Published online Sep 9, 2025. doi: 10.5409/wjcp.v14.i3.106524
Revised: March 28, 2025
Accepted: April 11, 2025
Published online: September 9, 2025
Processing time: 108 Days and 19.1 Hours
Phocomelia is a rare congenital disorder characterized by the absence or under
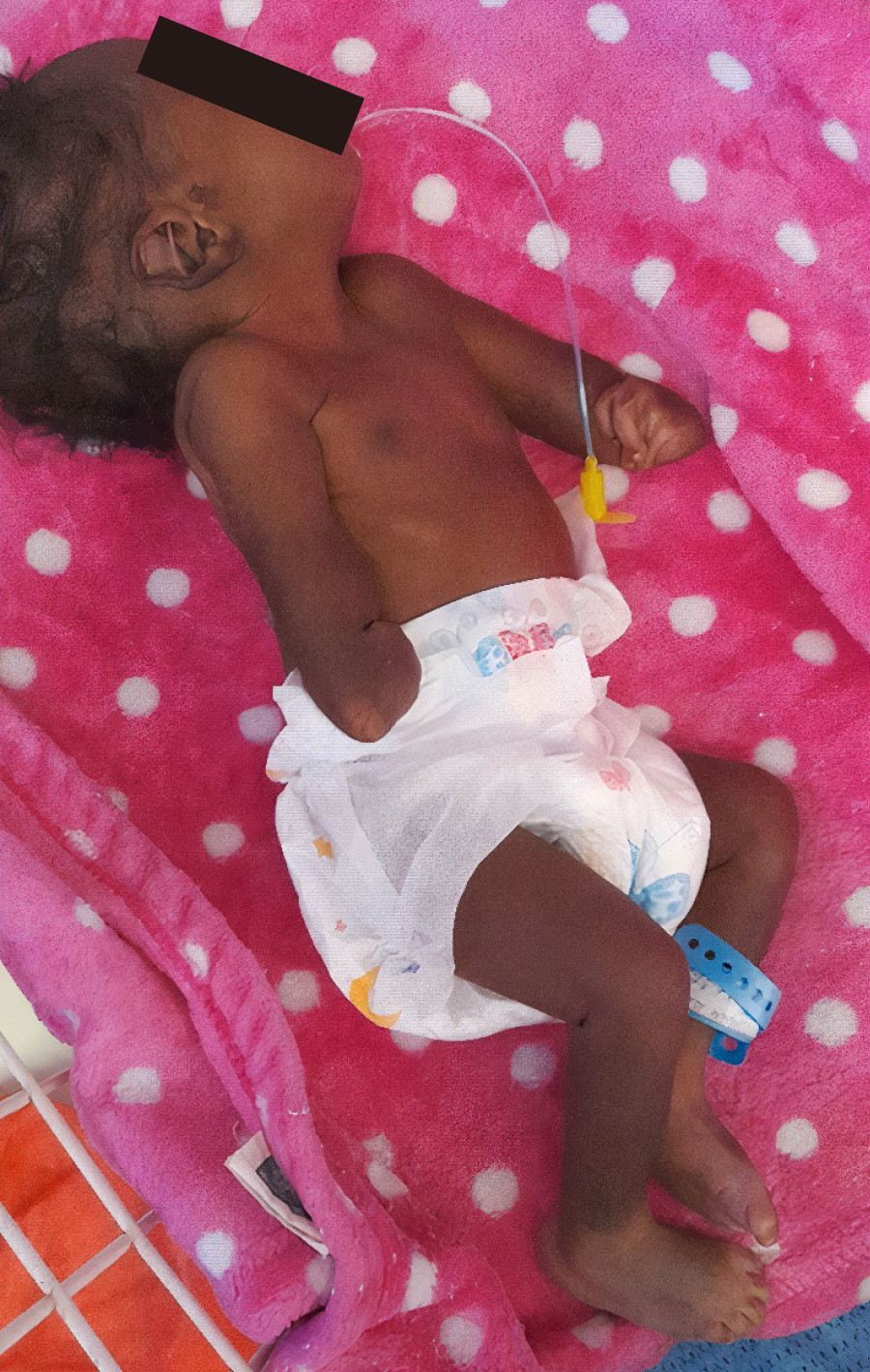
We report a 2-week-old term neonate with bilateral phocomelia, micrognathia, jaundice, and low birth weight. The pregnancy was unremarkable, with no tha
Bilateral phocomelia presents significant functional challenges. Comprehensive diagnostic workups and early rehabilitation strategies are essential for optimizing patient outcomes.
Core Tip: This rare case of bilateral phocomelia in a neonate highlights the diagnostic and rehabilitative challenges in resource-limited settings and underscores the value of early detection, genetic workup, and multidisciplinary care.
- Citation: Omullo FP, Shahabi K, Kitheghe TK, Mutuku B, Simiyu BW. Phocomelia: Bilateral limb deficiency in a neonate: A case report. World J Clin Pediatr 2025; 14(3): 106524
- URL: https://www.wjgnet.com/2219-2808/full/v14/i3/106524.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i3.106524
Phocomelia” is derived from the Greek words “foke”, meaning seal, and “melos”, meaning limbs[1]. Affected limbs in phocomelia bear a seal-like appearance[2]. Phocomelia affects approximately 0.62 per 100000 live births globally, with a higher incidence in males and left-sided limb involvement. Most phocomelia cases are isolated (53.2%), while 9.9% are syndromic. Most non-syndromic cases are monomelic (55.9%) with a predilection for upper limb (64.9%) involvement. Also, unilateral deformities are more common than bilateral ones[3]. This condition gained prominence during the thalidomide crisis of the 20th century. However, non-thalidomide causes have been implicated, including vascular disruption, genetic mutations, and environmental teratogens[4,5]. This report presents a neonate with bilateral upper limb phocomelia without a history of teratogen exposure, micrognathia, short neck, low birth weight, and neonatal jaundice, underscoring the need for genetic and antenatal evaluation. This case report explores phocomelia's clinical approach, diagnostic process, and management strategies.
A 2-week-old male neonate was admitted for respiratory distress, jaundice, and congenital upper limb malformations.
The neonate was born at term via spontaneous vaginal delivery to a para 3+2 mother. The mother had 7 antenatal visits at a peripheral health facility but lacked a detailed obstetric ultrasound. The mother presented in the second stage of labor and delivered a male neonate weighing 2400 g. The neonate required resuscitation for seven minutes due to poor Apgar scores of 4, 5, and 6 at one, five, and ten minutes, respectively. He was subsequently admitted to the newborn unit with signs of respiratory distress, including central cyanosis, nasal flaring, grunting, and marked intercostal retraction.
The pregnancy was unremarkable, with no reported infections, medication use, radiation exposure, or known genetic disorders. Moreover, the mother denied any exposure to local herbs or traditional medicine. Genetic testing was not performed due to financial constraints. Tripple serology screening was negative.
The mother had two healthy children and two early pregnancy losses at 12 weeks. The etiology of the losses was not in
Initial vital signs included a pulse rate of 164 beats/min, respiratory rate of 70 breaths/min, temperature of 35.1 ℃, and oxygen saturation of 72% on room air. The neonate had micrognathia, a short neck, scleral and body jaundice, and signs of respiratory distress. Cardiovascular examination revealed a low-grade murmur. Musculoskeletal assessment showed bilateral upper limb phocomelia with absent humeri and malformed forearms. Neurological examination was normal except for lethargy.
Included in Table 1.
| Parameter | Findings |
| Blood group | B+ |
| White blood cell count | 36000 × 109/L (neutrophils 60%, lymphocytes 19.1%) |
| Haemoglobin | 9.6 g/dL |
| Platelets | 161000 |
| Direct bilirubin | 78.65 mmol/L |
| Total bilirubin | 278.6 mmol/L |
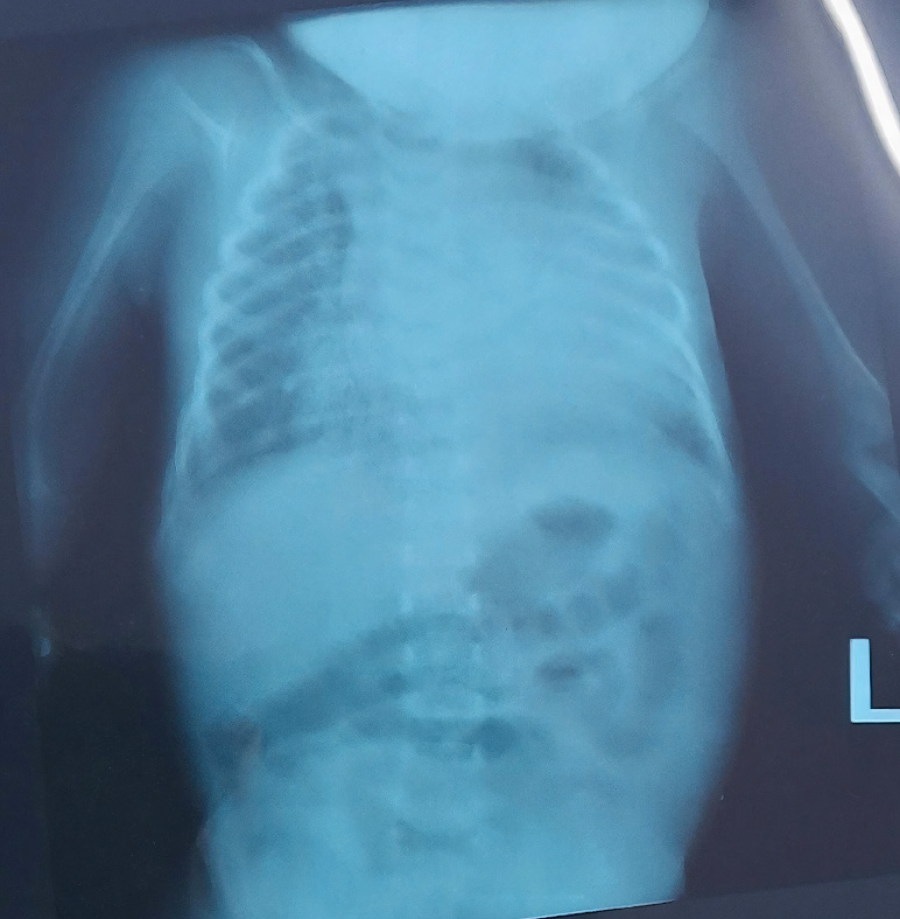
Radiographs confirmed bilateral short humeri and malformed forearms (Figure 1). Abdominal ultrasound findings were normal, and no associated skull anomalies or pelvic hypoplasia were detected (Figure 2).
A multidisciplinary team was involved in the management of the neonate. The neonatology team provided initial stabilization, closely monitored for complications such as respiratory distress and neonatal jaundice, and initiated supportive care. A genetics consultation was recommended to evaluate potential hereditary syndromes, though genetic testing was not performed due to resource constraints. A pediatric orthopedic surgeon assessed the upper limb malformations and outlined possible future reconstructive and prosthetic interventions. Rehabilitation specialists were consulted early to plan for prosthetic limb fitting and adaptive support. Physiotherapists and occupational therapists initiated early interventions to enhance residual limb strength, motor function, and sensory adaptation. Additionally, psychologists and social workers provided parental counseling.
The neonate was diagnosed with bilateral upper limb phocomelia in the absence of teratogenic exposure. Additional diagnoses included low birth weight, early-onset neonatal jaundice, perinatal asphyxia, and early-onset neonatal sepsis. The absence of genetic testing limited confirmation of an underlying syndromic association, emphasizing the need for further evaluation and long-term follow-up.
The neonate received oxygen therapy, phototherapy, intravenous antibiotics, and maintenance fluids. His condition stabilized, and prosthetic planning was initiated. Family counseling and psychosocial support were provided.
The neonate’s condition stabilized following supportive management, with improved oxygenation, resolution of respiratory distress, and successful treatment of neonatal jaundice and sepsis. Feeding tolerance improved, and weight gain was monitored. While the bilateral upper limb malformations remained unchanged, early prosthetic planning and physiotherapy were initiated to promote motor function and adaptation. For long-term management, the neonate was scheduled for regular follow-up with neonatology, genetics, orthopedic surgery, and rehabilitation specialists. Developmental assessments were planned to track motor and neurological progress, while prosthetic consultations aimed at early fitting for improved functional independence. The family received ongoing psychosocial support.
Phocomelia is a rare congenital anomaly affecting the normal growth and development of the musculoskeletal system[1]. In phocomelia, long bones are often absent, and rudimentary hands and feet are attached to the trunk by small, irregularly shaped bones[6]. Etienne Geoffroy Saint-Hilaire coined the term phocomelia in 1836[3]. Its prevalence is 0.62 in 100000 births[1].
Limb buds begin to develop from the 26th day of intrauterine life and are fully formed by the end of the 14th week[3]. Any aberrations in this normal pattern culminate into different types of deformities, including partial (meromelia) or total (amelia) absence of limbs[1]. Potential causes of phocomelia are thalidomide, substance use (cocaine or alcohol), radiation exposure, vascular compromise (particularly reduced blood flow through the abnormal subclavian artery), and gestational diabetes[1]. Thalidomide has been attributed to many cases of phocomelia, especially in the 1960s[6]. Its antiangiogenic properties have been implicated in the pathogenesis of phocomelia[4,7].
However, phocomelia can either be hereditary or caused by genetic mutations. Several genes play critical roles in the normal development of limbs[8]. The Wnt-7, Shh, and FGF genes have been implicated in limb embryogenesis. The Wnt-7 gene regulates the ventral-dorsal axis and determines the anterior and posterior surfaces of the limbs. On the other hand, Shh controls the anterior-posterior axis and determines the position of fingers and toes[3].
Additionally, the FGF gene facilitates limb development and elongation. Mutations in these genes often cause phocomelia[9]. Consanguinity plays a critical role in the pathogenesis of phocomelia, especially Al-Awadi/Raas-Rothschild syndrome[1]. The increased homozygosity resulting from consanguineous unions raises the likelihood of autosomal recessive conditions manifesting[10]. Furthermore, studies in populations with high consanguinity rates have shown a stronger correlation between such genetic disorders and familial marriages[11].
Approximately 9.9% of phocomelia cases occur together with other anomalies such as horseshoe kidney, polycystic kidney, cleft palate, retrognathia, and hypertelorism[12,13]. Thus, associated anomalies suggest that phocomelia may arise as part of broader syndromic presentations involving multiple organ systems[13]. Our patient had micrognathia and a short neck. Various associated clinical patterns of malformation facilitate the classification of phocomelia into Al-Awadi/Raas-Rothschild syndrome, Roberts syndrome, Schinzel phocomelia, and Zimmer phocomelia[1,14]. The unique aspect of this case is the bilateral upper limb involvement, low birth weight, jaundice, and absence of thalidomide or any other drug involvement. Differential diagnoses of phocomelia include sporadic phocomelia, Holt-Oram syndrome, thrombocytopenia-absent radius syndrome, Roberts syndrome, and thalidomide-induced phocomelia[1].
Antenatal care should be attended to as per the World Health Organization protocol. Adequate attendance allows early diagnosis and possible termination of pregnancies with lethal congenital anomalies[14]. In low-resource settings, strengthening antenatal services significantly improves early identification and decision-making in affected pregnancies[15]. A fetal prenatal ultrasound diagnoses phocomelia syndrome between 18 and 22 weeks[1,16]. In high-risk pregnancies, prenatal diagnosis has been made as early as the 11th week of intrauterine life. Nevertheless, in our case, the mother did not undergo a detailed fetal ultrasound, culminating in failure of diagnosis before birth.
Managing phocomelia requires a multidisciplinary approach to optimize functional outcomes and improve the patient’s quality of life[3]. Early involvement of an interdisciplinary team is crucial for comprehensive assessment and care planning. Genetic evaluation is essential to identify underlying syndromic associations, especially in cases without a history of teratogenic exposure[16].
Long-term follow-up is necessary to monitor the child’s growth, musculoskeletal development, and adaptive abilities. Early physiotherapy and occupational therapy interventions promote motor development and independence by enhancing residual limb strength and functional adaptation[3]. Prosthetic planning should begin in infancy, focusing on early fitting of passive prostheses, followed by more advanced myoelectric or functional prostheses as the child develops motor skills and cognitive awareness[1]. Early prosthetic use improves long-term adaptability and reduces psychological distress in children with limb deficiencies.
This case of bilateral phocomelia in a neonate without thalidomide exposure highlights the challenges in diagnosing and managing rare congenital defects. The bilateral involvement, low birth weight, and micrognathia emphasize the importance of routine detailed ultrasounds in high-risk pregnancies for early detection and parental counseling. Moreover, genetic evaluation should be prioritized to identify potential causes of non-thalidomide phocomelia. In this case, the absence of gene testing underscores the need for comprehensive postnatal investigations. This case highlights the need for structured diagnostic and intervention strategies to optimize outcomes. Future research should explore genetic and environmental contributors to phocomelia, particularly in cases without thalidomide exposure.
The authors thank Dr. Nick Mutisya, the newborn unit staff, and the patient’s family for their cooperation and support in preparing this case report.
| 1. | Abu Isneina S, Karaki M, Salah R, Rasheed B, Atrash M. Non-syndromic phocomelia: A rare case report signifying prenatal screening. SAGE Open Med Case Rep. 2024;12:2050313X241271868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Rehman W, Arfons LM, Lazarus HM. The rise, fall and subsequent triumph of thalidomide: lessons learned in drug development. Ther Adv Hematol. 2011;2:291-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Bermejo-Sánchez E, Cuevas L, Amar E, Bianca S, Bianchi F, Botto LD, Canfield MA, Castilla EE, Clementi M, Cocchi G, Landau D, Leoncini E, Li Z, Lowry RB, Mastroiacovo P, Mutchinick OM, Rissmann A, Ritvanen A, Scarano G, Siffel C, Szabova E, Martínez-Frías ML. Phocomelia: a worldwide descriptive epidemiologic study in a large series of cases from the International Clearinghouse for Birth Defects Surveillance and Research, and overview of the literature. Am J Med Genet C Semin Med Genet. 2011;157C:305-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Kim JH, Scialli AR. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol Sci. 2011;122:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 5. | Ridings JE. The thalidomide disaster, lessons from the past. Methods Mol Biol. 2013;947:575-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Yesender M, Anjum A, Saritha S, Sadananda Rao B, Ramani TV, Ericson P. Limb defects: A spectrum of correlated study. IJAR. 2016;4:1810-1818. [DOI] [Full Text] |
| 7. | Collins MD, Scott WJ. Thalidomide-induced limb malformations: an update and reevaluation. Archives of Toxicology. Arch Toxicol. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Jin L, Wu J, Bellusci S, Zhang JS. Fibroblast Growth Factor 10 and Vertebrate Limb Development. Front Genet. 2018;9:705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Pakkasjärvi N, Syvänen J, Wiro M, Koskimies-Virta E. Amelia and phocomelia in Finland: Characteristics and prevalences in a nationwide population-based study. Birth Defects Res. 2022;114:1427-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Fareed M, Afzal M. Genetics of consanguinity and inbreeding in health and disease. Ann Hum Biol. 2017;44:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Khayat AM, Alshareef BG, Alharbi SF, AlZahrani MM, Alshangity BA, Tashkandi NF. Consanguineous Marriage and Its Association With Genetic Disorders in Saudi Arabia: A Review. Cureus. 2024;16:e53888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 12. | Shukla AK, Sanjay SC, Krishna L, Krishnappa N. Tetra-phocomelia: a rarest of rare case. J Clin Diagn Res. 2015;9:TD03-TD04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Osadsky CR. Phocomelia: Case report and differential diagnosis. Radiol Case Rep. 2011;6:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Lavanya C, Devi TR, Gayathri D. An interesting case of Phocomelia. Int J Reprod Contracept Obstet Gynecol. 2020;9:866. [DOI] [Full Text] |
| 15. | Gamberini C, Angeli F, Ambrosino E. Exploring solutions to improve antenatal care in resource-limited settings: an expert consultation. BMC Pregnancy Childbirth. 2022;22:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 16. | Samal SK, Rathod S, Ghose S. Tetra-phocomelia: the seal limb deformity - a case report. J Clin Diagn Res. 2015;9:QD01-QD02. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |