Published online Sep 9, 2025. doi: 10.5409/wjcp.v14.i3.101476
Revised: February 13, 2025
Accepted: March 25, 2025
Published online: September 9, 2025
Processing time: 273 Days and 18.4 Hours
Functional gastrointestinal disorders (FGIDs), defined as ‘Disorders of Gut-Brain Interaction’, are now considered a global health problem. There is a dearth of concepts and scales to assess the severity of the different symptoms encountered while dealing with the variety of FGIDs as described in the ROME IV classification. We introduced a novel scoring system with the incorporation of 16 different symptoms called Bacharyya’s Questionnaire Scale and started using it while dealing with children suffering from FGIDs.
To verify the usefulness and applicability of this recently developed scale, this study was undertaken with the objectives to establish the validity of this scoring system in assessing the severity of symptoms associated with a specific FGID in children and to determine the scoring system's applicability in assessing the treatment response.
The study included children aged 5 to 18 years diagnosed with any FGID based on ROME IV criteria. They completed the newly developed scale and a Visual Analog Scale at initial diagnosis and after a 2-month treatment period. A control group without FGID participated for comparative baseline purposes. Treatment response was defined as a less than or equal to 50% reduction in the total score, which is statistically significant.
Results from a comprehensive cohort of 190 cases and 90 controls indicated a female preponderance (57.9%) and prevalent disorders such as functional constipation (28%) and functional abdominal pain, not otherwise specified (21%). The grade of FGID (mild, moderate, severe) experienced by the patients was also derived. Post-treatment, 96 children demonstrated symptom improvement. The Spearman rank correlation coefficient for pre (r = 0.72, 95%CI: 0.65-0.77, P value < 0.0001) and post (r = 0.49, 95%CI: 0.3-0.64, P value < 0.0001) treatment data showed positive results with significant P values.
The novel scoring system shows high comprehensibility and gives an objective view of the symptomatology of FGIDs. The use of this novel score in clinical settings will be helpful to typify the FGIDs and may significantly improve decision-making processes to initiate appropriate treatment.
Core Tip: This study introduced and validated a novel scoring system, Bacharyya’s Questionnaire Scale (BQS), to assess the severity of symptoms in children with Functional Gastrointestinal Disorders (FGIDs). The BQS, developed to capture a broad range of symptoms outlined in the ROME IV criteria, was verified in 190 children. The results revealed a significant correlation between the BQS and symptom severity. This scale also effectively tracked the treatment responses. The BQS offers a comprehensive and practical tool for clinical decision-making in FGIDs, enhancing treatment personalization and improving health-related quality of life.
- Citation: Acharyya BC, Das P, Mukhopadhyay M. Validating a novel scoring system for the assessment and treatment of functional gastrointestinal disorders in children. World J Clin Pediatr 2025; 14(3): 101476
- URL: https://www.wjgnet.com/2219-2808/full/v14/i3/101476.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i3.101476
Functional gastrointestinal disorders (FGIDs) are defined as ‘Disorders of Gut-brain Interaction’[1]. Multifactorial etiologies of FGIDs, such as altered brain-gut interactions, genetic predisposition, dysbiosis, dysregulation of the intestinal barrier, and environmental factors, have been noted[2,3]. Children suffering from FGIDs, now considered a health problem found globally, have been shown to possess a lower health-related quality of life (HRQoL) and experience interference with sleep, school, and social activities, thereby imposing a psycho-socioeconomic burden on their families[4–7]. Although FGIDs are hard to define, they have a high prevalence of around 20%[8]. The most widely known and implemented of the FGID tools are the ROME IV assessments, which are symptom-based criteria with versions available for neonates/toddlers and children/adolescents[9,10]. Rome IV is a constellation of different symptoms grouped into different types of FGIDs. It does not have any subchapter or tool to assess the symptom intensity or the intensity of a particular type of FGID that they describe. Moreover, there is a substantial number of children who have overlapping symptoms between the different disorders.
Pediatric healthcare encounters a substantial challenge in effectively addressing FGIDs, with a significant gap in the availability of a specific scoring system to assess the severity of symptoms. There are a few scores like the Abdominal Pain Index (API) for Children[11], the Nausea Severity Scale (NSS) for Children[12], the IBS Severity Scoring Scale (IBS-SSS) for adults[13] and Constipation Scoring System (CSS) for adults[14], and those have been used in a few studies assessing the severity of a particular type of FGID in children. The Paediatric Quality of Life (PedsQL) Gastrointestinal Symptoms Scale and Gastrointestinal Worry Scales[15] are the scales used to assess the severity of pediatric FGIDs in some studies[7]. The PedsQL inventory at its gastrointestinal front encompasses 10 different symptoms such as stomach pain and hurt, stomach discomfort when eating, food and drink limits, trouble swallowing, heartburn and reflux, nausea and vomiting, gas and bloating, constipation, blood in poop as well as diarrhea. However, it does not cover all symptoms depicted in ROME IV. Moreover, blood in poop is often regarded as an alarm feature and those children may need further investigations before labeling them as suffering from FGIDs.
Keeping all these factors in mind, lately, the author developed a novel scoring system, the Bacharyya’s Questionnaire Scale (BQS) (Table 1). There is no single score incorporating all varieties of FGIDs, as depicted in ROME IV. ROME IV itself does not describe any score to assess the intensity/severity of each type of FGID mentioned. Thus the BQS score helps to encompass the diverse symptoms outlined in ROME IV for FGIDs in children. This score gives an accurate initial assessment of the symptoms of children with FGIDs. Moreover, it facilitates the evaluation of administered treatments and helps to indicate improvements in HRQoL. The BQS has been used at our clinic for the last 3 years. Thereby, to verify the usefulness and applicability of this recently developed scale, this study was undertaken with the objectives: (1) To establish the validity of this scoring system in assessing the severity of symptoms associated with specific FGID in children; and (2) To determine the scoring system's applicability in assessing the treatment response.
| Questions (is your child bothered by) | Not bothered | Not so bothered (once/10 days or more) | Slightly bothered (1-2 times/week) | Bothered (3 or more times/week) | Strongly bothered (1 or more times/ day) | Intolerably bothered (every day with school absence/play or activity interruption) |
| Regurgitation | 0 | 1 | 2 | 3 | 4 | 5 |
| Heart burn | 0 | 1 | 2 | 3 | 4 | 5 |
| Throat discomfort (globus) | 0 | 1 | 2 | 3 | 4 | 5 |
| Epigastric pain or burning | 0 | 1 | 2 | 3 | 4 | 5 |
| Early satiety | 0 | 1 | 2 | 3 | 4 | 5 |
| Nausea | 0 | 1 | 2 | 3 | 4 | 5 |
| Vomiting | 0 | 1 | 2 | 3 | 4 | 5 |
| Epigastric bloating | 0 | 1 | 2 | 3 | 4 | 5 |
| Foul smelling eructation | 0 | 1 | 2 | 3 | 4 | 5 |
| Belching | 0 | 1 | 2 | 3 | 4 | 5 |
| Peri umbilical pain | 0 | 1 | 2 | 3 | 4 | 5 |
| Constipation/hard stool Bristol 1 and 2 | 0 | 1 | 2 | 3 | 4 | 5 |
| Incomplete defecation | 0 | 1 | 2 | 3 | 4 | 5 |
| Diarrhea/soft stools | 0 | 1 | 2 | 3 | 4 | 5 |
| Stress related (Diarrhea/soft stool) | 0 | 1 | 2 | 3 | 4 | 5 |
| Any fecal urgency | 0 | 1 | 2 | 3 | 4 | 5 |
This prospective study was undertaken in a tertiary Pediatric Hospital in Kolkata from May 2022 to August 2024.
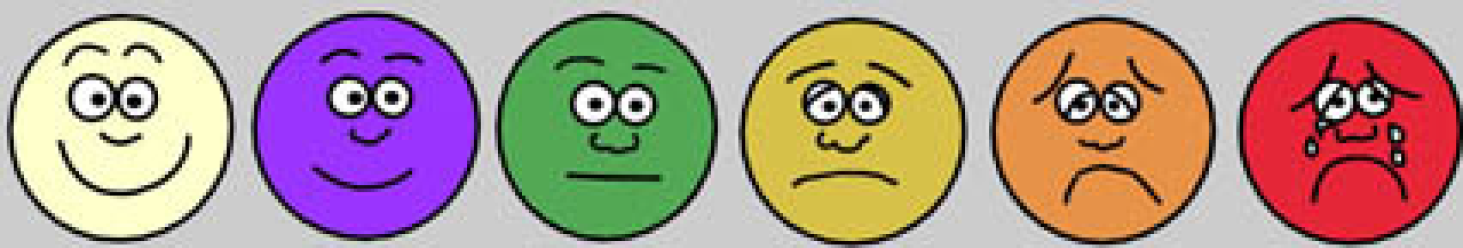
The BQS was developed by the author as shown in Table 1. The already established Visual Analog Score (Figure 1 and Table 2) was incorporated for comparison. The BQS assesses sixteen symptoms of FGIDs as per ROME IV, grading them according to their severity (episodes/week) and how bothered the patients feel by each symptom. Each score represents the severity of each symptom and the constellation of symptoms helped in characterizing the types of FGIDs.
| 0 | 1 | 2 | 3 | 4 | 5 |
| No problem at all | Mild | More than mild | Moderate | Severe | Sometimes very severe |
Participants were children who presented to the pediatric gastroenterology clinic of a tertiary care center due to various symptoms and were diagnosed with different FGIDs. The control group included all patients who did not have abdominal symptoms and came for routine check-ups or vaccinations. Patients were eligible for participation in the study group if no significant current organic disease was identified in the medical evaluation. Exclusion criteria included the presence of chronic diseases (like celiac disease, inflammatory bowel disease, chronic kidney disease, diabetes, and chronic neurological diseases, such as cerebral palsy, and learning disabilities) that may affect the gastrointestinal system. Children suffering from cyclical vomiting syndrome and abdominal migraine were also excluded.
Assuming a proportion of 40% and a power of 80% with a 95% confidence interval, the sample size was calculated as 188 participants and 94 controls.
The clinicians approached patients and their parents in the clinic who had expressed interest in the study. Patients were screened for eligibility by checking their medical records. Those children who fulfilled the inclusion criteria completed the questionnaires and the Visual Analog Scale during their initial and 2-month follow-up clinic visits. Children above 12 years filled the BQS on their own and children below 12 years filled it along with their parents. Study data were collected and further evaluated. The analysis reported in this study was based on baseline (pretreatment) data and 2-month follow-up data of the participants. Each symptom score was added up after completion of the questionnaire and the total score was noted. The constellation of symptoms helped us to note the type of FGID at the same time. A maximum score that could be obtained was 80, and a minimum score could be zero, which meant no symptoms. Treatment response was defined as a 50% reduction in the total score, which was established based on its correlation with scores of 1 or 2 on the Visual Analog Scale. This correlation was derived from our prior experience with the scale in clinical practice. Likewise, the control group also filled out these two questionnaires. Grading the severity of a particular FGID with a defined value (like below or above a number) was difficult due to the presence of various symptoms with different severity scores. However, regardless of the type of FGID, patients who experienced more than 50% of their symptoms at a high intensity (scores 4 and 5) were classified as having severe FGID. In contrast, those with more than 50% of symptoms of moderate intensity (scores 2 and 3) were classified as having moderate FGID. Patients with more than 50% of their symptoms scoring 0 and 1 were classified as having mild FGID.
Simple statistical calculations were undertaken for categorical variables. To verify the validity of this score, Spearman’s Rank correlation coefficient was calculated (between the children with FGIDs and the controls). The Spearman rank correlation coefficient was also calculated to verify the utility of the score for treatment response.
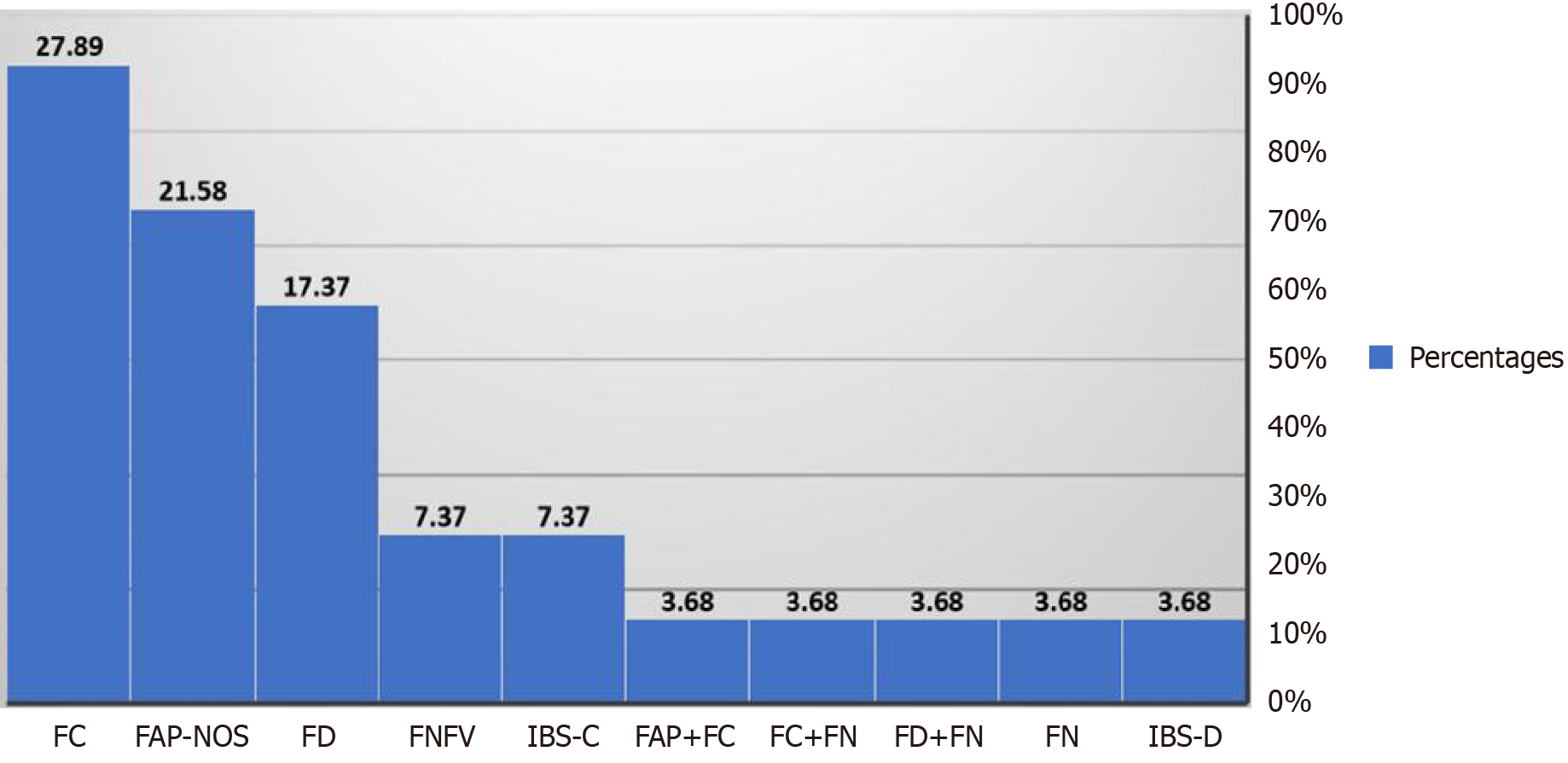
The results from a comprehensive cohort of 190 cases and 90 controls indicated a female preponderance (57.9%) and the median age of presentation was 9.7 years. The most prevalent disorders found were functional constipation (28%) and functional abdominal pain-not otherwise specified (21%) followed by functional dyspepsia (17.3%) (Figure 2 and Table 3). At post-treatment follow-up, 96 children again filled up the scoring card and demonstrated some improvement in their symptoms. To verify the validity of this score to assess the severity of any FGID, the Spearman rank correlation coefficient detected a large positive value between the cases and the control group (r = 0.72, 95%CI: 0.65-0.77, P value < 0.0001). To check the validity of the score to assess the treatment response, the rank correlation coefficient (r = 0.49. 95%CI: 0.3-0.64, P value (< 0.0001) also showed positive results with significant P-values.
| Type of FGID | Count of types of FGID (N190) | Percentages |
| FAP-NOS | 41 | 21.58 |
| FAP+FC | 7 | 3.68 |
| FC | 53 | 27.89 |
| FC+FN | 7 | 3.68 |
| FD | 33 | 17.37 |
| FD+FN | 7 | 3.68 |
| FN | 7 | 3.68 |
| FNFV | 14 | 7.37 |
| IBS-C | 14 | 7.37 |
| IBS-D | 7 | 3.68 |
FGIDs are defined as ‘Disorders of Gut-brain Interaction’, and they are characterized by chronic gastrointestinal symptoms such as regurgitation, heartburn, epigastric or periumbilical pain, nausea, vomiting, diarrhea, constipation, etc. Treatment of chronic symptoms depends on the availability of psychometrically sound tools to assess changes in the intensity and severity of symptoms over time. Thus, assessing the severity and prognosis of these FGID symptoms is of vital importance to specifically diagnose the type of FGIDs and the prognosis of clinical symptoms following treatment and to indicate the improvement in the HRQoL.
The present study depicted a novel score (BQS) incorporating various symptoms of all the FGIDs described in ROME IV except Cyclic Vomiting Syndrome and Abdominal Migraine. The study proved the validity of the score in assessing the severity of the FGIDs by correlating this score with the visual analog score (calculating the Spearman Rank Correlation Coefficient). Furthermore, at the end of treatment, a 50% or more reduction in the total score as well as individual symptomscore indicated a response to treatment. Additionally, the intenseness of any FGID can be classified as mild, moderate, or severe using the score as described above.
Laird et al established a valid scoring system, the API, using a revised scoring method for functional abdominal pain by evaluating the psychometric properties and concluded that the API (revised scoring method) is a useful, reliable, and valid measure of abdominal pain severity[16]. Russell et al[12] developed a psychometric measurement of the properties of chronic nausea in pediatrics, the NSS, and evaluated its reliability and validity in pediatric populations with abdominal pain-related FGID. Farrukh et al[17] discussed the presently available scoring systems. The IBS-SSS, the Irritable Bowel Syndrome Quality of Life, and how they are impacted by issues like ethnicity, gender, age, language and translation, ability to be read, etc. Froehner Junior et al[18] verified the CSS among the Brazilian population and concluded that it is a reliable instrument for measuring the severity of chronic constipation[18]. Unlike the aforementioned studies, which use scales to define a single symptom, our study stands out by incorporating a comprehensive questionnaire scale that encompasses most symptoms of FGIDs. This approach provides a broader perspective, acknowledging that FGIDs often manifest as a constellation of symptoms rather than a single, consistent symptom. This enhances the practical applicability of this scoring system over the others. To the best of our knowledge, there is no composite scoring system or prognostic model to evaluate the severity and intensity of almost all the symptoms of FGIDs to aid in clinical decision-making. Thus, we have developed this novel scoring system, the BQS, which encompasses these diverse symptoms.
In our study, it was observed that functional constipation (28%) and functional abdominal pain-not otherwise specified (21%) were most common. This is similar to the systemic review of FGIDs (ROME IV criteria) by Vernon-Roberts et al, which shows that the most common FGIDs in children aged over four years are functional constipation, functional dyspepsia, and irritable bowel syndrome[19]. Given the overlapping nature of symptoms in FGIDs, our study assessed the severity of each symptom to accurately classify different types of FGIDs, such as functional constipation, functional abdominal pain etc. This approach also helped identify which specific disorder predominated in individual patients. Post-treatment, 96 children demonstrated symptom improvement, which was recorded as a reduction in the severity scores in the questionnaire scale (i.e., ≤ 50% score reduction of the initial score).
Our study has some limitations, which include that of the 190 patients who were evaluated initially, only 96 patients filled out the questionnaire correctly on time at the end of the 2-month follow-up. This novel scoring system is highly comprehensible and provides an objective view of the symptomatology of FGIDs. Use of this novel score in clinical settings may significantly improve decision-making processes and select individualized treatment for patients with FGIDs to avoid inappropriate treatments. When the severity score is less than or equal to 50% of the initial score, then symptomatic improvement can be considered.
Diagnosing FGIDs remains a significant challenge due to their overlapping symptoms and the difficulty in objectively assessing their intensity. Our study highlights the necessity for a scoring system, like the BQS, to effectively classify FGID subtypes and provide an objective measure of symptom severity both before and after treatment. This approach not only aids in accurate diagnosis but also enhances treatment monitoring. To further validate this scoring system and ensure its applicability across diverse populations, larger-scale multicenter studies will be necessary.
| 1. | Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1036] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 2. | Giorgio V, Margiotta G, Stella G, Di Cicco F, Leoni C, Proli F, Zampino G, Gasbarrini A, Onesimo R. Intestinal Permeability in Children with Functional Gastrointestinal Disorders: The Effects of Diet. Nutrients. 2022;14:1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Carlson MJ, Moore CE, Tsai CM, Shulman RJ, Chumpitazi BP. Child and parent perceived food-induced gastrointestinal symptoms and quality of life in children with functional gastrointestinal disorders. J Acad Nutr Diet. 2014;114:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Lu PL, Saps M. Functional Gastrointestinal Disorders: All Roads Lead to Prevention. Clin Gastroenterol Hepatol. 2018;16:814-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Jansen J, Shulman R, Ward TM, Levy R, Self MM. Sleep disturbances in children with functional gastrointestinal disorders: demographic and clinical characteristics. J Clin Sleep Med. 2021;17:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Saps M, Velasco-Benitez CA, Blom PJJ, Benninga MA, Nichols-Vinueza DX. Prospective Study of Gastrointestinal Symptoms in School Children of South America. J Pediatr Gastroenterol Nutr. 2018;66:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Varni JW, Bendo CB, Denham J, Shulman RJ, Self MM, Neigut DA, Nurko S, Patel AS, Franciosi JP, Saps M, Yeckes A, Langseder A, Saeed S, Pohl JF. PedsQL™ Gastrointestinal Symptoms Scales and Gastrointestinal Worry Scales in pediatric patients with functional and organic gastrointestinal diseases in comparison to healthy controls. Qual Life Res. 2015;24:363-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Vernon-roberts A, Alexander I, Day AS. Prevalence of Functional Gastrointestinal Disorders (Rome IV Criteria) among a Cohort of New Zealand Children. Gastrointest Disord. 2023;5:261-272. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 9. | Benninga MA, Faure C, Hyman PE, St James Roberts I, Schechter NL, Nurko S. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology. 2016;6:1443-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 10. | Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional Disorders: Children and Adolescents. Gastroenterology. 2016;6:1456-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 812] [Article Influence: 90.2] [Reference Citation Analysis (5)] |
| 11. | Walker LS, Williams SE, Smith CA, Garber J, Van Slyke DA, Lipani T, Greene JW, Mertz H, Naliboff BD. Validation of a symptom provocation test for laboratory studies of abdominal pain and discomfort in children and adolescents. J Pediatr Psychol. 2006;31:703-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Russell AC, Stone AL, Wang A, Walker LS. Development and Validation of a Nausea Severity Scale for Assessment of Nausea in Children with Abdominal Pain-Related Functional Gastrointestinal Disorders. Children (Basel). 2018;5:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1223] [Article Influence: 43.7] [Reference Citation Analysis (1)] |
| 14. | Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996;39:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 851] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 15. | Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1938] [Cited by in RCA: 2153] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 16. | Laird KT, Sherman AL, Smith CA, Walker LS. Validation of the Abdominal Pain Index using a revised scoring method. J Pediatr Psychol. 2015;40:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Farrukh A. Measurement of Pain and Related Symptoms in Irritable Bowel Syndrome: The Use of Validated Pain Measurement Tools. Gastrointest Disord. 2022;4:22-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Froehner Junior I, Jorge JMN, Marques CFS, Santos VLCG, Jukemura J. The Portuguese-validated constipation scoring system (índice de gravidade da constipação intestinal): is it reliable in assessing the severity of chronic constipation in the intestinal tract of our population? Arq Bras Cir Dig. 2024;36:e1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Vernon-Roberts A, Alexander I, Day AS. Systematic Review of Pediatric Functional Gastrointestinal Disorders (Rome IV Criteria). J Clin Med. 2021;10:5087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |