Published online Jul 28, 2015. doi: 10.5320/wjr.v5.i2.166
Peer-review started: November 26, 2014
First decision: January 20, 2015
Revised: April 13, 2015
Accepted: July 7, 2015
Article in press: July 8, 2015
Published online: July 28, 2015
Processing time: 252 Days and 17.1 Hours
To analyze the current methods of primary staging and repeated staging (restaging) of the mediastinal nodes in non-small-cell lung cancer (NSCLC), all methods currently used for staging of NSCLC are analyzed. These methods include imaging techniques [computer tomography (CT), positron emission tomography (PET) combined with CT (PET/CT)], endoscopic/ultrasound techniques (endobronchial ultrasound/transbronchial needle aspiration) and endoscopic ultrasound/fine needle aspiration and surgical techniques [standard cervical mediastinoscopy, video-assisted mediastinoscopy, extended mediastinoscopy, video-assisted mediastinoscopic lymphadenectomy, transcervical extended mediastinal lymphadenectomy, anterior mediastinotomy (Chamberlain procedure) and video-assisted thoracic surgery]. The diagnostic yield of Chest CT is regarded insufficient for both, primary staging and restaging. The PET/CT became a standard imaging technique preceding curative surgery of radical chemo-radiotherapy. The issue of intraoperative staging is also described. Finally, the author’s proposed algorithm of staging, both for primary staging and restaging after neoadjuvant therapy is presented. Detailed staging of NSCLC enables selection of patients with early stage disease for curative surgical/multimodality treatment and helps to avoid unnecessary surgery in advanced disease.
Core tip: All methods currently used for staging of non-small-cell lung cancer are analyzed. These methods include imaging techniques [computer tomography (CT), positron emission tomography (PET) combined with CT (PET/CT)], endoscopic/ultrasound techniques endobronchial ultrasound/transbronchial needle aspiration and endoscopic ultrasound/fine needle aspiration and surgical techniques standard cervical mediastinoscopy, video-assisted mediastinoscopy, extended mediastinoscopy, video-assisted mediastinoscopic lymphadenectomy, transcervical extended mediastinal lymphadenectomy, anterior mediastinotomy (chamberlain procedure) and video-assisted thoracic surgery. The issue of intraoperative staging is also described. Finally, the author’s proposed algorithm of staging, both for primary staging and restaging after neoadjuvant therapy is presented.
- Citation: Zielinski M. Current methods of staging and restaging of the mediastinal nodes in non-small-cell lung cancer. World J Respirol 2015; 5(2): 166-175
- URL: https://www.wjgnet.com/2218-6255/full/v5/i2/166.htm
- DOI: https://dx.doi.org/10.5320/wjr.v5.i2.166
The prognosis of lung cancer is still very bad with only 16.3% of all patients with of treatment for individual patients in aim to provide the best chance of cure in the lung cancer surviving 5 years[1]. One of the main important issues is a proper choice early stage of the disease and to avoid unnecessary invasive surgical or multimodality treatment in cases with the advanced disease. Estimation of the TNM factors is the key of the process of staging. Because non-small-cell lung cancer (NSCLC) is often found in a disseminating phase this is most important to rule-out distant metastases (M1). Even in stage IV, however NSCLC is a treatable, although not curable disease due to the survival advantage and improvement in quality of life over best supportive care (BSC) after platinum-based chemotherapy[2]. In patients with M1 there is no role for any curative approach like surgery or radical chemo-radiotherapy and chemotherapy or best supportive care are the only reasonable options. The only exception from this rule are the isolated brain metastases without any other symptoms of loco-regional or distal dissemination in patients who are otherwise amenable to treatment with surgery or irradiation. In the part of these patients subsequent surgical treatment is undertaken with reported 5-year survival 13% (7%-21%)[3].
For patients with IIIB NSCLC with good performance status radical chemoradiotherapy is the preferred treatment option[4].
The limits of effectiveness of surgery are also clearly understood with more benefit than harm in stage I amenable to resection alone because radical surgery still offers the best chance of cure despite recent reports on effectiveness of stereotactic body radiotherapy (SBRT), also described as Stereotactic ablative radiotherapy (SABR) emerging as an alternative to surgery[5-8]. SABR was shown to be superior to conventional radiotherapy in regard to local control and overall survival[9]. In patients with stage II NSCLC surgery with adjuvant chemotherapy is regarded the standard of care with neoadjuvant chemotherapy followed by surgery as an alternative in patients with N1 nodes discovered preoperatively[10]. The most controversial group are patients with stage IIIa, N2 metastatic mediastinal nodes for whom multimodality treatment with or without subsequent surgery should be considered[3,11].
Therefore, the critical issue in planning of the treatment in patients with NSCLC is a proper staging allowing for choice of the best therapy for individual patients. Staging of the mediastinal nodes is critical to differentiate between patients in stage I and II who could benefit from curative surgery or radiotherapy, stage IIIA who should undergo multimodality treatment and stages IIIb-IV managed without operation. Repated staging (restaging) regards patients who underwent neoadjuvant treatment and are considered candidates for subsequent radical surgery.
In the clinical practice, mediastinal nodal staging include imaging, endoscopic and surgical techniques. Recently, there has been an increasing interest in genetic and proteomic which are still experimental, however. The results of current studies are promising and it is possible that in the future circulating subtypes of micro RNA, DNA and the other biomarkers might be very useful in staging of NSCLC[12-14]. However, currently, these techniques does not allow for accurate staging of the mediastinal nodes, however. Therefore the issue of biomarkers will not be addressed in this article.
The aim of this study is to summarize current experience on staging and restaging of NSCLC.
This article is based on the search made in PubMed for English language publications on staging of NSCLC from the period 2009-2014. Keywords: lung cancer, nsclc staging, nsclc invasive staging, ebus, eus, mediastinoscopy, vats were used. Some other important earlier publications were considered, as well. Only the publications in a peer-reviewed journals, the publications including large numbers of patients, with clearly presented methodology and results were used in this study. The results of the prospective randomized trials, practice guidelines, systematic reviews, and meta-analyses were regarded especially important were included preferentially. This is not a systematic review because the choice of the articles citated in this paper was dependent on the subjective opinion of the author. Therefore, the methodology of a Systematic Review was not obeyed, like the one presented by the PRISMA methodology[15]. All methods currently used for staging of NSCLC are analyzed. These methods include imaging techniques [computer tomography (CT), positron emission tomography (PET) combined with CT (PET/CT)], endoscopic/ultrasound techniques [endobronchial ultrasound/transbronchial needle aspiration (EBUS/TBNA) and endoscopic ultrasound/fine needle aspiration (EUS/FNA)] and surgical techniques [standard cervical mediastinoscopy (CM), video-assisted mediastinoscopy (VAM), extended mediastinoscopy, video-assisted mediastinoscopic lymphadenectomy (VAMLA), transcervical extended mediastinal lymphadenectomy (TEMLA), anterior mediastinotomy (Chamberlain procedure) and video-assisted thoracic surgery (VATS)].
Imaging studies: Chest CT is generally the preliminary step of diagnosis of NSCLC providing important information on staging of the disease. A staging process is continued in patients with clinical stages I-IIIb who are candidates for surgery or multimodality treatment. Chest CT is most often performed with the use of the contrast-enhanced (CE) technique allowing for differentiation of the mediastinal nodes from the mediastinal and hilar structures. The most common criterion of abnormality of the mediastinal nodes is the short-axis diameter of (> 1.0 cm) on a transverse scan. The reported pooled sensitivity, specificity and Negative Predictive Value (NPV) of chest CT were 55% (20%-91%), 81% (50%-97%) and 83% (54%-97%)[16]. There is a general agreement that Computer Tomography is insufficient in reliable staging of the mediastinal nodes.
PET: Introduction of PET has been a major progress in diagnosis and management of NSCLC. There are several advantages of use of PET including improvement of the primary staging and restaging after neoadjuvant chemotherapy or chemoradiotherapy. PET was useful in discovery of the clinically silent distant metastases which was dependent on the clinical stage of the disease: such metastases were found in 19% of patients, including 7.5% of those with stage I disease, 18% of those with stage II disease and 24% of those with stage III disease by CT[17]. Pooled sensitivity and specificity and NPV of PET/CT for staging of the mediastinal nodes were approximately 77% (33%-100%), 86% (43%-100%) and 91% (79%-100%), respectively[16]. Generally, PET was found to be more sensitive than CT for identifying the mediastinal lymph node involvement in patients with known or suspected NSCLC, although this advantage was denied by the Danish authors[18]. The limitation of PET was a small diameter of the malignant lesion (< 4 mm)[19,20]. Due to this limitation PET is less sensitive in normal size mediastinal nodes. In one study, the sensitivity was 32.4% for the small nodes (< 1 cm) and 85.3% for the nodes > 1 cm[21]. The reported sensitivity and specificity of PET in discovery of the mediastinal nodes metastases were 80% and 88%, respectively[16]. Mediastinal nodes which are positive on PET should be confirmed by biopsy due to the possible false positive results, especially in case of inflammatory process in the lungs. The use of PET is indicated especially in patients with large, centrally-located adenocarcinomas, as well as in patients with hilar nodal enlargement[4,20].
The therapeutic impact of PET/CT has been studied in regard to the number of futile thoracotomies, defined as surgery for a benign lesion, intraoperative detection of N2, N3 or other stage IIIB disease, exploratory thoracotomy for some other reason, or tumor recurrence or death within 1 year[21,22].
It was reported that the risk of death was twice as high when the Standardized Uptake Value (SUV) was above the median value and the greater FDG uptake was independently associated with a worse prognosis among patients with malignant nodules that were surgically resected, even after adjusting for age, tumor size, histology and type of resection[23-28].
Restaging of NSCLC after neoadjuvant treatment is another application of PET/CT.
In one review, sensitivity and specificity of PET for identifying residual N2 disease were only 64% and 85%, respectively which suggested that the diagnostic yield of PET/CT was inferior in restaging in comparison to the primary staging[29].
Another issue is reduction of SUV after neoadjuvant treatment. According to the results of one study, reductions in FDG uptake of at least 75% in the primary tumor and at least 50% in the involved lymph nodes were strongly associated with a complete response[30]. In the recent ACCP guidelines and ESTS guidelines PET/CT was recommended for staging of NSCLC before curative-intent treatment. In case positive results of PET tissue confirmation by biopsy is necessary[11,16,17].
Endoscopy/ultrasound: EBUS/TBNA and EUS/FNA are now considered the next phase of the mediastinal staging after CT and PET/CT. EBUS and EUS are complementary techniques allowing for visualization and biopsy of most of the mediastinal nodes. EBUS is better for examination of the right paratracheal nodes (stations 2R and 4R), for the upper paratracheal nodes (station 2L) and for the bilateral hilar nodes (N1). EUS is preferable for the lower paratracheal nodes (station 4L), and for the periesophageal and pulmonary ligament nodes (stations 8 and 9). Staging of the aorta-pulmonary window nodes (station 5) and paraaortic nodes (station 6) with EUS is a controversial issue. For the subcarinal nodes (station 7) both techniques are possibly equally good. In several studies combination of EBUS and EUS, so called combined ultrasound (CUS) were shown to be the best diagnostic yield[31,32]. With current EBUS endoscopes it is possible to perform both studies with one EBUS instrument introduced sequentially to the trachea and bronchi and then to the esophagus during one procedure performed in mild sedation. During the EBUS, EUS and CUS studies 1-4 nodal stations are usually needle-biopsied. Some authors recommend on site cytological examination to confirm that the proper cytological material has been taken during biopsy, the other authors questioned such policy, however[33-36]. Serious complications of EBUS and EUS were found in 0.3% and 0.05%, respectively in the review reported by von Bartheld et al[37]. The reported diagnostic yield for EBUS and EUS were dependent on the prevalence of the mediastinal metastases and was the lowest in the normal mediastinal nodes on CT and PET/CT. The reported sensitivity, specificity and NPV for EBUS were 89% (46%-97%) 100% (96%-100%) and 91% (60%-99%), and for EUS were 89% (45%-100%), 100% (90%-100%) and 86% (68%-100%), respectively[16].
Current ACCP and ESTS guidelines recommend to omit surgical staging in patients with small tumors (< 3 cm) localized in the peripheral (outer third of the lung) without positive mediastinal and hilar (N1) nodes on PET/CT. In these patients the risk of false negative mediastinal nodes is low and the patients can be referred directly to pulmonary resection. Surgical staging is necessary in patients with larger tumors (> 3 cm), centrally located, with positive N1 nodes and with the positive mediastinal nodes on CT or PET/CT (even if negative on EBUS/EUS). In such patients the risk of mediastinal nodes metastases is at least 20%-25% so it is necessary to confirm the absence on such metastases with surgical staging.
Cervical mediastinonoscopy formerly described as a gold standard of the mediastinal staging is still widely used[38-40]. Currently, VAM is a recommended version of mediastinoscopy, due to the improved technology, allowing for better view, simultaneous observation of the procedure on the screen with trainees and possible recording of the procedure. Both CM and VAM require general anaesthesia and can be performed as outpatient procedures. CM and VAM enable visualization and biopsy of the paratracheal nodes, bilaterally (station 2R,4R,2L,4L) and the subcarinal nodes (station 7). The other nodal stations are out the reach of CM and VAM. Reported sensitivity and NPV are 78% (32%-92%) and 91% (80%-97%) for CM and 89% (78%-97%) and 92% (83-96%) for VAM, respectively[16]. The advantage of VAM vs CM regards mainly better training and comfort of thr surgeon, diagnostic superiority of VAM over CM is less clear[41].
The diagnostic yield of EBUS/EUS and mediastinoscopy was compared in the prospective randomized multi-institutional ASTER study[42]. It was found that EBUS/EUS followed by mediastinoscopy had greater sensitivity for mediastinal nodal metastases in comparison to mediastinoscopy alone 94% (62/66; 95%CI: 85%-98%) vs 79% (41/52; 95%CI: 66%-88%) (P = 0.02) and resulted in fewer unnecessary thoracotomies 18%; (95%CI: 12%-26%) in the mediastinoscopy group vs 7%; (95%CI: 4%-13%) in the endosonography/mediastinoscopy group (P = 0.02)[39]. Contrary results were reported in the retrospective study comparing EBUS and EUS with TEMLA (see below)[43]. Primary staging was performed in 623 patients: EBUS in 351, EUS in 72 and CUS in 200 patients. TEMLA was performed for primary staging in 276 patients. There was no mortality and morbidity after EBUS/EUS. One patient died after TEMLA and morbidity rate after TEMLA was 7.2%. There was a significant difference between EBUS/EUS and TEMLA for sensitivity (87.8% and 96.2%; P < 0.01) and negative predictive value (NPV) (82.5% and 99.6%; P < 0.01) in favor of TEMLA. The undisputed benefit of EBUS is possibility to differentiate between N0 and N1 for NSCLC[44].
There are several techniques allowing for biopsy of the paraaortic nodes (station 6) and the aortopulmonary window nodes (station 5) including extended mediastinoscopy, anterior mediastinotomy, VATS and TEMLA.
Extended mediastinoscopy is technique added to the standard mediastinoscopy to reach and biopsy the station 5 and 6 nodes. The key of this procedure is to perform a finger dissection to create a tunnel in the mediastinum in front of the ascending aorta and to introduce a mediastinoscope through this tunnel to visualize and biopsy the stations 5 and 6 nodes. The pooled reported sensitivity and specificity of the Extended Mediastinoscopy were 71% and 91%, respectively[16,45,46].
The anterior mediastinotomy (chamberlain procedure) is performed in general anaesthesia to reach station 5 and 6 on the left side or the station 3A,4R and 10R on the right side. The mediastinum is entered from the front after resection of the second of third costal cartillage or intercostally, without resection of ribs. This technique does not allow to reach the other mediastinal nodal stations. The reported pooled sensitivity and specificity of the Chamberlain procedure were 71% (44%-81%) and 91% (89%-95%), respectively[16].
VATS is a technique allowing to reach virtually all mediastinal nodal stations but only unilaterally, although an access to the left paratracheal nodes is very challenging and limited. The disadvantages of VATS include greater invasiveness in comparison to mediastinoscopy, the use of general anaesthesia, selective lung ventillation and several VATS ports and the use of postoperative chest drainage. These reasons limit the use of VATS for preoperative staging. The additional advantage of VATS is the possibility to evaluate T stage and to rule-out pleural dissemination. The reported sensitivity and specificity and NPV of VATS for mediastinal staging were 99% (58%-100%), 100% and 96% (88%-100%), respectively[16,47].
VAMLA and TEMLA are new techniques intended for performance of the mediastinal lymphadenectomy (complete removal of the whole mediastinal nodes with the surrounding adipose tissue) to improve the accuracy of staging instead of obtaining the pieces of the nodes obtained with the CM[48-50].
Due to this advantage, the diagnostic yields of VAMLA and TEMLA were much higher in comparison to the standard mediastinoscopy (as was proved for TEMLA)[51]. VAMLA and TEMLA are performed through the neck incision (like mediastinoscopy). Both techniques became feasible after introduction of the two-blade Linder-Dahan mediastinoscope which enabled much wider access to the mediastinum, however, contrary to the VAMLA most part of the TEMLA procedure is performed in the open technique, without the use of a mediastinoscope. The nodal stations 1,3A, 3P, 5 and 6 are not removed with VAMLA but can be removed by TEMLA. In case of VAMLA, however stations 5 and 6 can be reached with use of additional Extended Mediastinoscopy. The other differences between VAMLA and TEMLA include more nodal stations and the mean number of nodes removed with TEMLA in comparison to VAMLA (11 vs 5 nodal stations and 20.8 vs 37.9 nodes, respectively) but also shorter mean operative time (54 min for VAMLA vs 128 min for TEMLA) and lesser invasiveness of VAMLA. There was no mortality and lower morbidity after VAMLA and 0.3% mortality and 6.6% morbidity for TEMLA, it was not clear however, if the results of VAMLA represented 30-d mortality and morbidity as was reported for TEMLA (the mortality of TEMLA was all due to no-surgical reasons). The diagnostic yield was slightly better for TEMLA than for VAMLA with reported sensitivity, specificity and NPV 96.2%, 100%, 98.9% and 93.8%, 100% and 96%, respectively[52,53]. It was not clear, however if the results for VAMLA were calculated for all nodal stations or only for those accessible for VAMLA. The other difference between VAMLA and TEMLA was the elevation of the sternum with a special retractor connected with the Rochard frame which widened the approach to the mediastinum and facilitated performance of TEMLA.
Restaging of the mediastinal nodes is an extremely important part of multimodality treatment of stage IIIA NSCLC. In several studies it was found that the results of survival in patients with residual metastatic nodes much inferior in comparison to the patients in whom the nodes are N0-1 after neoadjuvant therapy. This is especially pronounced in patients with residual multi-level metastatic nodes[54-56]. Therefore, a decision if to offer surgery to the patients after neoadjuvant therapy should be based on the reliable restaging. There are several methods of restaging of the mediastinal nodes after neoadjuvant treatment Imaging studies include CT which has relatively low diagnostic yield (sensitivity 41%-59%, specificity 62%-75% and accuracy 58%-60%) and PET combined with CT (PET/CT) with sensitivity 61%-77%, specificity 85%-90% and accuracy 78%-83%[16,29,30]. PET/CT was found to be superior to CT (accuracy 89% vs 36% for stage I)[16].
Restaging with endoscopic techniques include EBUS, EUS and combined EBUS/EUS. EBUS was used in the multi-institutional report (Copenhagen, Boston, Heidelberg, Chiba) on 124 patients restaged after induction therapy with sensitivity 76%, specificity 100%, PPV 100%, NPV 20%, accuracy 77%[57]. The results of the other study with use of EBUS were similar[19]. In the other study sensitivity and NPV of restaging with EBUS were 66.7% and 77.5%, respectively[58]. The reported sensitivity and NPV for restaging with EUS were 44% and 58%, respectively[59-61].
Surgical techniques of restaging include repeated mediastinoscopy (remediastinoscopy), VATS and TEMLA. Generally, remediastinoscopy seems to be a technique of moderate diagnostic yield with sensitivity of 61%-83% (with exception of sensitivity 29% in the study published by De Leyn et al[62]).
Restaging with VATS was described in the recent multi-institutional study reporting sensitivity of VATS of 67% (95%CI: 47-83), and negative predictive value (NPV) of 73% (95%CI: 56-86)[47].
The results of TEMLA in restaging of the mediastinal nodes reported sensitivity of 95.7% and NPV of 97.6%. In the retrospective study comparing the diagnostic yield of EBUS and EUS with TEMLA for restaging of NSCLC the endoscopic/ultrasound staging was performed in 88 patients and TEMLA in 78 patients. There was a significant difference between EBUS/EUS and TEMLA for sensitivity (64.3% and 100%, P < 0.01) and NPV (82.1% and 100%; P < 0.01) in favor of TEMLA[43,63].
Diagnostic yield of staging and restaging techniques for NSCLC are shown in Tables 1 and 2.
| Diagnostic technique | SensitivityMean (range) | SpecificityMean (range) | Negative predictive value Mean (range) |
| Chest CT | 55 (20-91) | 81 (50-97) | 83 (54-97) |
| PET/CT | 77 (33-100) | 86 (43-100) | 91 (79-100) |
| EBUS/TBNA | 89 (46-97) | 100 (96-100) | 91 (60-99) |
| EUS/FNA | 89 (45-100) | 100 (90-100) | 86 (68-100) |
| Mediastinoscopy | 78 (32-92) | 100 | 91 (80-97) |
| Video-mediastinoscopy | 89 (78-97) | 100 | 92 (83-96) |
| VATS | 99 (58-100) | 100 | 96 (88-100) |
| VAMLA | 93.8 | 100 | 96 |
| TEMLA | 96.2 | 100 | 98.9 |
| Diagnostic technique | SensitivityMean (range) | SpecificityMean (range) | Negative predictive valueMean (range) |
| Chest CT | 55 (20-91) | 81 (50-97) | 83 (54-97) |
| PET/CT | 77 (33-100) | 86 (43-100) | 91 (79-100) |
| EBUS/TBNA | 89 (46-97) | 100 (96-100) | 91 (60-99) |
| EUS/FNA | 89 (45-100) | 100 (90-100) | 86 (68-100) |
| Mediastinoscopy | 78 (32-92) | 100 | 91 (80-97) |
| Video-mediastinoscopy | 89 (78-97) | 100 | 92 (83-96) |
| VATS | 99 (58-100) | 100 | 96 (88-100) |
| VAMLA | 93.8 | 100 | 96 |
| TEMLA | 96.2 | 100 | 98.9 |
Intraoperative biopsy or removal of the mediastinal nodes described as lymphadenectomy is a final step of staging. According to the ESTS guidelines there are several methods of intraoperative staging including selective biopsy of the piece of the nodes or nodes, sampling (removal of the whole node), systematic sampling (removal of the whole nodes from the nodal stations predetermined before an operation), systematic nodal dissection (systematic lymphadenectomy), lobe-specific nodal dissection and extended lymphadenectomy. Systematic nodal dissection is recommended in all cases to ensure complete resection[64]. This technique includes removal of at least three mediastinal nodal stations with the surrounding fatty tissue are removed (the subcarinal nodes must be removed in every case). Additionally, hilar and intrapulmonary nodes should removed, as well. There is an a general agreement that systematic lymphadenectomy enables the most detailed study of the mediastinum due to the largest number of the removed nodes, the therapeutic benefit of systematic lymphadenectomy has not been proved unequivocally, however. Is the studies of Wu et al[65] and Keller et al[66] the authors found that systematic nodal dissection (SND) was superior to mediastinal lymph nodal sampling (MLS) in surgical treatment of non-small cell lung cancer (NSCLC).
The survival benefit was not confirmed by the results of the prospective randomized American College of Surgery Oncology Group Z0030 Trial reported by Darling et al[67] who concluded that in the clinical stage I NSCLC if systematic and thorough presection sampling of the mediastinal and hilar lymph nodes was negative, mediastinal lymph node dissection did not improve survival in patients with early stage non-small cell lung cancer, but these results were not generalizable to patients staged radiographically or those with higher stage tumors.
Lobe-specific systematic nodal dissection is a technique in which specific nodal stations according to the location of the tumor are removed. This procedure is acceptable for peripheral squamous T1 tumors, if hilar and interlobar nodes are negative on frozen section studies; it implies removal of, at least, three hilar and interlobar nodes and three mediastinal nodes from three stations in which the subcarinal is always included[68]. Ma et al[69] found no difference between Systematic Lymphadenectomy (SL) and Lobe-Specific Lymphadenectomy (LL) in regard to migration of N staging, Overall Survival and Disease-Free Survival for cT1aN0M0 tumors with high rate of Ground-Glass Opacity (GG0). Shapiro et al[70] found that lobe-specific N2 nodal evaluation resulted in a recurrence rate similar to that of complete mediastinal evaluation. The authors concluded that lobe-specific mediastinal nodal evaluation appeared acceptable in patients with early-stage NSCLC.
Contrary results were reported by Maniwa et al[71] who found that the recurrence of mediastinal node cancer in patients undergoing Systematic Lymphadenectomy was significantly greater than that in those undergoing Lobe-Specific Lymphadenectomy. Selected lymph node biopsies and sampling are justified only to prove nodal involvement when resection is not possible, not for radical surgery[64].
Staging of NSCLC is currently an increasingly complex process with staging of the mediastinal nodes being a central part of this process. There is a general agreement that chest CT is insufficiently accurate to predict metastatic involvement in patients with a discrete enlargement of the nodes or normally looking mediastinum. PET/CT emerged as a standard of staging in the patients considered candidates for surgical treatment. The main value of PET/CT is discovery of possible clinically silent metastasis[32]. PET/CT will probably never replace CT completely, because anatomical details of the chest are visualized much more precisely on good quality CT than on PET/CT. In the clinical stage IA peripheral tumors negative PET/CT is possibly sufficient to refer patients directly to surgery. In all other patients with possibly curable tumors, invasive staging is necessary, however. During the last decade the role of EBUS and EUS rose substantially. These studies are currently recognized as the second step of staging after CT and PET/CT due to minimal invasiveness. It seems reasonable to combine endoscopic/ultrasound and surgical staging, this approach has been recently supported by results of our group[31]. The results reported by the leading experts on EBUS/EUS are impressive and lead them to claim that due to the advantages and possible superiority of EBUS and EUS in comparison to mediastinoscopy the latter one is no longer necessary. Herth et al[32] concluded that the combination of EBUS and EUS “may be able to replace more invasive methods as a primary staging method for patients with lung cancer”. Tournoy et al[72] concluded that “EUS-FNA reduces the need for surgical staging procedures in patients with (suspected) lung cancer in whom a mediastinal exploration is needed”. According to Vilmann et al[73] “It seems therefore logical to assume that the combination of EUS-FNA and EBUS-TBNA will replace more invasive methods such as mediastinoscopy for diagnosis and staging of lung cancers in the near future”[73]. The same authors called the combination of EBUS and EUS “the complete medical mediastinoscopy” and claimed that “A recent publication from our group has documented a sensitivity and specificity of 100% when EUS-FNA and EBUS-TBNA is used in combination for staging of the mediastinum”. However, surgical staging is not the past history. Even in some recent publication cervical mediastinoscopy was still regarded the gold standard of the mediastinal staging (Shrager)[40]. It is not clear if the results reported by the most experienced endoscopists could be achieved also by the average performers of EBUS and EUS. The reported results of mediastinoscopy pooled in the ACCP publication included broader number of publication, not only the best experts but also some poorer results that were compared to EBUS/EUS in aim to show superiority of endoscopy/ultrasound over surgical staging[16]. Therefore, the better results of EBUS/EUS might not be the proof that this modality was really better than mediastinoscopy. The final step of mediastinal nodal staging is a systematic lymphadenectomy performed during pulmonary resection of preoperatively, by means of VAMLA or TEMLA.
The importance of lymphadenectomy is limited not only to staging but this procedure may has also a therapeutic role, although the reported results are equivocal. The results of American College of Surgery Oncology Group Z0030 Trial did not confirm any beneficial influence of lymphadenectomy in comparison to sampling in clinical stage I NSCLC but it is still possible that there might be such influence in stage II and III as was reported by Wu et al[65] and Keller et al[66] who found that lymphadenectomy improved the results of survival in comparison to sampling.
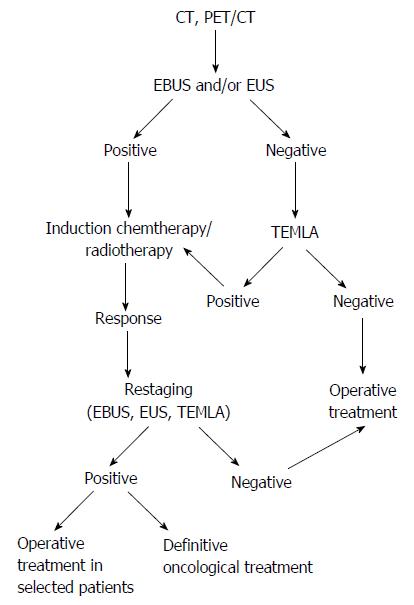
Due to the extremely large number of publication regarding mediastinal staging it is an imperative for every practitioner involved in diagnosis and treatment of NSCLC to form his/her own opinion how to choose the best possible way of staging. What was presented in this paper is a subjective view differing from the data presented in the most comprehensive systematic reviews[16]. For example, in my opinion, the value of relatively new techniques as PET/CT, EBUS or EUS is exaggerated, currently. In the past, the same happened to the chest CT. In the early 1980, it has been reported that sensitivity of chest CT in staging of lung cancer exceeded 80% to fall down to about 55%, according to the recent publications[74,75]. The time will solve if sensitivity of EBUS will still be around 90% as is being currently reported by the best experts. The real value of this technique will be shown in hands of an average endoscopist, who do not publish their results. In this article I made an attempt to present the staging and restaging algorithm which I recommended (Figure 1).
Current staging for NSCLC which is a complex process including several imaging, endoscopy/ultrasound and surgical techniques enables optimal selection of patients with early stage disease for curative treatment and helps to avoid unnecessary surgical or multimodality treatment in the advanced disease.
The author thanks Mr Michael Clarc for language correction of the manuscript.
P- Reviewer: Boonsarngsuk V, Deng B, Hida T, Pereira-Vega A S- Editor: Tian YL L- Editor: A E- Editor: Wang CH
| 1. | Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e1S-29S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 487] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 2. | Socinski MA, Evans T, Gettinger S, Hensing TA, Sequist LV, Ireland B, Stinchcombe TE. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e341S-e368S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Kozower BD, Larner JM, Detterbeck FC, Jones DR. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e369S-e399S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 263] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 4. | Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, Diekemper R, Detterbeck FC, Arenberg DA. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e314S-e340S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 329] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 5. | Soldà F, Lodge M, Ashley S, Whitington A, Goldstraw P, Brada M. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; systematic review and comparison with a surgical cohort. Radiother Oncol. 2013;109:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 6. | Verstegen NE, Oosterhuis JW, Palma DA, Rodrigues G, Lagerwaard FJ, van der Elst A, Mollema R, van Tets WF, Warner A, Joosten JJ. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol. 2013;24:1543-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 7. | Mahmood S, Bilal H, Faivre-Finn C, Shah R. Is stereotactic ablative radiotherapy equivalent to sublobar resection in high-risk surgical patients with stage I non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. 2013;17:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e278S-e313S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 976] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 9. | Jeppesen SS, Schytte T, Jensen HR, Brink C, Hansen O. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol. 2013;52:1552-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Yasufuku K, Pierre A, Darling G, de Perrot M, Waddell T, Johnston M, da Cunha Santos G, Geddie W, Boerner S, Le LW. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142:1393-1400.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 379] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 11. | De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, Turna A, Van Schil P, Venuta F, Waller D. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45:787-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 563] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 12. | Markou A, Sourvinou I, Vorkas PA, Yousef GM, Lianidou E. Clinical evaluation of microRNA expression profiling in non small cell lung cancer. Lung Cancer. 2013;81:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Newman AM, Bratman SV, To J, Wynne JF, Eclov NC, Modlin LA, Liu CL, Neal JW, Wakelee HA, Merritt RE. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1341] [Cited by in RCA: 1644] [Article Influence: 149.5] [Reference Citation Analysis (0)] |
| 14. | Liu W, Wu Y, Wang L, Gao L, Wang Y, Liu X, Zhang K, Song J, Wang H, Bayer TA. Protein signature for non-small cell lung cancer prognosis. Am J Cancer Res. 2014;4:256-269. [PubMed] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9207] [Cited by in RCA: 8050] [Article Influence: 536.7] [Reference Citation Analysis (2)] |
| 16. | Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, Harris LJ, Detterbeck FC. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e211S-e250S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 1019] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 17. | Harders SW, Madsen HH, Hjorthaug K, Arveschoug AK, Rasmussen TR, Meldgaard P, Hoejbjerg JA, Pilegaard HK, Hager H, Rehling M. Mediastinal staging in Non-Small-Cell Lung Carcinoma: computed tomography versus F-18-fluorodeoxyglucose positron-emission tomography and computed tomography. Cancer Imaging. 2014;14:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | MacManus MP, Hicks RJ, Matthews JP, Hogg A, McKenzie AF, Wirth A, Ware RE, Ball DL. High rate of detection of unsuspected distant metastases by pet in apparent stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Verhagen AF, Bootsma GP, Tjan-Heijnen VC, van der Wilt GJ, Cox AL, Brouwer MH, Corstens FH, Oyen WJ. FDG-PET in staging lung cancer: how does it change the algorithm. Lung Cancer. 2004;44:175-181. [PubMed] |
| 20. | Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. The size of metastatic foci and lymph nodes yielding false-negative and false-positive lymph node staging with positron emission tomography in patients with lung cancer. J Thorac Cardiovasc Surg. 2004;127:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Hoekstra CJ, Stroobants SG, Smit EF, Vansteenkiste J, van Tinteren H, Postmus PE, Golding RP, Biesma B, Schramel FJ, van Zandwijk N. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:8362-8370. [PubMed] |
| 22. | Fischer B, Lassen U, Mortensen J, Larsen S, Loft A, Bertelsen A, Ravn J, Clementsen P, Høgholm A, Larsen K. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009;361:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 23. | Billé A, Pelosi E, Skanjeti A, Arena V, Errico L, Borasio P, Mancini M, Ardissone F. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothorac Surg. 2009;36:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Win T, Miles KA, Janes SM, Ganeshan B, Shastry M, Endozo R, Meagher M, Shortman RI, Wan S, Kayani I. Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non-small cell lung cancer. Clin Cancer Res. 2013;19:3591-3599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Xu N, Wang M, Zhu Z, Zhang Y, Jiao Y, Fang W. Integrated positron emission tomography and computed tomography in preoperative lymph node staging of non-small cell lung cancer. Chin Med J (Engl). 2014;127:607-613. [PubMed] |
| 26. | Koksal D, Demirag F, Bayiz H, Ozmen O, Tatci E, Berktas B, Aydoğdu K, Yekeler E. The correlation of SUVmax with pathological characteristics of primary tumor and the value of Tumor/ Lymph node SUVmax ratio for predicting metastasis to lymph nodes in resected NSCLC patients. J Cardiothorac Surg. 2013;8:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Amini A, Lou F, Correa AM, Baldassarre R, Rimner A, Huang J, Roth JA, Swisher SG, Vaporciyan AA, Lin SH. Predictors for locoregional recurrence for clinical stage III-N2 non-small cell lung cancer with nodal downstaging after induction chemotherapy and surgery. Ann Surg Oncol. 2013;20:1934-1940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Paesmans M, Berghmans T, Dusart M, Garcia C, Hossein-Foucher C, Lafitte JJ, Mascaux C, Meert AP, Roelandts M, Scherpereel A. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol. 2010;5:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 29. | Rebollo-Aguirre AC, Ramos-Font C, Villegas Portero R, Cook GJ, Llamas Elvira JM, Romero Tabares A. Is FDG-PET suitable for evaluating neoadjuvant therapy in non-small cell lung cancer Evidence with systematic review of the literature. J Surg Oncol. 2010;101:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Cerfolio RJ, Bryant AS, Ojha B. Restaging patients with N2 (stage IIIa) non-small cell lung cancer after neoadjuvant chemoradiotherapy: a prospective study. J Thorac Cardiovasc Surg. 2006;131:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Szlubowski A, Zieliński M, Soja J, Annema JT, Sośnicki W, Jakubiak M, Pankowski J, Cmiel A. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging--a prospective trial. Eur J Cardiothorac Surg. 2010;37:1175-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Herth FJ, Krasnik M, Kahn N, Eberhardt R, Ernst A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest. 2010;138:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 33. | Oki M, Saka H, Kitagawa C, Kogure Y, Murata N, Adachi T, Ando M. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer: a randomized study. Respiration. 2013;85:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 34. | Nakajima T, Yasufuku K, Saegusa F, Fujiwara T, Sakairi Y, Hiroshima K, Nakatani Y, Yoshino I. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for nodal staging in patients with lung cancer. Ann Thorac Surg. 2013;95:1695-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 35. | Monaco SE, Schuchert MJ, Khalbuss WE. Diagnostic difficulties and pitfalls in rapid on-site evaluation of endobronchial ultrasound guided fine needle aspiration. Cytojournal. 2010;7:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Joseph M, Jones T, Lutterbie Y, Maygarden SJ, Feins RH, Haithcock BE, Veeramachaneni NK. Rapid on-site pathologic evaluation does not increase the efficacy of endobronchial ultrasonographic biopsy for mediastinal staging. Ann Thorac Surg. 2013;96:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | von Bartheld MB, van Breda A, Annema JT. Complication rate of endosonography (endobronchial and endoscopic ultrasound): a systematic review. Respiration. 2014;87:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Hammoud ZT, Anderson RC, Meyers BF, Guthrie TJ, Roper CL, Cooper JD, Patterson GA. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg. 1999;118:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 187] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Lemaire A, Nikolic I, Petersen T, Haney JC, Toloza EM, Harpole DH, D’Amico TA, Burfeind WR. Nine-year single center experience with cervical mediastinoscopy: complications and false negative rate. Ann Thorac Surg. 2006;82:1185-1189; discussion 1189-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Shrager JB. Mediastinoscopy: still the gold standard. Ann Thorac Surg. 2010;89:S2084-S2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Citak N, Buyukkale S, Kok A, Celikten A, Metin M, Sayar A, Gurses A. Does video-assisted mediastinoscopy offer lower false-negative rates for subcarinal lymph nodes compared with standard cervical mediastinoscopy. Thorac Cardiovasc Surg. 2014;62:624-630. [PubMed] |
| 42. | Annema JT, van Meerbeeck JP, Rintoul RC, Dooms C, Deschepper E, Dekkers OM, De Leyn P, Braun J, Carroll NR, Praet M. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. 2010;304:2245-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 378] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 43. | Zielinski M, Szlubowski A, Kołodziej M, Orzechowski S, Laczynska E, Pankowski J, Jakubiak M, Obrochta A. Comparison of endobronchial ultrasound and/or endoesophageal ultrasound with transcervical extended mediastinal lymphadenectomy for staging and restaging of non-small-cell lung cancer. J Thorac Oncol. 2013;8:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Yasufuku K, Nakajima T, Waddell T, Keshavjee S, Yoshino I. Endobronchial ultrasound-guided transbronchial needle aspiration for differentiating N0 versus N1 lung cancer. Ann Thorac Surg. 2013;96:1756-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Ginsberg RJ, Rice TW, Goldberg M, Waters PF, Schmocker BJ. Extended cervical mediastinoscopy. A single staging procedure for bronchogenic carcinoma of the left upper lobe. J Thorac Cardiovasc Surg. 1987;94:673-678. [PubMed] |
| 46. | Obiols C, Call S, Rami-Porta R, Iglesias M, Saumench R, Serra-Mitjans M, Gonzalez-Pont G, Belda J. Extended cervical mediastinoscopy: mature results of a clinical protocol for staging bronchogenic carcinoma of the left lung. Eur J Cardiothorac Surg. 2012;41:1043-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Jaklitsch MT, Gu L, Demmy T, Harpole DH, D’Amico TA, McKenna RJ, Krasna MJ, Kohman LJ, Swanson SJ, DeCamp MM. Prospective phase II trial of preresection thoracoscopic mediastinal restaging after neoadjuvant therapy for IIIA (N2) non-small cell lung cancer: results of CALGB Protocol 39803. J Thorac Cardiovasc Surg. 2013;146:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Hürtgen M, Friedel G, Toomes H, Fritz P. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA)--technique and first results. Eur J Cardiothorac Surg. 2002;21:348-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Zieliński M. Transcervical extended mediastinal lymphadenectomy: results of staging in two hundred fifty-six patients with non-small cell lung cancer. J Thorac Oncol. 2007;2:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Zieliński M. Technical pitfalls of transcervical extended mediastinal lymphadenectomy--how to avoid them and to manage intraoperative complications. Semin Thorac Cardiovasc Surg. 2010;22:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Kuzdzał J, Zieliński M, Papla B, Urbanik A, Wojciechowski W, Narski M, Szlubowski A, Hauer L. The transcervical extended mediastinal lymphadenectomy versus cervical mediastinoscopy in non-small cell lung cancer staging. Eur J Cardiothorac Surg. 2007;31:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Witte B, Wolf M, Huertgen M, Toomes H. Video-assisted mediastinoscopic surgery: clinical feasibility and accuracy of mediastinal lymph node staging. Ann Thorac Surg. 2006;82:1821-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Zieliński M. Video-assisted mediastinoscopic lymphadenectomy and transcervical extended mediastinal lymphadenectomy. Thorac Surg Clin. 2012;22:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Sawabata N, Keller SM, Matsumura A, Kawashima O, Hirono T, Osaka Y, Maeda H, Fukai S, Kawahara M. The impact of residual multi-level N2 disease after induction therapy for non-small cell lung cancer. Lung Cancer. 2003;42:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Bueno R, Richards WG, Swanson SJ, Jaklitsch MT, Lukanich JM, Mentzer SJ, Sugarbaker DJ. Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. Ann Thorac Surg. 2000;70:1826-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 177] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 56. | Decaluwé H, De Leyn P, Vansteenkiste J, Dooms C, Van Raemdonck D, Nafteux P, Coosemans W, Lerut T. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg. 2009;36:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 57. | Herth FJ, Annema JT, Eberhardt R, Yasufuku K, Ernst A, Krasnik M, Rintoul RC. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol. 2008;26:3346-3350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 58. | Szlubowski A, Herth FJ, Soja J, Kołodziej M, Figura J, Cmiel A, Obrochta A, Pankowski J. Endobronchial ultrasound-guided needle aspiration in non-small-cell lung cancer restaging verified by the transcervical bilateral extended mediastinal lymphadenectomy--a prospective study. Eur J Cardiothorac Surg. 2010;37:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Annema JT, Veseliç M, Versteegh MI, Willems LN, Rabe KF. Mediastinal restaging: EUS-FNA offers a new perspective. Lung Cancer. 2003;42:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 60. | von Bartheld MB, Versteegh MI, Braun J, Willems LN, Rabe KF, Annema JT. Transesophageal ultrasound-guided fine-needle aspiration for the mediastinal restaging of non-small cell lung cancer. J Thorac Oncol. 2011;6:1510-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Szlubowski A, Zieliński M, Soja J, Filarecka A, Orzechowski S, Pankowski J, Obrochta A, Jakubiak M, Węgrzyn J, Cmiel A. Accurate and safe mediastinal restaging by combined endobronchial and endoscopic ultrasound-guided needle aspiration performed by single ultrasound bronchoscope. Eur J Cardiothorac Surg. 2014;46:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | De Leyn P, Stroobants S, De Wever W, Lerut T, Coosemans W, Decker G, Nafteux P, Van Raemdonck D, Mortelmans L, Nackaerts K. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 Non-small-cell lung cancer: a Leuven Lung Cancer Group Study. J Clin Oncol. 2006;24:3333-3339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 63. | Zieliński M, Hauer L, Hauer J, Nabiałek T, Szlubowski A, Pankowski J. Non-small-cell lung cancer restaging with transcervical extended mediastinal lymphadenectomy. Eur J Cardiothorac Surg. 2010;37:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B, Zielinski M, Lerut T, Weder W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. 2006;30:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 503] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 65. | Wu Yl, Huang ZF, Wang SY, Yang XN, Ou W. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer. 2002;36:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 236] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 66. | Keller SM, Adak S, Wagner H, Johnson DH. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg. 2000;70:358-365; discussion 366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 239] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 67. | Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI, Jones DR, McKenna RJ, Landreneau RJ, Rusch VW. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 583] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 68. | Asamura H, Nakayama H, Kondo H, Tsuchiya R, Naruke T. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg. 1999;117:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Ma W, Zhang ZJ, Li Y, Ma GY, Zhang L. Comparison of lobe-specific mediastinal lymphadenectomy versus systematic mediastinal lymphadenectomy for clinical stage T1a N0 M0 non-small cell lung cancer. J Cancer Res Ther. 2013;9 Suppl 2:S101-S105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Shapiro M, Kadakia S, Lim J, Breglio A, Wisnivesky JP, Kaufman A, Lee DS, Flores RM. Lobe-specific mediastinal nodal dissection is sufficient during lobectomy by video-assisted thoracic surgery or thoracotomy for early-stage lung cancer. Chest. 2013;144:1615-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 71. | Maniwa T, Okumura T, Isaka M, Nakagawa K, Ohde Y, Kondo H. Recurrence of mediastinal node cancer after lobe-specific systematic nodal dissection for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2013;44:e59-e64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 72. | Tournoy KG, De Ryck F, Vanwalleghem LR, Vermassen F, Praet M, Aerts JG, Van Maele G, van Meerbeeck JP. Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: a randomized trial. Am J Respir Crit Care Med. 2008;177:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Vilmann P, Puri R. The complete “medical” mediastinoscopy (EUS-FNA + EBUS-TBNA). Minerva Med. 2007;98:331-338. [PubMed] |
| 74. | Rea HH, Shevland JE, House AJ. Accuracy of computed tomographic scanning in assessment of the mediastinum in bronchial carcinoma. J Thorac Cardiovasc Surg. 1981;81:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 75. | Modini C, Passariello R, Iascone C, Cicconetti F, Simonetti G, Zerilli M, Tirindelli-Danesi D, Stipa S. TNM staging in lung cancer: role of computed tomography. J Thorac Cardiovasc Surg. 1982;84:569-574. [PubMed] |