Revised: April 23, 2013
Accepted: May 7, 2013
Published online: July 24, 2013
Processing time: 140 Days and 13 Hours
AIM: To determine peri-operative, oncological, functional and safety profiles of extraperitoneal robot-assisted radical prostatectomy (eRARP) vs transperitoneal robot-assisted radical prostatectomy (tRARP) in a single centre.
METHODS: A total of 120 consecutive patients underwent 50 eRARP and 70 eRARP operations respectively by the same surgical team. Peri-operative and post-operative outcomes including blood loss, hospitalization, complications (Clavien grade), positive surgical margin (PSM) rates, continence and erectile function were compared. The performance of eRARP required several technical modifications. These included development of Retzius’ space by balloon insufflation, laparoscopic dissection of lateral extensions of this area; caudal port positioning; cranial digital stripping of peritoneum for sucker port and lodging the bagged prostate specimen adjacent to the lateral assistant port to permit space for urethro-vesical anastomosis.
RESULTS: Robotic console times were shorter with eRARP vs tRARP (145.1 min vs 198.3 min, P < 0.0001). There were no significant differences in blood loss, PSM rates (eRARP 17.7% vs tRARP 22%) or complications (eRARP 8.5% vs tRARP 8%). A drain was used in all patients after tRARP and in 25/70 eRARP cases. Length of hospital stay was shorter after eRARP (mean 1.94 d vs 3.6 d, P < 0.0002). There were no differences between techniques in continence or potency at 6 mo. eRARP required several technical modifications: development of Retzius’ space by balloon insufflation, laparoscopic dissection of lateral extensions of this area; caudal port positioning; and lodging the bagged prostate specimen adjacent to the lateral assistant port to permit space for urethro-vesical anastomosis.
CONCLUSION: eRARP demonstrated advantages in surgical times, hospital stay and equivalence in PSM rates, complications and functional outcomes. eRARP is a useful alternative to tRARP especially in patients with adhesions, pre-existing inguinal hernias, or those unable to withstand steep Trendelenburg position.
Core tip: Extraperitoneal robot-assisted radical prostatectomy (RARP) is a feasible alternative to transperitoneal RARP with equivalent complication rates, and pathological and functional outcomes. This approach replicates the principles of open radical prostatectomy with minimal requirement for Trendelenberg position or post-operative drain. It is particularly suited for patients with adhesions, pre-existing inguinal herniae and those unable to stand robotic surgery in steep Trendelenburg position.
- Citation: Anderson C, Ayres B, Issa R, Perry M, Liatsikos E, Stolzenburg JU, Ghani KR. Extraperitoneal robot-assisted radical prostatectomy: Comparison with transperitoneal technique. World J Clin Urol 2013; 2(2): 3-9
- URL: https://www.wjgnet.com/2219-2816/full/v2/i2/3.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v2.i2.3
Robotic-assisted radical prostatectomy (RARP) using the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA, United States) has become the predominant method for the surgical treatment of prostate cancer in the United States[1]. Compared to traditional open and laparoscopic methods, RARP results in similar oncological outcomes with some studies demonstrating improved recovery of continence and potency[2]. RARP may be performed by either a transperitoneal (tRARP) or extraperitoneal approach (eRARP). However, the overwhelming majority of surgeons use the transperitoneal (TP) approach based on its early description by pioneers of the technique[3]. Comparative studies of TP vs extraperitoneal (EP) approaches to RARP are limited to only four studies[4-7] and it is not clear whether these theoretical advantages translate into better clinical outcomes. The current study aims to determine the peri-operative, functional, oncological and safety profiles for both techniques in a single centre. We also discuss important technical modifications learnt from our experience of eRARP.
Between August 2008 and May 2011, 120 patients underwent RARP by a single surgeon (CA) in a tertiary centre. The first 50 consecutive patients underwent tRARP. The technique was then changed to an EP approach and the next 70 patients (eRARP) analysed. The cases were performed by an experienced pelvic laparoscopic and robotic surgeon but represented the early robotic experience of the personnel at this institution.
For the TP approach, we used the technique described by Menon et al[8]. For the EP approach we used our own modifications of the endoscopic extraperitoneal radical prostatectomy (EERPE) technique described by Stolzenburg et al[9]. A 20 F drain was placed in all patients following tRARP. The steps for access and port placement for eRARP are described below. In both techniques the assistant was situated on the left side of the patient with two robotic ports placed on the right side of the camera. This configuration was preferred in order to have the ability to use robotic graspers simultaneously on the left and right sides.
Equipment: All cases were performed using a 6-port technique with the four-arm da Vinci system[10]. Three standard 8 mm robotic ports are used along with a threaded 5/150 mm length blunt tipped port for the sucker, a 12/150 mm port with stability cone for the camera and a 12/100 mm assistant port. The camera and suction instrument port have an extra long shaft to overcome the oblique trajectory required to cross the posterior rectus sheath as well as to avoid clashing of the instruments outside the body. A round pre-peritoneal distension balloon is used for creation of the extraperitoneal space (PDB®1000 Balloon, Covidien, Mansfield, MA, United States). As the diameter of the robotic camera is larger than the balloon insufflation port, a laparoscopic camera was used for EP space creation.
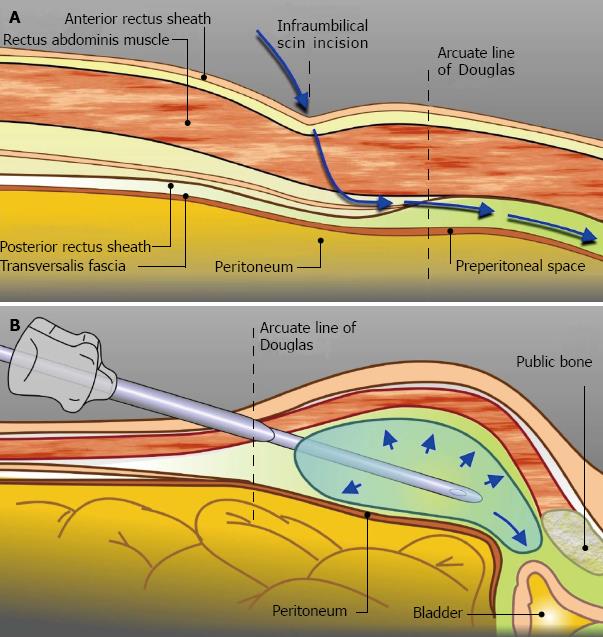
Extraperitoneal access: EP access was undertaken with the patient supine. An oblique incision is made 2 cm infero-lateral to the right of the umbilicus. The anterior rectus sheath is incised and the rectus muscle separated to expose the posterior sheath. An index finger is inserted above the posterior sheath in the direction of the pubis to create space for the pre-peritoneal distension balloon. The balloon is manually inflated a small amount to secure it within the retropubic space (Figure 1). Further insufflation is done under direct vision to create the EP space. Once satisfactory the balloon is deflated and removed.
Port placement: An index finger is inserted through the infra-umbilical incision to strip and release left-sided fascial attachments of the peritoneum to anterior abdominal wall. The suction instrument port incision is made 3 cm left of the umbilicus. The port is inserted through the abdominal wall with the tip of the index finger of the other hand, via the infra-umbilical incision, protecting its entry. Next, the blunt tipped camera port is inserted via the infra-umbilical incision between the rectus muscle fibres and anterior to the posterior rectus muscle sheath with a retractor elevating the anterior sheath to facilitate its insertion. Sutures placed in the anterior sheath tighten the seal around the stability cone of the camera port and secure it in place.
CO2 insufflation is commenced to a pressure of 12 mmHg. Via the suction instrument port, the suction or blunt grasper is used to release any remaining left-sided fascial attachments. At approximately two thirds of the distance from the pubic symphysis to umbilicus and three finger-breadths lateral from the midline, the left robotic port is inserted under direct vision. Using the suction and left robotic ports as working channels, peritoneum on the right side is mobilised off the anterior abdominal wall. Dissection begins lateral to the inferior epigastric vessels and superolateral to the arcuate line.
The first right-sided robotic port (4th arm port) is inserted at point two to three finger-breadths in the line from the right anterior superior iliac spine to umbilicus. The second right-sided robotic port is placed at the same level as the left robotic port, equidistant from the midline. The inferior epigastric vessels must be noted to avoid injury during its insertion. Once all three robotic ports are placed, dissection is continued from the right side with stripping of peritoneum away from the left anterior abdominal wall. The 12 mm assistant port is placed three finger-breadths in the line between the left anterior superior iliac spine and umbilicus (Figure 2).
Prostatectomy: The patient was placed in a 10°-15° Trendelenburg position and the patient side-cart docked. Standard pelvic lymphadenectomy was done if needed. Prostatectomy was begun with dissection of the endopelvic fascia. The remainder of the procedure was similar to the TP approach with the only modification being fixation of the bagged prostate specimen adjacent to the assistant’s 12 mm port, to enable adequate space for urethro-vescial anastomosis (UVA). A 20 F drain was placed in the retropubic space in selected patients who underwent concomitant lymphadenectomy or the UVA was difficult or blood oozing was present.
Data were collected prospectively into a database. Complications were graded using the modified Clavien classification[11]. Outcomes for erectile function and continence were evaluated using the International Index of Erectile Function 5 (IIEF-5) and ICS male Short Form (SF) questionnaires respectively. These were provided to patients preoperatively and quarterly postoperatively by a nurse specialist. We defined continence as being either pad free or using one security pad at 6 mo. Initial tests for normality were carried out and appropriate statistical tests chosen (paired t test and Kruskal-Wallis test). Commercially available statistics programs were used (GraphPad InStat, version 3.05, GraphPad Software, United States; Medcalc version 7.0, Medcalc Software, Belgium) for statistical comparisons.
Table 1 compares the peri-operative data and complications between the two groups. Patient pathological characteristics are provided in Table 2. There were no significant differences in the prostate specific antigen, prostate TRUS volume, Gleason score, pathological stage or positive surgical margin (PSM) rates between TP and EP groups. Fourteen patients had pelvic lymph node dissection and four had mesh repair for concurrent inguinal hernia in the EP-group. In the TP-group seven patients had lymph node dissection and one had a mesh hernia repair. The hernia repair was done similarly in both groups and the mesh was placed in the EP space at the end of the TP operation in order to avoid its contact with the bowel. Adjusting for these concurrent procedures resulted in mean console times of 145.1 min for eRARP vs 198.3 min for tRARP (P < 0.0001). The overall operating time was also shorter in the EP-group but this did not reach statistical significance. There were no significant differences in blood loss between eRARP and tRARP techniques (372 mL vs 342 mL respectively, P < 0.0008). In total there were two blood transfusions in the eRARP group, whilst one transfusion in the TP-group.
| Characteristic | tRARP (n = 50) | eRARP (n = 70) |
| Mean total operative time (min) | 255.7 (155-490) | 236.8 (170-375) |
| Prostatectomy console time (min) | 198.3 ± 64 (120-420) | 145.1 ± 38.7 (96-293) |
| Mean blood loss (mL) | 342 ± 320 (50-2000) | 372 ± 368 (100-2500) |
| Drain use | 50/50 | 25/70 |
| Length of stay (mean) | 3.52 ± 2 (2-12) | 1.94 ± 1.38 (1-8) (P < 0.0002) |
| Intra-operative complications (Clavien Grade) | 1 Ureteric injury (Grade 2) | 1 Rectal injury (Grade 2) |
| Post-operative complications (Clavien Grade) | 1 Arm neuropraxia (Grade 1) | 1 Urinary retention (Grade 1) |
| 1 Blood transfusion (Grade 2) | 2 Blood transfusions (Grade 2) | |
| 1 Anastomotic leak requiring suprapubic catheter insertion on readmission (Grade 3b) | 2 Pelvic collection/haematoma requiring percuataneous drainage (Grade 3a) |
| Variables | tRARP (n = 50) | eRARP (n = 70) |
| Age (yr) | 60.5 ± 7.5 (42-72) | 62.1 ± 6 (47-72) |
| PSA (ng/mL) | 8.67 ± 6.1 (2.8-34.8) | 8.66 ± 8.58 (1.3-71.8) |
| Prostate volume (cc) | 39.5 ± 14.66 (15-70) | 44.9 ± 17.4 (18-82) |
| Biopsy Gleason score | ||
| 6 | 26 | 30 |
| 7 | 20 | 38 |
| 8 | 3 | 2 |
| Pathological stage | ||
| T2 | 37 | 51 |
| T3a | 11 | 12 |
| T3b | 2 | 7 |
| Specimen Gleason score | ||
| 5 | 1 | |
| 6 | 11 | 10 |
| 7 | 30 | 52 |
| 8 | 4 | 3 |
| 9 | 4 | 4 |
| Cancer volume (cc) | 3.9 ± 3.2 (0.06-14.7) | 3.2 ± 3.58 (0.2-23.7) |
| Positive surgical margin | ||
| T2 | 13.5% of 37 patients | 12.7% of 48 patients |
| T3 | 30.5% of 13 patients | 22.7% of 22 patients |
The complication rates were similar amongst both techniques (Table 1). Complication rate after tRARP was 8% vs 8.5% after eRARP (P = 1.0). A drain was used in only 25 patients (36%) in the eRARP vs in all patients in the TP group (P < 0.0007). The length of stay was shorter with eRARP (mean 1.94 d vs 3.52 d, P < 0.0001). The proportion of patients discharged on the first postoperative day was significantly higher following an EP procedure (49% vs 0%, P < 0.0001).
Although the EP approach was completed in all patients, small peritoneal breaches and subsequent intraperitoneal insufflation were encountered in 4 (6%) patients. These did not significantly hamper the dissection and by placing a 14 G venous cannula into the abdominal cavity the pneumoperitoneum was kept to a minimum thereby avoiding diminution in the EP space.
At 6 mo the continence rate in the two groups was equivalent with 93% continent in the tRARP group and 94% in the eRARP group. There were no differences in potency outcomes using either technique. At 6 mo, patients undergoing nerve-sparing RARP achieved satisfactory erections (IIEF-5 ≥ 17), with or without oral pharmacotherapy in 67% and 69% for eRARP and tRARP respectively.
Table 3 summarises the published data on eRARP[4-7,12-14] including those studies that directly compare eRARP and tRARP[4-7]. Studies where the data has been duplicated in larger series are excluded from this analysis[13,15-18]. Our study is the first report from a United Kingdom centre performing eRARP and demonstrates overall operative times, PSM and complication rates consistent with previously published studies. We found the console time was significantly shorter with the EP approach although there were no significant differences in total operative times between the two techniques. During eRARP console time is saved by avoiding the need to release adhesions if present, and mobilise the bladder to get into the retropubic space. However extra time is required to create EP space and access and this is why overall times were not statistically different between groups. It is possible that with increasing experience of EP access for RARP, access times could be shortened. Indeed, two studies have demonstrated reductions in total operative time for eRARP when compared to tRARP[4,5].
| Reference | Centre | Level of evidence | No. of patients | Mean operative time (min) | Mean blood loss (mL) | Complication rate (transfusion rate) | PSM rate | Hospital stay (d) | Conversions |
| Joseph et al[12] | Rochester, United States | 4 | 325 | 180 | 196 | 9.8% (1.3%) | 13% | 96% < 24 h | 2 converted to TP |
| Atug et al[4] | New Orleans, United States | 4 | 40 | 229 | 221 | 12.5% (NS) | 20% | Mean 1.2 | none |
| Rozet et al[13] | Institut Montsouris, France | 4 | 133 | 166 | 609 | 19.4% (9.8%) | 19.50% | Mean 5.4 | 4 converted to LRP |
| Capello et al[6] | Rochester, United States | 2b | 31 | 181 | 199 | 0% (0%) | 3.20% | NS | none |
| Madi et al[5] | Ann Arbor, United States | 4 | 34 | 214 | 125 | 5.9% (0%) | 23.50% | Median 1 | none |
| Ploussard et al[16] | Henri Mondor, France | 4 | 206 | 160 | 504 | 8.3% (3.4%) | 27.70% | Mean 4 | 1 converted to LRP |
| Chung et al[7] | South Korea | 4 | 155 | 150 | 351 | 7.1% (NS) | 22.60% | Mean 5.1 | none |
| This study | St George’s, United Kingdom | 4 | 70 | 145.1 | 372 | 8.5% (2.8%) | 15.70% | Mean 2 | none |
In our study, blood loss was equivalent between patients undergoing eRARP and tRARP. Also, there were no significant differences in transfusion rates between groups. Previous comparisons between tRARP and eRARP have not demonstrated differences in blood loss. Despite using the same discharge criteria for both groups, the length of stay following eRARP in our study was significantly shorter than after tRARP. A drain was used considerably less after eRARP and it is possible that this may have had an influence on the length of stay. However the ability to avoid a drain in the EP approach, as the peritoneal cavity is not breached, is one of the advantages of eRARP.
We found no significant differences in complications between TP and EP approaches to RARP. In a recent comparison by Chung et al[7] of 105 TP-RARP with 155 eRARP’s over a two-year period, no significant differences in total operative time or blood loss were demonstrated while console times were significantly shorter with eRARP. Interestingly they found postoperative pain scores were significantly lower in patients undergoing eRARP. Also, TP patients developed more ileus (× 7) as well as a significant increase in the incidence of postoperative hernias. Although TP patients had prolonged ileus in some studies[4,5], these were smaller studies and we suggest that further large studies might prove the beneficial effect of eRARP in avoiding ileus.
One of the risks of the TP approach to minimally invasive radical prostatectomy (RP), regardless of the use of a robotic system is the possibility of bowel-related complications. In a series of 567 patients undergoing TP-laparoscopic RP by Guillonneau et al[19], 2% of patients had intraperitoneal complications requiring re-intervention. The exact risk of bowel injury during tRARP is difficult to determine and is probably affected by the experience of the robotic surgeon. In a recent study from the Vattikuti Urology Institute, 9 of 3317 patients undergoing tRARP had a bowel injury requiring enterotomy[20]. eRARP results in the avoidance of peritoneal entry and bowel contact thereby reducing bowel-related morbidity.
There were no differences in the PSM rates between the different approaches in our study. This finding is corroborated by the existing literature which reveals PSM rates varying from 3.2% to 27.7% after eRARP with no significant differences between eRARP and tRARP. As the working space in eRARP is smaller, there had been concerns that larger prostates may make it more difficult to remove the prostate and therefore contribute to higher PSM rates. So far there is no evidence that larger prostates (> 75 g) result in differences in PSM rates after eRARP[18].
In our study there was no difference between the two groups for continence at 6 mo with 93% continent in the tRARP group and 94% in the eRARP group. Although, continence rates over a period of 12 mo was available for patients of the TP group, the number of patients of the EP group with 12 mo follow-up data was not adequate for a comparison. In the largest series of eRARP, 96% of 179 patients at 6 mo follow up were continent (without pads)[12]. In a more recent study by Ploussard et al[16] of 206 patients, the 12-mo continence rate (no pad use) was 74% whilst it was 98% when patients used a safety pad. In our series, there was no difference in patients who had nerve-sparing technique at 6 mo while 67% and 69% achieved satisfactory erections, with or without oral pharmacotherapy, for eRARP and tRARP respectively. Potency rates after eRARP have been reported between 39% to 70% in previous studies[12-14].
We confirm the advantage of using the 4th arm in eRARP[10] and found it particularly helpful during difficult anastomosis in a narrow pelvis by allowing the surgeon to switch to the 4th arm as the working right-sided instrument for suturing instead of the more medial right-sided 3rd arm which can be restrictive in that circumstance. There is also an assumption that the EP approach can sometimes increase the tension on the VUA. This was not borne out in our experience but in cases where there was perceived difficulty, the VUA was facilitated by applying perineal pressure, and in some cases, freeing the bladder attachments.
In eRARP the peritoneum acts as a natural bowel retractor thereby preventing bowel falling into the operative field. Therefore only 10-15 degrees of Trendelenburg position is necessary. In contrast there are considerable effects of a steep Trendelenburg position on physiology during T-RARP[21-25]. Patients with cardiovascular or respiratory co-morbidities may not be able to maintain the steep Trendelenburg position and therefore the EP approach which affords a less-steep position may be preferred in such patients. Furthermore, in institutions where long operative times are anticipated, either due to early experience of the surgeon or the requirement for training, the physiological effects of steep Trendelenburg will be more significant and indeed negated with the EP approach. It follows too that the risk of compartment syndrome in the limbs from prolonged operating is also reduced with the EP approach.
Table 4 lists the advantages of both approaches to RARP. One limitation of our study is that some of the improvements in eRARP may have been due to improved performance of robotic surgery as a result of increasing experience. Also, we did not assess functional outcomes at 12 mo although, the primary purpose of this study was a feasibility to determine peri-operative and short-term operative outcomes including PSM rates. In this regard, eRARP performed no worse than tRARP with certain advantages over tRARP as outlined. It is now our standard of care.
| Advantages of tRARP | Advantages of eRARP |
| Larger working space | Reduction in robotic console time |
| Allows extended pelvic lymphadenectomy | Reduction in bowel related morbidity |
| Lower incidence of lymphocele | Physiological effects of laparoscopy less marked due to minimal Trendelenburg position |
| Preferred in patients with mesh hernia repairs | Containment of leak (urine, blood) within retropubic space |
| Preferred in patients with pre-existing inguinal hernia (allows mesh repair) | |
| Preferred in patients with intra-abdominal adhesions (reduces peritoneal viscera interference) |
In conclusion, our experience and the literature to date demonstrate no differences on the performance of the EP approach for RARP. Console times may be shorter with eRARP. One advantage of eRARP is reduction in bowel-related complications such as ileus. In particular, patients who may benefit from eRARP include those with extensive adhesions, pre-existing inguinal hernias, or unable to withstand a steep Trendelenburg position.
Robot-assisted radical prostatectomy (RARP) is currently the most common way for removing prostate cancer in the United States. The technique of RARP usually involves an approach which is transperitoneal which predisposes a risk of intraoperative bowel injury or contact of intraperitoneal contents with urine in case of post-operative urine leak. While an extraperitoneal approach is feasible, it is less commonly performed. In this article, the authors study the comparison of an extraperitoneal with transperitoneal approach to RARP.
This technique of extraperitoneal RARP is feasible and the authors discuss points of technique for its successful adoption.
The authors provide the first analysis of an extraperitoneal approach to RARP from a United Kingdom cancer centre.
The authors demonstrate equivalent pathological and functional outcomes with the extraperitoneal approach, with added advantages of shorter stay due to less ileus and lower requirement to use a post-operative drain.
Extraperitoneal RARP involves entry into the peritoneal cavity, and mobilization of bowel away from the pelvis in order to access the prostate. Transperitoneal RARP replicates principles of open retropubic radical prostatectomy without breaching the peritoneal cavity and no contact with bowels. Not surprisingly, extraperitoneal RARP had lower rates of ileus, lower post-operative stay in hospital and less requirement for post-operative drain.
This is a well written paper on a timely topic.
P- Reviewer Hakenberg OW S- Editor Song XX L- Editor A E- Editor Ma S
| 1. | Menon M. Robot-assisted radical prostatectomy: is the dust settling. Eur Urol. 2011;59:7-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, Guazzoni G, Guillonneau B, Menon M, Montorsi F. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 705] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 3. | Tewari A, Peabody J, Sarle R, Balakrishnan G, Hemal A, Shrivastava A, Menon M. Technique of da Vinci robot-assisted anatomic radical prostatectomy. Urology. 2002;60:569-572. [PubMed] |
| 4. | Atug F, Castle EP, Woods M, Srivastav SK, Thomas R, Davis R. Transperitoneal versus extraperitoneal robotic-assisted radical prostatectomy: is one better than the other. Urology. 2006;68:1077-1081. [PubMed] |
| 5. | Madi R, Daignault S, Wood DP. Extraperitoneal v intraperitoneal robotic prostatectomy: analysis of operative outcomes. J Endourol. 2007;21:1553-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Capello SA, Boczko J, Patel HR, Joseph JV. Randomized comparison of extraperitoneal and transperitoneal access for robot-assisted radical prostatectomy. J Endourol. 2007;21:1199-1202. [PubMed] |
| 7. | Chung JS, Kim WT, Ham WS, Yu HS, Chae Y, Chung SH, Choi YD. Comparison of oncological results, functional outcomes, and complications for transperitoneal versus extraperitoneal robot-assisted radical prostatectomy: a single surgeon’s experience. J Endourol. 2011;25:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Menon M, Tewari A, Peabody J. Vattikuti Institute prostatectomy: technique. J Urol. 2003;169:2289-2292. [PubMed] |
| 9. | Stolzenburg JU, Do M, Rabenalt R, Pfeiffer H, Horn L, Truss MC, Jonas U, Dorschner W. Endoscopic extraperitoneal radical prostatectomy: initial experience after 70 procedures. J Urol. 2003;169:2066-2071. [PubMed] |
| 10. | Esposito MP, Ilbeigi P, Ahmed M, Lanteri V. Use of fourth arm in da Vinci robot-assisted extraperitoneal laparoscopic prostatectomy: novel technique. Urology. 2005;66:649-652. [PubMed] |
| 11. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] |
| 12. | Joseph JV, Rosenbaum R, Madeb R, Erturk E, Patel HR. Robotic extraperitoneal radical prostatectomy: an alternative approach. J Urol. 2006;175:945-950; discussion 951. [PubMed] |
| 13. | Rozet F, Jaffe J, Braud G, Harmon J, Cathelineau X, Barret E, Vallancien G. A direct comparison of robotic assisted versus pure laparoscopic radical prostatectomy: a single institution experience. J Urol. 2007;178:478-482. [PubMed] |
| 14. | Ploussard G, Xylinas E, Salomon L, Vordos D, Hoznek A, Abbou CC, De La Taille A. Robot-assisted extraperitoneal laparoscopic radical prostatectomy: experience in a high-volume laparoscopy reference centre. BJU Int. 2010;105:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Xylinas E, Ploussard G, Salomon L, Paul A, Gillion N, Laet KD, Vordos D, Hoznek A, Abbou CC, de la Taille A. Intrafascial nerve-sparing radical prostatectomy with a laparoscopic robot-assisted extraperitoneal approach: early oncological and functional results. J Endourol. 2010;24:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Ploussard G, Xylinas E, Paul A, Gillion N, Salomon L, Allory Y, Vordos D, Hoznek A, Yiou R, Abbou CC. Is robot assistance affecting operating room time compared with pure retroperitoneal laparoscopic radical prostatectomy. J Endourol. 2009;23:939-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Madeb R, Golijanin D, Knopf J, Vicente I, Erturk E, Patel HR, Joseph JV. Patient-reported validated functional outcome after extraperitoneal robotic-assisted nerve-sparing radical prostatectomy. JSLS. 2007;11:443-448. [PubMed] |
| 18. | Boczko J, Erturk E, Golijanin D, Madeb R, Patel H, Joseph JV. Impact of prostate size in robot-assisted radical prostatectomy. J Endourol. 2007;21:184-188. [PubMed] |
| 19. | Guillonneau B, Rozet F, Cathelineau X, Lay F, Barret E, Doublet JD, Baumert H, Vallancien G. Perioperative complications of laparoscopic radical prostatectomy: the Montsouris 3-year experience. J Urol. 2002;167:51-56. [PubMed] |
| 20. | Agarwal PK, Sammon J, Bhandari A, Dabaja A, Diaz M, Dusik-Fenton S, Satyanarayana R, Simone A, Trinh QD, Baize B. Safety profile of robot-assisted radical prostatectomy: a standardized report of complications in 3317 patients. Eur Urol. 2011;59:684-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Kalmar AF, Foubert L, Hendrickx JF, Mottrie A, Absalom A, Mortier EP, Struys MM. Influence of steep Trendelenburg position and CO(2) pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. Br J Anaesth. 2010;104:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Lestar M, Gunnarsson L, Lagerstrand L, Wiklund P, Odeberg-Wernerman S. Hemodynamic perturbations during robot-assisted laparoscopic radical prostatectomy in 45° Trendelenburg position. Anesth Analg. 2011;113:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Gutt CN, Oniu T, Mehrabi A, Schemmer P, Kashfi A, Kraus T, Büchler MW. Circulatory and respiratory complications of carbon dioxide insufflation. Dig Surg. 2004;21:95-105. [PubMed] |
| 24. | Lee JR, Lee PB, Do SH, Jeon YT, Lee JM, Hwang JY, Han SH. The effect of gynaecological laparoscopic surgery on cerebral oxygenation. J Int Med Res. 2006;34:531-536. [PubMed] |