Published online Nov 9, 2021. doi: 10.5409/wjcp.v10.i6.192
Peer-review started: November 11, 2020
First decision: April 6, 2021
Revised: April 18, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: November 9, 2021
Processing time: 361 Days and 20.2 Hours
Alopecia areata (AA) is an inflammatory disease with autoimmune, environmental, and inherited components directed at the hair follicle, either limited to patchy hair loss over the scalp (Focalis, AF), total loss of scalp hair (Totalis, AT), or total loss of both scalp and body hair (Universalis, AU). Despite multiple treatment modalities, no therapy exists. Vitamin D deficiency in patients with AA/AT/AF influences disease severity and duration, inversely correlating with inflammation histologically.
Three girls presented with AT (P1), AU (P2), and AF (P3) at the ages of 1, 5, and 5 years, respectively. For P1-P2, all available treatments implemented for 2 years had failed. We started an initial 6-mo repletion with oral cholecalciferol 2000/4000 IU/d, with no apparent effect. Then we attempted immunomodulation using oral calcitriol and its analog paricalcitol. On calcitriol, 0.5 mcg/d P1 regrew hair within 6 mo. After 4 years, a relapse with loss of eyebrow hair was resolved after doubling the calcitriol dose to 0.5 mcg × 2/d; the results have been maintained for 6 years to date. On calcitriol, 0.25 mcg × 3/d P2 led to the development of asymptomatic hypercalcemia-hypercalciuria, which was immediately resolved by switching to paricalcitol 2 mcg × 3/d; mild tolerable hypercalciuria was main
Vitamin D may have immunomodulating therapeutic impact on AT/AU/AF, which needs to be explored with further pilot clinical trials.
Core Tip: Alopecia areata (AA) is an inflammatory disease with autoimmune, environmental, and inherited components directed at the hair follicle, either limited to patchy hair loss over the scalp (Focalis, AF), total loss of scalp hair (Totalis, AT), or total loss of both scalp and body hair (Universalis, AU). Despite multiple treatment modalities, there is no current therapy. Three girls aged 3, 7, and 5 years with AT, AU, and AF were treated with oral calcitriol, paricalcitol, and cholecalciferol, showing hair regrowth at 6, 6, and 3 mo, respectively but only as fur for P2 with AU. Vitamin D may have an immunomodulating therapeutic impact on AT/AU/AF, which needs to be explored with further pilot clinical trials testing the effectiveness and establishing the optimal form and dosage of vitamin D.
- Citation: Papadimitriou DT, Bothou C, Dermitzaki E, Alexopoulos A, Mastorakos G. Treatment of alopecia totalis/universalis/focalis with vitamin D and analogs: Three case reports and a literature review. World J Clin Pediatr 2021; 10(6): 192-199
- URL: https://www.wjgnet.com/2219-2808/full/v10/i6/192.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v10.i6.192
Alopecia areata (AA) is a non-scarring T-cell mediated autoimmune disease directed at the hair follicle (HF), either limited to patchy hair loss over the scalp (Focalis, AF), total loss of scalp hair (Totalis, AT), or loss of both scalp and body hair (Universalis, AU). Its prevalence among the young and adult population is 0.7%-3.8%, significantly affecting patients’ lives and having psychosocial implications. Management of the disease can be challenging, and despite multiple treatment modalities, no successful treatment is available. Pediatric age and more extensive disease with resistance to initial therapies with corticosteroids may sometimes benefit from a cocktail of established therapies. The likelihood of complete spontaneous regrowth in AA is estimated to be less than 10%, but even then, relapses are common and frustrating[1].
HF is a micro-organ with its own immune and hormonal microenvironment. During the anagen segment of the hair cycle, HF epithelium generates and maintains an area of immune privilege, which is mainly characterized by the low expression of major histocompatibility complex class Ia antigens and local production of immunosuppressive agents. This HF immune privilege (HFIP) is important for the protection of anagen- and melanogenesis-associated antigens from immune recognition by autoreactive CD8+ T cells. The collapse of mechanisms that maintain the HFIP renders the HF susceptible to inflammatory assault, contributing to the development of AA, while growing evidence implicates interferon gamma in triggering HFIP collapse[2].
The role of vitamin D in the proliferation and differentiation of keratinocytes has been extensively studied and well established in the literature. Vitamin D is syn
Recent studies in mice and in vitro support the pivotal role of VDR in the postnatal maintenance of the HF. In the late anagen and catagen phases, there is an increase in VDR expression, which is associated with the decreased proliferation and increased differentiation of keratinocytes, making the presence of VDR a prerequisite for maintenance of the normal hair cycle[6]. However, the roles of vitamin D and the VDR in the hair cycle have not been completely elucidated, and clinical therapies for hair disorders have not been established. However, vitamin D is an important immuno
We present three cases with AT/AU/AF that emphasize the pivotal role of treatment with cholecalciferol, the active hormone calcitriol, and its analogue parical
Sudden and total hair loss in the scalp, both the scalp and body, and in multiple focalized areas of the scalp in three girls aged 1, 5, and 5 years, respectively.
Two girls diagnosed with AT and AU based on clinical examination[10], who ex
A third girl aged 5 years (patient #3, P3) presented with sudden (within the last month) hair loss compatible with AF.
None of the patients were suffering from other chronic dermatological diseases (vitiligo and psoriasis) or other systemic diseases such as diabetes mellitus, anemia, hypothyroidism or hyperthyroidism, systemic lupus, rheumatoid arthritis, chronic renal or liver disease, also autoimmune polyendocrinopathy type 1 was also excluded with the necessary laboratory testing. In P3, although there was normal thyroid function with negative anti-thyroid peroxidase (TPO) and anti-thyroglobulin (Tg) antibodies, signs of Hashimoto’s thyroiditis were shown in thyroid ultrasonography (U/S) performed by a pediatric radiologist. All three girls were vitamin D-deficient with vitamin D levels (25OHD3) of 60 nmol/L (24 ng/mL) in P1, 50 nmol/L (20 ng/mL) in P2, and 42.5 nmol/L (17 ng/mL) in P3, and normal calcium metabolism and parathyroid hormone (PTH) (PTH < 45 ng/mL)[11]. Zinc, B12, vitamin A, vitamin E, and ferritin levels were within normal range in all patients, also with negative celiac serology.
None of the patients nor any first-degree family members were suffering from other chronic dermatological diseases (vitiligo and psoriasis).
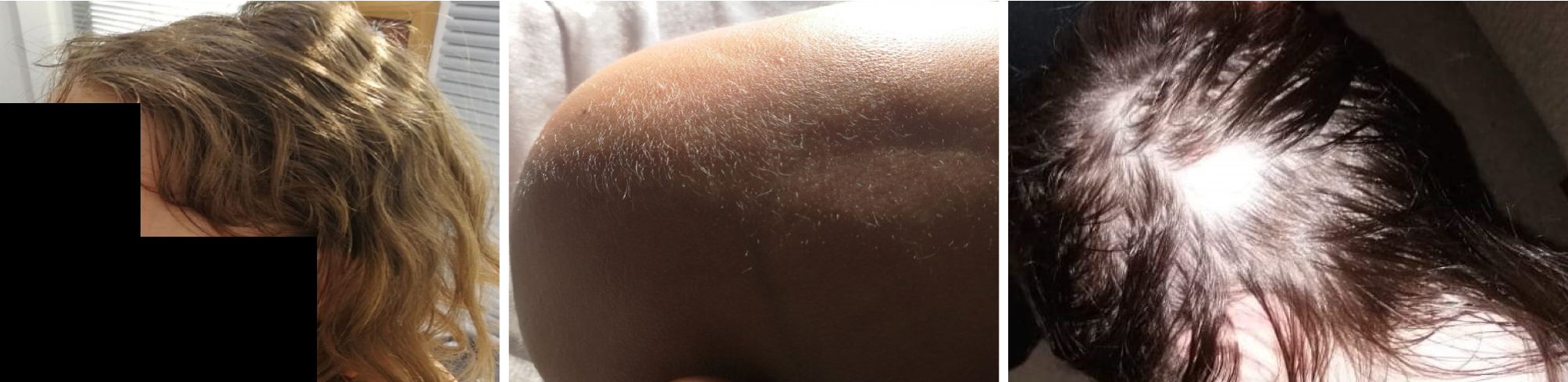
In P1, there was complete absence of scalp hair and eyebrows. In P2, there was complete absence of body hair. In P3, five localized areas had complete hair loss at the scalp, with a diameter of 3-5 cm, along with a palpable goiter (Figure 1). An expe
All three girls were vitamin D-deficient with vitamin D levels (25OHD3) found 60 nmol/L (24 ng/mL) in P1, 50 nmol/L (20 ng/mL) in P2 and 42.5 nmol/L (17 ng/mL) in P3, with the rest of the calcium metabolism and PTH being normal (PTH < 45 ng/mL)[11]. Zinc, vitamin B12, vitamin A, vitamin E, and ferritin levels were within normal range in all patients, also having a negative serology negative for celiac disease.
A thyroid ultrasound was performed by a pediatric radiologist. In P3, although there was normal thyroid function with negative anti-TPO and anti-Tg abs, signs of Hashimoto’s thyroiditis were found.
P1 had AT, P2 AU and P3 AF.
As P1 and P2 were vitamin D-deficient, we started an initial 6-mo repletion with oral cholecalciferol 2000/4000 IU/d at the upper tolerable daily dose, according to the Endocrine Society Clinical Practice Expert Guideline Committee, i.e. infants < 1-year 2000 IU daily and children 1-18 years 4000 IU daily[12] (https://www.endocrine.org/clinical-practice-guidelines/vitamin d deficiency), with no apparent effect on hair growth. Then, based on the previous experience of our group we attempted to induce immunomodulation by oral calcitriol[13-15] in P1 and P2, while both girls were continuously supplemented with cholecalciferol 2000 and 4000 IU p.o., respectively.
Active forms of vitamin D, such as calcitriol (1,25(OH)2 D, the biologically active form of vitamin D), and its up to 10 times less calcemic analog paricalcitol[16], are used to treat secondary hyperparathyroidism occurring in patients with kidney disease, leading to bone disease. Since they have different effects on calcium meta
Treatment with 0.5 mcg/d P1 grew hair within the first 6 mo of treatment (except a small region at the rear of the scalp; Figure 1). After 4 years, there was a relapse with loss of eyebrow hair, which was resolved within 3 mo after raising calcitriol dose at 0.5 × 2 mcg/d. The result has been maintained for 7 years now since treatment initiation with normal calcium metabolism: calcium (Ca) 10.1 mg/dL (normal range: 8.5-10.5 mg/dL), phosphorus (P) 5.1 mg/dL (normal range: 3.5-5.5 mg/dL), alkaline phosphatase (ALP) 318 IU/L (normal range: 199-440 U/L), parathyroid hormone (PTH) 26 pg/mL (normal < 45 pg/mL), 25OHD3 41 ng/mL (normal range: 30-150 ng/mL), 1-25 (OH)2D3 30 ng/mL (normal range: 18-80 pg/mL) and normal 0.08 Ca/Cr ratio in a 2 h morning urine sample (normal range: < 0.22).
Treatment with 0.25 mcg × 3/d p.o. P2 developed asymptomatic hypercalcemia – hypercalciuria (Ca 14 mg/dL, Urine Ca/Cr 1.37 in a 2-h morning sample) within 1 mo and was immediately switched to an even higher corresponding dose of paricalcitol[17] at 2 mcg × 3/d p.o. Then calcium metabolism normalized: Ca 9.8 mg/dL, P 3.8 mg/dL, ALP 146 IU/L, PTH 22.4 pg/mL, 25(OH)D 152.5 nmol/L (61 ng/mL), 1-25 (OH)2 D3 38 ng/mL, apart from mild hypercalciuria (Ca/Cr 0.5 in a 2-h morning urine sample), closely monitored and with normal kidney U/S every 6 mo. Hair regrowth including scalp hair, eyebrows and eyelashes was noted by 6 mo but maintained at 12 mo only as fur (Figure 1). With no further improvement, paricalcitol treatment was discontinued at 12 mo with a complete subsequent relapse of AU.
In P3, treatment with high dose cholecalciferol p.o. (8000 IU/d) completely resolved all focalized alopecia areas within 3 mo with normal hair regrowth at all sites and 25(OH)D levels restored at 155 nmol/L (62 ng/mL). At 6 mo dermatological exami
We present three cases of AT/AU/AF treated with oral calcitriol, its analogue paricalcitol, and high-dose cholecalciferol. Almost complete hair regrowth including scalp hair and eyebrows was accomplished in the girl with AT on calcitriol treatment. A relapse was avoided by raising the calcitriol dose and the patient can be considered cured, with the result being maintained for 7 years now, having a beneficial effect on the girl’s well-being. Treatment with calcitriol is being continued though, as calcium metabolism is completely normal, and the family wishes to maintain it being afraid of a possible relapse. In the AU case, calcitriol caused hypercalcemia – hypercalciuria and was switched to paricalcitol, a less calcemic analog. While hair regrowth was noted by 6 mo of treatment with even eyelashes being temporarily restored, at 12 mo scalp hair was still as fur, leading to treatment discontinuation and subsequent complete AU relapse. In the AF case, early onset high dose daily cholecalciferol treatment was successful, restoring completely alopecia areas with no further relapses. Undoubtedly, just three cases do not suffice to suggest generalized use of the presented approach. Nevertheless, the possible implications of vitamin D in the clinical care of patients with AT/AU/AF, as in autoimmune disorders in general, are being examined and dis
It is well established that vitamin D reduces the function and differentiation of T-helper 17 cells, down-regulates the T-helper 1 cells and increases the action of T-regs, resulting in immunomodulation[7,20]. AT/AU, as an inflammatory disease with autoimmune, environmental, and inherited components, is characterized by imbalance of the above-mentioned parts of the immune system. Previous work of our group has shown the negativation of Type 1 associated autoantibodies after treatment with oral calcitriol[13] but also practically the cure of severe atopic dermatitis, also an auto
Regarding the role of vitamin D and its receptor (VDR) in hair, it is well established that VDR is expressed in the outer root sheath (ORS), HF bulb, and the sebaceous gland in the HF and participates in differentiation of HFs[6]. VDR knock out mice (VDR KO) have been proved to suffer from alopecia areata[22]. VDR expression is decreased in HF and epidermal keratinocytes in AA leading to suppression of Wnt/ beta catenin signals and cell differentiation[23]. This downregulation of VDR could be explained either due to the local inflammation that leads to loss of the VDR expression or due to the vitamin D deficiency. This is supported by the hypothesis that vitamin D deficiency is a stimulus for the local inflammation and vice versa, which could lead to a vicious cycle in the chronic status of the disease. Re-appearance of the VDR on HF was detected after topical calcipotriol treatment, a synthetic derivative of calcitriol, used in the treatment of psoriasis[24]. Similarly with other studies presenting small series of patients, using local treatments containing calcipotriol, over 50% experienced improvement of the alopecia manifestations[9,25].
On the other hand, vitamin D deficiency among AA patients is a common finding. Many studies reveal significantly reduced 25(OH)D concentrations among this population[26,27]. Another recent prospective study comparing 30 patients with AA with 30 controls showed that vitamin D deficiency in AA influences disease severity and duration[28]. Simultaneously, VDR expression was reduced in AA and as hypo
The study from Daroach et al[28] was – to the best of our knowledge – the first effort of systematic supplementation of the vitamin D deficient AA. They used oral cholecalciferol 60.000 IU once weekly for 12 wk and detected clinical improvement and VDR upregulation, even though statistically significant results were not acquired[28]. The reason for this might be that, according to many studies, serum 25(OH)D above a certain cut-off may be required for its immunomodulatory actions but also a minimum duration of treatment for the upregulation of the VDR expression is required[7]. The dosage that has been used in this study would assure normal (30-150 ng/mL) 25(OH)D concentrations, above or around 40-60 ng/mL, as in our AF patient. Though, as in our cases, a pharmacological therapeutic intervention, as the individualized schemes with the active hormone calcitriol and its analog paricalcitol we used, may be required to obtain positive therapeutic results. This is because cholecalciferol is subjected to internal transformation to the active hormone calcitriol to exert most of its’ immunomodulatory actions and this counterbalance has its limitations[30].
Even if not finally successful in resolving AU in our case, the active hormone calcitriol and its analog paricalcitol had some undeniable and visible effect on scalp and body hair – even as fur -, on eyebrows’, and eyelashes’ regrowth, indicating that vitamin D possesses an immunomodulating capability that interferes with the me
Treatment with vitamin D in the form of cholecalciferol, as well the active hormone calcitriol and its analogs, such as the already marketed paricalcitol, may be envisioned for patients with AA/AT/AF, however with close monitoring of Ca metabolism parameters. Pilot clinical trials and RCTs are required to prove the effectiveness and safety of this therapeutic approach, as to establish the optimal form and dosage of vitamin D administration, alone or in combination with other treatments.
We thank Konstantinos Karkavitsas MD, Consultant Pediatric and Adult Dermatologist for his valuable contribution providing details on the previous history and treatments in P1 and P2, as well as for discussing with our multidisciplinary team our treatment modalities.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sideris N S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Hordinsky MK. Overview of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S13-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest. 2007;117:2019-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Bikle DD. Vitamin D and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Holick MF. Vitamin D: a d-lightful solution for health. J Investig Med. 2011;59:872-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Malloy PJ, Pike JW, Feldman D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Rev. 1999;20:156-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Sassi F, Tamone C, D'Amelio P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 484] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 8. | Bakry OA, El Farargy SM, El Shafiee MK, Soliman A. Serum Vitamin D in patients with alopecia areata. Indian Dermatol Online J. 2016;7:371-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Darwin E, Hirt PA, Fertig R, Doliner B, Delcanto G, Jimenez JJ. Alopecia Areata: Review of Epidemiology, Clinical Features, Pathogenesis, and New Treatment Options. Int J Trichology. 2018;10:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 11. | Stagi S, Cavalli L, Ricci S, Mola M, Marchi C, Seminara S, Brandi ML, de Martino M. Parathyroid Hormone Levels in Healthy Children and Adolescents. Horm Res Paediatr. 2015;84:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6974] [Cited by in RCA: 6844] [Article Influence: 488.9] [Reference Citation Analysis (0)] |
| 13. | Papadimitriou DT, Marakaki C, Fretzayas A, Nicolaidou P, Papadimitriou A. Negativation of type 1 diabetes-associated autoantibodies to glutamic acid decarboxylase and insulin in children treated with oral calcitriol. J Diabetes. 2013;5:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Bothou C, Alexopoulos A, Dermitzaki E, Kleanthous K, Papadimitriou A, Mastorakos G, Papadimitriou DT. Successful Treatment of Severe Atopic Dermatitis with Calcitriol and Paricalcitol in an 8-Year-Old Girl. Case Rep Pediatr. 2018;2018:9643543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Papadimitriou DT. High Doses of Oral Calcitriol (up to 6μg/day) and Paricalcitol (up to 72 μg/day) Have Successfully Intercepted Progression to Clinical Type 1 Diabetes for over 3 Years in a 10-Year-Old Boy. Endocr Rev. 2017;. |
| 16. | Slatopolsky E, Finch J, Ritter C, Denda M, Morrissey J, Brown A, DeLuca H. A new analog of calcitriol, 19-nor-1,25-(OH)2D2, suppresses parathyroid hormone secretion in uremic rats in the absence of hypercalcemia. Am J Kidney Dis. 1995;26:852-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 133] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Ono K, Yoshida A, Saito N, Fujishima T, Honzawa S, Suhara Y, Kishimoto S, Sugiura T, Waku K, Takayama H, Kittaka A. Efficient synthesis of 2-modified 1alpha,25-dihydroxy-19-norvitamin D3 with Julia olefination: high potency in induction of differentiation on HL-60 cells. J Org Chem. 2003;68:7407-7415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Webb NJA, Lerner G, Warady BA, Dell KM, Greenbaum LA, Ariceta G, Hoppe B, Linde P, Lee HJ, Eldred A, Dufek MB. Efficacy and safety of paricalcitol in children with stages 3 to 5 chronic kidney disease. Pediatr Nephrol. 2017;32:1221-1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 19. | Seeherunvong W, Nwobi O, Abitbol CL, Chandar J, Strauss J, Zilleruelo G. Paricalcitol versus calcitriol treatment for hyperparathyroidism in pediatric hemodialysis patients. Pediatr Nephrol. 2006;21:1434-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | da Costa DS, Hygino J, Ferreira TB, Kasahara TM, Barros PO, Monteiro C, Oliveira A, Tavares F, Vasconcelos CC, Alvarenga R, Bento CA. Vitamin D modulates different IL-17-secreting T cell subsets in multiple sclerosis patients. J Neuroimmunol. 2016;299:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Sochorová K, Budinský V, Rozková D, Tobiasová Z, Dusilová-Sulková S, Spísek R, Bartůnková J. Paricalcitol (19-nor-1,25-dihydroxyvitamin D2) and calcitriol (1,25-dihydroxyvitamin D3) exert potent immunomodulatory effects on dendritic cells and inhibit induction of antigen-specific T cells. Clin Immunol. 2009;133:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Sakai Y, Kishimoto J, Demay MB. Metabolic and cellular analysis of alopecia in vitamin D receptor knockout mice. J Clin Invest. 2001;107:961-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Lim YY, Kim SY, Kim HM, Li KS, Kim MN, Park KC, Kim BJ. Potential relationship between the canonical Wnt signalling pathway and expression of the vitamin D receptor in alopecia. Clin Exp Dermatol. 2014;39:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Kim DH, Lee JW, Kim IS, Choi SY, Lim YY, Kim HM, Kim BJ, Kim MN. Successful treatment of alopecia areata with topical calcipotriol. Ann Dermatol. 2012;24:341-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Narang T, Daroach M, Kumaran MS. Efficacy and safety of topical calcipotriol in management of alopecia areata: A pilot study. Dermatol Ther. 2017;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Tsai TY, Huang YC. Vitamin D deficiency in patients with alopecia areata: A systematic review and meta-analysis. J Am Acad Dermatol. 2018;78:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Lee S, Kim BJ, Lee CH, Lee WS. Increased prevalence of vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:1214-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Daroach M, Narang T, Saikia UN, Sachdeva N, Sendhil Kumaran M. Correlation of vitamin D and vitamin D receptor expression in patients with alopecia areata: a clinical paradigm. Int J Dermatol. 2018;57:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Tsai TY, Huang YC. Reply to: "Serum vitamin D level and disease severity of alopecia areata: A meta-regression analysis". J Am Acad Dermatol. 2018;79:e51-e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner Res. 2007;22 Suppl 2:V64-V68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Yang SW, Tsai CY, Pan YC, Yeh CN, Pang JH, Takano M, Kittaka A, Juang HH, Chen TC, Chiang KC. MART-10, a newly synthesized vitamin D analog, represses metastatic potential of head and neck squamous carcinoma cells. Drug Des Devel Ther. 2016;10:1995-2002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |