Published online Aug 8, 2016. doi: 10.5409/wjcp.v5.i3.330
Peer-review started: April 6, 2016
First decision: May 17, 2016
Revised: May 26, 2016
Accepted: June 1, 2016
Article in press: June 3, 2016
Published online: August 8, 2016
Processing time: 127 Days and 22.9 Hours
AIM: To identify a hypothesis on: Supine sleep, sudden infant death syndrome (SIDS) reduction and association with increasing autism incidence.
METHODS: Literature was searched for autism spectrum disorder incidence time trends, with correlation of change-points matching supine sleep campaigns. A mechanistic model expanding the hypothesis was constructed based on further review of epidemiological and other literature on autism.
RESULTS: In five countries (Denmark, United Kingdom, Australia, Israel, United States) with published time trends of autism, change-points coinciding with supine sleep campaigns were identified. The model proposes that supine sleep does not directly cause autism, but increases the likelihood of expression of a subset of autistic criteria in individuals with genetic susceptibility, thereby specifically increasing the incidence of autism without intellectual disability.
CONCLUSION: Supine sleep is likely a physiological stressor, that does reduce SIDS, but at the cost of impact on emotional and social development in the population, a portion of which will be susceptible to, and consequently express autism. A re-evaluation of all benefits and harms of supine sleep is warranted. If the SIDS mechanism proposed and autism model presented can be verified, the research agenda may be better directed, in order to further decrease SIDS, and reduce autism incidence.
Core tip: An earlier article presents evidence that supine sleep is a stressor, with sympathetic arousal that protects infants with defects in auto-resuscitation from sudden infant death syndrome. This article argues that a possible side-effect in the population being subjected to supine sleep is an increase in the expression of features contributing to diagnosis of autism spectrum disorder. In a literature search, five countries were identified (Denmark, United Kingdom, Australia, Israel, United States) with published time trends of autism, and with change-points coinciding with supine sleep campaigns. The stressor hypothesis for both conditions are amenable to testing, a better understanding of both is likely to improve outcomes.
- Citation: Bergman NJ. Hypothesis on supine sleep, sudden infant death syndrome reduction and association with increasing autism incidence. World J Clin Pediatr 2016; 5(3): 330-342
- URL: https://www.wjgnet.com/2219-2808/full/v5/i3/330.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v5.i3.330
Supine sleep campaigns have successfully achieved global reductions in sudden infant death syndrome (SIDS), which is well documented in 13 countries[1]. In all these countries the reduction was fairly immediate and in proportion to the population uptake of supine sleep following the public health messages. Reductions achieved a plateau, somewhat higher in the United States than in Scandinavia[2]. Continued intensive supine sleep messages have not lowered mortality further. Indeed, in the United States unexplained infant deaths appear to have increased[3]. The underlying mechanism for SIDS has not been explained, nor the mechanism whereby prone sleep exerts a potentially harmful effect. That prone sleep is harmful has indeed been the assumption, since the association was so clear.
This author has recently presented a hypothesis for the mechanisms of SIDS and the protective mechanism of supine sleep[4]. Briefly, this hypothesis elaborates on the Triple Risk Model for SIDS[5], the three aspects being an underlying vulnerability, a critical developmental period, and an exogenous stressor or risk factor. The key element of this model is that there is an underlying defect: The great majority of SIDS cases have identified brainstem abnormalities, which have not yet been found in controls[5,6]. This author proposes that these defects are specific to various stages of auto-resuscitation[7], and it is the failure of these which is the proximal or immediate cause of SIDS. Tobacco specifically augments defects in the gasping mechanism that initiates auto-resuscitation[8]. However, at an intermediate level of causation, auto-resuscitation is a necessary response to “adverse autonomic events” (AAE), whereby the autonomically immature organism responds in a primitive reptilian autonomic defence style, through a purely parasympathetic discharge orchestrated by the ventrolateral periaqueductal gray matter[9]. This is not compatible with mammalian physiology, therefore a robust auto-resuscitation mechanism is activated. Distal mechanisms include those that induce the AAEs, or conversely reduce them, along with the critical period and the risk factors of the Triple Risk Model. In healthy infants without defect, such AAE’s appear to be common, and auto-resuscitation is robust. REM sleep processes negative emotions[10], and is likely the key period of SIDS risk[11,12] with increasing frequency of AAEs. In SIDS subjects, neural gliosis suggests that there have been repeated episodes of “near-misses” with hypoxic damage, prior to a lethal event[13]. It is possible that the same defects in serotonin metabolism identified in SIDS cases may contribute also to sleep disruption, increased REM sleep and decreased quiet sleep has been documented in such cases. Further, serotonin is also involved in anxiety and autonomic arousal[14], which may contribute further to increased AAE.
The association with prone sleep has made it the focus of research as to mechanisms of harm. No such harmful effects have been ascertained. This author argues from a biologically based developmental paradigm, that prone sleep is in fact the normal and healthy form of sleep[4], as a near universal mammalian phenomenon (exceptions include bats and sloths). Supine sleep in human infants qualifies as a stressor in a number of respects, primarily in producing a raised level of state organisation, and autonomic arousal[4]. This is indeed protective for such infants that have a brainstem defect, the effect may be due to decreased frequency of REM sleep, but perhaps more through an element of sympathetic autonomic stressor arousal, that counteracts the primitive parasympathetic dissociation defence mechanism[4].
The defect has not been found in post-mortem control cases[6], and SIDS qualifies as a “rare disease”[15]. The lethality of the defect is clearly not absolute, otherwise it would not be responsive to supine sleep. Conjecture can be based on existing data: in the United States neonatal mortality has halved, this would fit a population with a defect prevalence of 1.0/1000 with a 50% lethality. Sweden had a mortality around 1.0/1000, this has fallen to 0.25/1000; perhaps a 5/1000 defect prevalence with a 20% lethality reduced to 5% by supine sleep. The number needed to treat from such a “treatment” is high, perhaps above 1000. Clearly, current information does not allow an exact figure, but this is likely the order of magnitude, or else controls with defect would be found.
Consideration should then be given to potential side-effects of such an intervention applied to a whole population. Plagiocephaly was identified early[16], occurring in 1 of 60 supine sleeping infants, but will only rarely have long lasting major impact. More importantly, supine sleep in the first months of life leads to delayed motor development at 6 mo and up to a year[4,17]. Recent developments in epigenetics and developmental neuroscience have relevance here. Prolonged stressor effects result in elevated cortisol levels that mediate gene methylation changes during sensitive periods of early development. “Perinatal life is a critical time for DNA methylation and for susceptibility to environmental factors”[18], methylation generally down-regulates genes, with adverse effects[18]. Sleep cyclicity is another factor essential for the development of healthy neuronal circuits[19]. Supine sleep may therefore have two separate mechanisms that disrupt early development, as evidenced by delayed motor development.
The first two months of life are a critical period for socio-emotional development[20]. This entails neural circuitry from the amygdala and associated limbic structures (emotional brain) to the medial and orbito-prefrontal cortex and executive function, also called the social brain[20]. The establishment of early resilience requires that social oxytocin circuits are connected also to reward-related dopamine circuits[21], a likely consequence of early bonding and secure attachment. A predictable consequence of such disruption is autism spectrum disorder (ASD), from here just “autism”. Autism has recently been redefined in the DSM-5[22], encompassing persistent deficits in social communication and interaction, along with restricted and repetitive patterns of behaviour, beginning in early childhood and impairing everyday functioning. The emotional social deficit has been attributed to methylation of oxytocin receptors[18,23], and repetitive behaviours may be attributable to dopamine pathway disruption[24,25].
There has been extensive debate in the literature, with some arguing that the increase in autism is due to diagnostic changes and other factors[26,27]. These include methodological variations in conducting surveys[26,28], definitions of autism displaying variability[29] (including new definition in DSM-5, predicted by some to decrease the identified incidence[30,31], or make little difference[32-34]), broadening of diagnostic concepts[27], increased awareness[35-37], diagnostic substitution[38,39], and altered ranking of co-morbidities[28,40]. This debate has led to some relevant reflection: Hrdlicka and Dudova[41] argue there is a need for a “broader model of social disorders”. While “autism” as a diagnosis may be welcomed by parents seeking economic support for care of a challenged child, autism could be seen as a smaller piece of a broader group of “social inhibition disorders”[41], all of which require support without discrimination[28,40,42].
The above notwithstanding, “a significant portion of the time trend remains unexplained”[43], an actual increase cannot be ruled out[43-47]. Keyes et al[48] analysed Californian data by birth cohorts, showing a consistent increase over time, with no evidence of an characteristic factor contributing to increase. The Autism and Developmental Disabilities Monitoring Network (ADDM)[49] likewise reports on birth cohorts, using a standardised approach in case finding and diagnosis for self-selected sites in the United States, incidence has risen from 6.6 to 14.7 (1994 to 2002 birth cohorts).
The hypothesis presented in this paper assumes that a portion of the increased incidence of autism is real, and proposes that supine sleep is contributing to that real increase. Since supine sleep campaigns have been introduced in many countries, with measurable change in infant sleeping position in the community, such change should according to this hypothesis be reflected in change points in the incidence of autism, attributable to “change in risk factor prevalence”[50]. Further, only susceptible infants will express such autism, therefore an incidence plateau should be achieved within a time period that matches the sleeping behaviour change in the community. Establishing this requires accurate data based on birth cohorts. Most cases are believed to be diagnosed by the age of 8 years[49], although current trends do show that additional cases are diagnosed in the teen years[51].
A literature search was undertaken for published incidence or prevalence data on autism for countries with clear dates for supine sleep campaigns, and with time line series that straddle a period before and after such campaigns. The focus period was the decade before supine sleep campaigns (1980’s), through the campaign decade, and for the decade after (2000’s), allowing for full expression of incidence. Data on actual sleep position in community over time are scarce[42], as reported in author’s previous paper[4], aligning to such data would be preferable otherwise. Search was conducted through PubMed, using terms “autism” or “ASD”, with “incidence”, “prevalence” and “trends”. This was followed up in Google Scholar, and subsequent internet searches on key words found in articles. Data were collated in Excel in country-specific graphs. Statistical analysis was not undertaken, merely identification of change points aligned to supine sleep campaigns.
Based on the putative insight that supine sleep is a stressor, a mechanistic hypothesis for increase in autism was generated. This integrates genetics, epigenetics, stress biology and developmental neuroscience with current theories and understanding of ASD.
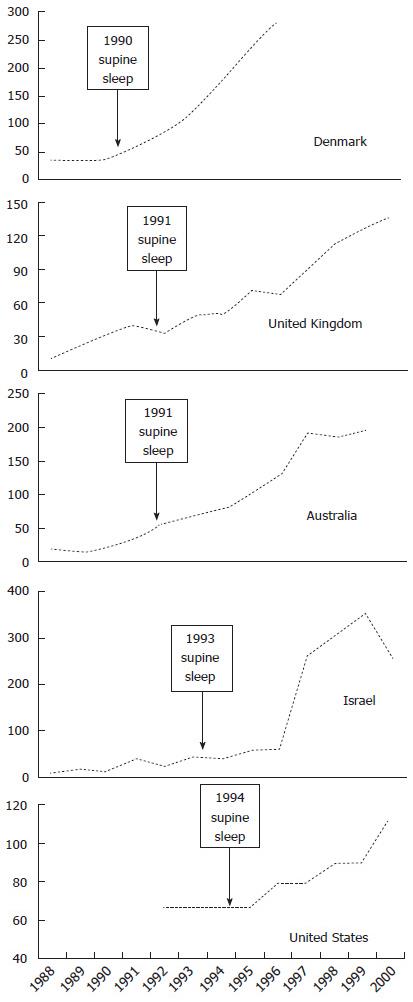
Data for autism incidence from five countries are presented in Table 1 and Figure 1, with incidence time series straddling supine sleep campaigns.
| Country | Campaign | Supine sleep data | Ref. |
| Denmark | 1990 | - | 1Madsen et al[45] Parner et al[109] |
| United Kingdom | 1991 | Gilbert et al[42] | 1Taylor et al[52] Blaxill[64] Smeeth et al[112] |
| Australia | 1991 | - | 1Nassar et al[53] Atladottir et al[51] Parner et al[109] |
| Israel | 1993 | Inbar et al[115] Tauman et al[116] | 1Gal et al[55] |
| United Sates | 1994 | Willinger et al[65] | Blaxill[64] Boyle et al[56] 1MMWR[49] Keyes et al[48] |
There is a broadly consistent temporal relationship between supine sleep campaigns and the change-points for autism increase for Denmark in 1990[45], United Kingdom in 1991[52], Australia in 1991[53], Israel in 1993[54,55], and the United States in 1994[49,56]. Note the data for Israel are as reported from a national database for medical insurance cover, so rather than the usual 8 years, the mean age at diagnosis was 39 mo[54], providing a close match of change-point with supine sleep campaign date. Uptake of supine sleep following launch of campaigns[42] correlates with rates of later autism increase. The change-points span a five-year period in five separate countries, making any other extrinsic or secular factor less likely.
The quality of supine sleep data is poor[42], but where such exists there is an improved correlation, since population supine sleep increase started before actual campaigns in the United States, United Kingdom and Australia. Norway has long term supine sleep data, the only data known to author that precedes safe sleep campaigns, based on a retrospective survey of parent recall conducted in 1992 and going back 25 years[57]. This showed a correlation of decreasing supine sleep with increasing SIDS, and a corresponding decrease of SIDS following supine sleep campaign[58]. Autism data from earlier years was not found, published data does not cover the putative change-point[59-61], a comment from such later reports is that this represents a “tenfold increase in all ASD” compared to previous reports[59].
McDonald and Paul[62] “used data sets from Denmark, California, Japan, and a worldwide composite of studies” on autism, seeking change-points that may assist in identifying an “exposure to controllable exogenous stressors”. They identified a worldwide change-point around 1988-1989. They identify Japan as being alone in having no change-point, this may reflect the patchy uptake of supine sleep from independent prefectures[63], with no standardised denominators for comparisons. Blaxill[64] provides a detailed review of time trends in autism prevalence in the United Kingdom and the United States. These results show slight increases preceding the formal campaigns, which may reflect population uptake of supine sleep prior to formal campaigns, or other factors. For the United Kingdom, Gilbert et al[42] shows change before 1990, for the United States this can be seen in the NISP data for sleeping position[65] and CDC data for SIDS[66]. Note however that countries with clear change-points all had supine sleep campaigns with launch dates.
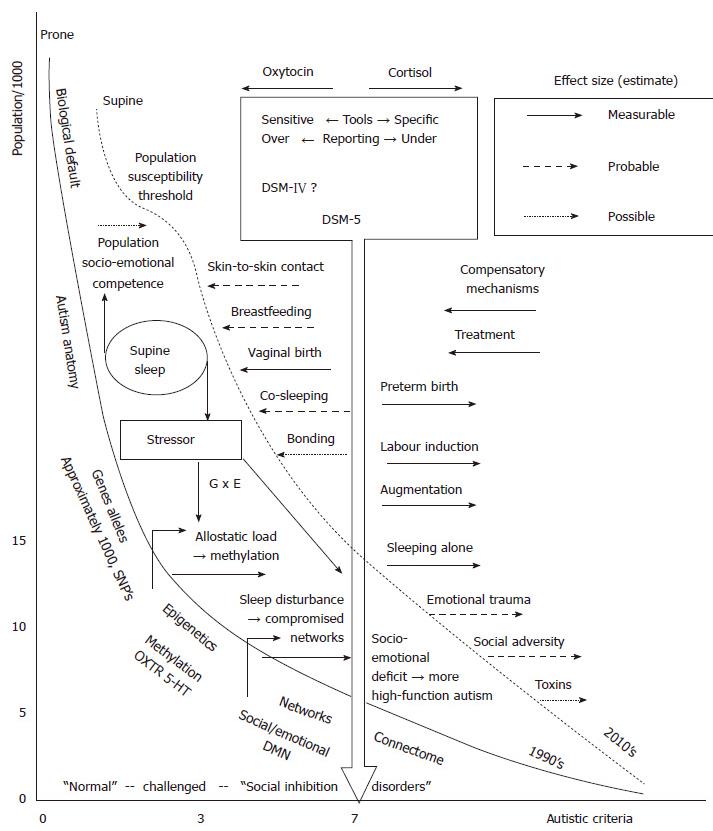
It is acknowledged that the quality of this data leaves much to be desired. However, the original case for association with prone sleep and SIDS was based on similar quality data. In either case, an epidemiological association requires a biologically plausible hypothesis of causation, which in turn can be tested. The hypothesis for supine sleep’s effect on autism incidence is presented as an integrated mechanism model in Figure 2. The “busyness” of the model is commensurate with the complexity of the subject. The population incidence is on the Y axis, plotted against the sum of individual symptom criteria of autism on the X axis. The majority of the population may have one or no autistic feature. However numbers of “healthy” people may have autistic features[67], but are coping well and without “impaired everyday functioning” (criteria D, DSM-5). Some may have impaired functioning and have some autistic features, perhaps part of another “social inhibition disorder”[41]. Given the high heritability and variable expression of “autism genes”, relatives of confirmed cases are likely to be clustered to the right of the larger population, closer to the diagnostic cut-off[68].
The DSM-5 does not provide diagnostic cut-off as a numeric, nor any sense of severity, but an arbitrary score of 7 is here marked by the blocked arrow as the diagnostic cut-off point for autism. The diagnostic cut-off line can be (administratively) shifted separately from the reality in the population. The new DSM-5 is believed to decrease the incidence[30], and is therefore more to the right than the DSM-IV cut-off. Diagnostic and screening tools, and new International Classification of Diseases editions, with various sensitivity and specificity, may be to the left or right of the block arrow. This could be depicted as a zone, and even be quantified, but makes little difference to the hypothesis presented here.
The curves represent the “population autistic criteria”, but could equally be seen as the “population emotional and social competence curves”; the focus of this paper is however on the extreme right of the curve where autism is diagnosed. Aligning with the ADDM, the model is regarded as applying to 8 years old, assuming all cases of autism are expressed or diagnosed by this age. The solid curve represents the “true” or real incidence of “population autistic criteria” in the population in the early 1990’s, the dashed line the real incidence in the 2010’s. Prior under-diagnosis would provide incidence to the left of solid line curve, later over-diagnosis provides figures to the right of the dashed curve. For realistic scale the curves are approximately aligned to the DSM-5 diagnostic cut-off with reported ADDM incidence data of 1992 and 2002 birth cohorts[49]. The curves should however be seen as a generic and conceptual model. Effectively this presents autism as having doubled in incidence during this period, conveyed by a right shift of the “population autistic criteria” curve. Note this is a modest increase in comparison to other data sources, factoring in secular artefactual increases, as described in the introduction.
To the left of the solid curve are itemised basic components of autism etiology and pathology. Space precludes a description of these, review references are provided on “autism anatomy”[69], “genes and alleles”[70-74], “epigenetics”[18,23], “networks”[75], and the “connectome”[76,77]. The latter suggests that autism is a neural network disease, providing a unifying view of the genes, epigenes, effects of environment, hormones and receptors and all the anatomical parts identified. Underlying genetic defects are necessary, and in some cases sufficient, to “cause” autism[72,78].
To the right of the block arrow are external factors other than the hypothesised effect of supine sleep that are known or believed to increase autism incidence (right shift of curve, dashed line). This hypothesis does not address prenatal adverse factors.
Being born preterm exposes the developing brain to an environment that makes profound changes to a broad range of genes, even in children who do not go on to develop autism[79], and even those born late preterm[80]. The incidence of preterm birth has increased in the ten year period depicted, but so have also efforts to improve the quality of care: Preterm birth may therefore account for a small portion of the increase depicted.
In a population sample (North Carolina) a significant effect on autism was found following induction and augmentation of labour[81]. Caesarean birth can for similar reasons be included in this argument[82]. Though of relatively short duration, these interventions directly disrupt key oxytocin circuits of the connectome in autism[83,84]. These interventions may interfere with the normal pulsatile function of oxytocin[85]. Oxytocin administration has been in use for many years preceding 1994, only in so far as the use has increased after 1994 may this have made a minor contribution to the observed increase. In terms of gene-environment interaction[86], induction and augmentation involve approximately a one day “adversity dose”. A single anaesthetic and surgical procedure for an infant does not appear to be enough “adversity dose” to impact the curve[87]. Schieve et al[50] review evidence on factors associated with autism as above, and have quantified the possible impact the above factors may have had on the increase in autism, concluding their contribution has been negligible.
“Toxic stress” as defined in early childhood development[88] would also produce a right shift. However, much of the underlying etiology of autism begins in the uterine and early birth period[78]. Even in great adversity, if the early uterine environment and the early perinatal period was “good-enough”, some resilience will be in place. The contribution to increasing autism may thus be relatively small, even if many other developmental and social ills follow. This hypothesis requires that “toxic stress” be expressed early to increase the incidence of autism. Repeated emotional and social traumas during the first year of life have been linked to autism[89]. Toxic stress during the First 1000 Days will certainly further exacerbate early changes that took place[90], and so the sum contribution of childhood adversity to right shift is at most moderate, but more likely mild as other factors preceded.
A large number of environmental factors and toxins have been proposed as contributing to the increase in autism. Many of them are plausible in so far as they can impact on epigenetic mechanisms and neurodevelopmental processes. However none of them can easily be linked to the increase since the early 1990’s. A possible contributor is advancing parental age[91], which may be acting through increased genetic and allelic changes.
To the left of the block arrow are protective factors. Skin-to-skin contact and breastfeeding support oxytocin networks[92], so shift the curve to the left. The paradigm could however be that they represent the basic biologically normal condition of the original and normative curve for “population autistic criteria” (prone sleep, solid curve), in which autism is infrequently caused solely by adverse genomic phenomena. Prone sleep can be regarded as part of a package of normal biological expectation of human reproduction. Co-sleeping is controversial, but is an integral part of human life course sciences[93]. In the context of preterm birth, and perhaps family history of autistic features, consciously increasing the dose of leftward factors (enhancing oxytocin) may be an informed choice for some parents. Higher maternal intake of folate and some other nutrients may lower autism risk[94]. In the ecobiodevelopmental model presented by Shonkoff et al[88], “life science theory” is presented as a key concept alongside epigenetics and neuroscience. In the model, all of the protective factors listed are in fact directly out of “life science theory”. This encompasses a holistic approach to reproduction, where no single factor acts in isolation.
Compensatory mechanisms develop in the majority of autism cases later in life[95], but some may be apparent and effective at 8 years, and provide a left shift to the curve. Successful treatment likewise, if only by accomplishing everyday functioning: It appears the underlying neurology does not change that much[96].
The hypothesis that supine sleep produces rightward shift, i.e., increases autism incidence, is depicted between the solid and dashed curves. Supine sleep can be seen as a population-wide novel environmental factor introduced in the early 1990’s, before which the solid line of the model represents the baseline “population autism criteria”.
Supine sleep may bring two separate and distinct stressor disruptions to early development. In the context of the current public health recommendations, it does so over an extended and critical period, more so than many of the “right shifting” factors described above. First is the autonomic stressor effect, sufficient to cause motor developmental delay[17]. High sympathetic tone elevates cortisol and other mediators, which may lead to gene methylation[90,97]. Oxytocin receptor gene methylation has been measured accurately[89], showing a correlation with autism severity, with methylation reported as percentages[98]. Changes may be acute or act over time, as described in the allostasis and allostatic load concept[86]. The Developmental Origins of Health And Disease concept clarifies that developmental disruptions caused by stressors occur at critical periods during development, impacting only the specific developmental goal of that time[99]. One result of stress in the period from 0-2 mo may be disruption of socio-emotional networks, and other parts of the connectome implicated in autism[76]. The default mode network is implicated in autism, this may likely be disturbed antenatally[72,78,100], but could be further dysregulated by the stress of supine sleep.
Second, good quality sleep cycling is necessary for consolidation of memory in adults[101], and even more for neurodevelopment in infants and children[19]. Supine sleep disturbs sleep architecture, with autonomic effects equivalent to anxious arousal and with adverse effect on normal sleep cyclicity[102]. The consolidation and integration of diverse neural networks is necessary for developing the capacities required for Theory of Mind[103]. Oxytocin is core to developing emotional and social networks, and future Theory of Mind[104]. Birth itself and early bonding are highly reliant on oxytocin, which is critical to the parturition process, to early breastfeeding and to bonding[105,106]. Breastfeeding and early bonding are maintained by skin-to-skin contact, which in and of itself supports oxytocin, and the neurobiological processes associated with oxytocin. Continued contact allows mother to be sensitive and attuned to her infant’s cues[107], and the infant to establish a trajectory toward a secure attachment.
Ecologically, supine sleep may be part of a package that acts synergistically to disrupt development. Supine sleep and swaddling often go together, the latter per se increases stress, even when practised only the first day there is a measurable adverse impact one year later[108]. Life sciences theory affirms infants should never sleep alone, and maternal-infant separation has been shown to increase autonomic arousal[102]. Other co-factors do undoubtedly exist, but for the current paper, supine sleep is identified as a likely contributor to developmental disruption leading to the increase in autism.
Supine sleep does not “cause” autism in and of itself, the model proposes it as one of many external risk factors, operating during a critical period, and requiring underlying vulnerability (genetic susceptibility), analogous to the Triple Risk Model for SIDS. In the model therefore, it is proposed that supine sleep is exerting at least a moderate effect in shifting the curve to the right, thereby increasing the incidence of autism.
This hypothesis is consistent with the changing profile of the autism spectrum in the last two decades. The actual numbers of cases with lower IQ and profound developmental disruption has stayed approximately the same[109,110], but the proportion with high functioning and high IQ has increased[48,53]. The first four months are a critical period for socio-emotional development, not IQ. This is also consistent with the observation that many cases are only diagnosed after some years, despite public health efforts at “early diagnosis”. The Theory of Mind concept comes with a prolonged “latent” period[103], and when the primary stressor only starts after birth, as opposed to early and midfetal life[72,78], the expression and recognition of autism may be similarly delayed.
Denmark[45] and Japan[110] are countries that have specifically studied autism in relation to vaccines, demonstrating no effect of the latter. Both however document similar increase in autism incidence (after thimerosal cessation), and in contexts where similar diagnostic criteria have been used consistently. The Danish data is robust, being based on total population inpatient and outpatient psychiatric records, with the population register as denominator. Incidence prior to 1990 was stable, after which there was an increase[45], such an increase may indeed be caused by increased community awareness. Hansen et al[111] attribute 60% of increase in Denmark to change in diagnostic criteria in 1994 and inclusion of outpatients in 1995. In Yokohoma Japan, in a defined catchment with dedicated mental health services and standardised tools, reported incidence increased from 40/1000 between 1988 and 1992, to 117.2 for those born in 1996[110]. Robust data also come from the United Kingdom (United Kingdom General Practice Research Database)[112], showing a fivefold increase from 1988 through to 2001, after which “incidence and prevalence rates in 8-year old children reached a plateau… and remained steady through 2010”[52]. This appears to apply also for Israel and Australia, showing a levelling off of the autism increase[52-54], possibly also in the United States since 2001[113], with supine sleep rates remaining stable. This is consistent with the hypothesis presented in that the population dose of supine sleep cannot increase much more (96% in 1996 in the United Kingdom)[42], and the full effect of susceptibility from this one proposed contributory factor is maximised. The hypothesis presented here for autism may equally apply to all or some of social inhibition disorders.
In parallel to autism, SIDS reduction reached a plateau in the United States and elsewhere. Apart from the finite stressor dose effect, another reason for this may be that for both SIDS and autism there are rare underlying genetic susceptibilities. Under the most ideal conditions of low environmental risk factors, both autism and SIDS would therefore still occur, though rarely. Expression of autism and SIDS genes are exacerbated by adverse environments. In autism, supine sleep thereby exerts an epigenetic and developmental mechanism that disrupts the connectome. In SIDS, supine sleep is working as a protective mechanism on already disrupted neural networks. Since supine sleep is a stressor, and is acting at an intermediate level of causation, it is an imperfect intervention, and can only prevent a finite portion of SIDS mortality, hence the plateau. In the absence of new risk factor changes, it is likely that all current increase in autism is secular, as presented for Sweden[28].
In presenting this integrated mechanism review as a hypothesis to this readership, the intention is not to make any kind of public health recommendation, this would be premature, and beyond the scope of this paper. The implications are however considerable, and merit urgent attention: A reassessment is warranted[114]. The epidemiological arguments presented should be scrutinised in data sources globally, with respect to sleep position, autism and SIDS. The proposed model identifies some of the complexity involved, in that exacerbating factors over and above supine sleep need to be teased out, as well as protective factors.
The primary contention that supine sleep is a stressor is amenable to testing. Current clinical and physiological studies already provide ample evidence that supine sleep causes autonomic arousal, and other stressor effects, but this finding has been interpreted as healthy. It may be interpreted as harmful if methylation of specific receptors related to autism could be correlated to supine sleep position. A purely epidemiological approach could be to select cohorts that complied or did not comply to supine sleep recommendations, and compare autism rates, first retrospectively, and then perhaps prospectively. Ethically the latter might be possible if the prone cohort had additional protective measures against SIDS. Genome-wide sequencing for methylation, and focusing on methylation of specific genes identified (e.g., for oxytocin receptors), could establish presence or absence of harmful stress. Other measures of stress or allostatic load, and socio-emotional outcome measures, may confirm or refute the hypothesis. The prevalence of SIDS defects and autism genes should be quantified. This may allow a new perspective on the risk benefit ratio in terms of quantifying SIDS decrease against possible autism increase. More research could focus on practical methods to identify neonates with SIDS and autism susceptibility, allowing for differentiated care options.
In conclusion, it is proposed that there may be an association between supine sleep and autism incidence increase. No other potential stressor than supine sleep is known to have been introduced globally in widely separated regions, nor one that matches the temporal patterns described here. The biological rationale proposed is that supine sleep may be a stressor, increasing gene methylation in, and disrupting needed sleep cyclicity for developing socio-emotional neural circuits. As stated above, it would be premature to offer any kind of clinical or parenting advice based on this hypothesis. Rather, the SIDS mechanism proposed and autism model presented should be urgently examined and researched, then the future research agenda may be better directed, toward better care and advice to parents and health departments in order to further decrease SIDS, and reduce autism incidence.
I am grateful to colleagues, and several reviewers, who have provided me with literature and information, challenged my thinking, and commented on aspects of this paper. I am especially grateful to my wife Jill, for assistance with the figures and on-going support and proof-reading this manuscript.
Autism has increased since the early 1990’s. Sudden infant death syndrome (SIDS) decreased only to level off after supine sleep campaigns in the 1990’s. Currently supine sleep for neonates and infants is very strongly encouraged by public health authorities. However, the mechanism whereby supine sleep achieves SIDS reduction is totally unknown. This article suggests that supine sleep achieves SIDS reduction through a stressor mechanism, which will have the unintended side-effect of increasing autistic criteria in sensitive individuals of the population. The relevance of this article is that a re-evaluation of the fields of both SIDS and autism may lead to research that improves outcomes for both.
Current research into SIDS includes identifying the mechanism of harm from prone sleep; this article suggests such research is fruitless. In terms of autism, a more fruitful direction of research suggested by this study is developmental stress biology.
The major innovative thinking of this article lies in its re-appraisal of prone sleep as the healthy physiological sleep. This can be seen as an application of “rare disease epidemiology”. For example, the rare side-effects of vaccines given to the whole population are accepted since the risks of those are greatly outweighed by the benefits. In the case of supine sleep campaigns, the potential risks have not been properly evaluated. Increasing autistic criteria in the population should be regarded as a major risk factor, which requires urgent and accurate quantification in order to properly balance benefit and risk of supine sleep.
These findings emphasise that for both conditions, rare underlying genetic susceptibility is fundamental, and this will be a fruitful direction of research. The mechanistic model published previously on SIDS, and the model in this article on autism, allow more focused preventive and therapeutic application. In SIDS for example, a cardiorespiratory based physiological screening test is a possibility. In autism, genetic screening could identify a smaller part of the population for which family counselling allowing may result in advice to provide prone sleep, based on “informed choice”.
The term autism is used for brevity, where the correct terminology is autism spectrum disorder. This is currently best understood as a “connectome” disorder, this term refers to brain networks and their interactions, shared areas of high neural network traffic are referred to as hubs.
The topic is really interesting and the manuscript is clear and well organized.
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: South Africa
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ciccone MM, Verrotti A S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Hauck FR, Tanabe KO. International trends in sudden infant death syndrome: stabilization of rates requires further action. Pediatrics. 2008;122:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Alm B, Norvenius SG, Wennergren G, Skjaerven R, Øyen N, Milerad J, Wennborg M, Kjaerbeck J, Helweg-Larsen K, Irgens LM. Changes in the epidemiology of sudden infant death syndrome in Sweden 1973-1996. Arch Dis Child. 2001;84:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:e1341-e1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Bergman NJ. Proposal for mechanisms of protection of supine sleep against sudden infant death syndrome: an integrated mechanism review. Pediatr Res. 2015;77:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Randall BB, Paterson DS, Haas EA, Broadbelt KG, Duncan JR, Mena OJ, Krous HF, Trachtenberg FL, Kinney HC. Potential asphyxia and brainstem abnormalities in sudden and unexpected death in infants. Pediatrics. 2013;132:e1616-e1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr Pulmonol. 2003;36:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Dergacheva O, Griffioen KJ, Neff RA, Mendelowitz D. Respiratory modulation of premotor cardiac vagal neurons in the brainstem. Respir Physiol Neurobiol. 2010;174:102-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage. 2012;60:505-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 295] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 10. | Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 389] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 11. | Kohyama J, Shimohira M, Itoh M, Fukumizu M, Iwakawa Y. Phasic muscle activity during REM sleep in infancy-normal maturation and contrastive abnormality in SIDS/ALTE and West syndrome. J Sleep Res. 1993;2:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Kahn A, Groswasser J, Rebuffat E, Sottiaux M, Blum D, Foerster M, Franco P, Bochner A, Alexander M, Bachy A. Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep. 1992;15:287-292. [PubMed] |
| 13. | Kinney HC. Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies. Dev Psychobiol. 2009;51:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 655] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 15. | Heemstra HE, van Weely S, Büller HA, Leufkens HG, de Vrueh RL. Translation of rare disease research into orphan drug development: disease matters. Drug Discov Today. 2009;14:1166-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Biggs WS. Diagnosis and management of positional head deformity. Am Fam Physician. 2003;67:1953-1956. [PubMed] |
| 17. | Majnemer A, Barr RG. Association between sleep position and early motor development. J Pediatr. 2006;149:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | LaSalle JM. Epigenomic strategies at the interface of genetic and environmental risk factors for autism. J Hum Genet. 2013;58:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Peirano P, Algarín C, Uauy R. Sleep-wake states and their regulatory mechanisms throughout early human development. J Pediatr. 2003;143:S70-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Schore AN. Effects of a secure attachment relationship on right brain development, affect regulation, and infant mental health. Infant Ment Health J. 2001;22:7-66. [DOI] [Full Text] |
| 21. | Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 876] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 22. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders - dsm-iv-tr. Arlington: American Psychiatric Publishing 2013; 1-85. |
| 23. | Mbadiwe T, Millis RM. Epigenetics and autism. Autism Res Treat. 2013;2013:826156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16:e92-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 258] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 25. | Radulescu E, Minati L, Ganeshan B, Harrison NA, Gray MA, Beacher FD, Chatwin C, Young RC, Critchley HD. Abnormalities in fronto-striatal connectivity within language networks relate to differences in grey-matter heterogeneity in Asperger syndrome. Neuroimage Clin. 2013;2:716-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45:601-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 642] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 27. | Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, Montiel-Nava C, Patel V, Paula CS, Wang C. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5:160-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1937] [Cited by in RCA: 1434] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 28. | Lundström S, Reichenberg A, Anckarsäter H, Lichtenstein P, Gillberg C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ. 2015;350:h1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Pennington ML, Cullinan D, Southern LB. Defining autism: variability in state education agency definitions of and evaluations for autism spectrum disorders. Autism Res Treat. 2014;2014:327271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Kulage KM, Smaldone AM, Cohn EG. How will DSM-5 affect autism diagnosis? A systematic literature review and meta-analysis. J Autism Dev Disord. 2014;44:1918-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Maenner MJ, Rice CE, Arneson CL, Cunniff C, Schieve LA, Carpenter LA, Van Naarden Braun K, Kirby RS, Bakian AV, Durkin MS. Potential impact of DSM-5 criteria on autism spectrum disorder prevalence estimates. JAMA Psychiatry. 2014;71:292-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 32. | Kim YS, Fombonne E, Koh YJ, Kim SJ, Cheon KA, Leventhal BL. A comparison of DSM-IV pervasive developmental disorder and DSM-5 autism spectrum disorder prevalence in an epidemiologic sample. J Am Acad Child Adolesc Psychiatry. 2014;53:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 33. | King BH, Navot N, Bernier R, Webb SJ. Update on diagnostic classification in autism. Curr Opin Psychiatry. 2014;27:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Huerta M, Bishop SL, Duncan A, Hus V, Lord C. Application of DSM-5 criteria for autism spectrum disorder to three samples of children with DSM-IV diagnoses of pervasive developmental disorders. Am J Psychiatry. 2012;169:1056-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 35. | Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC. Changes in prevalence of parent-reported autism spectrum disorder in school-aged U.S. children: 2007 to 2011-2012. Natl Health Stat Report. 2013;1-11, 1 p following 11. [PubMed] |
| 36. | Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, Cheon KA, Kim SJ, Kim YK, Lee H. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 795] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 37. | Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, Bebbington P, Jenkins R, Meltzer H. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 507] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 38. | Bishop DV, Whitehouse AJ, Watt HJ, Line EA. Autism and diagnostic substitution: evidence from a study of adults with a history of developmental language disorder. Dev Med Child Neurol. 2008;50:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Shattuck PT. The contribution of diagnostic substitution to the growing administrative prevalence of autism in US special education. Pediatrics. 2006;117:1028-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Gillberg C, Fernell E. Autism plus versus autism pure. J Autism Dev Disord. 2014;44:3274-3276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Hrdlicka M, Dudova I. Controversies in autism: is a broader model of social disorders needed? Child Adolesc Psychiatry Ment Health. 2013;7:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Gilbert R, Salanti G, Harden M, See S. Infant sleeping position and the sudden infant death syndrome: systematic review of observational studies and historical review of recommendations from 1940 to 2002. Int J Epidemiol. 2005;34:874-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Bresnahan M, Li G, Susser E. Hidden in plain sight. Int J Epidemiol. 2009;38:1172-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | King M, Bearman P. Diagnostic change and the increased prevalence of autism. Int J Epidemiol. 2009;38:1224-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 45. | Madsen KM, Lauritsen MB, Pedersen CB, Thorsen P, Plesner AM, Andersen PH, Mortensen PB. Thimerosal and the occurrence of autism: negative ecological evidence from Danish population-based data. Pediatrics. 2003;112:604-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Rutter M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr. 2005;94:2-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Williams JG, Higgins JP, Brayne CE. Systematic review of prevalence studies of autism spectrum disorders. Arch Dis Child. 2006;91:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 256] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 48. | Keyes KM, Susser E, Cheslack-Postava K, Fountain C, Liu K, Bearman PS. Cohort effects explain the increase in autism diagnosis among children born from 1992 to 2003 in California. Int J Epidemiol. 2012;41:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Morbidity and Mortality Weekly Report (MMWR). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1-21. [PubMed] |
| 50. | Schieve LA, Rice C, Devine O, Maenner MJ, Lee LC, Fitzgerald R, Wingate MS, Schendel D, Pettygrove S, van Naarden Braun K. Have secular changes in perinatal risk factors contributed to the recent autism prevalence increase? Development and application of a mathematical assessment model. Ann Epidemiol. 2011;21:930-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Atladottir HO, Gyllenberg D, Langridge A, Sandin S, Hansen SN, Leonard H, Gissler M, Reichenberg A, Schendel DE, Bourke J. The increasing prevalence of reported diagnoses of childhood psychiatric disorders: a descriptive multinational comparison. Eur Child Adolesc Psychiatry. 2015;24:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 52. | Taylor B, Jick H, Maclaughlin D. Prevalence and incidence rates of autism in the UK: time trend from 2004-2010 in children aged 8 years. BMJ Open. 2013;3:e003219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 53. | Nassar N, Dixon G, Bourke J, Bower C, Glasson E, de Klerk N, Leonard H. Autism spectrum disorders in young children: effect of changes in diagnostic practices. Int J Epidemiol. 2009;38:1245-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Senecky Y, Chodick G, Diamond G, Lobel D, Drachman R, Inbar D. Time trends in reported autistic spectrum disorders in Israel, 1972-2004. Isr Med Assoc J. 2009;11:30-33. [PubMed] |
| 55. | Gal G, Abiri L, Reichenberg A, Gabis L, Gross R. Time trends in reported autism spectrum disorders in Israel, 1986-2005. J Autism Dev Disord. 2012;42:428-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, Visser S, Kogan MD. Trends in the prevalence of developmental disabilities in US children, 1997-2008. Pediatrics. 2011;127:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1015] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 57. | Irgens LM, Markestad T, Baste V, Schreuder P, Skjaerven R, Oyen N. Sleeping position and sudden infant death syndrome in Norway 1967-91. Arch Dis Child. 1995;72:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Arntzen A, Samuelsen SO, Daltveit AK, Stoltenberg C. Post-neonatal mortality in Norway 1969-95: a cause-specific analysis. Int J Epidemiol. 2006;35:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Isaksen J, Diseth TH, Schjølberg S, Skjeldal OH. Observed prevalence of autism spectrum disorders in two Norwegian counties. Eur J Paediatr Neurol. 2012;16:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Nilsen RM, Surén P, Gunnes N, Alsaker ER, Bresnahan M, Hirtz D, Hornig M, Lie KK, Lipkin WI, Reichborn-Kjennerud T. Analysis of self-selection bias in a population-based cohort study of autism spectrum disorders. Paediatr Perinat Epidemiol. 2013;27:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 61. | Surén P, Bakken IJ, Aase H, Chin R, Gunnes N, Lie KK, Magnus P, Reichborn-Kjennerud T, Schjølberg S, Øyen AS. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in Norwegian children. Pediatrics. 2012;130:e152-e158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 62. | McDonald ME, Paul JF. Timing of increased autistic disorder cumulative incidence. Environ Sci Technol. 2010;44:2112-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Sawaguchi T, Namiki M. Recent trend of the incidence of sudden infant death syndrome in Japan. Early Hum Dev. 2003;75 Suppl:S175-S179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Blaxill MF. What’s going on? The question of time trends in autism. Public Health Rep. 2004;119:536-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Willinger M, Hoffman HJ, Wu KT, Hou JR, Kessler RC, Ward SL, Keens TG, Corwin MJ. Factors associated with the transition to nonprone sleep positions of infants in the United States: the National Infant Sleep Position Study. JAMA. 1998;280:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 177] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Colson ER, Rybin D, Smith LA, Colton T, Lister G, Corwin MJ. Trends and factors associated with infant sleeping position: the national infant sleep position study, 1993-2007. Arch Pediatr Adolesc Med. 2009;163:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 67. | Jung M, Kosaka H, Saito DN, Ishitobi M, Morita T, Inohara K, Asano M, Arai S, Munesue T, Tomoda A. Default mode network in young male adults with autism spectrum disorder: relationship with autism spectrum traits. Mol Autism. 2014;5:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 68. | Saito Y, Suga M, Tochigi M, Abe O, Yahata N, Kawakubo Y, Liu X, Kawamura Y, Sasaki T, Kasai K. Neural correlate of autistic-like traits and a common allele in the oxytocin receptor gene. Soc Cogn Affect Neurosci. 2014;9:1443-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Stigler KA, McDonald BC, Anand A, Saykin AJ, McDougle CJ. Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Res. 2011;1380:146-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 70. | Fakhoury M. Autistic spectrum disorders: A review of clinical features, theories and diagnosis. Int J Dev Neurosci. 2015;43:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 71. | Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, Mahajan M, Manaa D, Pawitan Y, Reichert J. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46:881-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 906] [Cited by in RCA: 812] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 72. | Hormozdiari F, Penn O, Borenstein E, Eichler EE. The discovery of integrated gene networks for autism and related disorders. Genome Res. 2015;25:142-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 73. | Radua J, El-Hage W, Monté GC, Gohier B, Tropeano M, Phillips ML, Surguladze SA. COMT Val158Met × SLC6A4 5-HTTLPR interaction impacts on gray matter volume of regions supporting emotion processing. Soc Cogn Affect Neurosci. 2014;9:1232-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Rosti RO, Sadek AA, Vaux KK, Gleeson JG. The genetic landscape of autism spectrum disorders. Dev Med Child Neurol. 2014;56:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Zielinski BA, Anderson JS, Froehlich AL, Prigge MB, Nielsen JA, Cooperrider JR, Cariello AN, Fletcher PT, Alexander AL, Lange N. scMRI reveals large-scale brain network abnormalities in autism. PLoS One. 2012;7:e49172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 76. | Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, Bullmore ET. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137:2382-2395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 810] [Cited by in RCA: 835] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 77. | Goch CJ, Stieltjes B, Henze R, Hering J, Poustka L, Meinzer HP, Maier-Hein KH. Quantification of changes in language-related brain areas in autism spectrum disorders using large-scale network analysis. Int J Comput Assist Radiol Surg. 2014;9:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Casanova EL, Casanova MF. Genetics studies indicate that neural induction and early neuronal maturation are disturbed in autism. Front Cell Neurosci. 2014;8:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Ball G, Boardman JP, Aljabar P, Pandit A, Arichi T, Merchant N, Rueckert D, Edwards AD, Counsell SJ. The influence of preterm birth on the developing thalamocortical connectome. Cortex. 2013;49:1711-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 80. | Guy A, Seaton SE, Boyle EM, Draper ES, Field DJ, Manktelow BN, Marlow N, Smith LK, Johnson S. Infants born late/moderately preterm are at increased risk for a positive autism screen at 2 years of age. J Pediatr. 2015;166:269-75.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 81. | Gregory SG, Anthopolos R, Osgood CE, Grotegut CA, Miranda ML. Association of autism with induced or augmented childbirth in North Carolina Birth Record (1990-1998) and Education Research (1997-2007) databases. JAMA Pediatr. 2013;167:959-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 82. | Polo-Kantola P, Lampi KM, Hinkka-Yli-Salomäki S, Gissler M, Brown AS, Sourander A. Obstetric risk factors and autism spectrum disorders in Finland. J Pediatr. 2014;164:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 83. | Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 424] [Cited by in RCA: 417] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 84. | LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry. 2015;20:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 239] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 85. | Olza Fernández I, Marín Gabriel M, Malalana Martínez A, Fernández-Cañadas Morillo A, López Sánchez F, Costarelli V. Newborn feeding behaviour depressed by intrapartum oxytocin: a pilot study. Acta Paediatr. 2012;101:749-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1191] [Cited by in RCA: 1033] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 87. | Ko WR, Huang JY, Chiang YC, Nfor ON, Ko PC, Jan SR, Lung CC, Chang HC, Lin LY, Liaw YP. Risk of autistic disorder after exposure to general anaesthesia and surgery: a nationwide, retrospective matched cohort study. Eur J Anaesthesiol. 2015;32:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 88. | Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232-e246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2721] [Cited by in RCA: 2579] [Article Influence: 198.4] [Reference Citation Analysis (1)] |
| 89. | Kumsta R, Hummel E, Chen FS, Heinrichs M. Epigenetic regulation of the oxytocin receptor gene: implications for behavioral neuroscience. Front Neurosci. 2013;7:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 90. | McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2696] [Cited by in RCA: 2126] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 91. | Idring S, Magnusson C, Lundberg M, Ek M, Rai D, Svensson AC, Dalman C, Karlsson H, Lee BK. Parental age and the risk of autism spectrum disorders: findings from a Swedish population-based cohort. Int J Epidemiol. 2014;43:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 92. | Uvnäs-Moberg K. Neuroendocrinology of the mother-child interaction. Trends Endocrinol Metab. 1996;7:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Gettler LT, McKenna JJ. Evolutionary perspectives on mother-infant sleep proximity and breastfeeding in a laboratory setting. Am J Phys Anthropol. 2011;144:454-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. 2014;43:443-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 281] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 95. | Jones EJ, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci Biobehav Rev. 2014;39:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 359] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 96. | Canitano R. New experimental treatments for core social domain in autism spectrum disorders. Front Pediatr. 2014;2:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 97. | Wang D, Szyf M, Benkelfat C, Provençal N, Turecki G, Caramaschi D, Côté SM, Vitaro F, Tremblay RE, Booij L. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS One. 2012;7:e39501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 98. | Jack A, Connelly JJ, Morris JP. DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Front Hum Neurosci. 2012;6:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 99. | Hochberg Z, Feil R, Constancia M, Fraga M, Junien C, Carel JC, Boileau P, Le Bouc Y, Deal CL, Lillycrop K. Child health, developmental plasticity, and epigenetic programming. Endocr Rev. 2011;32:159-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 421] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 100. | Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr. 2015;169:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 101. | Born J, Wagner U. Sleep, hormones, and memory. Obstet Gynecol Clin North Am. 2009;36:809-829, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Morgan BE, Horn AR, Bergman NJ. Should neonates sleep alone? Biol Psychiatry. 2011;70:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 103. | Kana RK, Libero LE, Hu CP, Deshpande HD, Colburn JS. Functional brain networks and white matter underlying theory-of-mind in autism. Soc Cogn Affect Neurosci. 2014;9:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 104. | Parker KJ, Garner JP, Libove RA, Hyde SA, Hornbeak KB, Carson DS, Liao CP, Phillips JM, Hallmayer JF, Hardan AY. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci USA. 2014;111:12258-12263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 105. | Feldman R. Oxytocin and social affiliation in humans. Horm Behav. 2012;61:380-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 409] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 106. | Olza-Fernández I, Marín Gabriel MA, Gil-Sanchez A, Garcia-Segura LM, Arevalo MA. Neuroendocrinology of childbirth and mother-child attachment: the basis of an etiopathogenic model of perinatal neurobiological disorders. Front Neuroendocrinol. 2014;35:459-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 107. | Bigelow AE, Littlejohn M, Bergman N, McDonald C. The relation between early mother-infant skin to skin contact and later maternal sensitivity in South African mothers of low birth weight infants. Infant Ment Health J. 2010;31:358-377. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 108. | Bystrova K, Ivanova V, Edhborg M, Matthiesen AS, Ransjö-Arvidson AB, Mukhamedrakhimov R, Uvnäs-Moberg K, Widström AM. Early contact versus separation: effects on mother-infant interaction one year later. Birth. 2009;36:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 109. | Parner ET, Thorsen P, Dixon G, de Klerk N, Leonard H, Nassar N, Bourke J, Bower C, Glasson EJ. A comparison of autism prevalence trends in Denmark and Western Australia. J Autism Dev Disord. 2011;41:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 110. | Honda H, Shimizu Y, Rutter M. No effect of MMR withdrawal on the incidence of autism: a total population study. J Child Psychol Psychiatry. 2005;46:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 111. | Hansen SN, Schendel DE, Parner ET. Explaining the increase in the prevalence of autism spectrum disorders: the proportion attributable to changes in reporting practices. JAMA Pediatr. 2015;169:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 112. | Smeeth L, Cook C, Fombonne PE, Heavey L, Rodrigues LC, Smith PG, Hall AJ. Rate of first recorded diagnosis of autism and other pervasive developmental disorders in United Kingdom general practice, 1988 to 2001. BMC Med. 2004;2:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Rosenberg RE, Daniels AM, Law JK, Law PA, Kaufmann WE. Trends in autism spectrum disorder diagnoses: 1994-2007. J Autism Dev Disord. 2009;39:1099-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Pelligra R, Doman G, Leisman G. A reassessment of the SIDS Back to Sleep Campaign. ScientificWorldJournal. 2005;5:550-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 115. | Inbar Z, Meibar R, Shehada S, Irena V, Rubin L, Rishpon S. “Back to sleep”: parents compliance with the recommendation on the most appropriate sleeping position of infants, Haifa District, Israel, 2001. Prev Med. 2005;40:765-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 116. | Tauman R, Reisner SH, Amitai Y, Wasser J, Nehama H, Sivan Y. Sleep position in Israeli Jewish infants following the “back to sleep” campaign. Isr Med Assoc J. 2004;6:540-545. [PubMed] |