Published online Nov 20, 2014. doi: 10.5321/wjs.v3.i4.30
Revised: November 3, 2014
Accepted: November 17, 2014
Published online: November 20, 2014
Processing time: 74 Days and 6.5 Hours
Pierre Robin sequence (PRS) is a triad of micrognathia, glossoptosis, and cleft palate that results in an obstruction of the airway on inspiration and impeding feeding. The tongue of infants with PRS fall back toward the posterior pharyngeal wall (glossoptosis) due to receding chin produced by mandibular micrognathia (small jaw) or retrognathia. This causes a serious condition with potentially severe, life-threatening airway obstruction. If untreated, this problem can lead to exhaustion, cardiac failure, and ultimately death, especially during the early months of life. Actually, in the majority of PRS infants, these symptoms can be managed by placing the infant in the prone position until adequate growth of the jaw occurs. If this type of treatment fails, the infant then should be considered for other conservative therapies or surgical interventions. This paper reviews surgical interventions such as tongue-lip adhesion, mandibular traction, mandibular distraction, tracheotomy and conservative orthodontic approaches, and presents a baby treated successfully with an orthodontic appliance.
Core tip: Pierre Robin sequence is a severe congenital condition characterized by triad of micrognathia, glossoptosis, and cleft palate. Glossoptosis and micrognathia may result in obstruction of the airway on inspiration and impeding feeding. If untreated, this problem can lead to exhaustion, cardiac failure, and ultimately death, especially during the early months of life. This paper give detailed reviews supported with figures for surgical interventions and conservative orthodontic approaches, and also presents a baby treated successfully with an orthodontic appliance. Orthodontic nutrition plate appears to be a viable alternative in treatment of Pierre Robin sequence to surgical treatment modalities that are more aggressive in nature.
- Citation: Kılıç SC, Kılıç N, Oktay H, Kiki A. Pierre Robin sequence from orthodontic and surgical perspective. World J Stomatol 2014; 3(4): 30-37
- URL: https://www.wjgnet.com/2218-6263/full/v3/i4/30.htm
- DOI: https://dx.doi.org/10.5321/wjs.v3.i4.30
Infants with congenital craniofacial anomalies often display associated severe mandibular hypoplasia causing obstruction of the airway through retro-positioning of the tongue-base into the posterior pharyngeal airway. Pierre Robin[1], a French Stomatologist at French School of Stomatology, defined a new syndrome in 1923 which involves mandibular micrognathia, glossoptosis and respiratory distress. In 1934, Robin[2] revised the characteristics of the syndrome and included cleft palate as an additional factor that could be present. An incomplete cleft of the palate is associated with the Robin sequence in approximately 50% of these patients. Formerly, it was named Pierre Robin syndrome, anomalad, or complex. Today, it is referred as Pierre Robin sequence because the underdeveloped lower jaw initiates a sequence of events (i.e., the micrognathia resulting in glossoptosis, which prevents the palatal shelves to fuse at intra-uterin growth)[3].
The main clinical problems faced by clinicians include upper airway obstruction and feeding difficulties. The tongue of infants with pierre robin sequence (PRS) fall back toward the posterior pharyngeal wall (glossoptosis) due to receding chin produced by mandibular micrognathia (small jaw) or retrognathia. This results in an obstruction of the airway on inspiration and impeding feeding. If untreated, this problem can lead to exhaustion, cardiac failure, and ultimately death, especially during the early months of life[4].
In normal intra-uterine growth and development, the tongue moves downward and goes away from the roof of the mouth between nine to eleven weeks of gestation. This movement of the tongue allows an accurate space for two palatal shelves to shift towards to the midline and become integrated (palatal closure). In PRS cases, however, micrognathic or retrognathic lower jaw results in failure of the tongue to descend and thus keeps the tongue positioned higher in the mouth than normal, thereby interfering with the normal closure of the palate. As a conclusion, a wide U-shaped cleft occurs in the soft palate, and sometimes it may involve posterior part of hard palate. To varying degrees, glossoptosis contributes to tongue-base obstruction, sleep apnea, and respiratory distress. Additional factors such as tongue prolapse into the cleft area, lack of voluntary control of the tongue musculature, and negative pressure pull of the tongue into hypopharynx may also contribute to dysphagia[5]. Three pathophysiological theories exist to explain the occurrence of micrognathia: mechanical or positional theory, neurological maturation theory, and dysregulation theory. The most widely accepted one is mechanical or positional theory although the etiopathogenesis of mandibular micrognathia itself remains a matter of considerable debate. According to mechanical or positional (compression) theory, mandibular micrognathia is a result of intrauterine molding against sternum, possibly associated with oligohydramnios[6]. If this theory is true, it would appear logical to expect some rebound growth of mandible shortly after birth, reducing facial convexity and perhaps allowing the mandible to “catch up” with maxilla.
Since the major symptoms included glossoptosis, upper airway obstruction and feeding difficulties are definitely or at least mostly related to micrognathia, clinicians’ special interest are focused upon growth of and/or lengthen the mandible in these infants. Actually, in the majority of PRS infants, these symptoms can be managed by placing the infant in a prone position until adequate mandibular growth occurs. This traditional treatment method causes the jaw and tongue to fall forward, opening the airway[7].
If this type of treatment fails, the infant then should be considered for other conservative therapies and/or surgical interventions. Conservative interventions can be performed with different orthodontic methods until adequate mandibular growth occurs. Surgical options include tongue-lip adhesion (a procedure to pull the tongue forward), release of the musculature of mouth floor, mandibular traction, and mandibular distraction or tracheotomy[3].
Surgical interventions are really more aggressive in nature. Currently there are no undisputed practical guidelines for surgical management of airway obstruction in patients with PRS who fail conservative treatment[8]. The PRS literature is unclear as to which surgical intervention is most effective. According to Mackay[9], it is even unclear if there is a ‘‘one surgery fits all’’ type of approach that is superior to a more adaptable and patient-dependent approach. Each surgical intervention has significant potential complications that must be considered. Other factors such as surgeon’s training and experience may also play key roles in decision process[9]. Level and severity of airway obstruction or presence of multiple levels of airway narrowing demonstrated clinically and endoscopically, should guide the intervention[5].
Tracheostomy is a surgically created opening through the neck into the trachea (breathing tube) for the purpose of assisting breathing. With the exception of patients who are seen as candidates for a first-line surgical therapy by some surgeons, the traditional approach in the infants with PRS is tracheostomy[10]. Tracheostomy is the definitive and often a reserved procedure for the treatment of airway obstruction of patients with PRS whose condition fails to respond to other measures. It should be applied particularly for the patients with lower airway obstruction who require chronic ventilator support[11].
Tracheostomy may be associated with frequent and serious adverse effects, complications, and even death[12,13]. Up to 60% of the infants undergoing tracheostomy may experience some type of complications such as suprastomal granulation and collapse, tracheal stenosis, tube obstruction, fistulas, accidental decannulation, creation of false passages, cellulitis, neck scarring and loss of airway[12-14]. Recurrence of airway obstruction or feeding difficulties may also occur following tracheostomy.
As stated previously, although tracheostomy is still a first-line surgical therapy for some surgeons and the technique improved over the last 20 years, morbidity and mortality associated with tracheostomy are undeniable. This explains why it has now become a last resort for the treatment of PRS[15].
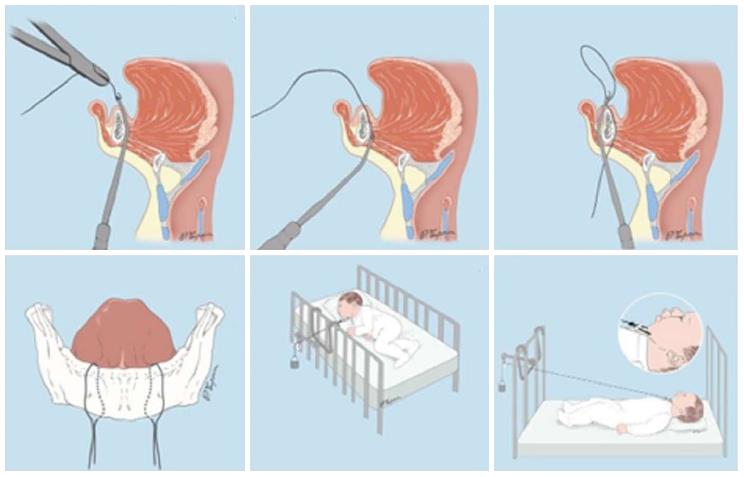
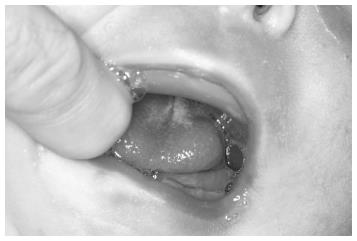
Introduction of this technique to the literature is far away up to approximately 80 years ago[11]. Documentation of high incidence of temporomandibular joint ankylosis was the primary reason why the first attempts regarding mandibular traction by wires were abandoned[16]. Although this technique is currently gaining great interest and popularity among surgeons[17,18], it is considerably aggressive in nature and requires an intensive care. Mandibular traction is accomplished by positioning two circum-mandibulary wires on both sides of the symphysis under local anesthesia (Figure 1). Continuous mandibular tractions are performed by using weights ranged from 50 to 200 g, except feeding[17-19]. Duration of the traction therapy has been reported to vary between 26.6[17] and 40[18] d. Position of the infant is changed every 2 h during this period[18].
The data obtained from the results of these studies suggest that mandibular traction with wires may be an effective treatment for upper airway obstruction with no major complication. The procedure immediately alleviates patients’ respiratory problems and apnoea[17-19]. The piercing of the skin by traction wires may cause small scars on the chin.
Glossopexy or tongue-lip adhesion can be effective in relieving tongue-base obstruction. In this technique, anterior ventral part of the tongue is anchored to lower lip (mucosa plus or minus muscle), and posterior part to mandible. Main adverse outcomes are dehiscence and need for subsequent procedures[20]. Other complications of tongue-lip adhesion are infection, submaxillary duct obstruction, lip scarring, postoperative obstructive sleep apnea, severe dysphagia, and growth retardation[20,21]. Although some authors have observed weight gain and improved feeding after glossopexy[22], tongue-lip adhesion may result in airway obstruction or feeding difficulties due to altered tongue mobility and swallowing.
Distraction osteogenesis (DO) is the surgical technique in which new bone formation is induced by gradual separation of bony segments after an osteotomy. This technique increases pharyngeal airway size by gradual mandibular lengthening. Distraction osteogenesis generates not only new bone but also new soft tissue in the distraction area. Mandibular distraction osteogenesis is becoming more common in management of the infants with PRS, and overcorrection of mandibular position is currently recommended to maximize the mandibular length and airway size[23].
The first maxillofacial application of DO was carried out by McCarthy[24], in 1992 when he used this method to lengthen a congenitally hypoplastic mandible. It is commonly used in medicine and dentistry for mandibular advancement in very severely affected (syndromic) children. In this regard, now, it gained common use to treat PRS infants. This procedure involves bilateral mandibular osteotomies and the placement of distraction devices (Figure 2)[25].
External or internal devices can be used, but both have pros and cons. External devices are easy to adjust and remove but can be dislodged and are associated with scarring. Internal devices are usually better tolerated but require repeat dissection for removal under general anesthesia. Activation of the distractor is usually done at a rate of 1 mm per day. However, distraction can be carried out at the rate of 1.5 mm per day in infants because of their fast healing response[26].
The fact that three-dimensional computed-tomography analysis indicates an increased mandibular length and volume after distraction may explain the airway improvement in the children who undergo MDO[27,28]. In a recent paper by Pfaff et al[28], the mean increase in the mandibular volume following distraction was measured as 113.3%. Denny et al[16] evaluating the effects of mandibular distraction on very young patients (from 3 mo to 8 years of age) with congenital micrognathia showed a normalization in the maxillo-mandibular relationships and 67.5% increase in cross-sectional area of the airway. Rachmiel et al[29] evaluated eighteen patients (between 6 mo and 14 years of age) with hypoplastic mandible and glossoptosis and found a mean of 22 mm forward mandibular elongation, an increase in SNB angle and pharyngeal airway after mandibular distraction.
These very short-term reports demonstrated favorable mandibular growth following distraction. Long-term effects of this procedure, however, on mandibular and also facial growth are not subjected to any research and remain unanswered due to being a relatively new procedure in infants and young children. In addition, a lot of severe complications regarding MD have been reported, which include wound infections, facial cellulitis, temporary paresthesia, facial nerve injury, scarring, cheek abscess, open bite deformity, tooth bud injury, jaw deformity, and dentigerous cyst formation[30]. As reported by some authors, the most common complication is loss or malformation of permanent teeth at a rate of 21%[31].
It is well known that PRS newborns often suffer from serious or even life-threatening airway obstructions in the respiratory tract resulting from anatomic malformations (mandibular micrognathia, glossoptosis and potentially a median cleft palate). Correction of the infant’s micrognathia and associated glossoptosis is possible by the previously mentioned interventions. Besides these treatment alternatives, orthodontists use various palatal plates and function-stimulating devices which enable the physicians to refrain from invasive surgery.
Traditionally, pioneer orthodontic plates used in PRS, also called feeding obturators, were used to facilitate feeding because it was assumed that feeding difficulties in these children were related with sucking inability due to the cleft[32]. These plates were designed to obturate the cleft area and close the opening between oral and nasal cavities. They created an artificial non-cleft palate which aided extraction of milk from a nipple. They successfully used to facilitate feeding, reduce nasal regurgitation, and shorten the time required for feeding[32].
Currently, function-stimulating devices have gained popularity among orthodontists and are commonly used for PRS infants. These devices can stabilize the infant’s vital parameters and ensure that it can be adequately fed during the appliance is placed. It is assumed that moving the tongue forward by a device that incorporates a tongue retaining and stimulating extension part result in mandibular growth promotion and thus orofacial musculature harmonization[4,33].
This kind of device was firstly introduced by Hotz et al[34] in 1982, and this appliance was later modified by Buchenau et al[35] in 2007 and called as “Pre-Epiglottic Baton Plate”. This palatal plate was made from a compound soft and hard acrylic covering both whole palate including alveolar ridges and velar extension approximately 2 to 3 cm in length, and a wire structure was added to extending acrylic in severe cases. The position of velar extension was endoscopically inspected and adjusted. According to these authors, this appliance reduced apnea indices of PRS infants by 71%[35]. In 2011, Bacher et al[36] introduced a new plate with velar extension, which was quite identical to the Pre-Epiglottic Baton Plate. This appliance stimulated mandibular growth and resolved airway obstruction by forward positioning of the tongue and mandible by applying posterior pressure on the root of tongue.
In 2006, “Tübingen soft palate plate” was described in the German literature by Brosch et al[37]. This appliance includes three parts: an acrylic palatal plate, an adjustable velar spur connected to the palatal plate with wires, and two frontal wire bows to keep the appliance in stable position with extra-oral fixation by applying adhesive tapes in the cheek and nose area.
In 2007, Oktay et al[4] introduced a modified nutrition plate including palatal plate and adjustable pharyngeal wire extension covered with an acrylic button. The rest of this paper describes this plate and a baby treated with this appliance. The following text and figures reproduced with kind “written permission” of the publisher.
A newborn girl with complaints of cleft palate, malnutrition, and respiratory distress was brought to the Pediatric Department at Research Hospital of the Faculty of Medicine in Atatürk University. The patient was diagnosed with Pierre Robin sequence and oxygen was provided to her so that the cyanosis in her legs and arms could be eliminated. In addition, a nasogastric catheter was inserted for nutrition. After the general condition of the patient improved, she was transferred to the Department of Orthodontics at the Faculty of Dentistry in Atatürk University for consultation and fabrication of a nutrition plate.
The mother stated that the baby was her first child. The mother had used some medicine for pharyngitis in the 3rd month of pregnancy and had a traffic accident in the 28th week of pregnancy, when she was slightly injured. It was stated also that there was no similar congenital or genetic anomaly in the grandparents. At another health center, where the parents had gone to get information about the baby’s condition, it was recommended that the baby’s mandible be brought forward by means of distraction osteogenesis. However, the parents refused this approach.
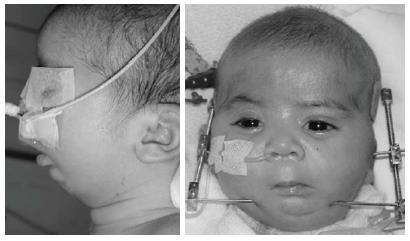
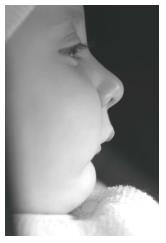
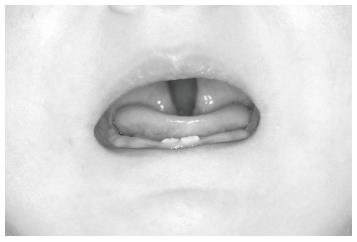
The baby was brought to our department when she was 13-day-old (Figure 3). Clinical examination showed that the baby had three characteristics of Pierre Robin sequence. Because the mandible was small and the tongue was located in the oropharyngeal area (Figure 4), there were severe difficulties with breathing and nutrition. It was decided that a modified nutrition plate should be applied so that vital functions could be restored and the tongue could be brought to its normal position within the mouth.
Impressions from the baby were taken with a silicone-based material in operating room conditions, and a fine study cast was created with hard plaster. To prevent the tongue from falling back into the oropharynx, a wire extension would be added to the nutrition plate. The slope and length of the wire extending toward the tongue root was determined with clinical experience. The borders of the nutrition plate were determined on the plaster cast, and after the waxing processes in the cleft region, the extension to be added to the rear part of the plate was prepared from 0.9-mm diameter stainless steel wire. The acrylic portions of the plate were prepared using typical methods. To prevent the wire extension from damaging the soft tissues, the end of the extension was covered with an acrylic button (Figure 5).
After construction of the nutrition plate and its extension was completed, the appliance was inserted in the mouth. The wire extension forced the tongue to displace anteriorly, and it returned to its normal position in the oral cavity as soon as the modified nutrition plate was positioned in the mouth (Figure 6). The obstruction caused by the tongue in the oropharynx was eliminated and the patient was able to breathe easily and comfortably. The baby began to feed comfortably with a bottle.
The parents were taught how to insert and to remove the nutrition plate and were informed of the importance of appliance care and cleaning, cleanliness of the cleft area and tongue, nutrition, and preferred sleeping positions. The baby was examined 1 d later, and no mucosal damage was seen to be caused by the plate and wire extension. However, the parents stated that the baby had felt disturbed initially due to the distal extension pressing on the tongue root anteriorly and that she had vomited a few times. However, they also reported that she got used to its presence in the following hours.
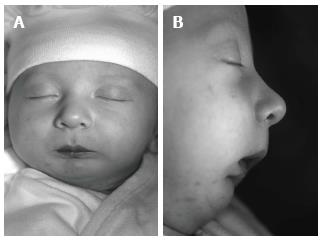
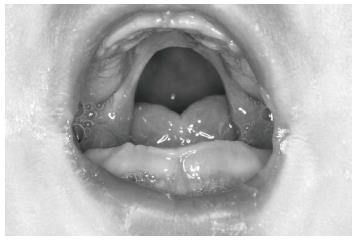
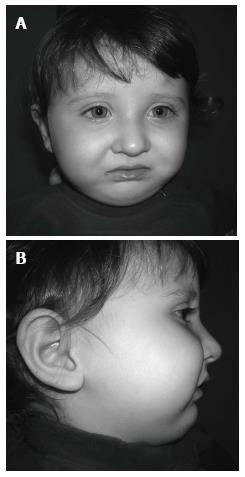
In the visit 1 mo later, the baby’s nutrition was observed to be fine. She was gaining weight normally and was breathing comfortably. The baby was seen monthly until the sixth month and her nutrition plate was changed every 2 mo. At the end of the sixth month, there appeared to be a correction in the baby’s profile (Figure 7), and the tongue had adapted to its normal position. Intraoral examination revealed that even after the appliance was removed, the tongue did not move back toward the oropharyngeal area (Figure 8). Because of this result, use of the appliance was discontinued. The parents’ pretreatment apprehension and concern about their baby’s state appeared to have subsided. The surgery was completed at another health center when the baby was 12 mo and 22-day-old.
The facial photographs, taken 18 mo after the modified nutrition plate application, showed that facial growth and development were returned to a normal pattern, and that the mandible had caught up to the maxilla (Figure 9).
In conclusion, the modified nutrition plate appears to be a viable alternative in the treatment of Pierre Robin sequence to surgical treatment modalities that are more aggressive in nature.
P- Reviewers: Ferreira MM, Gorseta K S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Robin P. La chute de la base de la langue consideree comme une nouvelle cause de gene dans la respiration nasopharyngienne. Bull Acad Med Paris. 1923;89:37-41. |
| 2. | Robin P. Glossoptosis due to atresia and hypotrophy of the mandible. Am J Dis Child. 1934;48: 541-547. |
| 3. | Jakobsen LP, Knudsen MA, Lespinasse J, García Ayuso C, Ramos C, Fryns JP, Bugge M, Tommerup N. The genetic basis of the Pierre Robin Sequence. Cleft Palate Craniofac J. 2006;43:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 4. | Oktay H, Baydaş B, Ersöz M. Using a modified nutrition plate for early intervention in a newborn infant with Pierre Robin sequence: A case report. Cleft Palate Craniofac J. 2006;43:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Evans KN, Sie KC, Hopper RA, Glass RP, Hing AV, Cunningham ML. Robin sequence: from diagnosis to development of an effective management plan. Pediatrics. 2011;127:936-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 6. | Daskalogiannakis J, Ross RB, Tompson BD. The mandibular catch-up growth controversy in Pierre Robin sequence. Am J Orthod Dentofacial Orthop. 2001;120:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Pasyayan HM, Lewis MB. Clinical experience with the Robin sequence. Cleft Palate J. 1984;21:270-276. [PubMed] |
| 8. | Collins B, Powitzky R, Robledo C, Rose C, Glade R. Airway management in pierre robin sequence: patterns of practice. Cleft Palate Craniofac J. 2014;51:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Mackay DR. Controversies in the diagnosis and management of the Robin sequence. J Craniofac Surg. 2011;22:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Runyan CM, Uribe-Rivera A, Karlea A, Meinzen-Derr J, Rothchild D, Saal H, Hopkin RJ, Gordon CB. Cost Analysis of Mandibular Distraction versus Tracheostomy in Neonates with Pierre Robin Sequence. Otolaryngol Head Neck Surg. 2014;151:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Perkins JA, Sie KC, Milczuk H, Richardson MA. Airway management in children with craniofacial anomalies. Cleft Palate Craniofac J. 1997;34:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Kremer B, Botos-Kremer AI, Eckel HE, Schlöndorff G. Indications, complications, and surgical techniques for pediatric tracheostomies--an update. J Pediatr Surg. 2002;37:1556-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Bath AP, Bull PD. Management of upper airway obstruction in Pierre Robin sequence. J Laryngol Otol. 1997;111:1155-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Pereira KD, MacGregor AR, Mitchell RB. Complications of neonatal tracheostomy: a 5-year review. Otolaryngol Head Neck Surg. 2004;131:810-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Meyer AC, Lidsky ME, Sampson DE, Lander TA, Liu M, Sidman JD. Airway interventions in children with Pierre Robin Sequence. Otolaryngol Head Neck Surg. 2008;138:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Denny AD, Talisman R, Hanson PR, Recinos RF. Mandibular distraction osteogenesis in very young patients to correct airway obstruction. Plast Reconstr Surg. 2001;108:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Dong CB, Zheng S, Shen C, Li H. Mandible traction with wires for the treatment of upper airway obstruction caused by Pierre Robin sequence in Chinese infants: Preliminary findings. J Craniomaxillofac Surg. 2014;Jan 15; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Baciliero U, Spanio di Spilimbergo S, Riga M, Padula E. Respiratory distress in Pierre Robin sequence: an experience with mandible traction by wires. Int J Oral Maxillofac Surg. 2011;40:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Pradel W, Lauer G, Dinger J, Eckelt U. Mandibular traction--an alternative treatment in infants with Pierre Robin sequence. J Oral Maxillofac Surg. 2009;67:2232-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Denny AD, Amm CA, Schaefer RB. Outcomes of tongue-lip adhesion for neonatal respiratory distress caused by Pierre Robin sequence. J Craniofac Surg. 2004;15:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Hoffman W. Outcome of tongue-lip plication in patients with severe Pierre Robin sequence. J Craniofac Surg. 2003;14:602-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Cruz MJ, Kerschner JE, Beste DJ, Conley SF. Pierre Robin sequences: secondary respiratory difficulties and intrinsic feeding abnormalities. Laryngoscope. 1999;109:1632-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Miloro M. Mandibular distraction osteogenesis for pediatric airway management. J Oral Maxillofac Surg. 2010;68:1512-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | McCarthy JG, Schreiber J, Karp N, Thorne CH, Grayson BH. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992;89:1-8; discussion 9-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1386] [Cited by in RCA: 1254] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 25. | Fariña R, Castellón L, Nagelash E, Valladares S. A new way to anchor the external device in mandibular distraction: three case reports with a Pierre Robin sequence. Int J Oral Maxillofac Surg. 2011;40:471-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Grago CA, Proffit WR, Ruiz RL. Maxillofaial distraction osteogenesis. White RP, Sarver DM, eds. Contemporary treatment of dentofacial deformity. St. Louis: Mosby 2003; 357-393. |
| 27. | Roy S, Munson PD, Zhao L, Holinger LD, Patel PK. CT analysis after distraction osteogenesis in Pierre Robin Sequence. Laryngoscope. 2009;119:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Pfaff MJ, Metzler P, Kim Y, Steinbacher DM. Mandibular volumetric increase following distraction osteogenesis. J Plast Reconstr Aesthet Surg. 2014;67:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Rachmiel A, Emodi O, Rachmiel D, Aizenbud D. Internal mandibular distraction to relieve airway obstruction in children with severe micrognathia. Int J Oral Maxillofac Surg. 2014;43:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Hong P. A clinical narrative review of mandibular distraction osteogenesis in neonates with Pierre Robin sequence. Int J Pediatr Otorhinolaryngol. 2011;75:985-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Scott AR, Tibesar RJ, Lander TA, Sampson DE, Sidman JD. Mandibular distraction osteogenesis in infants younger than 3 months. Arch Facial Plast Surg. 2011;13:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Radhakrishnan J, Sharma A. Feeding plate for a neonate with Pierre Robin sequence. J Indian Soc Pedod Prev Dent. 2011;29:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Ludwig B, Glasl B, Sader R, Schopf P. [Conservative orthodontic primary care of four newborns with the Pierre-Robin sequence triad]. J Orofac Orthop. 2007;68:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Hotz M, Gnoinski W. Clefts of the secondary palate associated with the “Pierre Robin syndrome”. Management by early maxillary orthopaedics. Swed Dent J Suppl. 1982;15:89-98. [PubMed] |
| 35. | Buchenau W, Urschitz MS, Sautermeister J, Bacher M, Herberts T, Arand J, Poets CF. A randomized clinical trial of a new orthodontic appliance to improve upper airway obstruction in infants with Pierre Robin sequence. J Pediatr. 2007;151:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Bacher M, Sautermeister J, Urschitz MS, Buchenau W, Arand J, Poets CF. An oral appliance with velar extension for treatment of obstructive sleep apnea in infants with Pierre Robin sequence. Cleft Palate Craniofac J. 2011;48:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Brosch S, Flaig S, Bacher M, Michels L, de Maddalena H, Reinert S, Mauz PS. [The influence of the Tübingen soft palate plate and early cleft closure on swallowing and Eustachian tube function in children with Pierre Robin sequence]. HNO. 2006;54:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |