Published online Nov 9, 2021. doi: 10.5409/wjcp.v10.i6.168
Peer-review started: May 9, 2021
First decision: June 17, 2021
Revised: June 30, 2021
Accepted: October 25, 2021
Article in press: October 25, 2021
Published online: November 9, 2021
Processing time: 183 Days and 8.4 Hours
Tidal breathing flow-volume (TBFV) analysis provides important information about lung mechanics in infants.
To assess the effects of breastfeeding on the TBFV measurements of infants who recover from acute bronchiolitis.
In this cross-sectional study, TBFV analysis was performed in infants with bronchiolitis prior to hospital discharge. The ratio of time to peak expiratory flow to total expiratory time (tPEF/tE) at baseline and after the administration of 400 mcg salbutamol was evaluated.
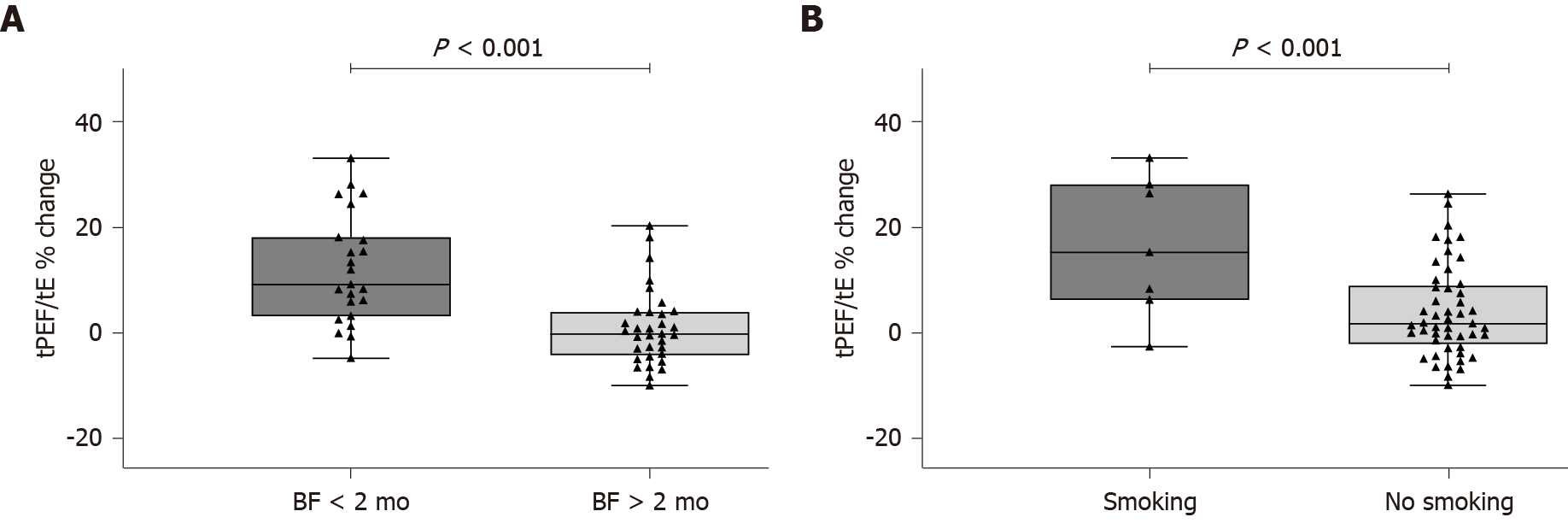
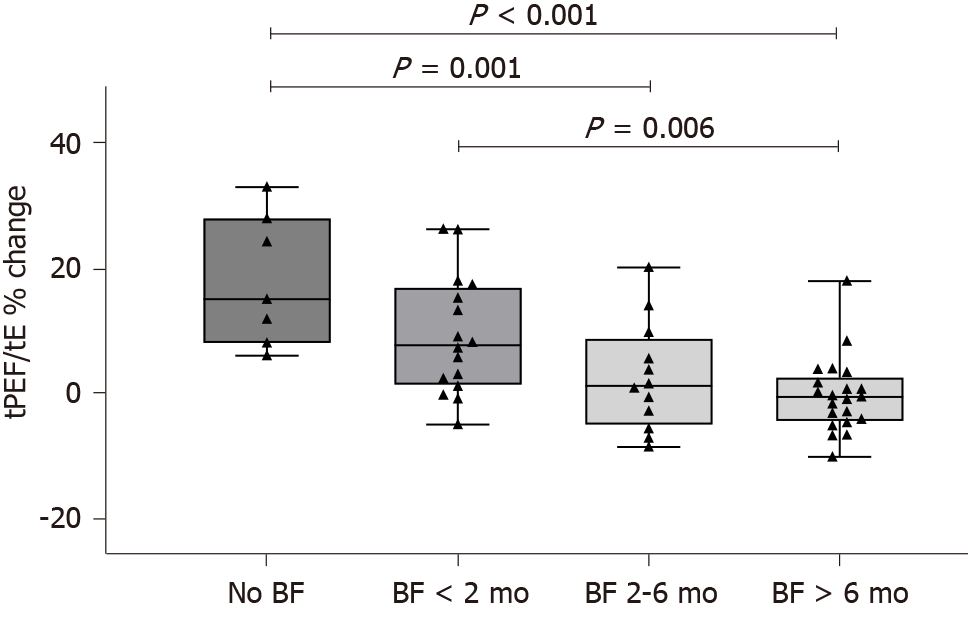
A total of 56 infants (35 boys), aged 7.4 ± 2.8 mo, were included. Of them, 12.5% were exposed to tobacco smoke and 41.1% were breastfed less than 2 mo. There were no differences in baseline TBFV measurements between the breastfeeding groups; however, those who breastfed longer than 2 mo had a greater change in tPEF/tE after bronchodilation (12% ± 10.4% vs 0.9% ± 7.1%; P < 0.001). Moreover, there was a clear dose-response relationship between tPEF/tE reversibility and duration of breastfeeding (P < 0.001). In multivariate regression analysis, infants who breastfed less (regression coefficient -0.335, P = 0.010) or were exposed to cigarette smoke (regression coefficient 0.353, P = 0.007) showed a greater change in tPEF/tE after bronchodilation, independent of sex, prematurity, and family history of asthma or atopy.
Infants who recover from bronchiolitis and have a shorter duration of breastfeeding or are exposed to cigarette smoke, have TBFV measurements indicative of obstructive lung disease.
Core Tip: Assessment of lung function using tidal breathing could be beneficial for infants and preschoolers in whom forced respiratory maneuvers cannot be performed. We examined the correlation between breastfeeding and tidal breathing analysis in infants with bronchiolitis, and demonstrated that those who were exposed to cigarette smoke and/or had a shorter duration of breastfeeding showed tidal breathing alterations indicative of obstructive pulmonary disease.
- Citation: Perikleous E, Fouzas S, Karageorgiou A, Steiropoulos P, Nena E, Chatzimichael A, Tsalkidis A, Paraskakis E. Association of breastfeeding with tidal breathing analysis in infants with bronchiolitis. World J Clin Pediatr 2021; 10(6): 168-176
- URL: https://www.wjgnet.com/2219-2808/full/v10/i6/168.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v10.i6.168
Bronchiolitis is a viral infection of lower airways that is characterized by substantial inflammation, increased mucus production, and necrosis of small airway epithelial cells[1]. It is the leading cause of infant morbidity and mortality worldwide[2], and represents a significant burden for the healthcare system, the family, and society[3]. Infants with co-existing conditions, such as prematurity and cardiopulmonary disorders, are at higher risk of developing more severe bronchiolitis[3]. Moreover, environmental factors such as smoking exposure, indoor and outdoor pollution[4], and lack of breastfeeding[5] may significantly increase susceptibility to the disease.
The favorable effects of breastfeeding are indisputable, and no other practice can drastically promote infant’s health in the short- and long-term[6]. Comprehensively, there is some evidence of the consistent advantageous impact of breastfeeding on increasing forced vital capacity (FVC)[7]. Early life nutrition with breast milk as the initial food for newborns is considered ‘the best’ due to its beneficial effects on overall health, along with improved lung function. A previous study showed a link between breastfeeding and lung function in school-age children, namely, greater forced expiratory flow at 50% (FEF50), particularly in those who breastfed longer than 3 mo including children of mothers with asthma[8]. Regarding bronchiolitis, current evidence suggests that breastfed infants have a clear immunological advantage compared with their formula-fed peers[9]; exclusive breastfeeding has been shown to decrease the requirement for oxygen supplementation, the length of hospital stay, and the risk of respiratory failure in infants with more severe forms of the disease[9]. However, despite the clear clinical advantages, less is known about the effects of breastfeeding on the pulmonary function of infants with acute bronchiolitis.
There is some evidence indicating that tobacco smoke exposure in children decreases lung function and augments airway hyperresponsiveness, predisposing infants to a more severe clinical course of infection compared to unexposed peers[4]. Similarly, studies have shown that maternal smoking during pregnancy is related to bronchiolitis[4,10]. Overall, pregnancy and subsequent parenthood can become major motivators for mothers and caregivers to permanently quit this detrimental practice.
Although lung function testing at bedside is notoriously difficult in infancy[11], recent evidence suggests that tidal breathing flow-volume (TBFV) measurement and analysis are feasible[11,12]. In particular, the ratio of time to peak expiratory flow to total expiratory time (tPEF/tE) decreases in obstructive lung disorders[10], providing important information on the underlying pathophysiological mechanisms and extent of lung injury[11,12].
The aim of this study was to assess the effects of breastfeeding on the lung function of infants who recovered from acute bronchiolitis. We hypothesized that breastfeeding may have favorable effects on baseline tPEF/tE values and/or tPEF/tE reversibility after bronchodilation, independent of other confounding factors.
This observational, cross-sectional study was performed between September 2016 and April 2018 in the Pediatric Department of the University General Hospital of Alexandroupolis (Alexandroupolis, Greece). All infants aged 2-12 mo and hospitalized with bronchiolitis were eligible to participate. Bronchiolitis was defined according to the relevant history and physical examination (fever, cough, tachypnoea, chest recession, wheeze or crackles during auscultation)[3]. Infants with genetic disorders, neuromu
On the day of hospital discharge, eligible infants underwent TBFV measurements in the pediatric lung function laboratory using the MasterScreen pediatric respiratory system (Jaeger/CareFusion, Hoechberg, Germany). All infants were tested according to relevant European Respiratory Society/American Thoracic Society guidelines[13-15] during natural sleep after feeding. A minimum of 30 s of natural breathing was recorded to acquire a set of at least 10 regular breaths. The ratio of tPEF/tE was automatically calculated by the system at baseline and 10 min after the administration of 300 mcg salbutamol inhaler via an appropriate holding chamber. The metadata of the study population were obtained from the medical records. The weight-for-length z-scores were estimated using Centers for Disease Control and Prevention/National Center for Health Statistics norms[16].
Continuous variables are expressed as the mean ± SD standard deviation and compared with the Student’s t-test or one-way analysis of variance (multiple comparisons). Multivariate regression analysis was used to determine the outcome of breastfeeding in terms of gender, history of prematurity, and family history of atopy. All analyses were performed using IBM SPSS version 25 (IBM Corp., Armonk, NY, United States).
A total of 56 infants (35 boys), aged 7.4 ± 2.8 mo, were included in this study. Their characteristics are presented in Table 1. Of them, 21.4% were born prematurely, 12.5% were exposed to tobacco smoke (during pregnancy and/or after birth), and 16.1% had a family history of asthma or atopy. No breastfeeding was reported in 7 infants (12.5%), whereas 23 infants (41.1%) were breastfed less than 2 mo (Table 1).
| Characteristics | |
| n | 56 |
| Age (mo) | 7.4 ± 2.8 |
| Male sex, n (%) | 35 (62.5) |
| Body weight, kg | 7.3 ± 1.6 |
| Body length, cm | 65 ± 8.1 |
| Weight-for-length z-score | -0.2 ± 2.0 |
| Gestational age, wk | 37.9 ± 1.5 (range 35-41) |
| Prematurity (< 37 wk) | 12 (21.4) |
| Breastfeeding | |
| No | 7 (12.5) |
| < 2 mo | 16 (28.6) |
| 2-6 mo | 12 (21.4) |
| > 6 mo | 21 (37.5) |
| Smoking exposure | |
| In pregnancy | 5 (8.9) |
| After birth | 7 (12.5) |
| In pregnancy and/or after birth | 7 (12.5) |
| Family history of asthma/atopy | 9 (16.1) |
There were no differences in baseline TBFV measurements between infants who did not breastfeed or breastfed less than 2 mo (Group 1) and those who breastfed longer than 2 mo (Group 2) (Table 2). Conversely, infants in Group 1 had a significantly higher change in tPEF/tE after bronchodilation compared with those in Group 2 (12% ± 10.4% vs 0.9% ± 7.1%; P < 0.001) (Figure 1A). The tPEF/tE reversibility was also higher in infants exposed to tobacco smoke during pregnancy and/or after birth (Figure 1B). There was a clear dose-response relationship between the reversibility of tPEF/tE and the duration of breastfeeding (P < 0.001) (Figure 2).
| BF < 2 mo (n = 23) | BF ≥ 2 mo (n = 33) | P value | |
| Tidal volume, mL/kg | 8.6 ± 1.8 | 8.3 ± 2.1 | 0.580 |
| Respiratory rate, bpm | 46.8 ± 20 | 44.7 ± 18.4 | 0.687 |
| Expiratory time, s | 0.57 ± 0.21 | 0.55 ± 0.22 | 0.735 |
| tPEF/tE, % | 35.4 ± 15.5 | 41.3 ± 13.7 | 0.139 |
Multivariate regression analysis showed that infants who breastfed less (beta -0.335, P = 0.010) or were exposed to cigarette smoke (beta 0.353, P = 0.007) had a greater change in tPEF/tE after bronchodilation, independent of sex, prematurity, and family history of asthma or atopy (Table 3).
| Regression coefficient β | P value | |
| Breastfeeding duration | -0.335 | 0.010 |
| Cigarette smoke exposure | 0.353 | 0.007 |
| Male sex | 0.005 | 0.974 |
| Prematurity | 0.031 | 0.833 |
| Family History of asthma/atopy | 0.121 | 0.379 |
In this study, we demonstrated that infants who recovered from acute bronchiolitis and had a shorter duration of breastfeeding or were exposed to cigarette smoke, had TBFV measurements indicative of obstructive lung disease. Specifically, these infants had a greater percent change in tPEF/TE after bronchodilation, and this effect was independent of other confounding factors such as premature birth and family history of asthma or atopy. Interestingly, there was a clear dose-response relationship between tPEF/tE reversibility and the duration of breastfeeding. Moreover, infants who were exposed to cigarette smoke showed a greater change in tPEF/tE after bronchodilation, independent of sex, prematurity, and family history of asthma or atopy.
Early life exposures may affect the outgrowth of pulmonary system, resulting in an immediate impact on later lung function. Previous studies have highlighted the key role of breastfeeding in terms of larger lung volumes at school age[8,17], suggesting the influence of breastfeeding on respiratory health. In addition, studies have shown that extended and exclusive breastfeeding reduces the risk of wheezing and asthma during infancy, early childhood[17-20], and even in youth[21], functioning as a shield against allergic predisposition. In a recent study of 555 children, forced expiratory volume in 1 s and FVC markedly increased in accordance with breastfeeding duration in those with asthma group[20]. However, in a novel birth cohort of 377 healthy term infants, a link between breastfeeding duration and obstructive or restrictive lung function was not shown[22]. Similarly, in a birth cohort of 620 infants, lung function was assessed at 12 and 18 years of age; duration of breastfeeding did not greatly influence lung function in children with a positive family history for allergic diseases[23]. Thus, whether breastfeeding protects against allergic disease in childhood remains a subject of debate, although exclusive breastfeeding for a duration of 6 mo is the keystone for the promotion of allergy health.
The evaluation of pulmonary function by TBFV analysis has certain benefits in infants in whom forced respiratory flows cannot be performed. Several studies have examined the application of TBFV measurements in a variety of lung disorders and have shown that a reduction in tPEF/tE ratio is suggestive of airway obstruction[11,12,15,24-26]. Zedan et al[25] reported that wheezing infants with a positive family history of asthma or who had never been breastfed, displayed significantly lower tPEF/tE compared with healthy controls. Similarly, children and infants with wheezing disorders have a reduced tPEF/tE ratio compared with control subgroups[27,28]. Moreover, studies of infants with chronic lung disease showed impaired lung compliance and reduced resistance during the first 12 mo of life[29].
Qi et al[30] found that wheezing infants had reduced lung function compared with those who were not wheezing, and that tPEF/tE was negatively associated with later poor respiratory outcomes; the deficit in tPEF/tE ratio remained after clinical improvement. However, a study in school-age children with asthma[24] showed no difference in tidal breathing parameters compared with control groups.
In accordance with our main findings, in a preliminary Norwegian study of infants with acute bronchiolitis, the tPEF/tE was reduced but improved after the administration of inhaled adrenaline[31]. By contrast, in another study in infants with bronchiolitis, the researchers did not find any significant differences in tPEF/tE after the administration of nebulized albuterol[32]. In a recent cross-sectional study, tPEF/tE was inversely related to the length of hospital stay and disease severity in infants with bronchiolitis[33], and was also significantly reduced in children exposed to parental smoking[33]. In another study of preschool wheezers, family history of asthma, breastfeeding duration less than 3 mo, and passive smoking, were all significant risk factors for bronchial hyperresponsiveness, defined as tPEF/tE increase > 20% following salbutamol administration[34].
Our study had a number of limitations. First, it was a single-center study with a small sample size; thus the findings cannot be generalized to all populations. Second, a control group was not included in the study design; consequently we could not compare our results with a subgroup of healthy peers. Third, the study design did not include some relevant confounding factors that could affect lung function, such as air pollution.
Infants who recovered from acute bronchiolitis and had a shorter duration of breastfeeding or were exposed to cigarette smoke, had TBFV measurements indicative of obstructive lung disease. Tidal breathing is undeniably a complex process, but its measurement during infancy appears promising. To understand the mechanisms by which acute bronchiolitis may affect lung function in infancy and beyond, additional large-scale research is required.
Bronchiolitis is a common viral infection of lower airways and a major cause of morbidity and mortality globally, especially among infants with concomitant medical conditions. The positive effects of breastfeeding are uncontested in infant’s health in the short- and long-term.
There are sufficient data suggesting the advantageous effects of breastfeeding on pulmonary function, but less information regarding the influence of breastfeeding on lung function in infants with acute bronchiolitis.
To assess the effects of breastfeeding on tidal breathing flow-volume (TBFV) measure
TBFV analysis was conducted in 56 infants with bronchiolitis prior to hospital discharge. The ratio of time to peak expiratory flow to total expiratory time (tPEF/tE) at baseline and after the administration of 400 mcg salbutamol was assessed using a MasterScreen pediatric respiratory system (Jaeger/CareFusion, Hoechberg, Germany). All infants were tested according to European Respiratory Society/American Thoracic Society guidelines in the middle of natural sleep following feeding. Multivariate regression analysis was used to investigate the outcome of breastfeeding in terms of gender, history of prematurity, and family history of atopy. All analyses were conducted in IBM SPSS version 25.
There were no differences in baseline TBFV measurements between breastfeeding groups; however, children who breastfed less than 2 mo had a greater tPEF/tE change after bronchodilation (12% ± 10.4% vs 0.9% ± 7.1%; P < 0.001). Additionally, a distinct dose-response relationship between tPEF/tE reversibility and duration of breastfeeding was shown (P < 0.001). In multivariable regression analysis, infants who breastfed less (beta -0.335, P = 0.010) or were exposed to cigarette smoke (beta 0.353, P = 0.007) exhibited a higher tPEF/tE change after bronchodilation, irrelevant of sex, prematurity, and family history of asthma or atopy.
Infants who recovered from acute bronchiolitis and had a shorter duration of breastfeeding or were exposed to cigarette smoke, had TBFV analyses indicative of obstructive lung disease, independently of other confounding factors. Tidal breathing is undoubtedly a complicated procedure, but its measurement during infancy is promising.
Additional large-scale studies are required to determine the mechanisms by which acute bronchiolitis may affect lung function in early infancy as well as later in life.
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigues AT S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Ali S, Plint AC, Klassen TP. Bronchiolitis. In: Wilmott RW, Kendig EL, Boat TF, Bush A, Chernick V. Kendig and Chernick’s disorders of the respiratory tract in children, 8th ed. Philadelphia: Elsevier Saunders, 2012: 443-452. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Global Burden of Disease Pediatrics Collaboration, Kyu HH, Pinho C, Wagner JA, Brown JC, Bertozzi-Villa A, Charlson FJ, Coffeng LE, Dandona L, Erskine HE, Ferrari AJ, Fitzmaurice C, Fleming TD, Forouzanfar MH, Graetz N, Guinovart C, Haagsma J, Higashi H, Kassebaum NJ, Larson HJ, Lim SS, Mokdad AH, Moradi-Lakeh M, Odell SV, Roth GA, Serina PT, Stanaway JD, Misganaw A, Whiteford HA, Wolock TM, Wulf Hanson S, Abd-Allah F, Abera SF, Abu-Raddad LJ, AlBuhairan FS, Amare AT, Antonio CA, Artaman A, Barker-Collo SL, Barrero LH, Benjet C, Bensenor IM, Bhutta ZA, Bikbov B, Brazinova A, Campos-Nonato I, Castañeda-Orjuela CA, Catalá-López F, Chowdhury R, Cooper C, Crump JA, Dandona R, Degenhardt L, Dellavalle RP, Dharmaratne SD, Faraon EJ, Feigin VL, Fürst T, Geleijnse JM, Gessner BD, Gibney KB, Goto A, Gunnell D, Hankey GJ, Hay RJ, Hornberger JC, Hosgood HD, Hu G, Jacobsen KH, Jayaraman SP, Jeemon P, Jonas JB, Karch A, Kim D, Kim S, Kokubo Y, Kuate Defo B, Kucuk Bicer B, Kumar GA, Larsson A, Leasher JL, Leung R, Li Y, Lipshultz SE, Lopez AD, Lotufo PA, Lunevicius R, Lyons RA, Majdan M, Malekzadeh R, Mashal T, Mason-Jones AJ, Melaku YA, Memish ZA, Mendoza W, Miller TR, Mock CN, Murray J, Nolte S, Oh IH, Olusanya BO, Ortblad KF, Park EK, Paternina Caicedo AJ, Patten SB, Patton GC, Pereira DM, Perico N, Piel FB, Polinder S, Popova S, Pourmalek F, Quistberg DA, Remuzzi G, Rodriguez A, Rojas-Rueda D, Rothenbacher D, Rothstein DH, Sanabria J, Santos IS, Schwebel DC, Sepanlou SG, Shaheen A, Shiri R, Shiue I, Skirbekk V, Sliwa K, Sreeramareddy CT, Stein DJ, Steiner TJ, Stovner LJ, Sykes BL, Tabb KM, Terkawi AS, Thomson AJ, Thorne-Lyman AL, Towbin JA, Ukwaja KN, Vasankari T, Venketasubramanian N, Vlassov VV, Vollset SE, Weiderpass E, Weintraub RG, Werdecker A, Wilkinson JD, Woldeyohannes SM, Wolfe CD, Yano Y, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, El Sayed Zaki M, Naghavi M, Murray CJ, Vos T. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatr. 2016;170:267-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 435] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 3. | Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, Johnson DW, Light MJ, Maraqa NF, Mendonca EA, Phelan KJ, Zorc JJ, Stanko-Lopp D, Brown MA, Nathanson I, Rosenblum E, Sayles S 3rd, Hernandez-Cancio S; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 1136] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 4. | Nenna R, Cutrera R, Frassanito A, Alessandroni C, Nicolai A, Cangiano G, Petrarca L, Arima S, Caggiano S, Ullmann N, Papoff P, Bonci E, Moretti C, Midulla F. Modifiable risk factors associated with bronchiolitis. Ther Adv Respir Dis. 2017;11:393-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Davisse-Paturet C, Adel-Patient K, Forhan A, Lioret S, Annesi-Maesano I, Heude B, Charles MA, de Lauzon-Guillain B. Breastfeeding initiation or duration and longitudinal patterns of infections up to 2 years and skin rash and respiratory symptoms up to 8 years in the EDEN mother-child cohort. Matern Child Nutr. 2020;16:e12935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC; Lancet Breastfeeding Series Group. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3459] [Cited by in RCA: 4102] [Article Influence: 455.8] [Reference Citation Analysis (0)] |
| 7. | Waidyatillake NT, Allen KJ, Lodge CJ, Dharmage SC, Abramson MJ, Simpson JA, Lowe AJ. The impact of breastfeeding on lung development and function: a systematic review. Expert Rev Clin Immunol. 2013;9:1253-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Dogaru CM, Strippoli MP, Spycher BD, Frey U, Beardsmore CS, Silverman M, Kuehni CE. Breastfeeding and lung function at school age: does maternal asthma modify the effect? Am J Respir Crit Care Med. 2012;185:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Dixon DL. The Role of Human Milk Immunomodulators in Protecting Against Viral Bronchiolitis and Development of Chronic Wheezing Illness. Children (Basel). 2015;2:289-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Morris MJ, Lane DJ. Tidal expiratory flow patterns in airflow obstruction. Thorax. 1981;36:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Hevroni A, Goldman A, Blank-Brachfeld M, Abu Ahmad W, Ben-Dov L, Springer C. Use of tidal breathing curves for evaluating expiratory airway obstruction in infants. J Asthma. 2018;55:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Lavizzari A, Zannin E, Ophorst M, Ciuffini F, Gangi S, Farolfi A, Colnaghi M, Dellacà RL, Mosca F. Tidal Breathing Measurements in Former Preterm Infants: A Retrospective Longitudinal Study. J Pediatr. 2021;230:112-118.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Frey U, Stocks J, Coates A, Sly P, Bates J. Specifications for equipment used for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/ American Thoracic Society. Eur Respir J. 2000;16:731-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Frey U, Stocks J, Sly P, Bates J. Specification for signal processing and data handling used for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur Respir J. 2000;16:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, Bisgaard H, Davis GM, Ducharme FM, Eigen H, Gappa M, Gaultier C, Gustafsson PM, Hall GL, Hantos Z, Healy MJ, Jones MH, Klug B, Lødrup Carlsen KC, McKenzie SA, Marchal F, Mayer OH, Merkus PJ, Morris MG, Oostveen E, Pillow JJ, Seddon PC, Silverman M, Sly PD, Stocks J, Tepper RS, Vilozni D, Wilson NM; American Thoracic Society/European Respiratory Society Working Group on Infant and Young Children Pulmonary Function Testing. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 849] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 16. | Medscape. CDC/NCHS Infant Weight for Length Percentiles (< 36 mo). [cited 1 May 2021]. In: Medscape [Internet]. Available from: https://reference.medscape.com/calculator/672/cdc-nchs-infant-weight-for-length-percentiles-lt-36-months. |
| 17. | van Meel ER, de Jong M, Elbert NJ, den Dekker HT, Reiss IK, de Jongste JC, Jaddoe VWV, Duijts L. Duration and exclusiveness of breastfeeding and school-age lung function and asthma. Ann Allergy Asthma Immunol. 2017;119:21-26.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Lodge CJ, Tan DJ, Lau MX, Dai X, Tham R, Lowe AJ, Bowatte G, Allen KJ, Dharmage SC. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 363] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 19. | Silvers KM, Frampton CM, Wickens K, Pattemore PK, Ingham T, Fishwick D, Crane J, Town GI, Epton MJ; New Zealand Asthma and Allergy Cohort Study Group. Breastfeeding protects against current asthma up to 6 years of age. J Pediatr. 2012;160:991-6.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Kim HS, Kim YH, Kim MJ, Lee HS, Han YK, Kim KW, Sohn MH, Kim KE. Effect of breastfeeding on lung function in asthmatic children. Allergy Asthma Proc. 2015;36:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Oh SS, Du R, Zeiger AM, McGarry ME, Hu D, Thakur N, Pino-Yanes M, Galanter JM, Eng C, Nishimura KK, Huntsman S, Farber HJ, Meade K, Avila P, Serebrisky D, Bibbins-Domingo K, Lenoir MA, Ford JG, Brigino-Buenaventura E, Rodriguez-Cintron W, Thyne SM, Sen S, Rodriguez-Santana JR, Williams K, Kumar R, Burchard EG. Breastfeeding associated with higher lung function in African American youths with asthma. J Asthma. 2017;54:856-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Gorlanova O, Appenzeller R, Mahmoud YS, Ramsey KA, Usemann J, Decrue F, Kuehni CE, Röösli M, Latzin P, Fuchs O, Soti A, Frey U; On Behalf Of The Bild Study Group. Effect of breastfeeding duration on lung function, respiratory symptoms and allergic diseases in school-age children. Pediatr Pulmonol. 2020;55:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Waidyatillake NT, Simpson JA, Allen KJ, Lodge CJ, Dharmage SC, Abramson MJ, De Livera AM, Matheson MC, Erbas B, Hill DJ, Lowe AJ. The effect of breastfeeding on lung function at 12 and 18 years: a prospective cohort study. Eur Respir J. 2016;48:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Cutrera R, Filtchev SI, Merolla R, Willim G, Haluszka J, Ronchetti R. Analysis of expiratory pattern for monitoring bronchial obstruction in school-age children. Pediatr Pulmonol. 1991;10:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Zedan M, Nasef N, El-Bayoumy M, El-Assmy M, Attia G, Zedan M, AlWakeel A, Kandil S, Laimon W, Fouda A. Does decline of lung function in wheezy infants justify the early start of controller medications? Indian J Pediatr. 2012;79:1176-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 455] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | van der Ent CK, Brackel HJ, van der Laag J, Bogaard JM. Tidal breathing analysis as a measure of airway obstruction in children three years of age and older. Am J Respir Crit Care Med. 1996;153:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Banovcin P, Seidenberg J, Von der Hardt H. Assessment of tidal breathing patterns for monitoring of bronchial obstruction in infants. Pediatr Res. 1995;38:218-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Lødrup Carlsen KC. Tidal breathing at all ages. Monaldi Arch Chest Dis. 2000;55:427-434. [PubMed] |
| 30. | Qi YY, Jiang GL, Wang LB, Wan CZ, Zhang XB, Qian LL. Lung Function in Wheezing Infants after Acute Lower Respiratory Tract Infection and Its Association with Respiratory Outcome. Chin Med J (Engl). 2017;130:4-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Lødrup Carlsen KC, Carlsen KH. Inhaled nebulized adrenaline improves lung function in infants with acute bronchiolitis. Respir Med. 2000;94:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Totapally BR, Demerci C, Zureikat G, Nolan B. Tidal breathing flow-volume loops in bronchiolitis in infancy: the effect of albuterol [ISRCTN47364493]. Crit Care. 2002;6:160-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Çelik E, Uysal P. Pulmonary function testing with tidal breath analyze technique is useful in predicting persistant small airway damage in infants with acute bronchiolitis. Pediatr Allergy Immunol. 2021;32:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Futrakul S, Deerojanawong J, Prapphal N. Risk factors of bronchial hyperresponsiveness in children with wheezing-associated respiratory infection. Pediatr Pulmonol. 2005;40:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |