INTRODUCTION
Lichen planus is a mucocutaneous disorder which involves various mucosal surfaces either alone or along with involvement of skin. It most commonly involves the oral mucosa when compared with other mucosal sites[1]. Oral lichen planus (OLP) is a disease of unknown etiology affecting stratified squamous epithelia[2]. In isolated OLP, only oral lesions will exist[3]. The disease affects 0.5%-2% of the general population. This disease most commonly involves middle aged patients of 30-60 years age group and females are more prone than males with a ratio of 1.4:1. OLP can be seen rarely in children and young adults[4,5]. OLP should be considered as a potentially malignant disorder because there is a relationship between oral cancer and OLP, although the degree of risk involved is variable[6].
The purpose of this review is to provide an update of the etiopathogenesis, clinical features, histological features, Diagnosis and management of OLP.
Clinical features of oral lichen planus
Oral Lichen planus was first described clinically by Erasmus Wilson in 1869 and histologically by Dubdreuilh in the year 1906[7]. Cutaneous lichen planus is recurrent, pruritic[8,9] and non-contagious[10]. Oral lichen planus rarely involves other sites like scalp, nails, esophagus, larynx and conjunctivae. OLP is gradual in onset and patients are unaware of the disease. Initially patients may present with roughening of oral mucosa, burning sensation and pain in oral mucosa to hot and spicy foods. Later red or white patches over the mucosa may appear which gradually progresses to oral ulcerations. The clinical history includes phases of remission and exacerbation[11].
The clinical presentation of oral Lichen planus resembles many other diseases. It can have many clinical presentations. In 1968, Andreasen divided OLP into 6 clinical forms: reticular, papular, plaque like, atrophic, erosive and bullous[12]. These forms may present either simultaneously or individually. Based on the predominant clinical morphology it will be labeled as specific form and the predominant morphology may change over time. Older individuals usually presents with more severe forms (erythematous/atrophic, erosive)[13].
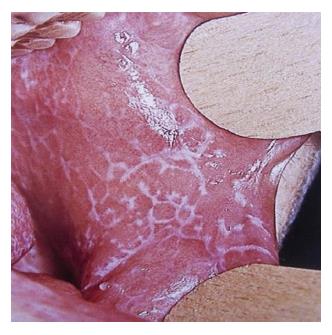
The clinical forms described by Andreasen were made simple by other authors who classified lichen planus grossly into three types: Reticular, atropic or erythematous and erosive[14]. The reticular form (Figure 1) is the most common type. It clinically presents as papules and plaques with interlacing white keratotic lines (wickham’s striae) surrounded by an erythematous border. Wickham’s striae are usually bilateral and seen on buccal mucosa, mucobuccal fold, gingiva and rarely on palate, tongue and lips. This type is reportedly more common in males than females and it is usually asymptomatic[15]. OLP usually present as a bilateral symmetrical lesion or involves multiple areas individually[16]. OLP involving the gingiva is termed as “desquamative gingivitis” which clinically manifest as a fiery red erythema of attached gingiva. OLP lesions which are associated with patchy brown melanin deposits in the oral mucosa are termed as inflammatory melanosis[5].
Figure 1 Reticular form of oral lichen planus.
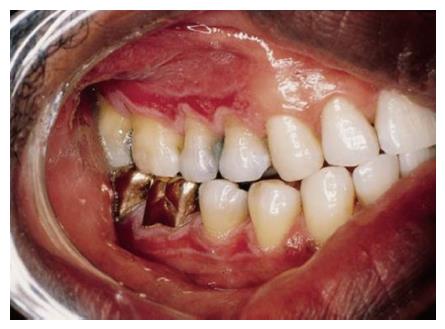
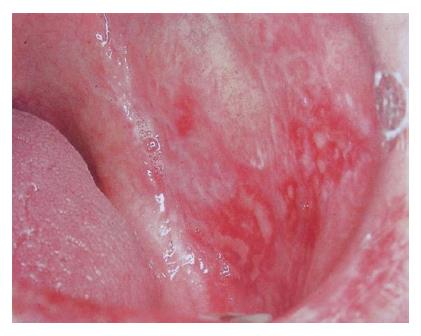
Reticular form of oral lichen planus is usually asymptomatic. Atrophic/erythematous (Figure 2) and erosive/ulcerative (Figure 3) lesions are symptomatic. Symptoms include mucosal sensitivity, burning sensation and continuous debilitating pain. Oral lichen planus lesions usually persist for many years. OLP patients have periods of exacerbation and quiescence. Periods of exacerbation are generally associated with psychological stress and anxiety and during this time there is increased erythema or ulceration with increased pain and sensitivity[5]. OLP resulting from mechanical trauma either during dental treatments or due to cheek biting is termed as koebner phenomenon[13].
Figure 2 Atrophic form of oral lichen planus.
Figure 3 Erosive form of oral lichen planus.
Malignant potential is high for atrophic and erosive forms of OLP[4,6], requiring regular follow up of patients. It should be done atleast 3 times in a year with more frequent examinations required for OLP with dysplasia. The symptoms of the disease such as burning sensation, loss of homogeneity in clinical appearance should be assessed thoroughly at each appointment and biopsy should be performed if required[17,18].
Etiology and pathogenesis
The exact etiology of this condition is unknown. Current literature suggests that T cell mediated immune mechanism is mainly implicated in the pathogenesis of OLP[5,13]. Pathogenesis of oral lichen planus may be antigen-specific and non-specific. Antigen-specific mechanisms include antigen presentation by basal keratinocytes and non-specific mechanisms include mast cell degranulation and matrix metalloproteinase (MMP) activation in OLP lesions. Both these mechanisms may combine which results in CD8+ cytotoxic T-cell accumulation in the superficial lamina propria followed by basement membrane disruption, intra-epithelial T-cell migration, and keratinocyte apoptosis. OLP chronicity may be due to deficient antigen-specific TGF-b1-mediated immunosuppression. This breakdown of normal oral mucosa could result in OLP[19].
Both endogenous and exogenous factors may cause cell-mediated immunity in a genetically susceptible patient and appears to play a major role in the pathogenesis of OLP[20]. The nature of the antigen implicated in OLP is uncertain, however numerous predisposing factors are known to induce OLP are identified. These are systemic medications, dental materials, chronic liver disease and hepatitis C virus, stress, genetics, tobacco chewing, Graft versus Host disease[16].
Systemic medications such as antimalarial drugs, non-steroidal anti-inflammatory drugs, antihypertensive agents, diuretics, oral hypoglycemic agents, beta blockers, pencilllins, sulfonamides, tetracyclines, heavy metals, thyroid preparations, antiretroviral medication have been reported to cause OLP[16,20-22].
The association of OLP with chronic liver disease was first suggested by Mokni et al[23] in 1991. Epidemiological evidences strongly suggest that Hepatitis C Virus may be an etiologic factor in OLP[24]. Association of OLP with several different autoimmune diseases such as alopecia areata, dermatitis herpetiformis, myasthenia gravis, etc. has been documented[20].
Periods of psychological stress and anxiety are associated with aggravation of OLP in most of the studies conducted so far[4,16,20,25,26]. Genetic predisposition also play a role in OLP pathogenesis[4,16]. Koebner phenomenon is a characteristic feature of cutaneous LP and is also observed in oral cavity. The erosive OLP lesions are most commonly seen in areas of trauma such as buccal mucosa and lateral surfaces of the tongue. These lesions may decrease in severity with the elimination of trauma[13,25]. Smoking, tobacco chewing, and betel nut chewing has been associated with the development of OLP in studies conducted in indian population[16,20]. Grinspan in 1963 found an interesting association between oral lichen planus, diabetes mellitus and hypertension, which he termed as Grinspan syndrome[27].
OLP is a T-cell mediated autoimmune disease in which the auto-cytotoxic CD8+ T cells trigger apoptosis of the basal cells of the oral epithelium. Initially keratinocyte antigen expression or unmasking of an antigen may occur followed by migration of T cells (mostly CD8+, and some CD4+ cells) into the epithelium. These migrated T cells are activated directly by antigen binding to major histocompatibility complex (MHC)-1 on keratinocyte or through activated CD4+ lymphocytes. In OLP, there will be up regulation of MHC-II expression along with increased number of Langerhan cells facilitating the antigen presentation to CD4+ cells, which activate CD8+ T cells through receptor interaction, interferon γ and IL-2. The activated CD8+ T cells trigger the apoptosis of basal keratinocytes by releasing tumor necrosis factor-α, granzyme B and by Fas–FasL mediated apoptosis. This results in loss of integrity of basement membrane. The MMP are principally involved in connective tissue matrix protein degradation[24].
DIAGNOSIS
The diagnosis can be made depending on the history, clinical and histopathological examination. However, in classical lesions, the diagnosis can be arrived based on clinical appearances (Wickham’s striae, erythematous area) only. When skin lesions are also present, the accuracy of diagnosis is strengthened[21,28].
Differential diagnosis of reticular OLP includes leukoplakia, lichenoid reactions, lupus erythematosus and graft vs host disease. The differential diagnosis of erosive OLP includes chronic cheek chewing, hypersensitivity mucositis, chronic candidiasis, discoid lupus erythematosus, squamous cell carcinoma, benign mucous membrane pemphigoid, pemphigus vulgaris and erythema multiforme[21,28].
It is sometimes difficult to clinically diagnose “desquamative gingivitis” when lesions in other sites are absent. Mucous membrane pemphigoid, pemphigus vulgaris and OLP may present as desquamative gingivitis of very similar clinical aspect[29]. Biopsy is the gold standard for the diagnosis of OLP. The biopsy should include marginal tissue containing both lesional and normal-appearing areas. OLP can be distinguished from other chronic white or ulcerative oral lesions including reactive keratoses, chronic hyperplastic candidosis, epithelial dysplasia, discoid lupus erythematosus, gastro-intestinal disease or anemic states with the help of histopathological examination[5,30].
Direct and indirect immunofluorescent studies, direct oral microscopy and enzyme linked immunosorbent assays can be helpful in reaching a diagnosis for problematic cases and to exclude malignancy. Among these, the most important being the Immunofluorescent studies which are helpful in making a diagnosis in cases of OLP that may resemble other diseases[20,28,29,31,32].
Histopathological features
The histological features of OLP are similar to cutaneous lichen planus. These were first described by Dubreuill in 1906 and later by Shklar[16,33]. The histopathological features of OLP are characterized by a dense sub-epithelial lympho-histiocytic infiltrate, increased numbers of intra-epithelial lymphocytes and degeneration of basal keratinocytes. Degenerating basal keratinocytes form colloid bodies which appear as homogenous eosinophilic globules[5]. Colloid/civatte/cytoid/hyaline bodies are round and are seen either in the lower layers of the epithelium or within the upper layers of the connective tissue. These represent degenerated epithelial cells or phagocytosed epithelial cell remnants within macrophages[33]. The ultrastructure of colloid bodies revealed that these are apoptotic keratinocytes and the end-labeling method demonstrated DNA fragmentation in these cells. Epithelial basement membrane changes are also common in OLP and consist of breaks, branches, duplications and disruption of the basal keratinocyte anchoring elements (hemidesmosomes, filaments and fibrils). These changes like degeneration of basal keratinocytes, disruption of the epithelial basement membrane and basal keratinocyte anchoring elements together lead to produce weakness at the epithelial-connective tissue interface which results in histological cleft formation (Max-Joseph space) and blisters in oral mucosa. Parakeratosis, acanthosis and “saw-tooth” rete peg formation may be seen[5].
Absence of basal cell liquefaction, atypical cytomorphology, heterogeneous population of infiltrate, nucleus enlargement, blunted rete ridges, increased mitotic figures, absence of civatte bodies, abnormal keratinization will help to rule out the definitive diagnosis of OLP[34]. One study suggested that Colloid bodies can be helpful to differentiate oral lichen planus from oral lichenoid reaction. The location of colloid bodies is either in epithelium or connective tissue but usually close to the epithelium-connective tissue junction in case of OLP, while these were mostly seen in lower spinous layer of epithelium in case of oral lichenoid reaction[35]. Certain times, the histopathological features are equivocal or do not agree with clinical picture. Another biopsy may be necessary to confirm the diagnosis of OLP by immunofluorescence[21].
Direct Immunofluorescent examination of tissue in case of OLP demonstrates deposition of fibrinogen along the basement membrane zone[21] and colloid bodies stain for immunoglobulins IgA, IgG, and IgM[33]. Although the existence of fibrin deposition at the mucosal submucosal interface, within vessels and the presence of colloid bodies is highly sensitive for a diagnosis of LP, but it lacks specificity[31]. The sensitivity of direct immunofluorescence is positive for 65.8% of the patients with OLP[28]. Direct immunofluorescence is most sensitive when the tissue taken from buccal floor, upper labial mucosa, hard palate and mucosa of the cheek. It is less sensitive when the tissue taken from the gingiva and the dorsum of the tongue. Use of punch biopsy technique instead of conventional biopsy is better to detect the disease in direct immunofluorescence[28]. There is no difference in the sensitivity of direct immunofluorescence between biopsies performed in perilesional tissue (radius of up to 1 cm from the lesion) and distant tissue (radius greater than 1 cm). This occurs because the immune deposit may be present in the entire oral tissue, not only close to the lesion. Distant sites also provide more sample options when tissue extraction is difficult[28].
Direct oral microscopy technique is noninvasive which helps in clinical examination of oral cavity. This is based on the principle of colposcopy used by gynecologists and dermoscopy used by the dermatologists. This is used in a study conducted by Drogoszewska et al[32] for determing the site for biopsy and for clinical diagnosis of OLP. The principle behind usage of oral microscopy is to reveal precancerous lesion of oral mucosa in subclinical phase in order to begin the treatment as early as possible and to prevent malignant transformation. In their study they have done the direct oral microscopy by using a Leisegang colposcope, model BG/LED Y/C type 3ML. The results of their study showed that direct oral microscopy provides an alternative to clinical examination with the naked eye for choosing most appropriate biopsy site so that it is helpful in early detection of malignant changes of OLP and helps in early intervention of malignancy[32].
Management of OLP
As the immunopathogenesis of OLP is unclear, the clinical management of OLP poses considerable difficulty to the dermatologist and oral physician[36]. Currently there is no cure for oral lichen planus[2,13,21,37].
Reticular OLP is often asymptomatic and require no treatment[4,16,36], whereas atrophic, erosive forms can cause symptoms. Symptomatic OLP require therapy and treatment of OLP should be initiated after careful evaluation of patient’s medical history, psychological state, treatment compliance and possible drug interactions while evaluating the cost effectiveness of any treatment modality[36]. When a medication is suspected that it is causing oral lichenoid lesions, then that drug should be discontinued[36,37]. OLP with involvement of the gingiva may be associated with deposition of plaque and calculus. Maintaining good oral hygiene by effective plaque control measures like supragingival scaling, oral hygiene instruction is essential which can enhance healing of the lesions and also decreases the painful symptoms of OLP[36,38]. Mechanical trauma of dental procedures, rough dental restorations, friction from sharp cusps and poorly fitting dental prostheses can be exacerbating factors of symptomatic OLP and these factors should be corrected[16,36].
Transformation of OLP to squamous cell carcinoma is most commonly seen in cases of OLP involving the palatal arch, tongue, labial mucosa and gingiva. Therefore, it is essential to differentiate lesions of OLP as OLP with dysplasia and without dysplasia[34]. It has been suggested that regular follow-up of patients with OLP without dysplasia should be performed for at least every 4 mo. More frequent examinations should be considered for patients of OLP with dysplasia[34]. Before initiating treatment for OLP, it should be confirmed by biopsy. Oral candidiasis can be caused by different treatment modalities used for OLP, therefore it is important to take care of oral candidiasis before initiating treatment and also during treatment of OLP[21]. Current treatment modalities are palliative and have varied efficacy. The usage of specific medication depends on the potential benefit vs side effect and it differs from patient to patient based on patient condition and physicians choice[13]. No treatment modality has proved to be curative for OLP. Therefore different drugs are used in a single patient which suggests the insufficiency of any one agent to provide relief to the patient[2]. Various treatment regimens are available for the management of symptomatic oral LP.
Treatment of inflammatory/symptomatic OLP
Corticosteroids: Corticosteroids till today remain the first line of treatment for OLP. These drugs can be administered topically, intralesionally or systemically.
The most widely accepted treatment of OLP involves use of topical or systemic corticosteroids[2]. Topical corticosteroids remain the mainstay and first line of OLP treatment[13]. The combination of systemic and topical steroid therapy is often effective in certain severe cases of OLP. Localized OLP lesions are treated with topical steroids either in the form of ointment or paste which can be applied two to four times daily after meals. Topical preparations are also available as lozenges or as a mouthwash or through an inhaler with a special adapter. The dosage and specific preparations are based on the individual patient’s needs. Steroid mouthrinse twice daily after food is effective method of treating generalized oral lesions[5,37,39]. Commonly used preparations include 0.025% or 0.05% clobetasol propionate gel, 0.1% or 0.05% betamethasone valerate gel, 0.05% fluocinonide gel, 0.05% clobetasol butyrate ointment or cream, 0.1% triamcinolone acetonide ointment[16,21,36,39-41], an aqueous suspension of triamcinolone acetonide 0.1% or 0.3% or 0.5% as oral rinse[37,42], dexamethasone elixir (5 mL of a 5 mg/5 mL suspension) as a mouth rinse[13] or 0.1% mouthwash[43], Hydrocortisone hemisuccinate in aqueous solution, betamethasone valerate pellets or aerosol or clobetasol propionate mouthwash[36,40].
Patients are adviced to apply a thin layer of the prescribed topical corticosteroid, 3 times a day. The gel or ointment can be applied either directly or indirectly by mixing with equal parts of Orabase, a gelatin–pectin–sodium carboxymethylcellulose-based oral adhesive paste which facilitates adhesion to the gingival tissues. The choice of delivery vehicle can be changed depending on clinician and patient preference. Oral application with a gel preparation is superior compared to other routes of administration. In patients with widespread symptomatic lesions, mouthwashes and aerosols are advised as direct mucosal application of topical medication will be uncomfortable to the patient. Patients should be instructed to gargle with 5 mL of the solution for 2 min after meals and at bedtime[21]. The topical steroid application is superior compared to systemic administration because of few side effects. Adverse effects include discomfort on application, thinning of the oral mucosa and candidiasis. Topical preparations of more potent corticosteroids can cause adrenal suppression. The signs and symptoms of OLP are usually improved within 8 wk of therapy with the use of topical steroid preparations[21,40]. Prolonged use of topical steroids mainly leads to development of oral candidiasis, so use of antifungal agents along with topical steroids is recommended. Fungal cultures also should be taken before, during and after the treatment[40,44].
Overall, topical steroids are used as a gel, cream, ointment with orabase, mouthwash, oral rinse, etc. The efficacy of the different topical steroid formulations are shown different results in various studies[36,40-44].
Persistent localized erosive OLP lesions are treated with Intralesional and perilesional injection of steroids with caution. Use of local anaesthetic with the preparation reduces the pain during injection. Candidiasis and atrophy of tissue are potential local complications. Intralesional injections of dexamethasone, hydrocortisone, triamcinolone acetonide, and methyl prednisolone are generally used[2,4,5,13,21,29,36,37,39].
Systemic corticosteroids should be reserved for diffuse erosive OLP, multisite disease and generalized atrophic or erosive OLP that do not respond to topical therapy. Depending on the severity of the disease, doses of prednisone 30-60 mg are given once daily for two to four weeks[36,39]. These drugs should be gradually tapered and potential adverse effects should be monitored during the treatment[13]. Clinical improvement of the OLP lesions is usually seen in majority of patients undergoing systemic prednisone therapy. Topical agent can be given in patients who are using prednisone once control is established[2].
Concurrently prescribing levamisole (150 mg/d) with prednisone will reduce the dose of prednisone. Use of Levamisole and prednisolone 25 mg/d for 3 consecutive days each week for 4-6 wk showed beneficial results in the management of erosive OLP[45,46].
Contraindications of steroid therapy include Hypersensitivity, hypertension, viral infection, tuberculosis, diabetes mellitus and stomach ulcers[5].
In summary, most of the patients can be managed with corticosteroids. Use of Topical or intralesional or systemic steroid preparation is based on severity of the disease, systemic condition and adverse effects during the treatment. Intralesional agents are used in cases of ulcerations which do not respond to topical agents. Systemic agents are restricted for multisite disease, diffused disease and for OLP lesions which do not respond to topical agents.
Calcineurin inhibitors
Calcineurin is a protein phosphatase which activates transcription of Inter Leukin-2 there by stimulates the growth and differentiation of T-cell response. Cyclosporine, tacrolimus and pimecrolimus are calcineurin inhibitors are generally used in treatment of OLP[24].
Cyclosporine A is an immunosuppressive agent which is beneficial in cutaneous lichen planus[36]. Cyclosporine (100 mg/mL solution, 5 mL swish and spit three times daily) can be used as a mouth rinse in OLP patients who do not respond to topical corticosteroids[47-49]. Oral Cyclosporin A (5 to 6 mg/kg per day) is very effective in recalcitrant severe forms of the disease[48]. Recent studies compared the efficacy of cyclosporine solution and triamcinolone acetonide 0.1% in orabase in oral lichen planus lesions, these studies concluded that cyclosporine was not effective when compared with triamcinolone acetonide 0.1% in orabase[50,51]. Side-effects with cyclosporine include transient burning sensation, itching, swelling lips and petechial haemorrhages. These side effects, cost of the drug and also questionable efficacy of cyclosporine limits its use in OLP[49-51].
Tacrolimus is a potent immunosuppressive agent which can be used in topical form that can control symptoms of refractory erosive OLP. Studies showed that Tacrolimus ointment 0.1% is well tolerated and it is very effective in erosive OLP that did not respond to topical steroids. Most common adverse effect is local irritation due to burning sensation. Tacrolimus can be used as safe alternate to steroids when the lesions are resistant to the conventional treatment as there are less adverse effects with this drug. Topical tacrolimus helps to release the stress and improves the quality of life of patients suffering from OLP. Topical tacrolimus should be used for short period of about one month, as relapse of the lesions are seen within 6 to 12 mo of treatment cessation. Therefore prolonged or intermittent use of topical tacrolimus ointment in patients with symptomatic OLP may be recommended with constant monitoring. The United States Food and Drug Administration have recommended tacrolimus to be used for short periods of time because of a potential cancer risk from prolonged use. The efficacy of usage of tacrolimus remains to be clearly established in large, well-designed clinical studies[52-56].
Studies using 1% topical cream of pimecrolimus showed significant results in reducing ulceration and inflammation of lesion with better tolerance and relief from pain. Pimecrolimus has significant anti-inflammatory activity with low systemic immunosuppressive potential. Burning sensation is the common complaint experienced by the patients with the use of pimecrolimus[52,57,58]. Ibrahim et al[58] also observed the decresased expression of Fas in the immunohistochemical specimens after the treatment with pimecrolimus. Fas is an important molecule which is involved in apoptosis.
Retinoids
Various Topical retinoids such as 0.1% vitamin A, 0.05% tretinoin ointment, isotretinoin 0.1% gel, etretinate and fenretinide, with their immunomodulating properties are effective in OLP. Irritation, burning sensation are commonly observed with application of topical retinoids. Temporary reversal of white striae can be achieved with topical retinoids[13,24,36,42]. Systemic retinoids such as isotretinoin, temarotene, tretinoin have been used in cases of severe lichen planus with varied degree of success. The positive effects of retinoids should be weighed against their significant side effects[36].
Azathioprine
Azathioprine has potent immunosuppressive effects, can been used in the treatment of erosive OLP. There is a risk of malignancy with the long-term use of this drug. Azathioprine cannot be considered as better alternative to systemic steroids alone or systemic steroids in conjunction with topical steroids[36,39].
Lycopene
Lycopene is a fat-soluble carotenoid. It has antioxidant activity, also acts by inhibition of cancer cell proliferation and interference with growth factor stimulation. It has shown to be effective in the management of oral leukoplakia and in chemoprevention of oral cancer. Supplementing with 8 mg/d of lycopene for 8 wk showed favorable results of reduced burning sensation and decreased signs and symptoms of OLP in patients, in a placebo controlled study[59].
Aloe vera
Aloe vera (Aloe barbadensis Miller) is cactus like plant and it is a member of the Liliaceae family. There are few studies conducted using aloe vera gel or aloe vera in a aqueous suspension and it is also compared with the triamcinolone gel which showed beneficial effects in relieving symptoms of OLP. Further studies are required to prove the efficacy of aloe vera in the treatment of OLP[60-62].
Hyaluronic acid
Hyaluronic acid (HA) is a linear polymer of glucuronic acid, N-acetylglucosamine disaccharide which helps in tissue healing. HA in the form of 0.2% gel showed transient improvement in decreasing the soreness associated with OLP in a placebo controlled double blind study[63].
Bacillus Calmette-Guerin polysaccharide nucleic acid
Bacillus Calmette-Guerin polysaccharide nucleic acid (BCG-PSN) is the third-generation BCG extract with various immunologic active materials including polysaccharide and nucleic acid. It has the ability to regulate the Th1/Th2 cytokine secretion in peripheral blood mononuclear cells (PBMC) of the OLP patients. In a study which compared the effectiveness of intralesional 0.5 mL BCG-PSN injection every alternative day with 10mg triamcinolone injection every week for about 2 wk showed equal effectiveness of both agents for erosive OLP. So BCG-PSN injections could be a promising therapeutic alternative for erosive OLP, especially for those insensitive or even resistant to glucocorticoids[64].
Anthocyanins
Anthocyanins are polyphenolic groups which block the spread of free radicals and are considered the main antioxidants of the plant kingdom. The extracts of grape seeds and grape skins contain anthocyanins. These are also present in other fruits, vegetables, chocolate, tea. Rivarola de Gutierrez et al[65] conducted a prospective, non-randomized study in 52 patients. Anthocyanins were administered in 100 mg/doses diluted in 5 mL of water, mouth rinses, during 5 min and spit, three times a day in 26 patients and control group received CP-NN cream (100 g of commercial preparation containing: 17-clobetasol propionate (micronized) 0.050 g, Neomycin (as sulfate) 0.350 g; Nystatin (micronized) 100.000 U/g. This was applied three times daily locally on lesions. There is improvement in the pain relief in patients with anthocyanins when compared with patients receiving CP-NN treatment[65].
Pharmacological agents like dapsone, doxycycline, griseofulvin, hydroxychloroquine sulphate, adalimumab, mycophenolates, efalizumab, cyclophosphamide, hydrochloroquine, phenytoin, mesalazine, interferon, glycyrrhizin, amitryptyline, amlexanox, curcuminoids, thalidomide, ignatia, purslane reported in the treatment of OLP. However, the main concerns with these are local and systemic side effects and lesion recurrence following withdrawal of treatment as fewer studies are reported with these agents. The cost-benefit and the safety profile of these drugs have to be more carefully considered and randomized controlled trials of these agents in larger groups of patients with OLP are recommended to clarify their effectiveness and safety profile[2,24,29,34,36,48,66,67].
NON-PHARMACOLOGICAL MODALITIES
Phototherapy or light therapy or heliotherapy has been widely used as an alternative therapy for the management of OLP. Different kinds of phototherapy include Ultra Violet (UV) phototherapy, photodynamic therapy and lasers[68].
UV Phototherapy
UVA treatment usually comprises UVA radiation (long wave length 315-400 nm Ultra Violet light) combined with a sensitizer (a chemical that increases the effect of UVA) called 8-methoxy psoralen. This form of treatment is referred to as PUVA (psoralen + UVA). To avoid PUVA side effects, photosensitization with topical 0.01% trioxsalen can be used for the treatment. Various side effects include nausea, eye symptoms, dizziness, paraesthesia and headache. PUVA therapy may be useful for severe forms of erosive OLP that do not respond to conventional treatment. Photochemotherapy with solar radiation has been introduced as an effective and cheaper alternative to PUVA. PUVA therapy has shown oncogenic potential, therefore it is not widely used and is discontinued for the treatment of OLP[4,16,36,68].
Photodynamic therapy
Photodynamic therapy (PDT) uses a photosensitizing compound (photosensitizer) which is activated at a specific wavelength of laser light which is known to destroy the targeted cell. PDT has shown positive results in management of head and neck tumors. The immunomodulatory activity of PDT also helpful in controlling the inflammation in OLP[2,16]. PDT with the use of different photosensitizers (methyl 5-aminolevulinate, phenothiazine dye methylene blue, Photolon®, a novel chlorin e6-derived photosensitizer) are used for the treatment of OLP and showed promising results in the treatment of OLP. The only PDT side effect reported was photosensitivity. However, further well-designed randomized controlled trials with larger numbers of patients with long follow-ups will be needed to evaluate the effectiveness of PDT in the treatment of OLP[68,69].
Lasers
Use of lasers for treatment of OLP is not recommended as the first choice of treatment, but it is suggested for use in patients who are unresponsive to topical corticosteroids[68]. Low-level laser therapy (LLLT; photobiostimulation, photobiomodulation) has physiological effects such as vasodilatation, enhancement of blood flow and lymph drainage, increased cellular metabolism, aggregation of prostaglandins, immunoglobulins and lymphokines, resulting in reduction of inflammation, immune response, and pain. Various low level lasers with different wavelenths, intensities, powers, durations, number of sessions, and therapeutic approaches (with or without tissue absorbent) have been used to treat oral lichen planus[70]. Few studies also reported the use of CO2 laser, excimer laser for the treatment of OLP[68,71,72]. Use of LLLT, CO2 lasers and excimer lasers are to be confirmed in well-designed controlled trials with large number of patients[68].
Surgery
Surgical excision has been recommended for isolated plaques or non-healing erosions as it may cure the disease and also provides tissue for histopathologic examination. Surgical excision is not recommended in erosive and atrophic forms because of erosions in these forms and also due to recurrence of inflammation. Cryosurgery has been successful in cases of erosive OLP resistant to other treatment modalities. Recurrences are common with the use of cryosurgery[2,37,36].
Treatment of dysplastic OLP
The inflammatory component of OLP is treated with various above mentioned methods and additional approaches are required for assessing and treating dysplastic component in these cases[34].