Published online Nov 8, 2015. doi: 10.5409/wjcp.v4.i4.148
Peer-review started: January 7, 2015
First decision: April 27, 2015
Revised: May 16, 2015
Accepted: September 10, 2015
Article in press: September 16, 2015
Published online: November 8, 2015
Processing time: 307 Days and 20.2 Hours
AIM: To evaluate the presentation patterns of a cohort of children diagnosed with coeliac disease (CD) at Christchurch Hospital, New Zealand.
METHODS: Children aged 16 years or less diagnosed with CD at Christchurch Hospital, Christchurch, New Zealand, over the 11 year period between 2000 and 2010 were identified retrospectively. Diagnosis of CD was based upon standard histological criteria of endoscopically-obtained duodenal biopsies. Overlapping search methods were used to identify all relevant diagnoses within the time period. Endoscopy reports and histology findings were reviewed to confirm diagnosis. The numbers of diagnoses per year were calculated and changes in annual rates over the study period were delineated. Available records were reviewed to ascertain presenting symptoms, baseline anthropometry and the indication for referral for each child. In addition, the results of relevant investigations prior to diagnosis were accessed and reviewed. These key investigations included the results of coeliac serology testing (including tissue transglutaminase and endomysial antibodies) as well as the results of tests measuring levels of micronutrients, such as iron. In addition, the histological findings of concurrent biopsies in the oesophagus and stomach were reviewed.
RESULTS: Over the 11 year study period, 263 children were diagnosed with CD at this New Zealand paediatric facility. Children were diagnosed from late infancy to 16.9 years: the largest subgroup of children (n = 111) were diagnosed between 5 and 12 years of age. The numbers of children diagnosed each year increased from 13 per year to 31 per year over the 11 years (P = 0.0095). Preschool children (aged less than 5 years) were more likely to have low weight, and to have diarrhoea and abdominal pain prior to diagnosis. Older children (over 5 years of age) most commonly presented with abdominal pain. Fifty-six (21.6%) of the 263 children were diagnosed following screening in high risk groups, with 38 of these children having no symptoms at diagnosis. Mean weight Z scores were lower in children aged less than five years than children aged 5-12 years or older children (-0.4096 ± 1.24, vs 0.1196 ± 0.966 vs 0.0901 ± 1.14 respectively: P = 0.0033).
CONCLUSION: Increasing numbers of children were diagnosed with CD in this New Zealand centre over this time, with varied presentations and symptoms.
Core tip: Coeliac disease (CD) is increasingly diagnosed throughout childhood. Physicians must be aware of the varied presentations of CD in children and consider this diagnosis accordingly.
- Citation: Kho A, Whitehead M, Day AS. Coeliac disease in children in Christchurch, New Zealand: Presentation and patterns from 2000-2010. World J Clin Pediatr 2015; 4(4): 148-154
- URL: https://www.wjgnet.com/2219-2808/full/v4/i4/148.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v4.i4.148
Coeliac disease (CD) is an autoimmune disease of the small bowel, triggered by dietary exposure to gluten in genetically susceptible individuals[1]. Ingestion of gluten provokes immune-mediated damage to the small intestinal mucosa. Following diagnosis of CD the optimal management currently involves a life-long gluten-free diet (GFD)[2].
Although traditionally felt to be a disease of infancy, presenting with classical symptoms of malabsorption (diarrhoea, abdominal bloating and failure to thrive), it is now clear that CD can develop at any age whilst on a gluten-containing diet[3]. A number of recent reports have documented increasing variability in presentation patterns with more patients presenting with non-specific complaints such as iron deficiency anaemia or lethargy[4-6]. In addition, more individuals are now identified consequent to active screening in groups at higher risk of CD (such as those with positive family history) and a number of these patients may be asymptomatic at diagnosis[7]. Although strategies such as active screening recognise more individuals with CD, it is felt that for every person diagnosed there are still many individuals who are undiagnosed in the community[8-10].
CD is now recognised as a common disease, with prevalence estimated to be at least 1: 100[7]. Locally, a population-based study performed in Christchurch, New Zealand, suggested a prevalence of one in 82 individuals[11]. In addition, a subsequent hospital-based study conducted in the same region demonstrated increasing numbers diagnosed each year over the last years of the twentieth century[12]. The primary aims of this retrospective study were to determine the rates of diagnosis and the presentation patterns of paediatric-onset CD at Christchurch Hospital in the first decade of the 21st century.
Children aged 16 years or less (defined as diagnosis before their seventeenth birthday) diagnosed with CD at Christchurch Hospital, Christchurch, New Zealand between January 1st 2000 and December 31st 2010 were identified retrospectively. Christchurch Hospital is the sole secondary-level public hospital for the Canterbury region and also provides tertiary paediatric services for surrounding regions. The study population did not include children diagnosed outside Christchurch Hospital.
The Department of Pathology database was searched for the keywords “coeliac disease”, “celiac disease” and “gluten sensitive enteropathy”. This search returned histology reports of patients who had been investigated for and/or diagnosed with CD. Records available from the Dietetics Department were also reviewed to identify children referred for dietetic education prior to commencement of a GFD. Each patient’s hospital records (hard-copy and electronic) was also retrieved and reviewed for the purpose of the study.
The duodenal biopsies taken at Christchurch Public Hospital from 2000-2010 were all analysed by the same medical laboratory, although by different pathologists. Lesions consistent with Marsh-Oberhuber criteria (including increased intraepithelial lymphocytes, partial to complete villous atrophy, crypt hyperplasia and increased lymphocytes in the lamina propria) were considered to be diagnostic[13]. The histological findings in other areas of the upper gastrointestinal tract were also reviewed.
Relevant background features, such as presenting signs and symptoms, risk group status, and anthropometry were extracted from hospital clinical records and where available, GP referral letters and blood test results. In the event of missing data, the patients’ last known GP was contacted and asked to provide relevant results and notes where available.
Endoscopy and pathology reports were reviewed. The results of serological tests [anti-gliadin (AGA), anti-endomysial antibodies (EMA), tissue transglutaminase (tTG) and deaminated gliadin peptide (DGP)] were obtained. The results of other tests conducted prior to diagnosis (haemoglobin level, iron studies, total IgA level and HLA DQ2/DQ8 status) were also noted where available. Patients were classified as iron deficient in the presence of one or more of the following: serum ferritin levels below normal reference range in the absence of an elevated C-reactive protein; transferrin saturation or serum iron levels below the normal reference range; total iron binding capacity and serum transferrin receptor levels above the normal reference range. Iron deficiency anaemia was defined as a microcytic hypochromic anaemia, with evidence of low iron stores as stated above.
Descriptive analyses were executed using Microsoft Excel 14.0.0. Weight and height Z scores were calculated using an online calculator employing data from the National Health and Nutrition Evaluation Survey - (http://www.emmes.com/study/ped/resources/htwtcalc.htm). Statistical analyses were performed using GraphPad InStat 3.0 (GraphPad Software, Inc., La Jolla, CA). One-way analysis of variance (ANOVA), t tests and chi-square tests were utilised for data analysis. Statistical significance was defined as a P value below 0.05.
A total of 263 children were diagnosed with CD over the time period. The median age of the study population was 7.88 years (range 0.8-16.9 years) and 169 (64%) were female. Two hundred and sixteen children were aged less than 12 years whilst 26 were aged less than 2 years of age. The largest number of children (42.3%) was aged between 6 and 12 years at diagnosis.
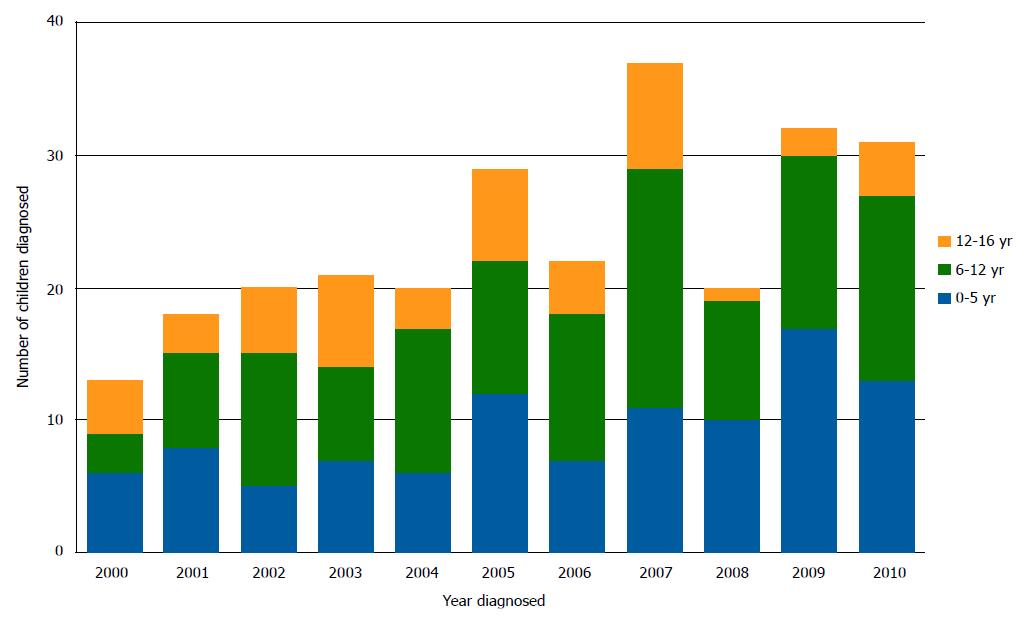
The gender and age distribution of the group did not vary over the study period. However, the numbers of children diagnosed each year increased from 13 in 2000 to 31 in 2010 (P = 0.009) (Figure 1).
At least one risk factor for CD was seen in 140 (53.4%) of the 263 children. Positive family history (n = 92), Trisomy 21 (n = 15) and type 1 diabetes mellitus (T1DM) (n = 22) were seen most commonly, whilst other risk groups included Turner’s syndrome, autoimmune thyroid disease and William’s Syndrome (n = 11). Two risk factors, such as positive family history and T1DM, were seen in 7 children. Diagnosis was made after screening in 56 (21%) children: 38 of whom were reported to be asymptomatic. One patient was found to have CD at the time of upper gastrointestinal endoscopy for suspected eosinophilic oesophagitis.
Details of presenting features were available for 260 of the 263 children. Thirty-eight of these 260 children had no reported symptoms at the time of diagnosis. Abdominal pain (44.2%) and diarrhoea (38.9%) were the most commonly reported symptoms in the 222 children presenting with symptoms (Table 1). Most children presented with a combination of intestinal and extra-intestinal symptoms. Whilst diarrhoea (51.5%) and failure to thrive (46.6%) predominated in the children aged less than 5 years of age, abdominal pain was the most common symptom in older children (P = 0.0032).
| Presenting sign or symptom | Children | |
| Gastrointestinal | Abdominal pain | 115 (43.6) |
| Diarrhoea | 101 (38.3) | |
| Bloating (± increased flatus) | 60 (22.7) | |
| Nausea and vomiting | 42 (15.9) | |
| Constipation | 17 (6.4) | |
| Nutritional | Iron deficiency (± anaemia) | 84 (31.8) |
| Poor weight gains/wasting | 65 (24.6) | |
| Weight loss | 27 (10.2) | |
| Micronutrient deficiency | 25 (9.5) | |
| Short stature | 10 (3.8) | |
| Extra-gastrointestinal | Lethargy | 36 (13.6) |
| Poor health/recurrent infections | 12 (4.6) | |
| Irritability/low mood | 11 (4.2) | |
| Headaches | 6 (2.3) | |
| Pubertal delay | 3 (1.1) | |
| Poor sleep | 2 (0.8) | |
| Neuropsychiatric symptoms | 1 (0.4) | |
| Asymptomatic | Identified following screening | 38 (14.4) |
Weight measurements were available for 229 of the 263 children, and height measurements available for 184 of the children. Overall, the mean Z score (± SD) for weight was -0.12 ± 0.22, whilst that for height was -0.24 ± 0.31. The children aged less than five years had a lower mean weight z score (-0.4096 ± 1.24) than the children aged 5-12 years (0.1196 ± 0.966) and the older group (0.0901 ± 1.14) (P = 0.0033, ANOVA). There were no differences in the mean height z scores for the three age groupings (Table 2).
| Age group(yr) | WeightZ score (± SD) | HeightZ score (± SD) | Iron deficiency (n) | Other deficiency (n) |
| 0-5 | -0.4096 ± 1.24 | -0.4210 ± 1.102 | 41 | 9 |
| 6-12 | 0.1196 ± 0.966 | -0.914 ± 1.125 | 25 | 8 |
| > 12 | 0.0901 ± 1.14 | -0.229 ± 1.347 | 21 | 7 |
Eighteen (7.8%) of the patients had a weight score more than 2 SD below the mean (wasting), and 15 (8.2%) had a height score more than 2 SD below the mean (stunting). More of the children with wasting were aged less than 5 years of age than older than this age (13 vs 5; P = 0.005). Stunting was not different across the age ranges (data not shown). Five children (2%) had a weight score more than 2 SD above the mean (overweight). Only three children (1.6%) had a height score more than 2 SD above the mean.
The results of serological tests were available in 261 children. AGA testing (IgA and IgG) was completed only prior to 2008: IgG AGA was positive in 82.4% of 193 children, whilst IgA AGA was positive in 50.8% of 185 children. EMA testing was positive in 177 (87.6%) of the 202 who had been tested for this antibody. tTG serology was positive in 183 (89.3%) of the 205 children who had been tested. Sixteen children had DGP testing (all since 2008): IgG DGP was positive in 75% and IgA DGP positive in 62.5% of this group.
Twelve children were negative for IgA-based tests (EMA, tTG and AGA): three of these children were IgA deficient. These three children all tested positive for IgG AGA and one was also IgG tTG positive.
Full blood count results were available in 178 children: 25 (14.0%) of these children were found to have a haemoglobin level below the normal reference range, and 12 (6.7%) had microcytosis. Four of the 25 anaemic children did not have assessment of iron status at the time of diagnosis: two were documented as having iron deficiency anaemia in their referral letters to hospital whilst the other two children had normocytic normochromic anaemia.
Iron status was assessed in 153 children: 84 (54.9%) of these children were iron deficient (Table 2). Measurement of other micronutrients was completed only in a small number of children: 24 were found to have deficiency of folate, vitamin B12, vitamin A, vitamin D, vitamin E, and/or vitamin K (Table 2). Genetic testing for HLA DQ2/DQ8 was not routinely undertaken during this period: only 28 children had undergone this investigation, all of whom had positive results. Detailed analysis of this HLA marker was therefore not conducted.
Duodenal biopsies were evaluated by pathologists and diagnosed according to Marsh-Oberhuber criteria[13]. Almost all the children diagnosed with CD also had concurrent biopsies of the oesophagus and the stomach taken during gastroscopy. Distinct abnormalities were seen in 86 children, with some children having more than one other finding. Common findings included reflux oesophagitis (seen in 21 children) and lymphocytic gastritis (n = 16) (Table 3).
| Histological diagnosis | Number of patients | Percentage of patients (%) | |
| Oesophageal findings | Reflux oesophagitis | 21 | 7.9 |
| Eosinophilic oesophagitis | 4 | 1.5 | |
| Candida oesophagitis | 1 | 0.4 | |
| Non-specific oesophagitis | 21 | 7.9 | |
| Gastric findings | Lymphocytic gastritis | 16 | 6.1 |
| Non-specific gastritis | 30 | 11.4 | |
| Reactive gastritis (bile reflux) | 11 | 4.2 | |
| Active chronic gastritis (H. pylori) | 1 | 0.4 | |
| Gastric metaplasia | 1 | 0.4 |
This retrospective study showed progressive increases in the number of children diagnosed with CD each year in this single New Zealand location over the 11 years of observation. Presenting symptoms varied widely in this group, and a substantial number of children were diagnosed following screening assessments in high risk groups. Preschool children were more likely to have nutritional impairment than older children. Although this study was not designed to define population-specific rates of CD, the results clearly emphasise that CD is an increasingly important condition in New Zealand children.
A prospective population-based study has previously documented that CD is common in adults in this region of New Zealand, with rates of 1:82[11], equivalent to prevalence studies in several other countries[7]. A further study documented increasing rates of diagnosis in children and adults from the same region over the last three decades of the 20th century[12]. That prospective study documented the diagnosis of CD in 62 children aged between 0 and 12 years of age, with increasing numbers in the last years of the 30 year period. In contrast, 216 children aged less than 12 years were diagnosed within the 11 year period of the current study, emphasising the increasing numbers of diagnoses in the same region of New Zealand.
Cumulative incidence rates in children (0-12 years old) in previous New Zealand studies have been low in comparison to corresponding adult rates: 0.10/1000 in Wellington[14], 0.35/1000 in Otago[15] and 0.40/1000 in Canterbury[12]. More recently the prevalence of childhood CD was ascertained in the New Zealand Asthma and Allergy Cohort[16]. This birth-cohort was established between 1997 and 2001, with infants recruited in two New Zealand centres (Christchurch and Wellington). The parents of 916 children in this cohort were asked in 2009 about doctor-diagnosed CD. Nine children were identified, giving a prevalence of 1%. However, this parental report was not validated by detailed review of each patient’s medical history.
This pattern of increasing numbers of young children diagnosed with CD in New Zealand may be related in part to enhanced awareness of CD and more recognition of groups at higher risk of developing this condition. Advances in serological tests and diagnostic approaches may be additional factors[17]. Environmental factors, such as infant breast-feeding practices, age at introduction of gluten, and the amount of gluten in the diet, could also be relevant to changes in rates of CD in recent years[18]. It is unlikely, however, that genetic risk will have altered over a period of just a few decades. There may also be a true rise in incidence of CD. Studies from Finland, in particular, indicate a true increase in the incidence of CD over time, with most recent rates estimated to be 2.4%[19]. In the New Zealand setting, deregulation of the New Zealand wheat industry and changes in infant feeding practices could be potential drivers of such a rise in incidence[11]. The retrospective nature of the current study did not allow any clarification of environmental risk factors, such as breast feeding, or of other details including family history of CD.
The current study clearly demonstrated that CD can be diagnosed at any age through childhood, with pain being a predominant presenting symptom, especially in older children. Overall, significant nutritional impairments were not commonly seen, with less than 10% percent being malnourished at diagnosis. However, nutritional impairment was more likely in preschool children than in those of school age. Unfortunately, the presentation patterns and nutritional state of the children diagnosed at the same centre in the preceding three decades were not documented[12]. However, the reduced number of children diagnosed prior to their second birthday in the second cohort compared to the earlier group (12% vs 21%) suggests less frequent presentation with classical malabsorptive symptoms.
Previous reports over the last decade from elsewhere in New Zealand[4] and Australia[5,20] also demonstrate similar findings in presentation patterns. Internationally, similar observations have been made, with less preschool children presenting with classical malabsorptive symptoms, and more with gastrointestinal or non-gastrointestinal symptoms[6].
A number of children in the current study had been diagnosed with CD following screening within a high risk group and more than two thirds of the children identified by screening were asymptomatic. Groups at highest risk of CD include those with a first degree family member, children with T1DM and Down syndrome (Trisomy 21)[7]. Children with a first degree family history of CD comprise the largest high risk group in the children diagnosed with CD in the current study. The increased identification of children following focused screening is noted elsewhere also[5,20].
The use of specific serological tests has evolved over the period of the current study. Whereas AGA and EMA were utilised in the last thirty years of the 20th century[12], tTG and EMA were the predominant tests utilised in the current cohort. AGA tests were available for part of the period, whilst DGP were utilised only in the latter years. In this setting, EMA and tTG were positive in the majority of those children tested.
The recently published European guidelines for the diagnosis of CD include a provision for the diagnosis of CD without obtaining duodenal biopsies[21]. The current study does not permit a detailed assessment of the validity of this protocol to the New Zealand setting. However, a number of children in the current cohort would not have reached diagnostic criteria without completion of duodenal biopsies. Furthermore, less than 15% of the current cohort had estimation of HLA DQ2/DQ8. Whilst best considered as a test to provide risk stratification (negative gene testing reflecting essentially no risk of developing CD), HLA testing is also included in the ESPGHAN guidelines as a requirement for considering diagnosis without duodenal biopsies[21].
A number of the current cohort had additional histological findings present in the upper gut. Whilst the presence of lymphocytic gastritis is well-recognised as a component of CD, the recognition of oesophageal findings is of interest. Several paediatric cohorts have shown an association between CD and eosinophilic oesophagitis[22-24]. Although the reason for this link is as yet unclear[25], it does further illustrate the evolving patterns of CD in children.
The limitations of this study relate principally to its retrospective nature. This limited the availability of all required data, with incomplete data across some key areas. This study did not consider children seen and diagnosed in the private sector, and consequently cannot be considered population-based. However, the small number of additional children diagnosed in the private sector would likely serve to further increase the total numbers diagnosed over time. The included population was seen and diagnosed within a single hospital setting over an 11 year period, and follows directly from an earlier assessment of CD in the same setting[12]. Together, these two datasets cover 41 years of children diagnosed with CD in Christchurch, New Zealand.
In conclusion, these data reflect a large increase in number of children and adolescents diagnosed with CD at this single New Zealand tertiary centre. Common to other reports, the presentation patterns of CD in children are many and varied. Practitioners must consider the diagnosis of CD in children seen with a variety of symptoms. The increased emphasis upon screening in groups at higher risk of CD is likely to lead to even more children diagnosed without symptoms.
Coeliac disease (CD) may present at any age after commencement of a gluten containing diet. Increasing rates of the diagnosis of CD was previously noted in the Christchurch region over the last three decades of the 20th century. The current study focused on the first 11 years of the 21st century and ascertained annual rates of diagnosis over this time.
CD may present throughout childhood, with a variety of symptoms. Increasing numbers of children are diagnosed after screening in groups at greater risk of developing CD. Such children may not have any symptoms at the time of screening or diagnosis.
Different symptom patterns may be seen in children of varying age. Screening for CD in children at increased risk of CD (such as those with a first degree family member) may lead to earlier diagnosis and avoid nutritional or other consequences of CD.
Physicians assessing children must be aware of the myriad different possible presentation patterns of CD. Early investigation in those with possible CD should be undertaken to avoid long term morbidity and adverse outcomes.
Genetic risk for the development of CD is attributed to particular HLA genes. Serological tests for CD include tissue transglutaminase antibodies (tTG: measured by immunoassay) and endomysial antibodies (EMA: assessed by immunofluorescence).
Increasing numbers of children were diagnosed with CD after screening exercises: a number of these children have no symptoms at the time of diagnosis. Assessment of HLA genetic status was completed in few of the children included in this retrospective study of CD. Consequently, few conclusions could be made about the relevance of these findings.
P- Reviewer: Sangkhathat S, Urganci N S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Tack GJ, Verbeek WH, Schreurs MW, Mulder CJ. The spectrum of celiac disease: epidemiology, clinical aspects and treatment. Nat Rev Gastroenterol Hepatol. 2010;7:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Kennedy NP, Feighery C. Clinical features of coeliac disease today. Biomed Pharmacother. 2000;54:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Westerbeek E, Mouat S, Wesley A, Chin S. Coeliac disease diagnosed at Starship Children’s Hospital: 1999-2002. N Z Med J. 2005;118:U1613. [PubMed] |
| 5. | Stone ML, Bohane TD, Whitten KE, Tobias VH, Day AS. Age related clinical features of childhood coeliac disease in Australia. BMC Pediatr. 2005;5:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Telega G, Bennet TR, Werlin S. Emerging new clinical patterns in the presentation of celiac disease. Arch Pediatr Adolesc Med. 2008;162:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Hill ID, Dirks MH, Liptak GS, Colletti RB, Fasano A, Guandalini S, Hoffenberg EJ, Horvath K, Murray JA, Pivor M. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 694] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 8. | Catassi C, Fabiani E, Rätsch IM, Coppa GV, Giorgi PL, Pierdomenico R, Alessandrini S, Iwanejko G, Domenici R, Mei E. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr Suppl. 1996;412:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 246] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Csizmadia CG, Mearin ML, von Blomberg BM, Brand R, Verloove-Vanhorick SP. An iceberg of childhood coeliac disease in the Netherlands. Lancet. 1999;353:813-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Ivarsson A, Persson LA, Juto P, Peltonen M, Suhr O, Hernell O. High prevalence of undiagnosed coeliac disease in adults: a Swedish population-based study. J Intern Med. 1999;245:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 128] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Cook HB, Burt MJ, Collett JA, Whitehead MR, Frampton CM, Chapman BA. Adult coeliac disease: prevalence and clinical significance. J Gastroenterol Hepatol. 2000;15:1032-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Cook B, Oxner R, Chapman B, Whitehead M, Burt M. A thirty-year (1970-1999) study of coeliac disease in the Canterbury region of New Zealand. N Z Med J. 2004;117:U772. [PubMed] |
| 13. | Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1200] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 14. | Ussher R, Yeong ML, Stace N. Coeliac disease: incidence and prevalence in Wellington 1985-92. N Z Med J. 1994;107:195-197. [PubMed] |
| 15. | Carrington JM, Hewitt CJ, Dowsett LR, Barbezat GO. The prevalence of coeliac disease in Otago. N Z Med J. 1987;100:460-462. [PubMed] |
| 16. | Tanpowpong P, Ingham TR, Lampshire PK, Kirchberg FF, Epton MJ, Crane J, Camargo CA. Coeliac disease and gluten avoidance in New Zealand children. Arch Dis Child. 2012;97:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | McGowan KE, Castiglione DA, Butzner JD. The changing face of childhood celiac disease in north america: impact of serological testing. Pediatrics. 2009;124:1572-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Ivarsson A, Hernell O, Stenlund H, Persson LA. Breast-feeding protects against celiac disease. Am J Clin Nutr. 2002;75:914-921. [PubMed] |
| 19. | Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, Lohi O, Bravi E, Gasparin M, Reunanen A. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 527] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 20. | Aurangzeb B, Leach ST, Lemberg DA, Day AS. Nutritional status of children with coeliac disease. Acta Paediatr. 2010;99:1020-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1834] [Article Influence: 141.1] [Reference Citation Analysis (1)] |
| 22. | Ooi CY, Day AS, Jackson R, Bohane TD, Tobias V, Lemberg DA. Eosinophilic esophagitis in children with celiac disease. J Gastroenterol Hepatol. 2008;23:1144-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Leslie C, Mews C, Charles A, Ravikumara M. Celiac disease and eosinophilic esophagitis: a true association. J Pediatr Gastroenterol Nutr. 2010;50:397-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Thompson JS, Lebwohl B, Reilly NR, Talley NJ, Bhagat G, Green PH. Increased incidence of eosinophilic esophagitis in children and adults with celiac disease. J Clin Gastroenterol. 2012;46:e6-e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Verzegnassi F, Bua J, De Angelis P, Dall’oglio L, Di Leo G, Ventura A. Eosinophilic oesophagitis and coeliac disease: is it just a casual association? Gut. 2007;56:1029-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |