Published online Jun 9, 2023. doi: 10.5409/wjcp.v12.i3.151
Peer-review started: February 20, 2023
First decision: April 8, 2023
Revised: April 13, 2023
Accepted: May 6, 2023
Article in press: May 6, 2023
Published online: June 9, 2023
Processing time: 107 Days and 16.2 Hours
Gastroesophageal reflux disease (GERD) might be either a cause or comorbidity in children with extraesophageal problems especially as refractory respiratory symptoms, without any best methods or criterion for diagnosing it in children.
To evaluate the prevalence of extraesophageal GERD using conventional and combined-video, multichannel intraluminal impedance-pH (MII-pH), and to propose novel diagnostic parameters.
The study was conducted among children suspected of extraesophageal GERD at King Chulalongkorn Memorial Hospital between 2019 and 2022. The children underwent conventional and/or combined-video MII-pH. The potential para
Of 51 patients (52.9% males), aged 2.24 years were recruited. The common problems were cough, recurrent pneumonia, and hypersecretion. Using MII-pH, 35.3% of the children were diagnosed with GERD by reflux index (31.4%), total reflux events (3.9%), and symptom indices (9.8%) with higher symptom recorded in the GERD group (94 vs 171, P = 0.033). In the video monitoring group (n = 17), there were more symptoms recorded (120 vs 220, P = 0.062) and more GERD (11.8% vs 29.4%, P = 0.398) by symptom indices. Longest reflux time and mean nocturnal baseline impedance were significant parameters for diagnosis with receiver operating characteristic areas of 0.907 (P = 0.001) and 0.726 (P = 0.014).
The prevalence of extraesophageal GERD in children was not high as expected. The diagnostic yield of symptom indices increased using video monitoring. Long reflux time and mean nocturnal baseline impedance are novel parameters that should be integrated into the GERD diagnostic criteria in children.
Core Tip: This was a retrospective and cross-sectional study with 51 children suspected extraesophageal gastrointestinal esophageal reflux disease (GERD). This study found the prevalence of GERD in these pediatric patients was 35.3% by using combined-video, multichannel intraluminal impedance-pH study. Moreover, longest reflux time and mean nocturnal baseline impedance were depicted as the significant parameters for GERD diagnosis with satisfied diagnostic value in children.
- Citation: Eiamkulbutr S, Dumrisilp T, Sanpavat A, Sintusek P. Prevalence of gastroesophageal reflux disease in children with extraesophageal manifestations using combined-video, multichannel intraluminal impedance-pH study. World J Clin Pediatr 2023; 12(3): 151-161
- URL: https://www.wjgnet.com/2219-2808/full/v12/i3/151.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v12.i3.151
Gastroesophageal reflux (GER) is a physiologic process that commonly occurs in infants. Gastroesophageal reflux disease (GERD) occurs when the refluxates cause troublesome symptoms. The incidence of GERD has increased in children (0.84 per 1000 persons-year)[1]. Its manifestation varies. Hence, a high index of suspicion is necessary, especially for extraesophageal manifestations[2]. In clinical practice, respiratory problems that are refractory to the standard treatment might be from the disease itself or are extraesophageal manifestations of GERD. Moreover, GERD can be a serious comorbidity that worsens those respiratory conditions. Consequently, the development of a standard tool to dia
There are many diagnostic tools for extraesophageal GERD, including pH monitoring, combined multichannel intraluminal impedance and pH (MII-pH) study, esophagogastroduodenoscopy (EGD) with biopsies, and laryngoscopy[3]. According to clinical practice guidelines for GERD diagnosis and management in children (North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition), the MII-pH study is the best diagnostic method for esophageal manifestations of GERD[4]. Recent studies in adults also propose that additional parameters from the MII-pH study, mean nocturnal baseline impedance (MNBI), and post-reflux swallow-induced peristaltic wave (PSPW), increase the diagnostic value of this tool[5]. However, there is scarce evidence to support the best method for diagnosing extraesophageal GERD in children. Further evaluation is necessary. The main purpose of this study is to determine the prevalence of GERD in children with extraesophageal symptoms using conventional and combined-video MII-pH studies. The secondary aim is to assess the diagnostic usefulness of combined-video MII-pH studies for GERD and other diagnostic parameters.
The present study is a retrospective and cross-sectional study in children and adolescents with respiratory symptoms and other extraesophageal manifestations suggestive of GERD. Participants were treated at King Chulalongkorn Memorial Hospital (KCMH) from February 2019 to December 2021. Patients who had extraesophageal symptoms (cough, apnea or brief resolved unexplained event, uncontrolled asthma, recurrent pneumonia, stridor, hoarseness, chronic sinusitis with unknown causes, allergic rhinitis with difficulty to treatment), and who were between 1 mo and 18 years old were included in the study. The exclusion criteria were patients who were on proton pump inhibitors or prokinetics during MII-pH monitoring, unwillingness to participate in the study, and patients who were already known the other causes that could explain those extraesophageal symptoms.
The Chulalongkorn University Institutional Review Board approved this study (IRB 029/64). Informed consents and assents were obtained from the patient’s guardians and patients, respectively, before recruitment to the study.
According to the protocol, patients had to fast for one to two hours before a nasal impedance catheter with a pH probe (Pediatric ZandorpH catheter with one antimony and six impedance sensors with 1.5 cm interval, Laborie, The Netherlands) was distributed throughout the esophagus. The Strobel formula[6] was used to calculate the proper position of the catheter. Chest X-ray was then used to confirm that the pH probe was located at 2–3 vertebral distance from the diaphragm. During the MII-pH monitoring, all patients had their regular meals. Video monitoring was conducted simultaneously with MII-pH monitoring in 17 patients. The total time of monitoring after excluding meal periods was at least 18 h. The number of reflux events, the reflux index (RI), the symptom index (SI)/symptom sensitivity index (SSI)/symptom association probability (SAP), longest reflux time (LRT), the MNBI, and PSPW were recorded.
Pathological reflux is defined according to the position statement by the British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) Motility Working Group[6] as RI > 7% in children aged ≥ 1 year, and > 10% in children aged < 1 year, or if reflux episodes occur ≥ 70 times in children aged ≥ 1 year, and ≥ 100 times in children aged < 1 year or positive SI/SSI/SAP. Regarding symptom recoding, SI is calculated as (reflux-related symptom occurrences/total symptom events) × 100. SI is considered positive if the value is ≥ 50%. SSI is calculated as (number of symptom-associated reflux/total number of reflux episodes) × 100. If the percentage of SSI is ≥ 10%, it is considered positive. SAP is calculated by dividing the total measuring time into 2-min intervals, and creating a four-field contingency table: The number of intervals with and without GER symptoms, number of intervals with and without GER symptoms, number of intervals without GER and with the symptoms, and number of intervals without GER and symptoms. Fisher’s exact test was performed for statistical examination of correlation. A percentage greater than 95% was considered positive.
Regarding the new parameters, MNBI was measured from the most distal impedance channel during sleep, with three 10-min time intervals that did not interfere with swallowing. The mean of these three values was then calculated. PSPW was defined as an antegrade 50% drop in impedance originating in the proximal esophagus within 30 s after the end of a reflux event and reaching the distal lumen. The PSPW index was calculated by the number of PSPW divided by the total number of refluxes[5,7].
Within three months of the MII-pH study esophagogastroduodenoscopy with biopsy was performed on selected patients from recruitment according to decisions by the doctors in charge. The Los Angeles (LA) Classification[8] was routinely used to record endoscopic findings. The esophageal histopathology finding was reported by a pathologist using the modified Esohisto criteria as described in our previous study[8]. In short, a calculated severity score of 0–0.25 was considered normal and a score of ≥ 0.5 was regarded as esophagitis.
Data of categorical variables were expressed as percentages or proportions, and continuous variables as median [interquartile range (IQR)]. These variables were compared using Fisher’s exact test and Wilcoxon signed ranks test as appropriate. Univariable and multivariable analyses were used to assess the independent factors of extraesophageal GERD from demographic variables and parameters from MII-pH analysis. Statistical significance was defined as P value < 0.05. Receiver operating characteristic analysis with calculation of the area under the curve was used to assess the diagnostic yield of the potential parameters of extraesophageal GERD. Statistical analysis was performed using SPSS software version 24.0.0 (SPSS Inc., Chicago, IL, United States) and Stata version 15.1 (Stata Corp, LLC, College Station, TX, United States).
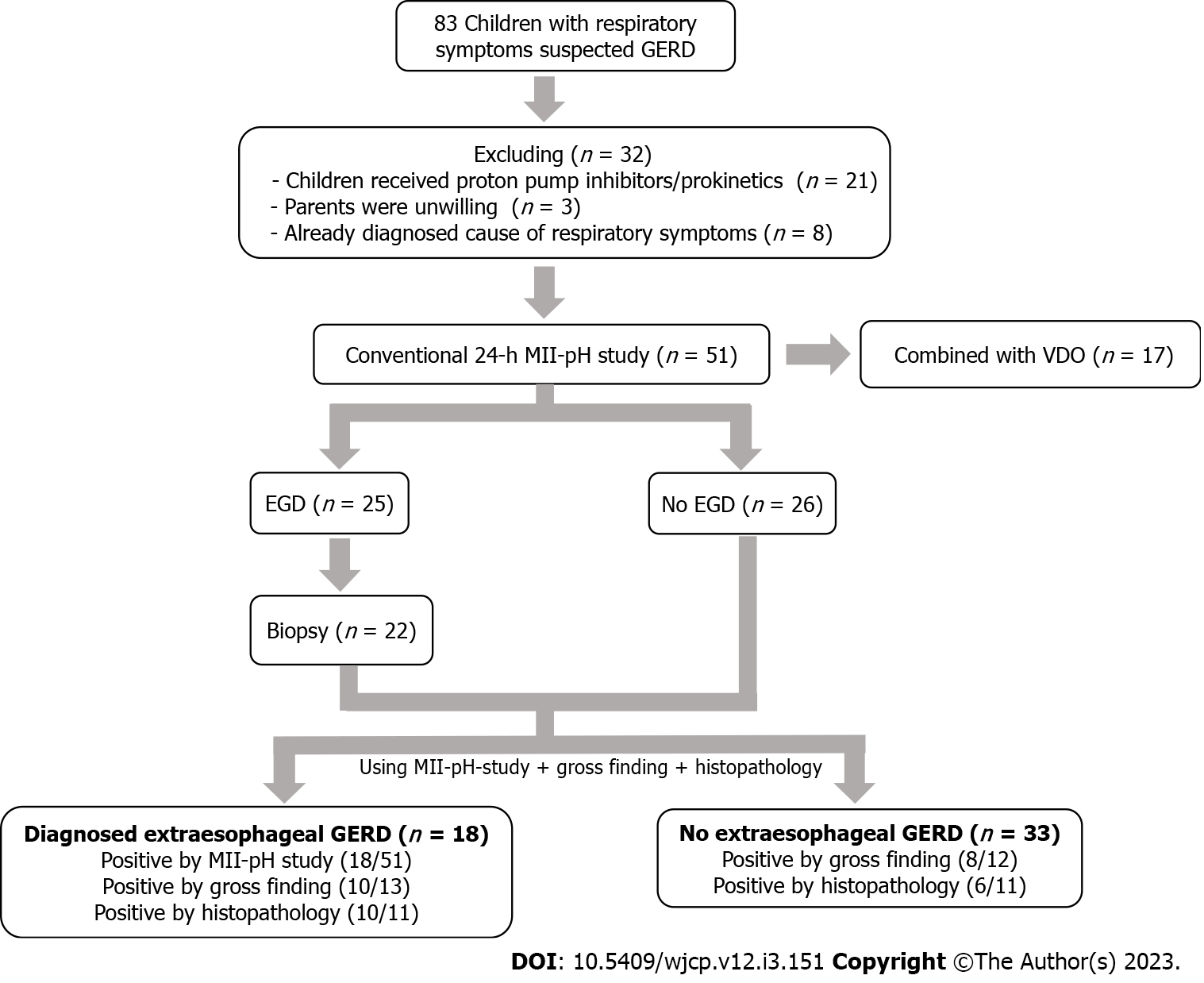
There were 83 children with suspected extraesophageal GERD at KCMH from February 2019 to July 2022. Thirty-two participants were excluded from the present study because they received proton pump inhibitors/prokinetics during the MII-pH study (n = 21), had unwilling guardians (n = 3), and because they had known causes of respiratory symptoms (n = 8). Consequently, a total of 51 children were recruited and 17 of them underwent both the MII-pH study and video monitoring. Twenty-five children underwent upper endoscopy, while 22 underwent esophageal biopsy for histopathology. The median age of these 51 participants was 2.24 (1.11, 7.67) years and 27 (52.9%) participants were males. Their underlying diseases were multiple anomalies (47%), respiratory disorders (35.3%), and neurological disorders (17.7%). The most common extraesophageal symptoms indicated in the MII-pH study were cough (41.2%), recurrent pneumonia (25.5%), and hypersecretion (11.8%). However, 16 (31.4%) participants also had concurrent gastrointestinal symptoms. The median duration of MII-pH recording was 22.09 (20.20, 23.41) h. Only 265 symptoms were recorded by guardians, including cough (n = 109, 41.1%), irritability (n = 54, 20.4%), apparent secretion (n = 24, 9.1%), vomiting (n = 34, 12.8%), and heartburn (n = 44, 16.6%). In total, 18 (35.3%) participants were diagnosed with GERD using the MII-pH study. In the subgroup of children who underwent upper endoscopy (n = 25) and 22 of them had received esophageal tissue biopsy, 18 (72%) and 16 (72.7%), respectively, were diagnosed with GERD using the LA classification and modified Esohisto, respectively (Figure 1).
According to the diagnostic criteria by the BSPGHAN Motility Working Group[6], 18 participants had parameters that were compatible with GERD. A comparison between the GERD and non-GERD groups did not reveal any statistical difference in the baseline characteristics. Besides the parameters from the MII-pH study that were used for diagnosing GERD (total reflux event, reflux index, and symptom index), total symptom record (94 vs 171 times, P = 0.033), LRT (1.3 (0, 4.3) vs 17.2 (9.43, 38.55), P < 0.001), and MNBI (1897.69 (806.89) vs 1300.48 (600.31) ohms, P = 0.008), were significantly different between the non-GERD and GERD groups, respectively. Meanwhile, PSPW, another novel metric, did not show a significant difference between the groups [53.57 (27.59) vs 56.11 (15.7), P = 0.721]. In the multivariable analysis, LRT and MNBI were the independent parameters that were significantly differences in participants diagnosed with GERD (P < 0.05) (Table 1).
| Characteristics | No extraesophageal GERD (n = 33) | Diagnosed extraesophageal GERD by MII-pH study (n = 18) | P value | |
| Univariable analysis | Multivariable analysis | |||
| Age (yr) (median, IQR) | 1.67 (0.91, 3.38) | 4.58 (1.70, 13.15) | 0.174 | |
| Age < 1 yr | 10 (30.3) | 2 (11.1) | ||
| Age ≥ 1 yr | 23 (69.7) | 16 (88.9) | ||
| Sex, male | 17 (51.5) | 10 (55.6) | 1 | |
| Underlying diseases | 0.441 | |||
| Respiratory disorder | ||||
| Bronchiectasis | 1 (3.03) | 0 | ||
| Chronic lung disease | 7 (21.21) | 2 (11.11) | ||
| Congenital hypoventilation syndrome | 0 | 2 (11.11) | ||
| Subglottic stenosis | 0 | 1 (5.56) | ||
| Tracheobronchomalacia | 1 (3.03) | 0 | ||
| Laryngomalacia | 1 (3.03) | 0 | ||
| BRUE | 1 (3.03) | 0 | ||
| Chronic cough | 1 (3.03) | 0 | ||
| Neurological disorder | ||||
| Swallowing dysfunction | 2 (6.06) | 1 (5.56) | ||
| Spastic cerebral palsy | 2 (6.06) | 3 (16.67) | ||
| Infantile spasm | 1 (3.03) | 0 | ||
| Multiple anomalies | ||||
| Syndromic disorder | 6 (18.18) | 2 (11.11) | ||
| Non-syndromic disorder | 9 (27.27) | 7 (38.89) | ||
| Indication for evaluation | ||||
| Extraesophageal symptoms | 0.679 | |||
| Recurrent pneumonia | 9 (27.3) | 4 (22.2) | ||
| Hypersecretion | 4 (12.1) | 2 (11.1) | ||
| Cough | 11 (33.3) | 10 (55.6) | ||
| Glossoptosis | 1 (3.0) | 0 | ||
| Tracheobronchomalacia | 1 (3.0) | 0 | ||
| Stridor | 1 (3.0) | 0 | ||
| Choking | 4 (12.1) | 1 (5.6) | ||
| Chronic rhinosinusitis | 0 | 1 (5.6) | ||
| BRUEs | 1 (3.0) | 0 | ||
| Apnea | 1 (3.0) | 0 | ||
| Esophageal symptoms | 0.293 | |||
| Vomiting | 8 (24.2) | 6 (38.9) | ||
| Heartburn | 1 (3.0) | 1 (5.6) | ||
| None | 24 (72.7) | 11 (61.1) | ||
| Total recorded symptoms (times, %) | 94 | 171 | 0.033 | 0.064 |
| Cough | 43 (45.75) | 66 (38.60) | 0.404 | |
| Irritability | 32 (34.04) | 22 (12.86) | 0.754 | |
| Apparent secretion | 7 (7.44) | 17 (9.94) | 0.346 | |
| GI symptoms (vomiting or heartburn) | 12 (12.77) | 66 (38.60) | 0.081 | |
| Impedance parameters (median, IQR) | ||||
| Total time (hours) | 22.12 (20.34, 24.04) | 21.34 (19.97, 22.62) | 0.391 | |
| Reflux index1 | 0.40 (0, 1.45) | 8.4 (4.43, 14.20) | 0.001 | |
| Longest reflux time (min) | 1.3 (0, 4.30) | 17.2 (9.43, 38.55) | 0.001 | |
| Total reflux events1 | 12 (5.00, 37.50) | 28 (18.50, 45.50) | 0.032 | 0.012 |
| Weakly acid reflux events | ||||
| Acid reflux events | 9 (2.50, 27.00) | 10 (3.75, 21.75) | 0.79 | |
| Nonacid reflux events | 1 (0, 4.50) | 15.5 (8.75, 25.25) | 0.001 | |
| Symptom indices | 0 (0, 4.00) | 0 (0,1.00) | 0.381 | 0.212 |
| Symptom index1 | 0 (0, 0.00) | 0 (0, 12.73) | 0.208 | |
| Symptom sensitivity index1 | 0 (0, 0.00) | 0 (0, 1.40) | 0.32 | |
| Symptom associated index1 | 0 (0, 0.00) | 0 (0, 85.63) | 0.168 | |
| MNBI (ohms) (mean, SD) | 1897.69 (806.89) | 1300.48 (600.31) | 0.008 | |
| PSPW (%) (mean, SD) | 53.57 (27.59) | 56.11 (15.70) | 0.721 | |
| Diagnosis GERD | ||||
| Gross finding (n = 25) | 8/12 (66.7) | 10/13 (76.9) | 0.673 | |
| Histopathology (n = 22) | 6/11 (54.5) | 10/11 (90.9) | 0.149 | |
The MII-pH study with video monitoring was performed in 17 participants. By the novel combined video-MII-pH study is different from the conventional MII-pH study. The video-MII-pH study had conducted simultaneously with MII-pH monitoring and given the clinical symptoms recorded by the investigator that can make more accurate of symptoms in children who cannot even report their symptoms. Then we compared the symptoms that were recorded by the participants’ guardians (conventional study) to the symptoms that were recorded by the video monitoring which is the same participants.
The total number of symptoms recorded from video by an investigator who simultaneously viewed throughout the recording, was detected higher than from participants’ guardians even though there’s no statistical significance (220 vs 120, P = 0.062. This led to an increase in the number of participants who were diagnosed with GERD by using symptom association indices (SI/SSI/SAP) (n = 2, 11.8% vs n = 5, 29.4%, P = 0.398) (Table 2).
| Characteristics | Conventional MII-pH study | Combined video-MII-pH study | P value |
| Total recorded symptoms | 120 | 220 | 0.062 |
| Cough | 31 (25.83) | 46 (20.90) | 0.259 |
| Irritability | 38 (31.67) | 101 (45.90) | 0.114 |
| Apparent secretion | 3 (2.50) | 43 (19.55) | 0.02 |
| GI symptoms (vomiting or heartburn) | 48 (40.00) | 33 (15.00) | 0.339 |
| Symptom indices (median, IQR) | |||
| Symptom index | 0 (0–5.00) | 0 (0–11.65) | 0.306 |
| Symptom sensitivity index | 0 (0–2.65) | 0 (0–5.80) | 0.306 |
| Symptom associated index | 0 (0–37.60) | 0 (0–93.15) | 0.306 |
| Diagnosis GERD using symptom indices | 2 (11.76) | 5 (29.41) | 0.398 |
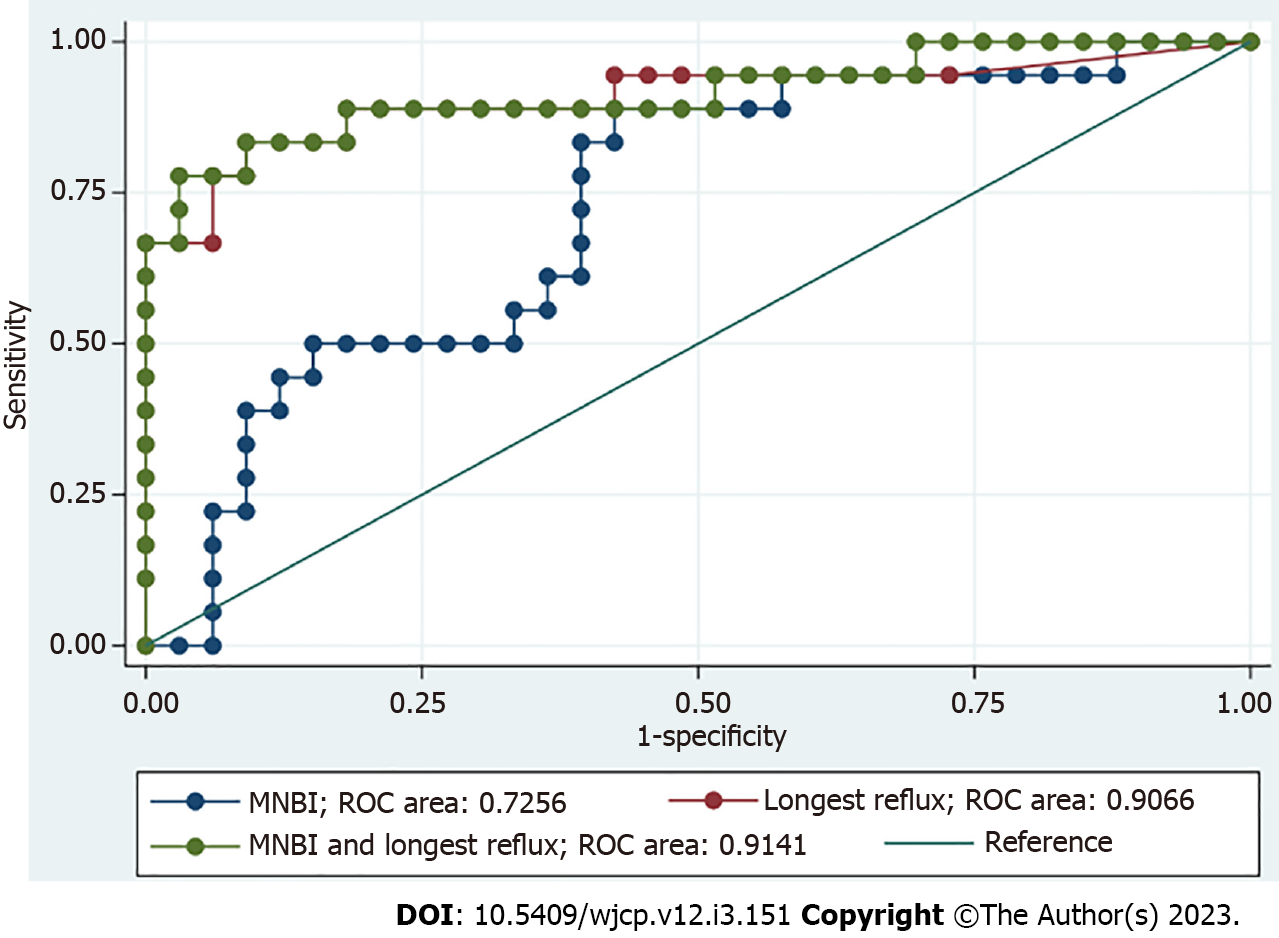
When using the significant parameters from multivariable analysis to identify GERD, longest reflux duration and MNBI yielded an area under the curve (AUC) of 0.907 (95%CI: 0.802–1) and 0.726 (95%CI: 0.581–0.870), correspondingly. Combining these two parameters yielded an AUC of 0.914 (95%CI: 0.819–1) (Figure 2). A cutoff value of eight minutes for longest reflux duration had a sensitivity of 83.33% and a specificity of 90.91%. A cutoff value of 1466 ohm for MNBI had a sensitivity of 50.0% and a specificity of 33.33%.
The prevalence of extraesophageal GERD was 35.3% by using the MII-pH study in this study. Interestingly, 31.4% of children who had extraesophageal manifestations of GERD also had gastro
There are several debates over clinical symptoms of extraesophageal reflux disease in children because of heterogeneity, non-specificity, and unreliability. Moreover, the prevalence of extraesophageal GERD reflects the real burden and depends on the modalities for diagnosis. Hence, finding the best diagnostic tool for extraesophageal GERD is crucial. Many proposed diagnostic tools, such as oropharyngeal pH monitoring/salivary pepsin, have been studied with unsatisfactory results[9-11]. As a result, the present study was designed using MII-pH study and histopathology—which are the gold standard for diagnosing esophageal GERD—to be the gold standard diagnosis for extraesophageal GERD. Moreover, combined-video monitoring was performed in 17 participants. The present study found that video monitoring increased the value of symptom-reflux association in extraesophageal GERD. This is congruent with our previous study which depicted a trend of symptom-reflux association with particular symptoms of GERD in children diagnosed with esophageal atresia[12]. The real-time video captured in MII-pH monitoring with physician symptom recordings had higher symptom indices. There were more symptoms recorded including cough, hypersecretion, irritability, and vomiting. There were also more GERD diagnoses secondary to the symptoms that were more trustworthy than conventional methods. These studies highlight that symptom recording by video monitoring in children increases the value of symptom association for GERD diagnosis.
Although identifying extraesophageal manifestation of GERD is challenging and needs evaluation regarding whether it represents true GERD or a mimicker, the present study found concomitant gastrointestinal symptoms or signs in a majority of these children. Moreover, esophagitis was also detected in a majority of children with extraesophageal symptoms in the present study. This might explain the gastrointestinal symptoms they experienced. The reflex theory is a possible explanation regarding why children with extraesophageal GERD also had gastrointestinal involvement. The theory suggests that reflux stimulates the vagus nerve in the esophagus leading to cough, bronchoconstriction, or other extraesophageal symptoms via vagally mediated reflexes[13,14]. However, previous studies demonstrated a lower prevalence of esophagitis in patients with extraesophageal GERD. A positive reflux-laryngitis varies from 5% up to 31%[15-18]. Routine upper endoscopy in all children with solely extraesophageal symptoms might be avoided. This invasive procedure should be preserved for patients who have both extraesophageal and esophageal manifestations of GERD. To increase the reliability of symptom assessment, we encourage the physician to have the dedicated history taking or possible video recording of symptoms during MII-pH study simultaneously with the routine symptom recorded by patients or their guardians. Upper endoscopy with biopsy is necessary and could increase the yield of GERD diagnosis in these selected children.
Besides using symptom association to diagnose extraesophageal GERD, we found that acid reflux was mainly present in the present study in accordance with Borrelli et al’s work. Borrelli et al[19] found that 66% of cough bursts were related to acid reflux episodes. However, different results were demonstrated by Zenzeri et al[20] who found predominantly weak acid and nonacid reflux in children with respiratory problems. Because of the lack of standardized protocol and children’s conditions in previous studies, a large multicenter study regarding children with different manifestations is necessary to extend the knowledge and narrow the specific treatment for them. In the present study, a majority of children had complicated underlying diseases (multiple anomalies, respiratory disease, or neurological deficit) that increased the risk of esophageal motility disorder and reflux. This could explain the high predominance of acid reflux.
The pathogenesis of GERD is complex and multifactorial. Though abnormal transient esophageal relaxation is the main pathogenesis, other factors such as-esophageal mucosal disease, esophageal dysmotility, gastroparesis, and anatomical defect (hiatal hernia, short segment of abdominal esophagus)-must be considered as aggravated risk factors of GERD[21]. Children with multiple comorbidities, especially neurological deficit, usually have sedentary lifestyles or are bedridden, and this affects gastrointestinal motility. We found that the LRT had statistically significant discriminate GERD and non-GERD children, reflexes the impairment of esophageal volume clearance as one of the pathogenesis of GERD[22,23]. However, there was no impairment of chemical clearance as shown by the insignificant difference in PSPW in both groups. MNBI is another parameter that can discriminate GERD from non-GERD children. MNBI represents esophageal integrity[5] and has a reasonably low value in GERD. To the best of our knowledge, PSPW and MNBI are the new impedance-pH parameters which are integral in the Lyon Consensus criteria for diagnosing GERD in adults[24]. The impact of PSPW and MNBI on increasing the yield of GERD diagnosis in children is rare. There is only one study concerning the impact of MNBI in children with GERD by Rosado-Arias et al[25]. That study found that a low MNBI is associated with a pathological AET. Our study is compatible with the study by Rosado-Arias et al[25] and confirmed the role of a low MNBI in helping the diagnostic yield of GERD. To the best of our knowledge, the present study is the first study to evaluate the PSPW parameter between GERD and non-GERD children. A previous study by Park et al[26] showed impairment of PSPW in adults with laryngopharyngeal reflux and esophageal GERD, but our study is incongruent with same. It is possible that the chemical clearance is important for the pathogenesis of extraesophageal GERD in adults vs in children. Further studies from multicenter or with the higher number of participants about PSPW and MNBI parameters, are crucial in aiding the diagnosis of GERD in children.
The certain strength of our study is that we integrated video monitoring into MII-pH study for diagnosing extraesophageal GERD in children. Moreover, the novel parameters (PSPW and MNBI) were evaluated to increase the yield of GERD diagnosis. However, there are some limitations. Firstly, it was a small number of children in a single center study. Therefore, the results are specific to the particular co-morbidities that were present and cannot be extrapolated to children with previously healthy or less co-morbidities. Secondly, because of the coronavirus disease 2019 pandemic, upper endoscopy was not performed in all children. Given this, there is the potential for selective bias and a high prevalence of children with extraesophageal GERD having esophagitis.
In conclusion, the prevalence of GERD was not as high as expected. Employing video monitoring into conventional MII-pH study increases the diagnostic yield of symptom indices. LRT and MNBI are novel parameters that should be integrated into the diagnostic criteria for GERD.
Gastroesophageal reflux disease (GERD) might be either a cause or comorbidity in children with extraesophageal problems especially as refractory respiratory symptoms, without any best methods or criterion for diagnosing it in children.
Recent studies in adults also propose that additional parameters from the multichannel intraluminal impedance (MII)-pH study, mean nocturnal baseline impedance (MNBI), and post-reflux swallow-induced peristaltic wave, increase the diagnostic value of this tool. However, there has been scarce evidence to support the best method for diagnosing extraesophageal GERD in children.
To study the prevalence of extraesophageal GERD, especially in children who presented with refractory respiratory problems by using combined video-MII-pH study. Furthermore, to identify other parameters from MII-pH study that can help the diagnosis of extraesophageal GERD.
Children with respiratory symptoms and other extraesophageal manifestations suggestive of GERD were enrolled to participate in the present study. MII-pH study and/or video monitoring and/or upper endoscopy with esophageal histopathology were performed. The prevalence of extraesophageal GERD and the novel diagnostic parameters to diagnose extraesophageal GERD were analyzed.
The prevalence of extraesophageal GERD was 35.3% by using the MII-pH study and 31.4% of children who had extraesophageal manifestations of GERD also had gastrointestinal symptoms. Total symptom record, longest reflux time (LRT), and MNBI were the parameters that were significantly different between the GERD and non-GERD groups. LRT and MNBI were the independent parameters from multivariable analysis. Using video monitoring during MII-pH study to depict more symptom record increases the diagnostic yield of extraesophageal GERD.
In conclusion, the prevalence of GERD was not as high as expected. Employing video monitoring into conventional MII-pH study increases the diagnostic yield of symptom indices. LRT and MNBI are novel parameters that should be integrated into the diagnostic criteria for GERD.
The diagnostic test for extraesophageal GERD in children is limited and there have been a few data support the favorable treatment outcome in these children. Hence, the extensive investigations in these difficult cases are needed and other mimic causes should be ruled out. Further study in aspect of esophageal manometry combined with video-MII-pH study and histopathology in various presentations of GERD should be initiated to extend the knowledge about the pathogenesis of GERD and hopefully, could tailor therapy for these patients.
We are grateful to all participants and their guardians in the present study; Voranush Chongsrisawat, Chomchanat Tubjareon, Sittichoke Prachuapthunyachart, Atikan Sirichoompun, Nattakoon Potjalongsin, all physician and nurses for the great care to our patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases, 174508; European Society of Pediatric Gastroenterology, Hepatology and Nutrition, 1135.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lv L, China; Xiao Y, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Ruigómez A, Wallander MA, Lundborg P, Johansson S, Rodriguez LA. Gastroesophageal reflux disease in children and adolescents in primary care. Scand J Gastroenterol. 2010;45:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 2. | Kleinman RE. Walker’s pediatric gastrointestinal disease. Sixth ed. Olivier-Jean Goulet GM-V IRS, Philip M, Sherman BLS, editor: House-USA; 2018. |
| 3. | Tolia V, Vandenplas Y. Systematic review: the extra-oesophageal symptoms of gastro-oesophageal reflux disease in children. Aliment Pharmacol Ther. 2009;29:258-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, Gupta S, Langendam M, Staiano A, Thapar N, Tipnis N, Tabbers M. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 529] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 5. | Frazzoni L, Frazzoni M, De Bortoli N, Ribolsi M, Tolone S, Russo S, Conigliaro RL, Penagini R, Fuccio L, Zagari RM, Savarino E. Application of Lyon Consensus criteria for GORD diagnosis: evaluation of conventional and new impedance-pH parameters. Gut. 2022;71:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 6. | Mutalib M, Rawat D, Lindley K, Borrelli O, Perring S, Auth MKH, Thapar N. BSPGHAN Motility Working Group position statement: paediatric multichannel intraluminal pH impedance monitoring-indications, methods and interpretation. Frontline Gastroenterol. 2017;8:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1518] [Cited by in RCA: 1653] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 8. | Schneider NI, Plieschnegger W, Geppert M, Wigginghaus B, Hoess GM, Eherer A, Wolf EM, Rehak P, Vieth M, Langner C. Validation study of the Esohisto consensus guidelines for the recognition of microscopic esophagitis (histoGERD Trial). Hum Pathol. 2014;45:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Mazzoleni G, Vailati C, Lisma DG, Testoni PA, Passaretti S. Correlation between oropharyngeal pH-monitoring and esophageal pH-impedance monitoring in patients with suspected GERD-related extra-esophageal symptoms. Neurogastroenterol Motil. 2014;26:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Dy F, Amirault J, Mitchell PD, Rosen R. Salivary Pepsin Lacks Sensitivity as a Diagnostic Tool to Evaluate Extraesophageal Reflux Disease. J Pediatr. 2016;177:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Plocek A, Gębora-Kowalska B, Białek J, Fendler W, Toporowska-Kowalska E. Esophageal Impedance-pH Monitoring and Pharyngeal pH Monitoring in the Diagnosis of Extraesophageal Reflux in Children. Gastroenterol Res Pract. 2019;2019:6271910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Maholarnkij S, Sanpavat A, Decharun K, Dumrisilp T, Tubjareon C, Kanghom B, Patcharatrakul T, Chaijitraruch N, Chongsrisawat V, Sintusek P. Detection of reflux-symptom association in children with esophageal atresia by video-pH-impedance study. World J Gastroenterol. 2020;26:4159-4169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 13. | Blondeau K, Sifrim D, Dupont L, Tack J. Reflux cough. Curr Gastroenterol Rep. 2008;10:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Pauwels A, Blondeau K, Dupont L, Sifrim D. Cough and gastroesophageal reflux: from the gastroenterologist end. Pulm Pharmacol Ther. 2009;22:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | de Bortoli N, Nacci A, Savarino E, Martinucci I, Bellini M, Fattori B, Ceccarelli L, Costa F, Mumolo MG, Ricchiuti A, Savarino V, Berrettini S, Marchi S. How many cases of laryngopharyngeal reflux suspected by laryngoscopy are gastroesophageal reflux disease-related? World J Gastroenterol. 2012;18:4363-4370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Qua CS, Wong CH, Gopala K, Goh KL. Gastro-oesophageal reflux disease in chronic laryngitis: prevalence and response to acid-suppressive therapy. Aliment Pharmacol Ther. 2007;25:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Reichel O, Issing WJ. Should patients with pH-documented laryngopharyngeal reflux routinely undergo oesophagogastroduodenoscopy? J Laryngol Otol. 2007;121:1165-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Suzuki T, Seki Y, Okamoto Y, Hoppo T. Hypopharyngeal multichannel intraluminal impedance leads to the promising outcome of antireflux surgery in Japanese population with laryngopharyngeal reflux symptoms. Surg Endosc. 2018;32:2409-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Borrelli O, Marabotto C, Mancini V, Aloi M, Macrì F, Falconieri P, Lindley KJ, Cucchiara S. Role of gastroesophageal reflux in children with unexplained chronic cough. J Pediatr Gastroenterol Nutr. 2011;53:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Zenzeri L, Quitadamo P, Tambucci R, Ummarino D, Poziello A, Miele E, Staiano A. Role of non-acid gastro-esophageal reflux in children with respiratory symptoms. Pediatr Pulmonol. 2017;52:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Rybak A, Pesce M, Thapar N, Borrelli O. Gastro-Esophageal Reflux in Children. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Sintusek P, Mutalib M, Thapar N. Gastroesophageal reflux disease in children: What's new right now? World J Gastrointest Endosc. 2023;15:84-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (4)] |
| 23. | Tack J, Pandolfino JE. Pathophysiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 24. | Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout AJPM, Vaezi M, Sifrim D, Fox MR, Vela MF, Tutuian R, Tack J, Bredenoord AJ, Pandolfino J, Roman S. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67:1351-1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 672] [Cited by in RCA: 943] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 25. | Rosado-Arias Y, Toro-Monjaraz EM, Cervantes-Bustamante R, Zarate-Mondragon F, Cadena-Leon J, Ignorosa-Arellano K, Loredo-Mayer A, Ramírez-Mayans J. Low Mean Nocturnal Baseline Impedance is Associated With a Pathological Acid Exposure Time in Children. J Pediatr Gastroenterol Nutr. 2022;74:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 26. | Park JS, Van der Wall H, Falk GL. Post-reflux swallow-induced peristaltic wave is impaired in laryngopharyngeal and gastro-oesophageal reflux disease. Clin Physiol Funct Imaging. 2022;42:8-14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |