugavalar B, Whelan P, Eardley I, Kiltie AE. Radiotherapy for node positive penile cancer: experience of the Leeds teaching hospitals. J Urol. 2011;186:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Mukenge SM, Catena M, Negrini D, Ratti F, Moriondo A, Briganti A, Rigatti P, Cipriani F, Ferla G. Assessment and follow-up of patency after lymphovenous microsurgery for treatment of secondary lymphedema in external male genital organs. Eur Urol. 2011;60:1114-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Vignes S, Trévidic P. [Lymphedema of male external genitalia: a retrospective study of 33 cases]. Ann Dermatol Venereol. 2005;132:21-25. [PubMed] |
| 16. | Champaneria MC, Workman A, Kao H, Ray AO, Hill M. Reconstruction of massive localised lymphoedema of the scrotum with a novel fasciocutaneous flap: A rare case presentation and a review of the literature. J Plast Reconstr Aesthet Surg. 2013;66:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Halperin TJ, Slavin SA, Olumi AF, Borud LJ. Surgical management of scrotal lymphedema using local flaps. Ann Plast Surg. 2007;59:67-72; discussion 72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 18. | Becker C, Pham DN, Assouad J, Badia A, Foucault C, Riquet M. Postmastectomy neuropathic pain: results of microsurgical lymph nodes transplantation. Breast. 2008;17:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Huang GK, Hu RQ, Liu ZZ, Pan GP. Microlymphaticovenous anastomosis for treating scrotal elephantiasis. Microsurgery. 1985;6:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Mukenge S, Pulitanò C, Colombo R, Negrini D, Ferla G. Secondary scrotal lymphedema: a novel microsurgical approach. Microsurgery. 2007;27:655-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Mihara M, Hara H, Narushima M, Todokoro T, Iida T, Ohtsu H, Murai N, Koshima I. Indocianine green lymphography is superior to lymphoscintigraphjy in imaging diagnosis of secondary lymphedema of the lower limbs. J Vasc Surg: Venous and Lym Dis. 2013;1:194-201. |
| 22. | Mukenge S, Negrini D, Catena M, Ratti F, Dosio F, Paesano P, Rigatti P, Ferla G. Development of functionally patent lymphatic meshes in postsurgical long-term resolution of peripheral secondary lymphedema. J Vasc Surg: Venous and Lym Dis. 2013;1:280-288. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Baumeister RG, Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg. 1990;85:64-74; discussion 75-76. [PubMed] |
| 24. | Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106:920-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 405] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 26. | Hong YK, Shin JW, Detmar M. Development of the lymphatic vascular system: a mystery unravels. Dev Dyn. 2004;231:462-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367-397. [PubMed] |
| 28. | Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Ylä-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593-4599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 485] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 29. | Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546-3556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 515] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 30. | Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 477] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 31. | Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 710] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 32. | Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769-778. [PubMed] |
| 33. | Rutkowski JM, Markhus CE, Gyenge CC, Alitalo K, Wiig H, Swartz MA. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol. 2010;176:1122-1129. [PubMed] |