Published online Mar 9, 2024. doi: 10.5409/wjcp.v13.i1.88783
Peer-review started: October 9, 2023
First decision: December 8, 2023
Revised: December 13, 2023
Accepted: January 4, 2024
Article in press: January 4, 2024
Published online: March 9, 2024
Processing time: 149 Days and 16.4 Hours
Infants' nutrition significantly influences their growth, development, and overall well-being. With the increasing demand for organic infant formula driven by the perception of health benefits and growing awareness of natural feeding options, it is crucial to conduct a comparative analysis of the gastrointestinal tolerability between organic and traditional infant formulas.
To provide a concise and precise analysis of the gastrointestinal tolerability of organic infant formula compared to traditional infant formula. Due to limited direct comparisons, the review synthesizes available literature on each formula type, presenting insights into their potential effects on infants' digestive health.
An extensive literature search was conducted, compiling studies on organic and traditional infant formulas, their compositions, and reported effects on gastrointestinal tolerability. We searched academic databases such as PubMed and Google Scholar and specialized nutrition, paediatrics, and infant health journals using relevant keywords till October 1, 2023.
Although specific comparative studies are scarce and formula heterogeneity is a significant limitation, this systematic review provides an in-depth understanding of organic infant formulas' composition and potential benefits. While scientific evidence directly comparing gastrointestinal tolerability is limited, organic formulas strive to use carefully selected organic ingredients to imitate breast milk composition. Potential benefits include imp
Despite limitations in direct comparisons, this systematic review provides insights into the composition and potential benefits of organic infant formulas. It emphasizes the need for further research to elucidate their gastrointestinal effects comprehensively.
Core Tip: This systematic review focuses on the growing demand for organic infant formula, highlighting its potential benefits and impact on gastrointestinal health compared to traditional infant formula. Although there are limited direct comparative studies, an analysis of available literature suggests that organic formulas aim to replicate the composition of breast milk, providing improved lipid profiles, higher methionine content, and potentially reducing antibiotic-resistant bacteria. To make informed decisions about infant nutrition, it is crucial to understand the digestive effects of these formulas. Therefore, further comprehensive research is needed to elucidate their gastrointestinal implications fully.
- Citation: Al-Beltagi M, Saeed NK, Bediwy AS, Elbeltagi R, Hamza MB. Gastrointestinal tolerability of organic infant formula compared to traditional infant formula: A systematic review. World J Clin Pediatr 2024; 13(1): 88783
- URL: https://www.wjgnet.com/2219-2808/full/v13/i1/88783.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i1.88783
Infants require proper nutrition for their growth, development, and overall well-being. Nutrients like protein, fat, carbohydrates, vitamins, and minerals are essential for the body to develop tissues, organs, and systems. Adequate nutrition strengthens their immune system and helps in brain development and cognitive function. Calcium and vitamin D assist in building strong bones. Breast milk or formula provides the necessary calories and nutrients, and introducing allergenic foods may reduce food allergy risk[1,2]. Inadequate nutrition increases the risk of illness in infants and children and is responsible for one-third of deaths in children below 5 years of age. Improper childhood nutrition can lead to obesity, a severe public health problem worldwide[3]. Malnutrition during early life, particularly in the first two years, leads to stunting, causing short stature during adulthood. Research has shown that malnourishment during early childhood can lead to long-term impaired intellectual performance during adulthood[4]. Breastfeeding promotes a strong emotional bond, and optimal nutrition reduces the risk of health problems later in life. Some infants may require specialized formulas due to medical conditions or specific dietary needs[5]. Infant formula is an essential alternative to breast milk for infants who cannot breastfeed or when it is unavailable. In such cases, healthcare professionals can help parents choose the most appropriate feeding option. Providing optimal nutrition during infancy is crucial for promoting healthy growth and development, supporting the immune system, and laying the groundwork for a healthy and thriving life. Parents, caregivers, and healthcare providers all play critical roles in ensuring that infants receive the nutrition they need to reach their full potential[6].
The demand for organic infant formula is increasing as parents become more aware of the potential health benefits. The term "organic" reflects a farming method that agrees with nature and is sustainable. Organic formula is considered a healthier option because it's free from synthetic additives, pesticides, genetically modified organisms (GMOs), and artificial additives[7]. Its market share has been steadily increasing in many regions. Organic infant formula is highly sought after as parents look for natural and safe baby products. More companies are entering the market, offering various products that meet strict regulations and certification standards. Organic infant formula is in higher demand in de
This systematic review aims to provide a concise and comprehensive analysis of the gastrointestinal tolerability of organic infant formula compared to traditional infant formula. The review examines the existing literature on both formula types and their impact on infants' digestive health. By comparing the gastrointestinal effects of organic and traditional infant formulas, the systematic review provides a comprehensive overview of the importance of proper nutrition for infants, the rising demand for organic infant formula, and the regulatory frameworks surrounding organic milk and infant formula. It also offers valuable insights for parents, caregivers, and healthcare professionals in making informed decisions when choosing between these two types of infant formulas.
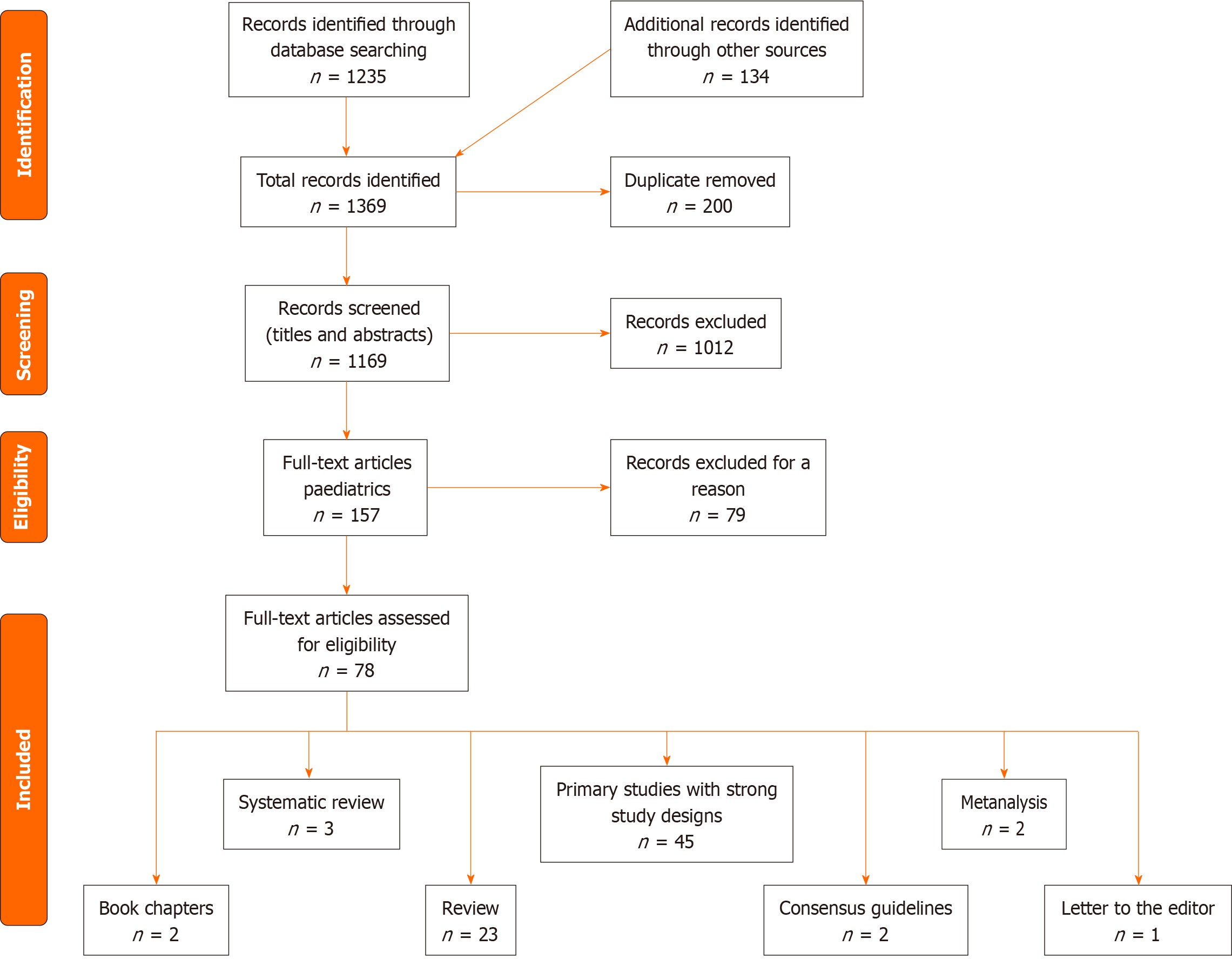
We conducted a comprehensive literature review to gather data on organic and conventional infant formula. We searched academic databases such as PubMed and Google Scholar and specialized nutrition, paediatrics, and infant health journals using relevant keywords till October 1, 2023. These keywords included "organic infant formula," "conventional infant formula," "gut tolerability," "nutritional composition," and related terms. Our review only included peer-reviewed articles, conference papers, and reputable publications. Our inclusion criteria focused on studies that examined the composition, gut tolerability, and nutritional aspects of organic and conventional infant formulas. We included studies that directly or indirectly compared these aspects, and only studies available in English were considered. We prioritized studies with transparent methodology, appropriate sample sizes, and a focus on infant populations. We also checked reference lists and conducted citation searches on the included studies. Articles with a possible commercial background were excluded. In total, we included 78 articles consisting of 45 research articles, two meta-analyses, three systematic reviews, 23 narrative reviews, two consensus guidelines, two book chapters, and one letter to the editor. We extracted data on formula compositions and nutritional profiles from producers' websites and end-products. Gut tolerability findings and related outcomes were extracted from the selected studies. We comprehensively compared the organic and conventional formulas, noting any discrepancies or variations in findings for further analysis and discussion. We evaluated the quality of each study, considering factors such as study design, sample size, methodology, and statistical significance. We identified and documented the limitations of each study. We assessed the overall quality of evidence for each aspect using established grading systems to ensure a robust analysis. Our comparative analysis focused on gut tolerability and nutritional composition differences between organic and conventional infant formulas. We paid specific attention to protein levels, lipids, lactose composition, and the presence of prebiotics and probiotics. We summarized the results, highlighting key findings from the literature. We identified research gaps and areas requiring further investigation based on the limitations and discrepancies observed in the reviewed studies. We also proposed recommendations for future research to enhance our understanding of gut tolerability and nutritional implications associated with organic and conventional infant formulas. We included a total of 175 full-text articles, including 102 research articles, nine meta-analyses, four systematic reviews, 58 narrative reviews, and two consensus guidelines. Figure 1 shows the PRISMA study flow chart.
From a systematic review of the included studies, we can summarize the findings into the following points as elaborated in Tables 1-5.
| European organic infant formula | American organic infant formula | |
| Regulations | EC has stricter standards than the United States FDA. For instance, all infant formulas must be organic, and there is a limit on certain ingredients, such as pesticides and antibiotics | The FDA regulates infant formulas through USDA & NOP, which have less strict standards than the EC. Not all infant formulas are required to be organic, & the FDA does not limit certain ingredients |
| Cultural attitudes toward the formula | Very strong positive attitude across the countries | Less than in Europe |
| Guidelines & regulations | Updated yearly | Not as frequent as European guidelines |
| Labelling and claims | Must meet standardized requirements, and products displaying the EU Organic Logo adhere to these regulations | Must comply with the NOP guidelines. The USDA Organic seal signifies adherence to these standards |
| Ingredients | It is more likely to be made with organic ingredients, such as milk from grass-fed cows or goats. They are also more likely to contain probiotics, beneficial bacteria that can help support gut health | It is more likely to contain added iron, which is essential for preventing iron deficiency anemia. They may also have other ingredients not allowed in European formulas, such as corn syrup solids |
| Probiotics | It is more likely to contain probiotics, which are beneficial bacteria that can help support gut health | Less likely to contain probiotics |
| Percentage of organic ingredients | Not less than 95% | Not less than 70% |
| Calories sources | Must have at least 30% of calories from lactose | Not required |
| The inclusion of sucrose is prohibited, except in small quantities in some specialty formulas, such as premature or hypoallergic formulas | It may contain Sucrose, glucose, and maltodextrins | |
| Added iron | It is less likely to contain added iron, which is vital for preventing iron deficiency anemia | More likely to contain added iron |
| Synthetic additives, pesticides, steroids, hormones, & GMOs | Strictly prohibited | Synthetic additives and GMOs are also prohibited, but specific regulations may differ |
| Taste | Some parents say that European formulas taste better than American formulas. This is likely because they are made with more natural ingredients | There is no consensus on whether European or American formulas taste better. Some parents prefer the taste of European formulas, while others prefer the taste of American formulas |
| Cost | Typically, they are more expensive than American formulas. This is because they are made with higher-quality ingredients & have stricter regulations | Typically, they are less expensive than European formulas. This is because they are made with less expensive ingredients and have less strict regulations |
| Brand | Country of origin | Protein source/100 mL | Fat source/100 mL | Carbohydrate source/100 mL | Other ingredients | |
| 1 | Arla Baby & Me Organic | Denmark | 1.4 g, Whey Protein Concentrate, Whey/ Casein Ratio 60/40 | 3.6 g, vegetable oils (sunflower, soy) | 6.7 g, Lactose | GOS, FOS, DHA of algal oil origin, ARA of fungal oil origin, Lecithin, choline, inositol, L-carnitine, tocopherol-rich extract |
| 2 | HiPP Organic | Germany | 1.2-1.5 g, Whey protein concentrate, W/C Ratio 60:40 | 3.5-4.4 g, Palm olein oil, rapeseed oil, coconut oil, sunflower oil | 6.5-7.5 g, Lactose | DHA, ARA, choline, taurine, nucleotides, lactoferrin, Metafolin, symbiotics (L. fermentum and GOS) |
| 3 | Kendamil Organic | United Kingdom | 1.4 g, Whey protein concentrate, W/C Ratio 60:40 | 3.5 g, Whole milk fat and reduced levels of Organic vegetable oils (sunflower, coconut, rapeseed). No palm oil | 7 g, Lactose | Marine algae-derived DHA, ARA, choline, taurine, nucleotides, lactoferrin, inositol, L-Carnitine, Organic GOS, 3'GL - Galactosyllactose |
| 4 | Holle Organic | Germany | 1.4 g, contains the A2 protein. W/C Ratio 60:40 | 3.4 g, vegetable oils (palm, sunflower, rapeseed oil), oil from the microalgae Schizochytrium sp.2, Mortierella Alpina oil (No palm oil) | 7.7 g, Lactose | Algae-derived DHA, ARA, choline, taurine, nucleotides, lactoferrin |
| 5 | Bellamy's Organic | Australia | 1.5 g, Whey protein concentrate, W/C Ratio 60:40 | 3.4 g, Palm olein oil, soybean oil, sunflower oil | 7.6 g, Lactose | Dried DHA and ARA oils [fish oil (tuna), choline, taurine, nucleotides, a Prebiotic GOS, 16 essential vitamins & minerals |
| 6 | Bubs Australia | Australia | 1.56 g, Organic Whey Protein Concentrate, Whey/ Casein Ratio 60/40 | 3.7 g, Organic Vegetable Oil Blend (High Oleic Sunflower, Coconut, Soy, Canola) | 7.3 g, Organic Lactose | Organic GOS, DHA, from Algae, ARA, Probiotic Bifidobacterium longum BB536 |
| 7 | Similac Organic with A2 milk | United States | 1.55 g, Whey Protein Concentrate, A2 beta-caseins, W/C of 48:52 | 4.2 g, Organic High Oleic Sunflower Oil, Soy Oil, Coconut Oil | 8 g, Organic Lactose | DHA, lutein, Choline, Beta-Carotene, Lycopene, Inositol, Nucleotides, Taurine, L-carnitine, L-methionine, Short-chain FOS |
| 8 | Happy Family Organics | United States | 1.38 g, Organic Whey Protein Concentrate, W/C ratio of 30:70 | 3.4 g, Organic Palm Olein or Palm Oil, Soy Oil, Coconut Oil, High Oleic (Safflower or Sunflower) Oil | 8 g, ORGANIC LACTOSE | DHA Algal Oil, Organic FOS and GOS, Choline, soy Lecithin, Beta-Carotene |
| 9 | Baby's Only Organic | United States | 1.54 g, Organic Whey Protein Concentrate, A2 Protein, W/C Ratio 60:40 | 4.2 g, Organic High Oleic Sunflower and/or Organic High Oleic Safflower Oils), Organic Soybean Oil, Organic Coconut Oil | 7.76 g, Organic Lactose | Choline, Taurin, Organic, Inositol, Non-Hexane Extracted Source of DHA & ARA |
| 10 | Plum Organics | United States | 1.38 g, Organic Whey Protein Concentrate W/C Ratio 60:40 | 3.7 g, Organic Palm Oil Or Palm Olein, Soy Oil, Coconut Oil, High Oleic (Safflower or Sunflower) Oi | 6.9 g, Organic Lactose | Plant-based DHA, & ARA. Tocopherol, Choline, Taurine, Lecithin |
| 11 | Honest Company Organic | United States, there was an issue about containing 11 non-organic elements | 1.6 g, Organic Whey Protein Concentrate, W/C Ratio 60:40 | 3.9 g, Organic Palm Oil or Palm Olein, Soy Oil, Coconut Oil, High Oleic (Safflower or Sunflower) Oil | 7.9 g, Organic Lactose, Organic Glucose Syrup Solids | Sodium Selenite, Taurine, Choline, Beta carotene, and Inositol Do not disclose the DHA/ARA extraction method |
| 12 | Enfamil Simply Organic | United States, the first organic formula that has certified USDA | 1.49 g, Organic nonfat milk W/C Ratio 20:80 | 3.6 g, organic vegetable oil (organic palm, organic coconut, organic soy, and organic high oleic sunflower oils) | 7.5 g, Organic Lactose, organic maltodextrin | Omega-3 DHA, Inositol, Choline, taurine, L-carnitine, & organic GOS |
| 13 | Nature’s One Baby’s Only | United States | 1.5 g, Organic Whey Protein Concentrate W/C Ratio 60:40 | 4.2 g, Organic High Oleic Sunflower, Soybean Oil, Coconut Oil | 7.7 g, Organic Lactose | Choline, Inositol |
| 14 | Bobbie Formula | United States | 1.47 g, organic whey protein concentrate, W/C Ratio 60:40 | 3.9 g, organic high oleic (sunflower or safflower) oil, canola oil, coconut oil, linoleic sunflower or safflower) oil | 8 g, Organic lactose | Choline, Inositol, Biotin, DHA, ARA |
| 15 | Earth's Best Organic | United States | 1.64 g, Whey protein concentrate, W/C Ratio of 70/30 | 4 g, Palm olein oil, soy oil, coconut oil, sunflower oil, Linolic acid 750 mg | 8 g, Lactose | DHA, ARA, choline, lutein, taurine, carnitine, selenium nucleotides, Iron, prebiotic FOS fiber |
| Feature | Human milk | Organic infant milk |
| Source | Produced by lactating mothers | Derived from organic cow's milk |
| Composition | Complex and ever-changing, tailored to the individual baby's needs | Mimics the composition of human milk but may not be identical |
| Nutrients | Contains all the nutrients a baby needs for optimal growth and development, including antibodies, enzymes, hormones, and growth factors | It contains most of the nutrients a baby needs but may not be as high in certain nutrients as human milk |
| Digestibility | Easily digestible, less strain on baby's digestive system, and well-absorbed | It may be more difficult to digest than human milk, especially for preterm babies. Generally easy to digest but may be more difficult to digest than human milk, especially for preterm babies. Some babies may have sensitivities |
| Allergies | It may help protect against allergies | Despite being organic, it may not offer the same protection against allergies as human milk |
| Infections | It may help protect against infections | It may not offer the same protection against infections as human milk |
| Growth Factors | Contains growth-promoting factors | Contains growth factors for the development |
| Probiotics | Contains beneficial bacteria | It may contain added probiotics |
| Cost | Free (if breastfeeding) | Varies, but typically more expensive than conventional infant formula |
| Availability | Available from any mother who is breastfeeding; no preparation is needed | Available at most grocery stores and online retailers, Requires preparation and storage |
| Environmental Impact | Minimal carbon footprint, no packaging waste | It may have a higher carbon footprint and packaging waste |
| Emotional Bonding | Promotes bonding between mother and baby | Less direct emotional bonding |
| Organic | Non-organic | |
| Dairy source | Milk often comes from organically raised cows or other organic animal sources | Milk may come from conventionally raised cows with potential hormone and antibiotic use |
| Ingredients | The certified organic formula must be at least 95% organic, including the milk, vitamins, minerals, and other nutrients—no synthetic pesticides, antibiotics, hormones, herbicides, GMOs, or artificial additives | It may include non-organic ingredients, synthetic pesticides, GMOs, artificial additives, non-organic corn syrup solids, soy oil, and palm oil |
| Nutrient levels | Provides essential nutrients for infant growth and development | Meets similar nutritional needs as the organic formula |
| Fat | 3.5-4.0 g/100 mL | 3.0-3.5 g/100 mL |
| Emphasis on organic and natural ingredients, including organic vegetable oils (palm, coconut, soy, sunflower, etc.) | Similar use of vegetable oils as fat sources may not be organic | |
| Aim for a closer resemblance to breast milk in terms of balanced omega-3 and omega-6 fatty acids. Slightly higher content of omega-3 fatty acids | Aim to provide appropriate ratios of fatty acids essential for infant development | |
| CHO | Formulated to meet the nutritional needs of infants. Organic lactose is the primary milk CHO mimicking breast milk in most organic formulas, especially the European formula. The American formula may add other CHO, such as corn syrup, glucose Syrup, and maltodextrin. The lactose amount is typically around 40% of the total calories, about 6-7 g/100 mL. Is easier to digest. Has a better texture & provides a creamy consistency | Formulated to meet the nutritional needs of infants. Lactose is the pr imary CHO source, designed to mimic the CHO composition of breast milk, especially the European formula. The American formula may add other CHO, such as corn syrup, glucose Syrup, Brown Rice Syrup, and sucrose. The lactose amount is typically around 40% of the total calories, about 8-9 g/100 mL |
| Proteins | It comes from organic dairy sources and contains easily digestible whey and casein proteins with smaller size molecules in a ratio (usually 60/40) and an amino acid pattern that mimics breast milk, supporting optimal digestion and balanced growth | Dairy sources are from conventionally raised cows, with whey and casein proteins with large-sized molecules, but the ratio might differ from breast milk. The amino acid pattern is designed to provide essential amino acids for infant growth |
| Flavors and colors | It may contain natural flavors & colors, such as vanilla or strawberry | It may contain artificial flavors and colors |
| Processing methods | Gentler processing to preserve nutrient content | It may undergo more intensive processing, potentially leading to some nutrient loss |
| Regulations | Subject to regulations set by health authorities (e.g., FDA in the U.S., EFSA in the EU | Subject to regulations set by health authorities (e.g., FDA in the U.S., EFSA in the EU) |
| Consumer preferences | Chosen by parents who prioritize natural and organic ingredients, absence of synthetic additives, pesticides, and GMOs | Chosen based on many factors like cost, availability, and a high standard of nutritional quality |
| Environmental considerations | Emphasizes organic farming practices and reduced chemical use | It may involve more intensive chemical use with potential environmental impacts |
| Item | Possible factor |
| Formula-related | Protein source and composition |
| Lactose content | |
| Fat source and composition | |
| Presence of prebiotics and/or probiotics | |
| Fiber content | |
| Osmolality and osmolarity | |
| Additives and nutrient density | |
| Infant-related factors | Presence of individual sensitivities |
| Medical Co-morbidities. e.g., prematurity | |
| Hydration status | |
| Feeding procedure factors | Milk temperature |
| The flow rate of the bottle | |
| The amount of the milk/feed | |
| The feeding technique |
A comparison between organic and non-organic infant formulas showed clear differences in various aspects. Organic formulas, usually obtained from organically raised animals, prioritize natural ingredients and strict regulations, ensuring a composition of at least 95% organic material. On the other hand, non-organic formulas may contain synthetic additives, GMOs, and non-organic components, which are typically sourced from conventionally raised animals and can have varying nutrient levels and processing methods.
Organic baby formulas are designed to replicate the composition of human milk, with a focus on using organic lactose as the primary source of carbohydrates and organic dairy-derived proteins, with a balanced ratio of whey and casein (usually 60/40). These formulas also prioritize organic and natural fats, particularly vegetable oils, to balance omega-3 and omega-6 fatty acids, similar to that found in breast milk.
Organic and non-organic formulas aim to fulfil infants' nutritional requirements, but there are some differences between them. Non-organic formulas maintain similar nutrient levels but may differ slightly in components like fat content (which can range from 3.0-3.5 g/100 mL), carbohydrate sources, and protein characteristics. Although non-organic formulas meet regulatory standards, they may not perfectly match the nutrient ratios found in organic formulations.
Different factors can affect the ability of an individual to tolerate food. These factors include the composition of the food, the presence of prebiotics and probiotics, and the amount of lactose in the food. Individual sensitivities, medical co-morbidities, and hydration levels are also factors that can influence gastrointestinal tolerance. Additionally, how the food is prepared and fed can impact its tolerability. Factors such as the milk's temperature, the bottle's flow rate, and the feeding technique can all play a role in how well someone can tolerate their food.
Organic formulas emphasize environmentally conscious farming practices and reduce chemical use, catering to parents prioritizing natural, organic ingredients and the absence of synthetic additives, pesticides, and GMOs. Conversely, non-organic formulas might involve more intensive chemical use, potentially impacting the environment, and were chosen based on diverse factors, including cost, availability, and perceived nutritional quality.
It is interesting to note that the use of animal milk for infant feeding dates back to around 2000 BC. However, the concept of organic milk is relatively new and emerged much later[10]. During the mid-1940s, there was a global need to increase agricultural practices due to a food shortage after World War II[11]. In dairy production, this was achieved through genetic selection for higher productivity and improved nutrition, including a greater proportion of grains in animal diets. This led to a significant increase in milk yields per cow in the United Kingdom, from 4099 liters per cow per year in 1975 to 7916 liters per cow per year in 2014[12]. Total milk production also increased by 9%, from 13407 million liters to 14649 million liters. However, this intensive farming had some drawbacks, such as poor fertility and longevity in dairy cows and an increased incidence of mastitis due to antibiotic use[13]. As a result, consumers in affluent, developed countries began to demand food from less intensive production systems, including milk and meat[14]. This new socio-economic marketplace provided an excellent platform for developing organic production and commercializing organic milk. Organic milk is produced under strict regulations prohibiting the use of synthetic pesticides, herbicides, fertilizers, and antibiotics on farms that meet specific animal welfare standards[15].
The organic food industry in Europe has grown significantly since the beginning of the 21st century. The dairy sector has been the largest and fastest-growing segment. In 2018, organic dairy sales in Great Britain represented 3.9% of total dairy sales, with the organic milk market valued at £351 million. This accounted for 29% of total organic food sales[16]. The organic liquid milk market is growing at an annual rate of 1.8%, and 25% of households in Great Britain buy organic milk. The United Kingdom's organic dairy market is expected to grow further due to various factors such as strong sales, high-profile private labels, improved distribution chains, rising export demand, and farm conversions. The number of organic farms and cows is also increasing all over Europe, with Germany, Austria, France, and Great Britain having the largest numbers of organic dairy cows[17]. People buy organic food for various reasons, including their beliefs that it is better for the environment, animals, and human health. A recent study found that people primarily purchase organic food because they perceive it as more nutritious and safer than conventional food[18]. However, other factors such as animal welfare, price, availability, freshness, appearance, and taste also play a role in consumer decision-making. In the United Kingdom, a case study found that the primary reason for purchasing organic milk is the perceived health benefits, with other important factors being better taste, perceived environmental benefits, and avoiding genetically modified ingredients[19]. In 2018, organic cow milk production in the European Union accounted for 3.40% of European dairy cows' production, which is double the figure since 2008[20]. On the other side of the ocean, retail purchases of organic milk products in the United States have increased fivefold since 2002, reaching more than $6 billion in 2020[21].
Organic milk production triggered the development of organic infant formula, which began to be produced and sold in Europe in the early 1990s due to the growing need for organic food products. Many people in Europe believe that organic infant formulas are healthier and more nutritious than conventional formulas. However, the United States did not introduce organic infant formula until 2006. Over the years, the organic infant formula market has grown significantly[22]. In 2000, the European Union introduced the Organic Food Regulation, establishing strict standards for ingredients, processing, and labeling of organic infant formulas. Due to these regulations, the European market for organic infant formula has grown significantly and was worth €2.5 billion in 2021. This growth is expected to continue due to the increasing demand for natural and sustainable products, rising awareness of the benefits of organic food, and health concerns about conventional infant formulas[23,24].
Regulation of organic infant formula in the United States was relatively delayed compared to Europe. The National Organic Program was established by the United States Department of Agriculture in 2009 to regulate the production and labeling of organic infant formula. The program enforces strict criteria for ingredients, production, and processing methods to ensure safety and nutritional value. Other federal regulations, including the Food, Drug, and Cosmetic Act, the Federal Trade Commission Act, and the Consumer Product Safety Improvement Act, also play crucial roles in maintaining the quality of organic infant formulas[21]. As demand for organic infant formula grows, regulatory agencies must remain vigilant and make necessary adjustments to ensure ongoing safety and quality. Countries such as China, Korea, Japan, and Australia have also developed their own standards and regulatory bodies for organic infant formula. Table 1 compares the European and American Organic Infant Formulas[25].
Organic and conventional infant formula manufacturers follow strict regulations and procedures to ensure their safety and nutritional adequacy. However, the processing methods and ingredients used in both types of formula may differ significantly due to differences in sourcing, production standards, and regulations[23]. Organic formula is made in a certified organic facility that meets specific environmental sustainability and social responsibility standards. The formula is made from ingredients sourced from organic farms that follow strict farming practices, excluding synthetic pesticides, GMOs, and synthetic fertilizers[26]. Organic processing methods emphasize natural and minimally processed methods, and the formula may undergo additional testing and quality control measures[27].
In contrast, conventional formulas can combine organic and non-organic ingredients, with some coming from farms that use synthetic pesticides, GMOs, and other conventional farming methods. Conventional formulas may include a broader range of additives, preservatives, and synthetic nutrients to achieve desired characteristics and shelf stability. Conventional processing methods may involve using various processing aids and solvents[28]. It is important to note that regulations and practices can vary between countries and regions[29]. When choosing an infant formula, parents should consider their values and priorities.
Organic infant formula is designed to provide infants with the essential nutrients for healthy growth and development. Although there may be slight variations in the specific ingredients among different brands or formulations, most organic infant formulas contain all the necessary nutritional components for infants and young children. The fatty acid profile in organic milk can vary significantly depending on the cows' diets, while protein composition has a more substantial genetic basis. Most studies on milk quality focus on fat composition, proteins, antioxidants, minerals, and other constituents such as terpenes and polyphenols[30]. Table 2 compares the composition of the main components of popular brands of organic first-stage infant formulas according to the companies' official sites. The primary source of protein in organic infant formulas is typically cow's milk, which may also be substituted with goat milk or plant-based proteins that have been modified and broken down to make them easier for infants to digest. Most organic formulas contain whey to a casein ratio of 60:40, which mimics breast milk. However, some organic formulas, especially United States ones, have higher casein ratios. Other formulas may contain type A2 beta-casein protein, which is known to be more digestible than type A1 beta-casein protein. The amino acid pattern in organic and conventional formulas differs significantly. Corbu et al[31] found that organic formulas had significantly higher Methionine content than conventional formulas. Methionine is essential for protein synthesis, cellular growth, repair of damaged cells, neurotransmitter synthesis, and antioxidant activity[32].
Organic lactose is commonly used as the main carbohydrate source due to its easy digestibility for infants and is naturally found in breast milk. It also enhances texture and adds a creamy consistency to products. Some organic companies have used organic brown rice syrup as a carbohydrate source. Still, it may contain inorganic arsenic levels up to six times the American standards for safe drinking water. To address this issue, these companies developed organic-compliant technology that filters and removes inorganic arsenic from organic brown rice syrup to undetectable levels[33].
Organic vegetable oils such as palm, coconut, soybean, or sunflower are added to provide essential fatty acids for brain development and energy. Tsiafoulis et al[34] discovered that organic milk has a significant increase in the percentage of (9-cis, 11-trans) 18:2 linoleic, linoleic, and α-linolenic acids - compounds that contain allylic protons and unsaturated fatty acids. Moreover, Tsiafoulis et al[34] found a significant decrease in the amount of caproleic acid found in organic milk compared to conventional milk. Additionally, Mazzei et al[35] found that organic milk has more unsaturated lipids and phosphatidylcholine than conventional milk.
Organic formulas are enriched with vital nutrients such as vitamins A, B, D, E, and K and essential minerals like calcium, phosphorus, iron, zinc, potassium, magnesium, and selenium. These nutrients support various body functions, promote overall growth, and facilitate bone development, red blood cell formation, and metabolic processes. Some organic formulas may contain prebiotics (like oligosaccharides) and probiotics (like Bifidobacteria and Lactobacillus) that help sustain a healthy gut microbiome and improve digestion. Nucleotides in breast milk are believed to promote infant immune development. Consequently, some formulas also include nucleotides and critical fatty acids like docosahexaenoic acid (DHA), arachidonic acid (ARA), and omega-6 fatty acids that are necessary for brain and eye development. Most organic formulas have DHA and ARA, which are often derived from algae and fungal sources[36].
Research studies have shown that organic milk contains higher levels of calcium, potassium, phosphorous, and molybdenum but lower levels of copper, iron, manganese, zinc, and aluminum when compared to conventional milk. There is also significant seasonal variation in the nutrient content of organic milk[37]. Additionally, some researchers have found that organic milk has lower levels of trace elements such as copper, zinc, iodine, and selenium when compared to conventional milk[38,39]. Organic infant formula should be supplemented with these trace elements, especially iodine, to prevent sub-optimal iodine status in infants. However, the iodine deficiency may depend on the location of organic farming[40]. Therefore, many companies fortify their organic formulas with organic iodine and selenium to ensure that infants receive adequate nutrition. It is important to note that the composition of organic infant formulas can vary between brands and formulations and is subject to change as manufacturers update their products[41]. However, government agencies regulate the composition of infant formulas to ensure that they meet the nutritional needs of infants and comply with organic certification standards. While organic formulas try to mimic human milk, their composition has significant differences, as shown in Table 3.
The organic formula is similar to the conventional formula in that it varies from company to company. However, certain minimum requirements must be met for a product to be labeled as organic. Table 4 compares the organic formula to conventional formulas. Although individual experiences may vary, the organic component of organic formulas offers several potential benefits. The fat profile is the most significant difference between organic and conventional milk. Organic milk has increased levels of mono- and poly-unsaturated fatty acids such as oleic, linoleic, conjugated linoleic, and α-linolenic acids. This leads to a reduction of atherogenic indices in organic milk. The improved lipid profile of organic milk is due to the excellent feeding strategies employed in organic dairy farms[42]. According to Ortman et al, organic milk also has higher levels of unsaturated fatty acids than conventional milk[43]. Gortzi et al[43] found that the milk fat and fatty acid profile are affected by animal feeding strategies, regardless of whether they are conventional or organic. Additionally, Ferreiro et al[44] discovered significantly higher levels of phosphatidylethanolamine, phos
The high methionine content found in organic baby formula allows for better formation of neurotransmitters, such as serotonin and dopamine. This improves brain development, mood regulation, and overall cognitive function[45]. Organic milk has been found to have better heat stability than conventional milk, according to a study by Čuboň et al[46]. This heat stability makes organic milk proteins more resistant to denaturation and provides better microbial control, resulting in a longer shelf-life than conventional milk[47]. Milk stability is also important to maintain consistent levels of DHA during formula storage[48].
Many organic infant formulas contain DHA derived from algae. A study by Yeiser et al[49] revealed that algal-derived DHA is safe, well-tolerated, and associated with normal growth in infants. DHA is crucial for developing brain grey matter and retinal photoreceptor cell membranes and accumulates considerably in the central nervous system[49]. Algal oil is preferred over fish oil due to its higher purity of DHA and safety. It is commonly used in food and healthcare products and interacts synergistically with other ingredients[50].
Evidence suggests that organic infant formula can reduce the risk of newborns developing bacterial resistance. Studies have shown that organic milk has lower levels of antibiotic-resistant bacteria than conventional milk[51]. In particular, a study by Neri et al[52] found that bacterial isolates in milk from organic farms had lower antibiotic resistance, especially to ampicillin and tetracycline, compared to milk from conventional farms[52]. This could be due to several factors, including the lower disease caseload in organic farms, lower use of antimicrobials, and exclusion of sick animals who were given antibiotics until no antibiotic excretion was expected in their milk[53]. However, the use of organic fertilizers may increase the risk of increasing antibiotic resistance gene abundance. Therefore, before using organic formula, it is important to conduct a thorough microbial evaluation to ensure the safety of the organic milk[54]. However, more research is needed to confirm whether the difference in bacterial resistance is due to the organic ingredients in the formula or other factors.
Several factors can affect how well an infant tolerates a particular formula. These factors can be related to the formula, how it is fed, or the infant's characteristics. It's essential to remember that each formula is unique, and each baby responds differently to it. Table 5 outlines the factors that can impact how well an infant tolerates a formula. The formula's type and source of protein can significantly affect gastrointestinal tolerability. Some babies may have difficulty digesting cow's milk protein, while formulas with whey protein or Casein A2 are easier to digest. Casein A2 has a different amino acid composition than Casein A1, making it easier to digest and more similar to breast milk[55]. Hydrolyzed protein formulas are recommended for babies with protein sensitivities[56]. Lactose-free or low-lactose formulas may be necessary for babies with lactose intolerance, but organic lactose is more tolerable than conventional lactose[57]. The type of fats in the formula also affects digestion, and a blend that resembles human milk is better tolerated. Prebiotics and probiotics can improve gastrointestinal function, and formulas with these additives may positively impact tolerability[58]. Specialized formulas with added dietary fibers can influence bowel movements, but fiber content must be balanced[59]. Osmolality and osmolarity affect gastrointestinal tolerability, with high osmolality leading to discomfort. Additives, vitamins, and minerals in the formula can also impact gastrointestinal function, and babies with sensitivities to specific additives may experience digestive issues[60].
It is important to note that not all infants are the same regarding formula feeding. Some infants may be sensitive to certain components, so finding the right brand may require trial and error[61]. This is especially true for babies with medical conditions such as reflux, colic, prematurity, or gastrointestinal disorders, who may need specialized formulas to manage their symptoms. Transitioning from breast milk to formula may take some time for the infant's gastrointestinal system to adjust, so it is essential to ensure that the infant is adequately hydrated while being fed formula[61]. By slowly introducing formula and monitoring the infant's response, you can determine the best formula suitable for their unique needs[1].
Effective formula feeding requires attention to several key factors that can impact gastrointestinal tolerability. Overfeeding or feeding too quickly can lead to discomfort or spitting up, while formula that is too hot or too cold can be challenging for infants to digest. It is best to warm the formula to body temperature before feeding to ensure an optimal temperature. The milk flow rate from the bottle is also crucial for gastrointestinal tolerability, as the formula that flows too quickly can cause gas and bloating. Adjusting the flow rate to ensure a slow and steady flow is important. Poor infant latching or hard sucking can also lead to air being swallowed along with the formula, causing gas and bloating. Proper latching and gentle sucking can help to achieve better milk tolerability. Finally, overfeeding can also cause gas and bloating, so it is essential to feed on demand and ensure that the proper feeding amount is achieved. Parents can help ensure that their infants are comfortable and well-nourished by paying attention to these factors[1,62-64].
Ensuring good tolerance in infant formula is of utmost importance as formula intolerance can lead to symptoms such as spit-ups, vomiting, fussiness, cramps, or constipation, which can significantly impact an infant's well-being and comfort. Commercially available formulas vary widely in their processing methods, sources, and levels of key components like protein, lipids, and micronutrients. These compositional differences are known to affect the formula's tolerability and can influence the health outcomes of the infants being fed[65]. It is worth noting that organic formulas are subject to regulatory guidelines and individual company protocols, which can affect their composition. Additionally, there are differences in the compositions of European and American organic formulas due to geographical and regulatory factors[66,67].
It has been found that the nutritional value of lactose in organic and conventional formulas is quite similar. However, new studies suggest that organic lactose may be better tolerated and more digestible, especially in cases of mild lactose intolerance. This could be because organic farming practices do not use synthetic pesticides and herbicides that may contaminate conventional milk[68]. However, there is insufficient evidence to prove that organic lactose is more beneficial than conventional lactose. Further research is needed to confirm these findings. Additionally, organic lactose is usually more expensive than conventional lactose[69]. However, conventional and organic formula producers provide low or free-lactose formula to manage severe lactose intolerance, a common cause of gastrointestinal problems in infants[70]. Unfortunately, no studies compare the effectiveness of organic and conventional lactose-free or low-lactose formulas in managing lactose intolerance.
Including organic zinc in formulas can improve the digestibility of essential components like crude protein, fat, and fiber compared to non-organic zinc[71]. Infant formula fat and protein contents are critical in determining stool consistency. For example, whey protein softens the stool, while casein makes it firmer. Moreover, the fat content in organic milk, especially if it is rich in polyunsaturated fatty acids (PUFA), can contribute to a softer stool consistency compared to standard fat content. Additionally, high lactose, magnesium, and galactooligosaccharides (GOS) can help to soften the stool consistency. Magnesium is a laxative and can stimulate intestinal motility by inducing cholecystokinin secretion and acetylcholine production[72].
It is essential to recognize that infant formula and human milk have different gut microbial colonization compositions that affect the gut microbiome of infants. Human milk, which is rich in nutritional and bioactive components such as lactoferrin, human milk oligosaccharides (HMOs), and immunoglobulins, is crucial in promoting growth and immunological development[73]. To make up for this difference, manufacturers often supplement formulas with prebiotics and probiotics to simulate the healthy gut microbiome seen in breastfed infants. These additives have bifidogenic properties and can regulate the immune system. Research has shown that adding prebiotic oligosaccharides to infant formula is well-tolerated by healthy infants. Including prebiotic oligosaccharides in infant formula results in softer stools, decreased fecal pH, and increased levels of Bifidobacteria when compared to formulas that lack this supplementation[74].
Table 2 indicates that many organic formulas contain prebiotics like organic GOS, FOS, 3'GL - Galactosyllactose, and probiotics such as L. fermentum and Bifidobacterium longum BB536. Conventional formulas have been using prebiotics and probiotics for a long time now to imitate breast milk. Some traditional formulas also add HMOs to aid gut microbiota development and facilitate gut maturation. However, HMOs are artificially produced in the lab with the same structure and function as those found in breast milk. Only Kendamil Organic formula is known to contain 3'GL – Galactosyllactose HMO, but its source is unclear. Researchers have found thirteen molecules in bovine colostrum that mimic breast milk HMOs, indicating that cow milk could be a potential source of organic HMOs[75].
Understanding and optimizing gut tolerability in infant formulas is paramount to ensure infants' well-being and comfort. Organic formulas may have better gut tolerability due to their composition and farming practices[76,77]. Still, comparative studies between organic and conventional formulas are needed to understand the nuanced effects of various components on the gastrointestinal system of infants. Including HMOs and using advanced prebiotic and probiotic supplementation can improve gut tolerability and overall health benefits of organic and conventional infant formulas[78].
Before recommending any infant formula, it is important to ensure that it meets specific ingredients, farming practices, and processing standards. A valid certification by a reputable certification body such as USDA, EFSA, or an equivalent organization should be available. The formula should also match the baby's nutritional needs according to their age and circumstances. The ingredient list should avoid artificial additives, synthetic preservatives, and unnecessary fillers. Whenever possible, ingredients should be sourced from organic farms. The formula should provide a balanced nutrition with essential nutrients such as vitamins, minerals, and fatty acids (e.g., DHA and ARA) to support the baby's physical growth and mental development.
The source of protein should be organic, for example, organically raised cows. This helps to minimize the risk of exposure to antibiotics, synthetic hormones, or pesticides. The formula should also use natural sweeteners like lactose and avoid any added sugars or high-fructose corn syrup. Supplementing the formula with prebiotics, probiotics, or both are recommended to support gut health and digestion. If the organic formula contains brown rice syrup, the healthcare professional should be sure of the arsenic content of the formula.
The list of non-organic ingredients allowed to be included in the formula should be stated, including its percentage, especially for the American formula. The packaging should be free from bisphenol-A to avoid potential human health risks, especially the increased risk of developmental disorders in the growing brains. It is also important to ensure that the formulas are labeled as non-genetically modified organisms (non-GMO), as this indicates that the ingredients used in the formula have not been genetically engineered.
There are several limitations to the current study. One of the biggest limitations is the lack of specific scientific studies directly comparing the gastrointestinal tolerability of organic and traditional infant formulas. The lack of available research restricts the depth and breadth of the review. Organic and traditional infant formulas are not standardized across brands or regions, resulting in different ingredients, nutrient composition, and processing methods. This variety makes it difficult to draw comprehensive conclusions that apply to all formulas within each category. Infants have different health conditions, dietary needs, and tolerances. The review may not include the full range of health conditions, which could result in bias or incomplete population representation. Some studies examining the gastrointestinal tolerability of infant formulas might have small sample sizes, reducing the statistical power and generalizability of their findings. This limitation affects the strength of the conclusions drawn from these studies. Studies assessing gastrointestinal tolerability may vary in duration, and some may be of short duration. Long-term effects and tolerability may not be adequately captured in shorter studies. Reports from parents on their experiences using infant formula could introduce reporting bias, as parents may perceive organic formulas more positively due to preconceived beliefs about their benefits. This bias could impact the interpretation of the results. Conducting controlled, randomized clinical trials directly comparing organic and traditional infant formulas can raise ethical concerns, particularly regarding exposing infants to possible risks associated with formula intolerance. Studies reporting significant differences or favorable outcomes may be more likely to be published, leading to publication bias. This could skew the overall conclusions of the review. The systematic-review is based on information available up to a specific knowledge cutoff date. More recent studies or developments in the field beyond that date may not be included, which could affect the comprehensiveness and accuracy of the review. It is essential to acknowledge these limitations to maintain transparency and ensure a balanced interpretation of the study's findings.
Understanding infant formula's impact on gastrointestinal tolerability is crucial for parents and healthcare providers. While factors like formula composition, feeding techniques, and individual differences affect how well infants tolerate formula, specific aspects of organic formulas might offer potential benefits. The comparison between organic and conventional formulas highlights differences in digestibility, prebiotic and probiotic contents, and potential advantages of organic lactose. However, more robust research is needed to establish these differences and their impact on infant health definitively. When selecting a formula, certifications, ingredient sources, nutritional balance, and the presence of natural additives should be considered. Organic formulas, often incorporating organic protein sources and beneficial supplements, represent a promising option, yet further studies are necessary to clarify their distinct advantages. Addressing these aspects holistically, alongside ongoing research and regulation enhancements, will support informed choices for parents seeking the best formula suited to their infant's unique needs and well-being.
The demand for organic infant formula has surged, driven by heightened parental awareness of health benefits and a growing organic product market. Differences in regulatory standards and cultural attitudes globally have shaped variations between European and American organic infant formula.
The increasing popularity of organic infant formula raises critical questions regarding its composition, regulatory frameworks, and potential impact on infants' gastrointestinal health, especially when compared to traditional formulas.
To conduct a comprehensive analysis comparing the gastrointestinal tolerability and nutritional compositions of organic and traditional infant formulas, exploring the existing literature and regulatory disparities between European and American organic formulas.
A systematic review was conducted, spanning multiple databases and reputable publications. Seventy-eight articles were included, comprising research papers, meta-analyses, systematic reviews, narrative reviews, and consensus guidelines. Data extraction covered formula compositions, nutritional profiles, and gastrointestinal tolerability findings from infant populations.
European organic infant formulas, regulated by the European Commission, exhibit stricter standards than American organic formulas regulated by the USDA & NOP. Variations were evident in regulations, ingredients, nutritional content, and cultural attitudes toward these formulas.
While both types of formulas aim to provide essential nutrients, disparities exist in ingredient sources, regulations, and nutrient levels. European formulas tend to prioritize organic ingredients and stricter regulations, while American formulas may contain additional ingredients like added iron and different carbohydrate sources.
The findings highlight the need for continued investigation into the long-term effects of organic versus traditional formulas on infants' gastrointestinal health. Future research could focus on refining regulations and examining the real-world impact of these differences on infant health outcomes.
We thank the anonymous referees and editors for their valuable suggestions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Guo F, China S-Editor: Liu JH L-Editor: A P-Editor: Zheng XM
| 1. | Martin CR, Ling PR, Blackburn GL. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 532] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 2. | Savarino G, Corsello A, Corsello G. Macronutrient balance and micronutrient amounts through growth and development. Ital J Pediatr. 2021;47:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 3. | Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4217] [Cited by in RCA: 3452] [Article Influence: 203.1] [Reference Citation Analysis (0)] |
| 4. | De Sanctis V, Soliman A, Alaaraj N, Ahmed S, Alyafei F, Hamed N. Early and Long-term Consequences of Nutritional Stunting: From Childhood to Adulthood. Acta Biomed. 2021;92:e2021168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 5. | Dieterich CM, Felice JP, O'Sullivan E, Rasmussen KM. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr Clin North Am. 2013;60:31-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 6. | Likhar A, Patil MS. Importance of Maternal Nutrition in the First 1,000 Days of Life and Its Effects on Child Development: A Narrative Review. Cureus. 2022;14:e30083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Glibowski P. Organic food and health. Rocz Panstw Zakl Hig. 2020;71:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Liu Y, Sam AG. The organic premium of baby food based on market segments. Agribusiness. 2022;38:533-556. [DOI] [Full Text] |
| 9. | Vigar V, Myers S, Oliver C, Arellano J, Robinson S, Leifert C. A Systematic Review of Organic Versus Conventional Food Consumption: Is There a Measurable Benefit on Human Health? Nutrients. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Stevens EE, Patrick TE, Pickler R. A history of infant feeding. J Perinat Educ. 2009;18:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. |
Salvatici S.
Writing the History of Food Security since 1945. |
| 12. | Rose R, Paparas D. Price Transmission: The Case of the UK Dairy Market. Commodities. 2023;2:73-93. [DOI] [Full Text] |
| 13. | Cheng WN, Han SG. Bovine mastitis: risk factors, therapeutic strategies, and alternative treatments - A review. Asian-Australas J Anim Sci. 2020;33:1699-1713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 14. | Saucier OR, Parsons RL. Refusing to "Push the Cows": The Rise of Organic Dairying in the Northeast and Midwest in the 1970s-1980s. Agricultural History. 2014;88:237-261 . [DOI] [Full Text] |
| 15. | Kaufmann S, Hruschka N, Vildozo L, Vogl CR. Alternative Food Networks in Latin America-exploring PGS (Participatory Guarantee Systems) markets and their consumers: a cross-country comparison. Agric Human Values. 2023;40:193-216. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Davis H, Stergiadis S, Chatzidimitriou E, Sanderson R, Leifert C, Butler G. Meeting Breeding Potential in Organic and Low-Input Dairy Farming. Front Vet Sci. 2020;7:544149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Bórawski P, Bórawski MB, Parzonko A, Wicki L, Rokicki T, Perkowska A, Dunn JW. Development of Organic Milk Production in Poland on the Background of the EU. Agriculture. 2021;11:323. [DOI] [Full Text] |
| 18. | Massey M, O'Cass A, Otahal P. A meta-analytic study of the factors driving the purchase of organic food. Appetite. 2018;125:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Hill H, Lynchehaun F. "Organic milk: attitudes and consumption patterns". British Food Journal. 2002;104:526-542. [DOI] [Full Text] |
| 20. | Manuelian CL, Vigolo V, Burbi S, Righi F, Simoni M, De Marchi M. Detailed comparison between organic and conventional milk from Holstein-Friesian dairy herds in Italy. J Dairy Sci. 2022;105:5561-5572. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Dimitri C, Nehring R. Thirty years of organic dairy in the United States: The influences of farms, the market and the organic regulation. Renewable Agriculture and Food Systems. 2022;37:588-602. [DOI] [Full Text] |
| 22. | Yang SP, Chang SC, Liang TC, Rospita Odorlina P. Situmorang, and Minhas Hussain. Sustainability. 2021;13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Grodkowski G, Gołębiewski M, Slósarz J, Grodkowska K, Kostusiak P, Sakowski T, Puppel K. Organic Milk Production and Dairy Farming Constraints and Prospects under the Laws of the European Union. Animals (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | European Union Law. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. EUR-Lex - 02018R0848-20220101 - EN - EUR-Lex (europa.eu) last accessed 12nd August 2023. |
| 25. | Lee J, Kim B, Lee SY, Choi J, Kang D, Lee H, Choi K, Sim HJ, Baek SY, Hyung SW, Ahn S, Seo D, Hwang J, Park JS, Kwak BM, Won J. Development of an infant formula certified reference material for the analysis of organic nutrients. Food Chem. 2019;298:125088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Murali AP, Trząskowska M, Trafialek J. Microorganisms in Organic Food-Issues to Be Addressed. Microorganisms. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Jukes TH. Organic food. CRC Crit Rev Food Sci Nutr. 1977;9:395-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Mariana RA, Aalaei K, da Silva DF, Barjon S, Añón MC, Abraham AG, Ahrné L. "Infant Milk Formulae Processing: Effect of Wet-mix Total Solids and Heat Treatment Temperature on Rheological, Emulsifying and Nutritional Properties. J Food Eng. 2021;290:110194 . [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Bakshi S, Paswan VK, Yadav SP, Bhinchhar BK, Kharkwal S, Rose H, Kanetkar P, Kumar V, Al-Zamani ZAS, Bunkar DS. A comprehensive review on infant formula: nutritional and functional constituents, recent trends in processing and its impact on infants' gut microbiota. Front Nutr. 2023;10:1194679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 30. | Butler G, Stergiadis S. Organic milk: Does it confer health benefits? In Milk and Dairy Foods-Their Functionality in Human Health and Disease, 1st ed.; Givens, I.D., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 121-143. Accessed 7 Aug. 2023. Available from: https://doi.org/10.1016/B978-0-12-815603-2.00005-X. |
| 31. | Corbu S, Pintus R, Dessì A, Puddu M, Cesare Marincola F, Fanos V. NMR-Based Metabolomics Analysis of Organic and Conventionally Produced Formula Milk: Preliminary Results. J Pediatr Neonat Individual Med 2019; 8: e080228 [DOI: 10. 7363/080228] . |
| 32. | Truswell AS. The A2 milk case: a critical review. Eur J Clin Nutr. 2005;59:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Holtcamp W. Suspect sweetener: arsenic detected in organic brown rice syrup. Environ Health Perspect. 2012;120:A204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Tsiafoulis CG, Papaemmanouil C, Alivertis D, Tzamaloukas O, Miltiadou D, Balayssac S, Malet-Martino M, Gerothanassis IP. NMR-Based Μetabolomics of the Lipid Fraction of Organic and Conventional Bovine Milk. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Mazzei P, Piccolo A. NMR-based metabolomics of water-buffalo milk after conventional or biological feeding. Chem Biol Technol Agric. 2018;5:3. [DOI] [Full Text] |
| 36. | Givens DI, Lovegrove JA. Higher PUFA and n-3 PUFA, conjugated linoleic acid, α-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: a systematic literature review and meta- and redundancy analyses. Br J Nutr. 2016;116:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Qin N, Faludi G, Beauclercq S, Pitt J, Desnica N, Pétursdóttir Á, Newton EE, Angelidis A, Givens I, Juniper D, Humphries D, Gunnlaugsdóttir H, Stergiadis S. Macromineral and trace element concentrations and their seasonal variation in milk from organic and conventional dairy herds. Food Chem. 2021;359:129865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Rey-Crespo F, Miranda M, López-Alonso M. Essential trace and toxic element concentrations in organic and conventional milk in NW Spain. Food Chem Toxicol. 2013;55:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Payling LM, Juniper DT, Drake C, Rymer C, Givens DI. Effect of milk type and processing on iodine concentration of organic and conventional winter milk at retail: implications for nutrition. Food Chem. 2015;178:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Bath SC, Button S, Rayman MP. Iodine concentration of organic and conventional milk: implications for iodine intake. Br J Nutr. 2012;107:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Ortman K, Pehrson B. Selenite and selenium yeast as feed supplements for dairy cows. Zentralbl Veterinarmed A. 1997;44:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Tzamaloukas O, Neofytou MC, Simitzis PE, Miltiadou D. Effect of Farming System (Organic vs. Conventional) and Season on Composition and Fatty Acid Profile of Bovine, Caprine and Ovine Milk and Retail Halloumi Cheese Produced in Cyprus. Foods. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Gortzi O, Malissiova E, Katsoulis K, Alibade A, Liappis D, Lalas S, Graikou K. Comparative Analysis of Fatty Acid Profile and Fat-Soluble Vitamin Content in Sheep and Goat Milk of Organic and Conventional Origin. Appl Sci. 2022;12:2809. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Ferreiro T, Gayoso L, Rodríguez-Otero JL. Milk phospholipids: Organic milk and milk rich in conjugated linoleic acid compared with conventional milk. J Dairy Sci. 2015;98:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Xi Y, Zhang Y, Zhou Y, Liu Q, Chen X, Liu X, Grune T, Shi L, Hou M, Liu Z. Effects of methionine intake on cognitive function in mild cognitive impairment patients and APP/PS1 Alzheimer's Disease model mice: Role of the cystathionine-β-synthase/H(2)S pathway. Redox Biol. 2023;59:102595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Čuboň J, Foltys V, Haščík P, Kačániová M, Ubrežiová I, Kráčmar S. The raw milk quality from organic and conventional agriculture. Acta Univ Agric Silvic Mendelianae Brun. 2008;56:25-30. [DOI] [Full Text] |
| 47. | Qian F, Sun J, Cao D, Tuo Y, Jiang S, Mu G. Experimental and Modelling Study of the Denaturation of Milk Protein by Heat Treatment. Korean J Food Sci Anim Resour. 2017;37:44-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Jia HX, Chen WL, Qi XY, Su MY. The stability of milk-based infant formulas during accelerated storage. CyTA-J Food. 2019;17:96-104 . [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Yeiser M, Harris CL, Kirchoff AL, Patterson AC, Wampler JL, Zissman EN, Berseth CL. Growth and tolerance of infants fed formula with a new algal source of docosahexaenoic acid: Double-blind, randomized, controlled trial. Prostaglandins Leukot Essent Fatty Acids. 2016;115:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Huang ST, Li HX, YU Y, Yuan B, Cao CJ, Cheng SJ. [Research Progress on Physiological Functions of DHA Algal Oil and Its Synergistic Application in Food]. Shipin Gongye Keji. 2023;44:468−476. |
| 51. | Roesch M, Perreten V, Doherr MG, Schaeren W, Schällibaum M, Blum JW. Comparison of antibiotic resistance of udder pathogens in dairy cows kept on organic and on conventional farms. J Dairy Sci. 2006;89:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Neri TAN, Park H, Kang S, Baek SH, Nam I. Antimicrobial Resistance and Prevalence of Methicillin-resistant Staphylococcus aureus in Bovine Mastitis Milk from Conventional and Organic Dairy Farms in South Korea. Preprints 2023; 2023061843 . [DOI] [Full Text] |
| 53. | Olmos Antillón G, Sjöström K, Fall N, Sternberg Lewerin S, Emanuelson U. Antibiotic Use in Organic and Non-organic Swedish Dairy Farms: A Comparison of Three Recording Methods. Front Vet Sci. 2020;7:568881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Guo Y, Qiu T, Gao M, Ru S, Gao H, Wang X. Does increasing the organic fertilizer application rate always boost the antibiotic resistance level in agricultural soils? Environ Pollut. 2023;322:121251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Nilsen H, Olsen HG, Hayes B, Sehested E, Svendsen M, Nome T, Meuwissen T, Lien S. Casein haplotypes and their association with milk production traits in Norwegian Red cattle. Genet Sel Evol. 2009;41:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Savino F, Cresi F, Maccario S, Cavallo F, Dalmasso P, Fanaro S, Oggero R, Vigi V, Silvestro L. "Minor" feeding problems during the first months of life: effect of a partially hydrolysed milk formula containing fructo- and galacto-oligosaccharides. Acta Paediatr Suppl. 2003;91:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Savino F, Palumeri E, Castagno E, Cresi F, Dalmasso P, Cavallo F, Oggero R. Reduction of crying episodes owing to infantile colic: A randomized controlled study on the efficacy of a new infant formula. Eur J Clin Nutr. 2006;60:1304-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Possner M, El-Neklaway I, Khater M, Fikry M, Alshahoud AN, Salah M, Said W, Tawfik E. Acceptability of "High sn-2" Infant Formula in Non-Breast Fed Healthy Term Infants Regarding Gastrointestinal Tolerability by Both Parents and Pediatrician: An Open-Label Pilot Study in the Gulf Cooperation Council (GCC) Countries. Pediatr Rep. 2021;13:639-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Vandenplas Y, De Greef E, Veereman G. Prebiotics in infant formula. Gut Microbes. 2014;5:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Mugambi MN, Musekiwa A, Lombard M, Young T, Blaauw R. Synbiotics, probiotics or prebiotics in infant formula for full term infants: a systematic review. Nutr J. 2012;11:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 61. | Almeida CC, Mendonça Pereira BF, Leandro KC, Costa MP, Spisso BF, Conte-Junior CA. Bioactive Compounds in Infant Formula and Their Effects on Infant Nutrition and Health: A Systematic Literature Review. Int J Food Sci. 2021;2021:8850080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 62. | Khoshoo V, Sun SS, Storm H. Tolerance of an enteral formula with insoluble and prebiotic fiber in children with compromised gastrointestinal function. J Am Diet Assoc. 2010;110:1728-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Le Huërou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. 2010;23:23-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 64. | Formula feeding information and support: Postnatal care: Evidence review T. London: National Institute for Health and Care Excellence (NICE); 2021 . [PubMed] |
| 65. | Lloyd B, Halter RJ, Kuchan MJ, Baggs GE, Ryan AS, Masor ML. Formula tolerance in postbreastfed and exclusively formula-fed infants. Pediatrics. 1999;103:E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | DiMaggio DM, Du N, Scherer C, Brodlie S, Shabanova V, Belamarich P, Porto AF. Comparison of Imported European and US Infant Formulas: Labeling, Nutrient and Safety Concerns. J Pediatr Gastroenterol Nutr. 2019;69:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Strzalkowski A, Black G, Young BE. Iron and DHA in Infant Formula Purchased in the US Fails to Meet European Nutrition Requirements. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 68. | Romero-Velarde E, Delgado-Franco D, García-Gutiérrez M, Gurrola-Díaz C, Larrosa-Haro A, Montijo-Barrios E, Muskiet FAJ, Vargas-Guerrero B, Geurts J. The Importance of Lactose in the Human Diet: Outcomes of a Mexican Consensus Meeting. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Brodziak A, Wajs J, Zuba-Ciszewska M, Król J, Stobiecka M, Jańczuk A. Organic versus Conventional Raw Cow Milk as Material for Processing. Animals (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 70. | Hafla AN, MacAdam JW, Soder KJ. Sustainability of US Organic Beef and Dairy Production Systems: Soil, Plant and Cattle Interactions. Sustainability. 2013;5:3009-3034 . [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Lasekan JB, Jacobs J, Reisinger KS, Montalto MB, Frantz MP, Blatter MM. Lactose-free milk protein-based infant formula: impact on growth and gastrointestinal tolerance in infants. Clin Pediatr (Phila). 2011;50:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Bongers ME, de Lorijn F, Reitsma JB, Groeneweg M, Taminiau JA, Benninga MA. The clinical effect of a new infant formula in term infants with constipation: a double-blind, randomized cross-over trial. Nutr J. 2007;6:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Vu MK, Nouwens MA, Biemond I, Lamers CB, Masclee AA. The osmotic laxative magnesium sulphate activates the ileal brake. Aliment Pharmacol Ther. 2000;14:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Balabánová M, Hošková Š, Zeman L. The effect of inorganic and organic form of zinc on digestibility of nutrients in dairy cows in three stages of reproductive cycle. Acta Univ Agric Silvic Mendel Brun. 2011;59:17-24. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 75. | Chong HY, Tan LT, Law JW, Hong KW, Ratnasingam V, Ab Mutalib NS, Lee LH, Letchumanan V. Exploring the Potential of Human Milk and Formula Milk on Infants' Gut and Health. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 76. | Ashley C, Johnston WH, Harris CL, Stolz SI, Wampler JL, Berseth CL. Growth and tolerance of infants fed formula supplemented with polydextrose (PDX) and/or galactooligosaccharides (GOS): double-blind, randomized, controlled trial. Nutr J. 2012;11:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Williams T, Choe Y, Price P, Katz G, Suarez F, Paule C, Mackey A. Tolerance of formulas containing prebiotics in healthy, term infants. J Pediatr Gastroenterol Nutr. 2014;59:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Aldredge DL, Geronimo MR, Hua S, Nwosu CC, Lebrilla CB, Barile D. Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology. 2013;23:664-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |