Published online Sep 9, 2025. doi: 10.5409/wjcp.v14.i3.105731
Revised: March 4, 2025
Accepted: April 14, 2025
Published online: September 9, 2025
Processing time: 131 Days and 16.1 Hours
This article examines the growing prevalence of pediatric obesity and its con
Core Tip: Understanding the impact of glucagon-like peptide-1 analogs in the pediatric population is crucial, as these medications have the potential to address a significant treatment gap in childhood metabolic disorders. Their effects on weight regulation, insulin sensitivity, and liver health could offer long-term benefits, particularly in condi
- Citation: Rehman R. Role of glucagon-like peptide-1 receptor agonists in pediatric obesity and metabolic dysfunction associated steatotic liver disease. World J Clin Pediatr 2025; 14(3): 105731
- URL: https://www.wjgnet.com/2219-2808/full/v14/i3/105731.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i3.105731
Pediatric obesity is defined as having a body mass index (BMI) at or above the 95th percentile for age and sex. Obesity has become a global health crisis, with prevalence rates reaching alarming levels worldwide. Recent data indicate that approximately 1 in 5 children and adolescents in the United States of America have obesity[1]. According to data from the World Health Organization, approximately 390 million children and adolescents aged 5-19 years were overweight in 2022, with a significant increase in overweight and obesity from previous decades (20% in 2022 vs 8% in 1990)[2]. This trend is particularly concerning in developed countries, where sedentary lifestyles and poor dietary habits contribute to the epidemic. However, low- and middle-income countries are also experiencing a rapid rise in childhood obesity rates, often coexisting with undernutrition.
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease, has emerged as a significant complication of pediatric obesity. MASLD is defined as hepatic fat accumulation in the absence of other liver diseases or excessive alcohol consumption[3]. Diagnosis typically involves a combination of clinical assessment, laboratory tests, and imaging studies (ultrasound abdomen, magnetic resonance elastography) with liver biopsy remaining the gold standard for staging of hepatitis and hepatic fibrosis. The prevalence of MASLD in the pediatric population has been estimated to range from 5% to 10% and rising to up to 47% in the obese population[4,5].
The development of obesity and MASLD in children involves a complex interplay of hormonal, metabolic, and inflammatory pathways. Excess caloric intake and reduced energy expenditure leads to adipose tissue expansion and dysfunction. This results in increased release of pro-inflammatory cytokines and altered adipokine profiles, contributing to systemic insulin resistance[6,7]. In the liver, insulin resistance promotes de novo lipogenesis and impairs fatty acid oxidation, leading to hepatic fat accumulation. This steatosis can progress to steatohepatitis, characterized by hepatocyte ballooning, inflammation, and fibrosis. The “multiple hit” hypothesis suggests that genetic predisposition, gut dysbiosis, and oxidative stress also play crucial roles in MASLD development and progression[6,7].
Diagnosing and treating obesity and MASLD in children is challenging due to its asymptomatic nature and lack of standardized screening for the latter. Traditional approaches to managing pediatric obesity and MASLD, such as lifestyle modifications and dietary interventions, have shown limited long-term success. Currently, the medications approved for use in pediatric obesity include orlistat, which inhibits pancreatic lipase, reducing the absorption of dietary fats and potentially leading to gastrointestinal side effects such as diarrhea. Another approved treatment is phentermine/topiramate, a combination of a sympathomimetic appetite suppressant (phentermine) and an anticonvulsant (topiramate), which acts as a centrally acting neuromodulator to reduce appetite and promote weight loss. However, these medications have not shown sustained long-term weight loss. The complex interplay of genetic, environmental, and behavioral factors in obesity and MASLD development necessitate more targeted and effective therapeutic strategies. This pressing need has led to increased interest in pharmacological interventions, with glucagon-like peptide-1 (GLP-1) receptor agonists or GLP-1 analogs emerging as a promising option for addressing both weight management and liver health in patients. For this review article, a comprehensive literature search was conducted using PubMed Central, Google Scholar, and Scopus databases.
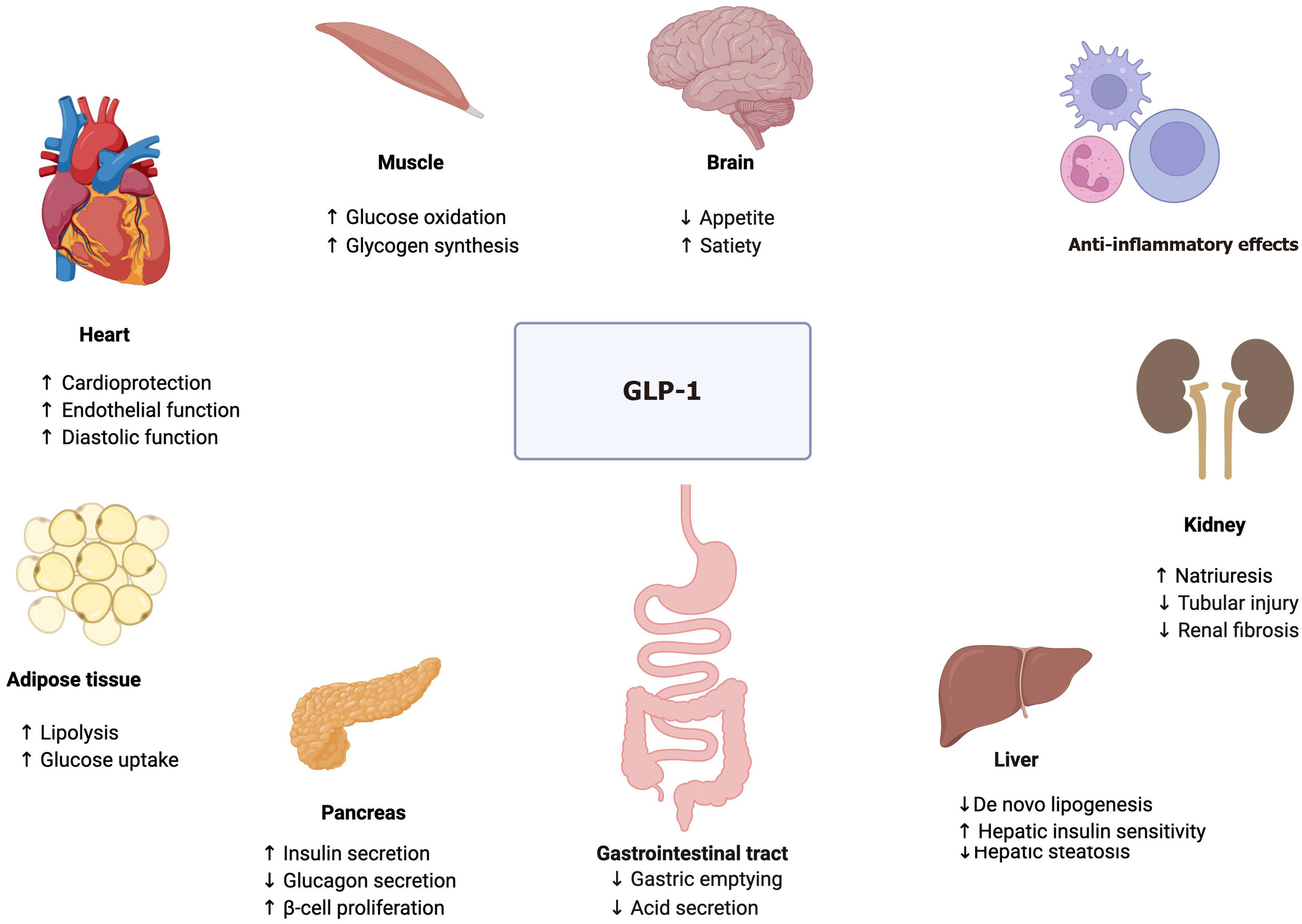
GLP-1 receptor agonists mimic the action of endogenous GLP-1, a gut-derived incretin hormone, and exert their effects through multiple mechanisms[8,9].
GLP-1 analogs act on the hypothalamus and brainstem to reduce hunger and increase satiety.
They decrease gastric motility and prolong the feeling of fullness leading to reduced food intake.
GLP-1 analogs enhance glucose-dependent insulin secretion and suppress glucagon release, improving overall glucose homeostasis.
These agents may reduce hepatic fat content by decreasing de novo lipogenesis and reducing inflammation.
Both the innate and innate-like cells express GLP-1 receptors. This activates multiple signaling pathways including protein kinase A/signal transducer and activator of transcription, phosphoinositide 3-kinases/protein kinase B, mitogen-activated protein kinase, and nuclear factor kappa B, and may exert anti-inflammatory effects in the liver and beyond.
Figure 1 summarizes the key effects of GLP-1 on target tissues.
As of 2025, the Food and Drug Administration (FDA) has approved a few GLP-1 receptor agonists for use in pediatric populations with several others under active investigation. This class of medication has been shown to have a good effect on BMI reduction along with diet and lifestyle modifications, in addition to improvements in glycemic control over time. Table 1 summarizes the key effects of these medications on obesity and MASLD.
| GLP-1 analog | Approval and indications | Key effects |
| Liraglutide | Approved for weight management in adolescents ≥ 12 years with BMI ≥ 30 kg/m2 | Significant reduction in BMI and improvements in liver enzymes in adolescents with obesity and MASLD |
| Semaglutide | Approved for adolescents ≥ 12 years with BMI ≥ 95th percentile | Significant reductions in BMI and waist circumference. Promising anti-fibrotic effects on liver injury |
| Exenatide | Approved for type 2 diabetes in children ≥ 10 years | Improved BMI, liver function, and metabolic measures in children and adolescents |
| Dulaglutide | Approved for managing type 2 diabetes in children ≥ 10 years and adults | Superior to placebo in improving glycemic control. Inferior to semaglutide in reducing body weight |
| Tirzepatide | Approved for weight management in adults with obesity and obstructive sleep apnea (Zepbound) and type 2 diabetes (Mounjaro) | Superior weight loss and BMI reduction compared to other available treatments, with potential future applications in pediatric obesity |
| Retatrutide | Under investigation for obesity and diabetes | Promising results expected due to the combined effects on GLP-1, GIP, and glucagon receptors |
Liraglutide was approved in December 2020 for weight management in adolescents aged 12 and older with a body weight above 60 kg and an initial BMI corresponding to 30 kg/m² or greater for adults. In a multicenter, randomized, double-blind trial of liraglutide including 251 adolescents, liraglutide with lifestyle therapy led to significant BMI reduction compared to placebo after 56 weeks of treatment. The study showed significant differences in BMI reduction between treatment groups. In the liraglutide group (n = 113), 51 participants (43.3%) achieved ≥ 5% BMI reduction compared to 20 participants (18.7%) in the placebo group (n = 105), with an estimated difference of -4.64 percentage points [95% confidence interval (CI): -7.14 to -2.14] in the intention-to-treat analysis. For more substantial weight loss, 33 participants (26.1%) in the liraglutide group achieved ≥ 10% BMI reduction, vs 9 participants (8.1%) in the placebo group[10]. These findings were also observed in a follow-up trial of patients aged 6 years to < 12 years with obesity where the mean percentage change from baseline in BMI was -5.8% with liraglutide vs 1.6% with placebo at week 56, an estimated difference of -7.4 percentage points (95%CI: -11.6 to -3.2, P < 0.001)[11]. Liraglutide has also been studied in MASLD and has been shown to improve liver function over time. Most of the literature includes randomized controlled trials in the adult population. In one randomized control trial (LEAN trial), 39% of patients on liraglutide had metabolic dysfunction-associated steatohepatitis resolution by liver histology compared to 9% who received a placebo (95%CI: 1.0-17.7, P = 0.019)[12]. In a meta-analysis of the Liraglutide Effect and Action in Diabetes program, liraglutide also improved hepatic function tests after 26 weeks of treatment[13]. In pediatrics, a small case series[14] of liraglutide in two adolescents with MASLD showed improvements in liver enzymes and liver fat fraction measured by magnetic resonance elastography over time. In another study, investigators used dulaglutide, liraglutide, and exenatide in nine adolescents with prediabetes/diabetes and concomitant fatty liver disease where they found that GLP-1 analog use was associated with a decrease in alanine aminotransferase (ALT) by 69% and A1c by 25.8% over an average of 11 months respectively. The investigators did not separate the results by choice of medication making specific medication-related interpretation difficult[15]. Pediatric studies are early but promising, though further large-scale research is needed to assess its use in pediatric MASLD.
Semaglutide received FDA approval in December 2022 for adolescents ≥ 12 years with BMI ≥ 95th percentile for age. Several studies have shown the beneficial effects of semaglutide in adult as well as pediatric patients with diabetes and obesity. In a large randomized controlled trial (STEP TEENS) in adolescents[16], 93/131 participants (73%) in the once-weekly semaglutide group had ≥ 5% weight loss at 68 weeks as compared with 11/62 participants (18%) in the placebo group, with an estimated difference of -16.7 percentage points (95%CI: -6.3 to 31.0, P < 0.001). This study also reported that there were greater improvements in waist circumference, glycemic control as well as alanine aminotransferase levels in the semaglutide group vs placebo. Van Boxel et al[17] also reported similar beneficial effects in their observational study. In their study, patients aged 10-18 years old with obesity who received once-weekly semaglutide (titrated over 8 weeks to a dose of 1 mg weekly) had statistically significant decreases in BMI. The mean percentage of total weight loss observed in the study was 6.4% ± 6.3% (P < 0.001). Semaglutide has also been shown to modulate extracellular matrix production and exert an anti-fibrotic effect, which has been shown to reduce liver injury and improve fibrosis in MASLD in early investigations[18]. A large phase 2 randomized controlled trial examined the effects of subcutaneous semaglutide on metabolic dysfunction-associated steatohepatitis in adults that showed that 40% of people in the 1mg weekly semaglutide group achieved resolution without worsening fibrosis, compared to only 17% in the placebo group (P < 0.01)[19]. The effect was dose-dependent, with higher doses leading to better outcomes over time. There are no recently published studies evaluating the role of semaglutide specifically in pediatric MASLD except for some promising liver function data reported in the STEP TEENS trial, as mentioned above. However, several studies evaluating the role of semaglutide specifically in MASLD in adults are currently underway including the ESSENCE trial[20], with future hopes to translate the data to pediatrics.
Exenatide is FDA-approved for type 2 diabetes in children ≥ 10 years, with encouraging evidence for obesity. In an earlier study by Kelly et al[21] use of exenatide in pediatric patients with severe obesity was associated with a greater reduction in BMI compared with placebo (-2.70%; 95%CI: -5 to -0.37, P = 0.03). This was further investigated in a randomized controlled trial that demonstrated several statistically significant improvements (P < 0.05) compared to placebo in adolescent participants receiving exenatide. Individuals treated with exenatide experienced reductions in BMI standard deviation score (-0.09), percentage of BMI at the 95th percentile (-2.9%), and body weight (-3 kg). While these changes were significant, the reduction in liver fat content did not reach statistical significance (P = 0.06)[22]. Choi et al[15] reported positive reductions in ALT in his small pediatric study for patients who used either exenatide, liraglutide or dulaglutide. These results suggest that exenatide may also be effective in improving various metabolic and anthropometric measures in addition to diabetes management.
Dulaglutide is approved in children > 10 years and adults for managing type 2 in addition to diet and lifestyle changes. Treatment with dulaglutide at a once-weekly dose of 0.75 mg or 1.5 mg was found to be superior to placebo in improving glycemic control through 26 weeks among adolescents with type 2 diabetes, without an effect on BMI[23]. When compared to semaglutide, dulaglutide was inferior to semaglutide in improving glycemic control and reducing body weight, with a similar safety profile[24]. Dulaglutide when used as treatment for type 2 diabetes was also shown to improve liver function, especially gamma-glutamyl transpeptidase values over time without significant reductions in ALT or aspartate aminotransferase levels[25].
Dual agonists with effects on GLP-1 receptors and glucose-dependent insulinotropic polypeptide receptors, like tirzepatide, and triple agonists with effects on GLP-1, glucose-dependent insulinotropic polypeptide, and glucagon receptors like retatrutide are currently being researched for their beneficial effects on obesity and diabetes in the adult population. Tirzepatide in particular is approved for weight management in adults with obesity and obstructive sleep apnea under the brand name Zepbound and approved for type 2 diabetes mellitus in adults under the brand name Mounjaro. In a large meta-analysis with a total of 11758 patients, tirzepatide significantly reduced the BMI of patients compared with GLP-1 receptor agonists, insulin, and placebo groups (P < 0.00001). When compared with placebo alone, tirzepatide had a prominent advantage in weight loss (≥ 20%; P < 0.00001) with a dose-dependent therapeutic effect[26]. These results offer promising new approaches to managing obesity in addition to diet and lifestyle modifications and potential future implications for pediatric obesity.
GLP-1 analogs are currently contraindicated in patients with medullary thyroid cancer and multiple endocrine neoplasia. Earlier studies in animal models have demonstrated abnormal alterations in thyroid C cells, with a gradual risk of adenomas. Despite initial reports of a potential increased risk of thyroid cancer in animal models, results from clinical studies have so far not shown similar effects. Caution may be warranted in patients with familial thyroid cancer or genetic predisposition for thyroid cancers[27]. In terms of side effect profile, gastrointestinal disorders are one of the most common reasons for discontinuing treatment with GLP analogs in adolescents[10,11,15,16]. Nausea, vomiting, and diarrhea are the most frequently reported adverse events, typically mild to moderate in severity and decreasing over time. Pancreatitis has also been reported to occur in patients on GLP-1 analogs to varying extent. Injection site reactions, including local irritation or discomfort at the injection site may also occur. While rare in non-diabetic patients, the risk of hypoglycemia should be monitored, especially in combination with other glucose-lowering medications[28]. In patients using GLP-1 analogs, including adolescents, it’s essential to monitor kidney function. While these are not directly linked to kidney damage, dehydration (from nausea, vomiting or diarrhea) can worsen kidney function in some individuals. Current studies have not demonstrated negative effects on bone mineral density with GLP-1 analogs, and they actually may have protective effects on bone through antiresorptive and anabolic properties. Long-term safety data in the pediatric population is still limited, and ongoing surveillance is crucial to identify potential long-term effects on growth and development.
Despite clinical benefits of GLP-1 receptor agonists and FDA approval for indications including weight loss, there remain challenges related to cost and insurance coverage. Many insurance providers impose strict eligibility criteria, requiring documentation of obesity-related comorbidities or previous unsuccessful weight loss attempts before approving coverage. Even when covered, high copays or prior authorization requirements may limit patient access, leading to treatment interruptions or discontinuation. Disparities in insurance coverage can disproportionately affect lower-income populations, exacerbating healthcare inequities. Future policy efforts should focus on improving affordability and streamlining access to these medications to ensure that all eligible patients can benefit from treatment.
It is also important to consider that while these medications have shown efficacy in promoting weight loss and glycemic control, their long-term success depends on a multifaceted approach that includes tailored dietary strategies, physical activity, and behavioral interventions. This is important especially in the pediatric population, to have close follow-up with dietitian. Nutritional counseling should focus on balanced, portion-controlled meals that provide adequate protein intake to support lean body mass while minimizing muscle loss. Strategies such as smaller, more frequent meals, adequate hydration, and the inclusion of more protein and fiber-rich foods may help mitigate some of the gastrointestinal side effects associated with these medications and improve adherence to therapy. Physical activity is another critical component, as regular exercise not only enhances weight management but also contributes to improved metabolic health, cardiovascular fitness, and muscle preservation. A structured program incorporating both cardiovascular exercises (e.g., brisk walking, cycling, swimming) and resistance training should be recommended, as strength training is particularly important in counteracting the loss of lean body mass associated with weight loss interventions. Furthermore, behavioral counseling should be integrated to address emotional eating, food cravings, and psychological factors that may hinder adherence. Identifying and addressing underlying stressors, anxiety, or depression can further enhance the long-term success of GLP-1 analog therapy.
Future research should focus on understanding the long-term metabolic adaptations with these therapies, strategies for sustained weight loss, and long-term safety profile in various age groups. Additionally, studies are needed to evaluate the role of digital health tools, such as mobile applications and remote monitoring, in enhancing adherence and providing real-time feedback to patients. By integrating pharmacologic, nutritional, behavioral, and technological advancements, GLP-1 analog therapy can be optimized to provide more effective and sustainable weight management solutions.
GLP-1 receptor agonists represent a promising therapeutic option for managing pediatric obesity with emerging data on its use in MASLD. Their ability to promote significant weight loss makes them an attractive adjunct to comprehensive lifestyle interventions. However, challenges related to cost, long-term safety, and adherence must be carefully considered. As research in this field continues to evolve, integrating GLP-1 analogs into comprehensive, multidisciplinary treatment approaches may offer new hope for addressing the growing epidemic of pediatric obesity and its metabolic complications.
| 1. | Stierman B, Afful J, Carroll MD, Chen TC, Davy O, Fink S, Frya CD, Gu QP, Hales CM, Hughes LP, Ostchega Y, Storandt RJ, Akinbami LJ. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files—Development of Files and Prevalence Estimates for Selected Health Outcomes. In: National Health Statistics Reports. United States: National Center for Health Statistics, 2021. |
| 2. | World Health Organization. Obesity and overweight. Mar 1, 2024. [cited 4 February 2025]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. |
| 3. | Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J Obes Metab Syndr. 2023;32:197-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 285] [Article Influence: 142.5] [Reference Citation Analysis (1)] |
| 4. | Parsa AA, Azama KA, Vawer M, Ona MA, Seto TB. Prevalence Study of MASLD in Adolescent and Young Adult Pacific Islanders and Asians Living in Hawai'i. J Endocr Soc. 2024;8:bvad165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Lee EJ, Choi M, Ahn SB, Yoo JJ, Kang SH, Cho Y, Song DS, Koh H, Jun DW, Lee HW. Prevalence of nonalcoholic fatty liver disease in pediatrics and adolescents: a systematic review and meta-analysis. World J Pediatr. 2024;20:569-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 6. | Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From "two hit theory" to "multiple hit model". World J Gastroenterol. 2018;24:2974-2983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 191] [Cited by in RCA: 267] [Article Influence: 38.1] [Reference Citation Analysis (5)] |
| 7. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2121] [Article Influence: 235.7] [Reference Citation Analysis (1)] |
| 8. | Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27:740-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 1106] [Article Influence: 158.0] [Reference Citation Analysis (1)] |
| 9. | Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. J Clin Invest. 2014;124:4223-4226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 10. | Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, Mastrandrea LD, Prabhu N, Arslanian S; NN8022-4180 Trial Investigators. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N Engl J Med. 2020;382:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 384] [Article Influence: 76.8] [Reference Citation Analysis (1)] |
| 11. | 11 Fox CK, Barrientos-Pérez M, Bomberg EM, Dcruz J, Gies I, Harder-Lauridsen NM, Jalaludin MY, Sahu K, Weimers P, Zueger T, Arslanian S; SCALE Kids Trial Group. Liraglutide for Children 6 to <12 Years of Age with Obesity - A Randomized Trial. N Engl J Med. 2025;392: 555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 12. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1471] [Article Influence: 163.4] [Reference Citation Analysis (1)] |
| 13. | Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrønd B, Gough SC, Tomlinson JW, Newsome PN. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther. 2013;37:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Kohut T, Panganiban J. Liraglutide with Lifestyle Intervention in Adolescents with Overweight/Obesity, Nonalcoholic Fatty Liver Disease, and Type II Diabetes Mellitus. JPGN Rep. 2021;2:e141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Choi E, Ramirez Tovar A, He Z, Soler Rodriguez DM, Vos MB, Arora S, Fadoju D. Glucagon-like Peptide-1 Receptor Agonists-A Potential New Medication for Pediatric Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD). Children (Basel). 2024;11:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 16. | Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK, Kelly AS, Mastrandrea LD, Sørrig R, Arslanian S; STEP TEENS Investigators. Once-Weekly Semaglutide in Adolescents with Obesity. N Engl J Med. 2022;387:2245-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 300] [Article Influence: 100.0] [Reference Citation Analysis (1)] |
| 17. | van Boxel EJ, Rahman S, Lai K, Boulos N, Davis N. Semaglutide treatment for children with obesity: an observational study. Arch Dis Child. 2024;109:822-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Scavo MP, Lisco G, Depalo N, Rizzi F, Volpe S, Arrè V, Carrieri L, Notarnicola M, De Nunzio V, Curri ML, De Pergola G, Piazzolla G, Giannelli G. Semaglutide Modulates Extracellular Matrix Production of LX-2 Cells via Exosomes and Improves Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Int J Mol Sci. 2024;25:1493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 1217] [Article Influence: 304.3] [Reference Citation Analysis (0)] |
| 20. | Newsome PN, Sanyal AJ, Engebretsen KA, Kliers I, Østergaard L, Vanni D, Bugianesi E, Rinella ME, Roden M, Ratziu V. Semaglutide 2.4 mg in Participants With Metabolic Dysfunction-Associated Steatohepatitis: Baseline Characteristics and Design of the Phase 3 ESSENCE Trial. Aliment Pharmacol Ther. 2024;60:1525-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 45] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 21. | Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, Fitch AK, Bomberg EM, Abuzzahab MJ. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 22. | Weghuber D, Forslund A, Ahlström H, Alderborn A, Bergström K, Brunner S, Cadamuro J, Ciba I, Dahlbom M, Heu V, Hofmann J, Kristinsson H, Kullberg J, Ladinger A, Lagler FB, Lidström M, Manell H, Meirik M, Mörwald K, Roomp K, Schneider R, Vilén H, Widhalm K, Zsoldos F, Bergsten P. A 6-month randomized, double-blind, placebo-controlled trial of weekly exenatide in adolescents with obesity. Pediatr Obes. 2020;15:e12624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Arslanian SA, Hannon T, Zeitler P, Chao LC, Boucher-Berry C, Barrientos-Pérez M, Bismuth E, Dib S, Cho JI, Cox D; AWARD-PEDS Investigators. Once-Weekly Dulaglutide for the Treatment of Youths with Type 2 Diabetes. N Engl J Med. 2022;387:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 24. | Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, Viljoen A; SUSTAIN 7 investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 519] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 25. | Kuchay MS, Krishan S, Mishra SK, Choudhary NS, Singh MK, Wasir JS, Kaur P, Gill HK, Bano T, Farooqui KJ, Mithal A. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: randomised controlled trial (D-LIFT trial). Diabetologia. 2020;63:2434-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 26. | Cai W, Zhang R, Yao Y, Wu Q, Zhang J. Tirzepatide as a novel effective and safe strategy for treating obesity: a systematic review and meta-analysis of randomized controlled trials. Front Public Health. 2024;12:1277113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (1)] |
| 27. | Capuccio S, Scilletta S, La Rocca F, Miano N, Di Marco M, Bosco G, Di Giacomo Barbagallo F, Scicali R, Piro S, Di Pino A. Implications of GLP-1 Receptor Agonist on Thyroid Function: A Literature Review of Its Effects on Thyroid Volume, Risk of Cancer, Functionality and TSH Levels. Biomolecules. 2024;14:687. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Shetty R, Basheer FT, Poojari PG, Thunga G, Chandran VP, Acharya LD. Adverse drug reactions of GLP-1 agonists: A systematic review of case reports. Diabetes Metab Syndr. 2022;16:102427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |