Published online Nov 28, 2013. doi: 10.5320/wjr.v3.i3.57
Revised: July 24, 2013
Accepted: August 16, 2013
Published online: November 28, 2013
Processing time: 165 Days and 18.7 Hours
The significant healthcare burden associated with chronic obstructive pulmonary disease (COPD) is driving us to improve our understanding of the natural history of this disease. Historically, the focus has been largely centred on diagnosing and treating individuals with moderate and severe disease. However, it is now recognised that the speed of decline in lung function as measured by forced expiratory volume in 1 s occurs faster in the earlier stages of the disease process. As a result, a clearer understanding of the potential benefits of treatment in early COPD is needed. It is recognised that many patients with COPD remain undiagnosed in the community which has prompted global case-finding initiatives. In this review we discuss the difficulties in diagnosing COPD in its early stages, examine the role of case-finding and look at the evidence for early intervention with therapeutic agents. There is a growing interest in the phenotypic variation amongst patients with COPD and we explore the role of phenotyping in early COPD and its potential benefits in providing a more individualised approach to COPD management. The majority of patients with COPD are known to die from non-respiratory causes such as cardiovascular disease. The mechanistic link is thought to relate to systemic inflammation, causing us to question whether earlier interventions could have a beneficial impact on the burden of co-morbidities for patients with COPD.
Core tip: In this review article we outline the difficulties in diagnosing chronic obstructive pulmonary disease (COPD) in its early stages and examine the role of case-finding initiatives. In addition we explore the evidence for early intervention with therapeutic agents and consider the impact of phenotyping in early disease, highlighting the potential benefits to a more individualized approach to COPD management.
- Citation: Brebner JA, Turner AM. Early chronic obstructive pulmonary disease: Beyond spirometry. World J Respirol 2013; 3(3): 57-66
- URL: https://www.wjgnet.com/2218-6255/full/v3/i3/57.htm
- DOI: https://dx.doi.org/10.5320/wjr.v3.i3.57
Chronic obstructive pulmonary disease (COPD) is a major global public health problem with a significant associated economic burden on both developing and higher income countries. In 2004, COPD was the fourth leading cause of death worldwide[1] and due to projected increases in tobacco use, it has been predicted by the World Health Organisation that it will become the third leading cause by 2030[2]. The Global Initiative for COPD (GOLD) has defined it as “a common preventable and treatable disease which is characterised by persistent airflow limitation that is usually progressive and associated with enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases”[3]. The pathophysiology of COPD is complex resulting from a variety of gene-environment interactions and there is considerable phenotypic heterogeneity expressed amongst disease sufferers[4].
There remains a lack of therapeutic interventions with strong evidence of disease modifying or curative potential, so early diagnosis and prevention strategies including smoking cessation are essential in our approach to COPD healthcare. Within the last decade studies have shown the prevalence of COPD to be much higher than previously realised[5,6] and the proportion of patients with COPD that remain undiagnosed has been reported to be as high as 66%-73%[7,8]. This has stemmed interest in “case-finding” initiatives by screening high risk individuals with the potential to diagnose COPD early and in some cases even before symptoms develop. As a result, it is likely that the number of patients diagnosed with “early” or “mild” disease is likely to increase, amplifying the need for clear management strategies for this cohort of COPD patients. In this article we will explore the definition of “early COPD” and examine the emerging concepts with respect to disease classification, monitoring of disease progression and therapeutic interventions, highlighting current controversies within these fields.
Spirometry is the primary tool utilised by respiratory physicians worldwide in the diagnosis of COPD. The GOLD and joint American Thoracic Society and European Respiratory Society guidelines advise physicians to consider spirometry in patients presenting with symptoms of chronic cough, dyspnoea or sputum production with a history of exposure to a risk factor such as smoking or occupational dust. In the context of such symptoms, the presence of a post-bronchodilator FEV1/FVC (forced expiratory volume in 1 s/forced vital capacity) ratio < 0.7 demonstrates incompletely reversible airflow limitation and hence a diagnosis of COPD[3,9]. The FEV1 as a percentage of its predicted value for the patient’s sex, age and height is used to sub-classify patients with respect to the severity of their airflow obstruction (mild FEV1≥ 80%, moderate ≥ 50% FEV1 < 80%, severe ≥ 30% FEV1 < 50%, very severe ≤ 30%)[3]. Advances in technology including the advent of simple to use hand held spirometers makes it a convenient test which can be easily performed in the outpatient clinical setting however, it is important to also recognise the potential drawbacks of using the fixed “FEV1/FVC ratio < 0.7” approach to diagnosing COPD.
Although this fixed cut-off is easy to remember it does not take in to consideration the fact that the FEV1/FVC ratio reduces with age[10] resulting in the potential over-diagnosis of COPD in the elderly population. Hardie et al[11] found 35% of healthy elderly never smokers to have a pre-bronchodilator FEV1/FVC ratio of less than 0.7, increasing to 50% of those over 80 years of age. Other approaches to interpreting spirometric values have therefore been advocated, in particular the use of statistically derived “lower limit of normal (LLN)”reference values[12]. In comparison to the fixed ratio method this has been shown to reduce the number of people potentially misclassified as having significant airflow obstruction[13-15]. The FEV1/FVC fixed ratio cut-off has also been found to underestimate airflow obstruction in younger adults[16]. This has the potential to create missed opportunities for early intervention and may impact on the success of smoking cessation[17].
Another potential pitfall of diagnosing COPD on the basis of spirometry alone is the recognition that emphysema and airflow obstruction do not necessarily go hand in hand, particularly in the early stages of disease. In a study of 80 current smokers who underwent high resolution computed tomography (HRCT) scanning and lung function tests, 20 were found to have radiological emphysema but only 5 of these subjects had evidence of airflow obstruction (defined by the authors as a low FEV1 and/or a low MEF50 using LLN cut-off)[18]. A more recent study followed up current and heavy smokers who had participated in a lung cancer screening trial. 1391 individuals had no evidence of airflow obstruction at baseline (FEV1/FVC > 0.7) but 21.9% progressed to developing obstruction over a mean period of 3 years. More severe baseline radiological emphysema (quantified by a lower Perc15 value) was found to be a risk factor for developing airflow obstruction at follow up[19]. The radiation and cost involved in performing HRCT scanning are likely to limit its utility in the early investigation of patients in clinical practice and many of the patients in these studies were asymptomatic. However, it does serve to highlight the point that radiological evidence of smoking related lung damage and lung function parameters can be discordant particularly in “early COPD” and this could potentially cause smokers with normal spirometry to be falsely reassured if no other investigations are undertaken.
The recognition that the majority of individuals with COPD remain undiagnosed has focused attention on methods of identifying these so called “missing millions” through case finding strategies[20-25]. There is a growing body of literature looking at different methods including utilisation of questionnaires to identify “at risk” patients and other screening tools such as peak flow (PEF) and spirometry, however at present there is no consensus of opinion on the best approach. Jithoo et al[22] used data from the Burden of Obstructive Lung Disease Study to compare combinations of questionnaire, PEF and spirometry strategies and concluded the pre- bronchodilator PEF followed by confirmatory spirometry to be the most cost effective approach. However this study was aimed only at the detection of moderate/severe COPD. Their reasoning for this was that the management of milder disease is only smoking cessation which should be offered to all smokers and the lack of proven benefit of pharmacological interventions in mild disease. Indeed one of the recommendations from a National Heart, Lung and Blood Institute workshop created to address case finding strategies in the United States was to target those with moderate to severe COPD[26]. Focus of attention on those with more severe disease is often highlighted due to the higher morbidity and mortality, the greater proven benefit of treatments and interventions for these groups and hence improved cost-effectiveness of a targeted approach. However, are we forgetting that all these individuals will previously have had mild disease? Could a more aggressive approach at an earlier stage not impact on the disease burden later on?
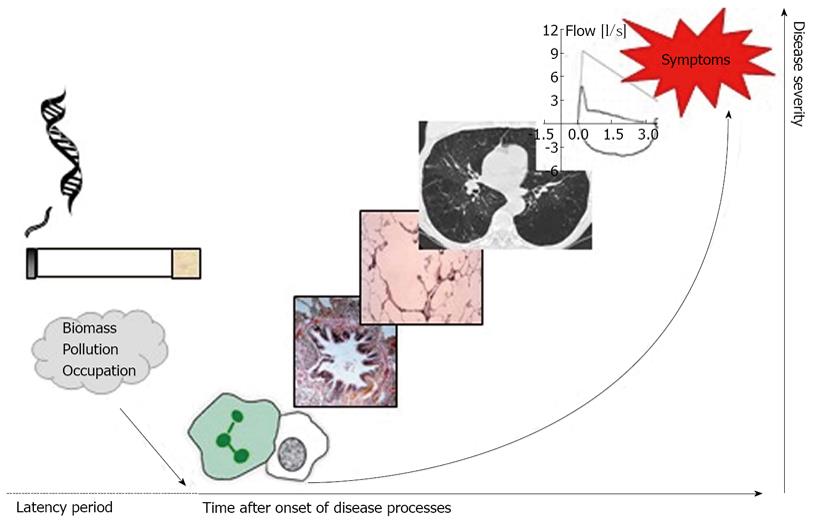
A previous review looking at the future for COPD made the important point that the terms “mild” and “early” COPD are not necessarily interchangeable given the vast variability in lung function decline in different individuals. The author highlighted the point that mild airflow obstruction in an older individual does not necessarily mean they have “early” disease[27]. The complexities of the pathophysiology of COPD and wide phenotypic variation means that at present we have no clear way of easily identifying patients early in the disease course, however a growing interest in developing biomarkers may serve to solve this problem in the future[28-30]. Decramer et al[31] proposed a concept of disease progression in COPD highlighting that physiological abnormalities are not necessarily present in the early stages of the disease process and that to truly identify patients with “early” COPD we need alternative diagnostic methods. This concept is shown in Figure 1.
The potential benefits and role of case finding in diagnosing patients with mild COPD does appear to be splitting opinion amongst healthcare professionals[32,33]. Some feel strongly that putting emphasis on diagnosing and treating mild COPD has the potential to divert limited resources away from interventions such as smoking cessation and from providing care for patients with “clinically-important” COPD[32]. Unfortunately we lack evidence from large randomized controlled studies (RCTs) that show efficacy of treatment in patients with mild COPD, and to meet the basic principles of a screening test we need to question “Does treatment of the developed clinical condition at an earlier stage than normal affect its course and prognosis?”[25]. As yet, we cannot prove that treatment of symptoms earlier in the disease prolongs life or quality adjusted life years conclusively, nor has any of the major drug trials proven reduced FEV1 decline in milder disease. Nevertheless appropriate interventional studies in milder disease are likely to occur over the next few years, such that the concept of earlier treatment may become better supported by RCT evidence, and ultimately support a screening based approach.
Symptoms, exacerbations and disease progression: There is considerable variability between individuals with respect to the timing of onset and nature of symptoms in early COPD. There can also often be discordance between symptomatic burden and severity of airflow limitation. A study specifically designed to explore whether symptoms predict the presence of COPD found that 92% of the smokers with airflow obstruction reported symptoms such as cough, dyspnoea, sputum production and wheeze. However, symptoms were also reported in 76% of smokers with normal spirometry[34]. The study concluded that the presence of symptoms added little to the predictive value of spirometry beyond age and smoking history but didn’t address the interesting question of whether the symptomatic smokers with normal spirometry are more likely to go on to develop airflow obstruction. The initial GOLD guidelines classified these individuals as GOLD stage 0 “at risk” however this classification has been removed from the more recent versions of the guidelines[3,35]. Interestingly, Miravitlles et al[8] found that these individuals have a significantly impaired health-related quality of life (QOL) compared to control subjects without COPD. A study aimed at validating whether individuals classified as GOLD stage 0 do go on to develop COPD, found that stage 0 was associated with a risk of excess FEV1 decline, but multivariate logistic regression analyses found the effect of GOLD stage 0 on the likelihood of developing COPD to be small[36]. One of the likely reasons for this was that it was found to be an “unstable” feature, as after 15 years follow up many individuals with stage 0 at baseline had subsequent resolution of symptoms and only 49.4% were still classified as having at least stage 0 disease. This study highlighted the need for us to be able to identify smokers “at particular risk” as assessing symptoms alone appears to be an inadequate predictor.
Large case finding studies are also highlighting the presence of undiagnosed, asymptomatic smokers with airflow obstruction. One such study in China reported that 35.5% of those diagnosed with COPD were asymptomatic and unsurprisingly over 90% of these individuals had no previous diagnosis[37]. The authors discussed the concept that common symptoms in COPD-cough, wheeze, sputum production and dyspnoea may represent different pathological processes such as airway hyperresponsiveness, mucus hypersecretion and emphysema. An individual’s perception of breathlessness in particular may be difficult to assess as those with early disease may unknowingly adapt to their dyspnoea symptoms or attribute this to being ‘‘unfit’’ or ageing. For these reasons some studies advocate that we should be screening all smokers over the age of 40 irrespective of symptoms[34,37]. It is also not clear whether certain symptoms are more likely to make patients seek healthcare advice - for example we might question whether “asymptomatic” COPD patients are more likely to have a “emphysema predominant” phenotype and therefore not be troubled by chronic sputum production and wheeze.
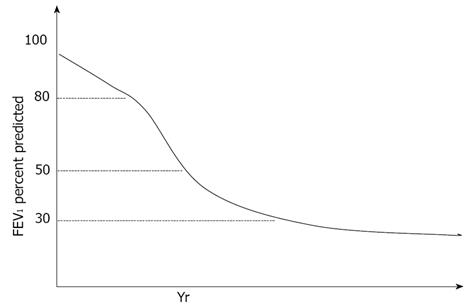
The ABCD assessment approach in the most recent GOLD guideline update recognises the importance of symptoms and exacerbation history when assessing a patient with established COPD and examining their future risk of exacerbations and decline[3]. Bridevaux et al[38] demonstrated in a population based cohort study that the presence of symptoms in individuals with GOLD stage 1 airflow obstruction has a significant impact on FEV1 decline, healthcare utilisation and quality of life measures. FEV1 decline is the most commonly used method for assessing COPD disease progression, and contrary to the classical belief that FEV1 decline accelerates with more advanced disease, it is now recognised that FEV1 decline is in fact faster in the earlier stages particularly GOLD stage 2 (Figure 2)[39]. It is therefore logical that we should be focusing more on investigating treatment at the earlier stages of COPD to impact on the natural history of the disease. The data available on FEV1 decline in mild disease is limited and further large scale prospective trials are needed to better evaluate this.
It is also apparent that there is a wide variation amongst individuals with respect to the rate of FEV1 decline stemming interest in finding ways to identify the so-called “rapid decliners” early, including the use of biomarkers and investigation for specific genetic variations[40]. However further evidence is needed to establish if early interventions can have a positive impact on disease progression in these individuals.
More advanced phenotyping methods: The concept of COPD as a heterogeneous disease is now well established, and assessing patients by simply examining the degree of airflow limitation is inadequate in reflecting this[41]. The concept of COPD ‘‘phenotypes’’ is not new, however there has been difficulty in creating clear, clinically relevant definitions. Han et al[42] emphasised that phenotyping patients should ideally provide prognostic information and defined a COPD phenotype as “a single or combination of disease attributes that describe individuals with COPD as they relate to clinically meaningful outcomes (symptoms, exacerbations, response to therapy, rate of disease progression or death)”. This provokes the interesting question of whether we should be aiming to phenotype patients with early COPD and by doing so whether we may be able to impact on their prognosis by having a more individualised approach to therapeutic strategies and interventions. They, and others, advocated the use of more detailed physiological tests as well as potentially CT scanning and biomarkers to define subgroups.
Initial work looking at proportions of COPD phenotypes and degrees of “overlap” focused on the three main subgroups of chronic bronchitis, emphysema and asthma[43]. For many Respiratory Physicians the “asthma overlap” group can often cause a diagnostic and therapeutic quandary and the true prevalence of this mixed phenotype is unknown. The fact that these patients are frequently excluded from both asthma and COPD studies means we may be doing these patients a disservice by blindly following COPD therapeutic guidelines based on evidence that may not be generalizable to this particular subgroup[44]. They will therefore be an important group for future RCTs to focus on, or provide as pre-specified subgroup analyses.
Other commonly discussed COPD phenotypes include the “frequent exacerbators”[45] and so-called “systemic inflammatory response”[46] patients. Frequent exacerbators are usually defined as those with more than 2 exacerbations per year, as used in the ECLIPSE study. The systemic inflammation group are of particular interest due to potential links with the development of comorbidities which will be discussed later. The validity of these subgroups will require prospective longitudinal studies and further research is needed to establish if they relate to differing underlying pathological processes. However, it is also realised that this method of defining phenotypes does rely on “a priori assumptions” with the potential for introducing bias[47]. This has spurred on a recent move towards utilising statistical methods such as principal component and cluster analyses as alternative approaches of identifying potential candidate phenotypes[47-50], although this has not been conducted specifically in a mild or early disease population.
Smoking cessation is an essential part of the treatment for early COPD, and is advocated unreservedly due to the strength of evidence for many health outcomes. Successful smoking cessation has been shown to halve the rate of FEV1 decline in patients with mild to moderate COPD returning it to a level comparable with never smokers[51]. However, it has also been demonstrated that inflammation can persist within the lung many years after smoking cessation causing speculation of an autoimmune or immune activating component to COPD[52-54]. Other treatments may also be worth considering; the evidence base for these is considered below.
Inhaled therapy: There is a paucity of large RCTs aimed specifically at effects of drug treatment in early COPD. Much of the evidence of potential benefit has been extrapolated from sub-group analyses of patients from large trials aimed primarily at patients with moderate to severe disease.
Bronchodilators: In early COPD, the mainstay of pharmacological treatment advised by the current guidelines for symptomatic patients is inhaled bronchodilator therapy in the form of short and long acting β2-agonists (LABA) and antimuscarinics (LAMA)[3]. The UPLIFT study examined the long-term effects of treatment with the LAMA tiotropium compared to placebo and found it reduced exacerbations and hospitalisations, improved lung function parameters and QOL but did not significantly reduce the rate of decline in FEV1[55]. However subsequent analyses looking specifically at the subgroup of patients with GOLD stage 2 disease found the rate of decline in the post-bronchodilator FEV1 to be lower in the tiotropium group compared to placebo[56]. The effect was small, calling into question the clinical relevance however it did raise the possibility that early intervention with LAMAs may have the potential to affect the course of disease in patients with moderate COPD. One limitation of this post-hoc analysis was that it only looked at a subcategory of GOLD stage 2 patients with an FEV1 of ≤ 70%. When a further analysis of individuals with an FEV1 of 60%-78% was undertaken, a trend for reduction in post-bronchodilator FEV1 decline was still seen (41 mL/year in tiotropium group, 49 mL/year in placebo group) but this no longer met statistical significance (P = 0.07)[57]. Significant improvements in symptoms, a reduction in exacerbation frequency and mortality were demonstrated however, supporting the use of tiotropium in patients with moderate airflow obstruction.
A small RCT specifically looking at efficacy in mild to moderate disease (FEV1≥ 60%) found that over a 12 wk period tiotropium significantly improved FEV1 and FVC compared to placebo[58]. Significant improvements in dyspnoea and QOL were not seen, perhaps due to study limitations such as the questionnaires used, sample size and length of study. Bronchodilator therapy may also benefit lung function in symptomatic GOLD stage 1 patients: in response to the administration of nebulised ipratropium bromide, O’Donnell et al[59] demonstrated improvements in forced expiratory flow rates, reduction in residual volumes and specific airway resistance and found a reduction in end expiratory lung volumes which was associated with less severe dyspnoea during exercise.
LABAs became a widely used treatment for COPD following studies that demonstrated their ability to improve lung function and provide symptomatic benefit to patients[60,61]. The most commonly used agents are salmeterol and formoterol, which are both twice daily LABAs with a 12 h duration of action. However, none of the major RCTs examining their efficacy in COPD included patients with an FEV1 of greater than 70% predicted[60-69]. It therefore remains unknown whether they could provide any benefit to patients with symptomatic GOLD stage 1 disease. Combining different classes of bronchodilators is also advocated in the guidelines and deemed preferential to increasing doses of a single agent due the potential for side effects. In recent years new ‘Ultra-LABAs’ such as indacterol and vilanterol have been developed which have a 24 h duration of action[70]. Clinical trials investigating their use in combination with once daily LAMAs are currently underway[71]. One small RCT published in 2010 assessed the efficacy of QVA149 (a once daily combination inhaler containing the LABA indacterol and LAMA glycopyrronium) and demonstrated significant improvements in FEV1 compared to monotherapy with indacterol or placebo. Again, GOLD stage 1 individuals were not included in this trial. However, it is interesting to speculate whether in the future these once-daily combination bronchodilators could play a role for patients with mild disease who remain symptomatic despite monotherapy with LAMAs or LABAs. The once daily administration is likely to be advantageous in terms of compliance and there may be less concern over the side effect profile in comparison to using inhaled corticosteroids.
One question that remains unanswered is whether patients found through case-finding initiatives with asymptomatic airflow obstruction should be commenced on bronchodilator therapy. There is no evidence to support this approach at present and issues of compliance with therapy and cost-effectiveness are likely to have an impact on treatment decisions for this cohort. However if future studies demonstrate the reduction in exacerbation frequency and mortality seen in the post-hoc analyses from the UPLIFT trial[57] then a more aggressive treatment approach may be warranted.
Inhaled corticosteroids: The current guidelines do not advocate the use of monotherapy with inhaled corticosteroids (ICS) in the treatment of COPD[3,9,72]. The Lung Health Study demonstrated that treatment with the ICS triamcinolone is associated with a reduction in airway reactivity and respiratory symptoms in patients with an FEV1 of 30%-90% predicted, however, there was no impact on FEV1 decline[73]. Similarly another placebo controlled trial examining the efficacy of inhaled budesonide in patients with an FEV1≥ 50% (corresponding to GOLD stage 1 and 2) who continued to smoke found that ICS use was associated with an initial increase in FEV1 but no effect on long term decline[74].
Combination inhalers containing ICS and LABA are in widespread use for COPD patients with more advanced disease, and evidence of their potential benefit at earlier stages appears to be building. The TORCH study was a large multicentre, randomised, placebo controlled trial examining the effect of combination LABA/ICS inhaler therapy (salmeterol and fluticasone proprionate) on survival in COPD[65]. One of the inclusion criteria was a pre-bronchodilator FEV1 of ≤ 60% however a post-hoc analysis established the benefits of a reduction in exacerbations, improved health status and FEV1 applied to patients across all the GOLD stages included in the study and therefore promoted it as an effective treatment for GOLD stage 2 disease[75]. However it is important to note that only GOLD stage 2 patients with an FEV1 of 50%-60% predicted were included in this study. A subgroup analysis from a smaller RCT comparing “triple therapy” with fluticasone/salmeterol and tiotropium vs monotherapy with tiotropium revealed that triple therapy was associated with a significant improvement in FEV1 and QOL scores with no increase in adverse events in GOLD stage 2 patients[76]. Neither of these studies included GOLD stage 1 patients but a recent study specifically aimed at examining the physiological derangements and impact of combination LABA/ICS treatment in mild to moderate COPD found there were significant improvements in airway function as measured by FEV1, functional residual capacity, inspiratory capacity and specific airways resistance both at rest and during exercise[77]. However these improvements did not translate into any symptomatic benefit in terms of dyspnoea or improved exercise tolerance.
One important consideration with ICS and ICS/LABA combination therapies is concern over the potential side effects such as reduction in bone mineral density, bruising and an increased risk of pneumonia[65,78,79]. In weighing up the risks and benefits for patients with mild disease we are hampered by the lack of firm evidence of benefit.
Will phenotyping alter our treatment approach? Proof of differential responses of COPD subgroups to therapeutic interventions is emerging. For example, in a subset of patients with chronic bronchitis and severe COPD, Roflumilast (a phostphodiesterase-4 inhibitor) has been shown to reduce exacerbation frequency and improve lung function[80]. Another study demonstrated that there was a greater reduction in the odds of exacerbations (45% vs 25%) in stable chronic bronchitis patients following the use of pulsed moxifloxacin if they had purulent or mucopurulent sputum at baseline. The presence of chronic bronchitis is known to be associated with a higher risk of exacerbations[81] which in turn puts patients at risk of a more rapid decline in lung function[82] which emphasises the clinical relevance of these findings. A subgroup of COPD patients that have been shown to gain greater benefit from higher dose ICS therapy are those with sputum eosinophilia[83-85], a controversial phenotype for many, and one which is key to differentiate from asthma. A recent article promoting the concept of treating by phenotype discusses the fact that COPD-asthma overlap patients should be treated with ICS in addition to LABAs irrespective of the severity of airflow obstruction[86]. Circulating eosinophils have also been found to predict response to steroid treatment during COPD exacerbations[87]; this marker may be more practical for many centres to use than sputum eosinophilia.
As with the majority of therapeutic studies in COPD, patients with mild disease were generally excluded from the studies discussed above, however the results should certainly cause us to question whether early characterisation of COPD patients with respect to phenotype using relatively simple methods such as history taking for chronic bronchitis symptoms, sputum colour charts and sputum induction for cell count analysis could allow for an earlier targeted approach to management with the potential to make a significant impact on the clinical course of the disease.
Systemic inflammatory response and comorbidities in early COPD: The systemic consequences of the chronic inflammatory response in COPD, means that it can no longer be considered a disease that only affects the lungs[88]. Similar to other chronic conditions such as diabetes, we should take a multisystem, holistic approach to assessing patients[89]. Previous studies have shown that the majority of patients with COPD die from non-respiratory causes[90,91]. Mannino et al[90] found that the increased risk of all-cause mortality spans all GOLD stages including individuals with respiratory symptoms but no airflow obstruction (previously GOLD stage 0). Indeed the majority of individuals with mild to moderate COPD died from cardiovascular or other causes. This study did not account for smoking, however there is evidence that demonstrates the link between COPD and co-morbidities, in particular cardiovascular disease goes beyond such common aetiological factors[92]. The mechanistic link is thought to relate to systemic inflammation[93]. Although the prevalence of systemic effects such as skeletal muscle dysfunction, osteoporosis and other co-morbidities increase along with the severity of airflow obstruction, it is recognised that they can occur even in the earlier stages of disease[94]. It remains unknown whether targeting treatment of systemic inflammation has the potential to influence the natural history of COPD[42] or indeed prevent the development of systemic complications. Some anti-inflammatory treatments, such as corticosteroids, have the potential to cause or worsen co-morbidities such as osteoporosis, diabetes and obesity. However new treatment paradigms are evolving with the development of novel agents and a growing interest in drugs with anti-inflammatory properties that are already licensed for other indications such as statins and macrolides[95]. Whether early treatment with anti-inflammatory drugs could improve outcomes for patients with COPD remains unknown but is an interesting prospect for future research.
To make a significant impact on the high global morbidity and mortality statistics related to COPD, it seems logical that we should move our focus to better understanding the early course of the disease. Studies in the last decade have changed our perspective of the natural history of COPD, and it is now evident that the rate of decline in lung function in the earlier stages is greater than previously realised. The early development of co-morbidities, impaired QOL and increased risk of mortality of patients with FEV1 > 50% predicted means we should not simply prescribe salbutamol and wait for these individuals to deteriorate. Symptoms may be inadequate to assess these mild patients, some of whom may also be early in their disease course; ideally we need biomarkers or genetic markers that can identify individuals at risk before significant physiological abnormalities become apparent. This information could be an additional tool in driving smoking cessation and lifestyle modification[96].
To date the majority of evidence relating to treatment in early COPD is from retrospective post-hoc analyses and there is a clear need of large scale prospective trials adequately powered to look at phenotypic subgroups. This would be in line the general move towards a more ‘individualised’ approach to treating patients with COPD, which requires a detailed assessment of disease severity, disease activity and impact on the individual. This can then allow us to tailor our therapeutic approach to maximise the benefit to the patient and hopefully impact on disease progression rather than taking a ‘‘one size fits all’’ approach to prescribing in early disease.
P- Reviewers: Araya J, Chen YH, Hattori N, Iftikhar IH S- Editor: Wen LL L- Editor: A E- Editor: Wang CH
| 1. | World Health Organization. The Global Burden of Disease. Available from: http: //www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html. |
| 2. | World Health Organization. World Health Statistics. Available from: http: //www.who.int/whosis/whostat/2008/en/index.html. |
| 3. | Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for the diagnosis, managment and prevention of chronic obstructive pulmonary disease: updated 2013. Available from: http: //www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. |
| 4. | Garcia-Aymerich J, Agustí A, Barberà JA, Belda J, Farrero E, Ferrer A, Ferrer J, Gáldiz JB, Gea J, Gómez FP. Phenotypic heterogeneity of chronic obstructive pulmonary disease. Arch Bronconeumol. 2009;45:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1397] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 6. | Menezes AM, Perez-Padilla R, Jardim JR, Muiño A, Lopez MV, Valdivia G, Montes de Oca M, Talamo C, Hallal PC, Victora CG. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 568] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 7. | Hvidsten SC, Storesund L, Wentzel-Larsen T, Gulsvik A, Lehmann S. Prevalence and predictors of undiagnosed chronic obstructive pulmonary disease in a Norwegian adult general population. Clin Respir J. 2010;4:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Miravitlles M, Soriano JB, García-Río F, Muñoz L, Duran-Tauleria E, Sanchez G, Sobradillo V, Ancochea J. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 454] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 9. | American Thoracic Society and European Respiratory Society. Standards for the diagnosis and managment of patients with COPD. Available from: http: //www.thoracic.org/clinical/copd-guidelines/resources/copddoc.pdf. |
| 10. | Stanojevic S, Wade A, Stocks J, Hankinson J, Coates AL, Pan H, Rosenthal M, Corey M, Lebecque P, Cole TJ. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med. 2008;177:253-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 498] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 11. | Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Mørkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002;20:1117-1122. [PubMed] |
| 12. | Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3399] [Cited by in RCA: 3858] [Article Influence: 203.1] [Reference Citation Analysis (0)] |
| 13. | Roberts SD, Farber MO, Knox KS, Phillips GS, Bhatt NY, Mastronarde JG, Wood KL. FEV1/FVC ratio of 70% misclassifies patients with obstruction at the extremes of age. Chest. 2006;130:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Schermer TR, Smeele IJ, Thoonen BP, Lucas AE, Grootens JG, van Boxem TJ, Heijdra YF, van Weel C. Current clinical guideline definitions of airflow obstruction and COPD overdiagnosis in primary care. Eur Respir J. 2008;32:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, Jensen RL, Falaschetti E, Schouten JP, Hankinson JL, Stocks J, Quanjer PH. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 344] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 16. | Cerveri I, Corsico AG, Accordini S, Niniano R, Ansaldo E, Antó JM, Künzli N, Janson C, Sunyer J, Jarvis D. Underestimation of airflow obstruction among young adults using FEV1/FVC & lt; 70% as a fixed cut-off: a longitudinal evaluation of clinical and functional outcomes. Thorax. 2008;63:1040-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Bednarek M, Gorecka D, Wielgomas J, Czajkowska-Malinowska M, Regula J, Mieszko-Filipczyk G, Jasionowicz M, Bijata-Bronisz R, Lempicka-Jastrzebska M, Czajkowski M. Smokers with airway obstruction are more likely to quit smoking. Thorax. 2006;61:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Clark KD, Wardrobe-Wong N, Elliott JJ, Gill PT, Tait NP, Snashall PD. Patterns of lung disease in a “normal” smoking population: are emphysema and airflow obstruction found together? Chest. 2001;120:743-747. [PubMed] |
| 19. | Mohamed Hoesein FA, de Hoop B, Zanen P, Gietema H, Kruitwagen CL, van Ginneken B, Isgum I, Mol C, van Klaveren RJ, Dijkstra AE. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011;66:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Falzon C, Soljak M, Elkin SL, Blake ID, Hopkinson NS. Finding the missing millions - the impact of a locally enhanced service for COPD on current and projected rates of diagnosis: a population-based prevalence study using interrupted time series analysis. Prim Care Respir J. 2013;22:59-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Haroon S, Adab P, Griffin C, Jordan R. Case finding for chronic obstructive pulmonary disease in primary care: a pilot randomised controlled trial. Br J Gen Pract. 2013;63:e55-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Jithoo A, Enright PL, Burney P, Buist AS, Bateman ED, Tan WC, Studnicka M, Mejza F, Gillespie S, Vollmer WM. Case-finding options for COPD: results from the Burden of Obstructive Lung Disease study. Eur Respir J. 2013;41:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Jordan RE, Lam KB, Cheng KK, Miller MR, Marsh JL, Ayres JG, Fitzmaurice D, Adab P. Case finding for chronic obstructive pulmonary disease: a model for optimising a targeted approach. Thorax. 2010;65:492-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Lange P, Marott JL, Dahl M, Ingebrigtsen TS, Vestbo J, Nordestgaard BG. Substantial need for early diagnosis, rehabilitation and treatment of chronic obstructive pulmonary disease. Dan Med J. 2012;59:A4396. [PubMed] |
| 25. | Wilson JM, Jungner YG. Principles and practice of mass screening for disease. Bol Oficina Sanit Panam. 1968;65:281-393. [PubMed] |
| 26. | NHLBI Workshop. A Case-finding Strategy for Moderate-to-Severe COPD in the United States. Available from: http: //www.nhlbi.nih.gov/meetings/workshops/case-finding-exesum.htm. |
| 27. | Agustí A, Vestbo J. Current controversies and future perspectives in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Casaburi R, Celli B, Crapo J, Criner G, Croxton T, Gaw A, Jones P, Kline-Leidy N, Lomas DA, Merrill D. The COPD Biomarker Qualification Consortium (CBQC). COPD. 2013;10:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Kostikas K, Bakakos P, Papiris S, Stolz D, Celli BR. Systemic biomarkers in the evaluation and management of COPD patients: are we getting closer to clinical application? Curr Drug Targets. 2013;14:177-191. [PubMed] [DOI] [Full Text] |
| 30. | Stockley RA. Biomarkers in COPD: time for a deep breath. Thorax. 2007;62:657-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Decramer M, Cooper CB. Treatment of COPD: the sooner the better? Thorax. 2010;65:837-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Enright P, White P. Detecting mild COPD: don’t waste resources. Prim Care Respir J. 2011;20:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Price D, Freeman D, Cleland J, Kaplan A, Cerasoli F. Earlier diagnosis and earlier treatment of COPD in primary care. Prim Care Respir J. 2011;20:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Ohar JA, Sadeghnejad A, Meyers DA, Donohue JF, Bleecker ER. Do symptoms predict COPD in smokers? Chest. 2010;137:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for the diagnosis, managment and prevention of chronic obstructive pulmonary disease. Available from: http: //www.goldcopd.org/uploads/users/files/GOLDWkshp2001.pdf. |
| 36. | Vestbo J, Lange P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2002;166:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Lu M, Yao WZ, Zhong NS, Zhou YM, Wang C, Chen P, Kang J, Huang SG, Chen BY, Wang CZ. Asymptomatic patients of chronic obstructive pulmonary disease in China. Chin Med J (Engl). 2010;123:1494-1499. [PubMed] |
| 38. | Bridevaux PO, Gerbase MW, Probst-Hensch NM, Schindler C, Gaspoz JM, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax. 2008;63:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 40. | Tashkin DP. Variations in FEV₁ decline over time in chronic obstructive pulmonary disease and its implications. Curr Opin Pulm Med. 2013;19:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, MacNee W, Miller BE, Rennard S, Silverman EK. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 816] [Cited by in RCA: 829] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 42. | Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 723] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 43. | Marsh SE, Travers J, Weatherall M, Williams MV, Aldington S, Shirtcliffe PM, Hansell AL, Nowitz MR, McNaughton AA, Soriano JB. Proportional classifications of COPD phenotypes. Thorax. 2008;63:761-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 44. | Miravitlles M, Calle M, Soler-Cataluña JJ. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol. 2012;48:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 45. | Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2034] [Cited by in RCA: 2018] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 46. | Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, Vestbo J, Lomas DA, Calverley PM, Wouters E. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7:e37483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 593] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 47. | Fingleton J, Weatherall M, Beasley R. Towards individualised treatment in COPD. Thorax. 2011;66:363-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Burgel PR, Paillasseur JL, Caillaud D, Tillie-Leblond I, Chanez P, Escamilla R, Court-Fortune I, Perez T, Carré P, Roche N. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. Eur Respir J. 2010;36:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 49. | Roy K, Smith J, Kolsum U, Borrill Z, Vestbo J, Singh D. COPD phenotype description using principal components analysis. Respir Res. 2009;10:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Weatherall M, Shirtcliffe P, Travers J, Beasley R. Use of cluster analysis to define COPD phenotypes. Eur Respir J. 2010;36:472-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Agustí A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax. 2003;58:832-834. [PubMed] |
| 53. | Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 585] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 54. | Stefanska AM, Walsh PT. Chronic obstructive pulmonary disease: evidence for an autoimmune component. Cell Mol Immunol. 2009;6:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1404] [Cited by in RCA: 1484] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 56. | Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 57. | Tashkin DP, Celli BR, Decramer M, Lystig T, Liu D, Kesten S. Efficacy of tiotropium in COPD patients with FEV1 ≥ 60% participating in the UPLIFT® trial. COPD. 2012;9:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Johansson G, Lindberg A, Romberg K, Nordström L, Gerken F, Roquet A. Bronchodilator efficacy of tiotropium in patients with mild to moderate COPD. Prim Care Respir J. 2008;17:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | O’Donnell DE, Laveneziana P, Ora J, Webb KA, Lam YM, Ofir D. Evaluation of acute bronchodilator reversibility in patients with symptoms of GOLD stage I COPD. Thorax. 2009;64:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 60. | Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821. [PubMed] |
| 61. | Mahler DA, Donohue JF, Barbee RA, Goldman MD, Gross NJ, Wisniewski ME, Yancey SW, Zakes BA, Rickard KA, Anderson WH. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957-965. [PubMed] |
| 62. | Aalbers R, Ayres J, Backer V, Decramer M, Lier PA, Magyar P, Malolepszy J, Ruffin R, Sybrecht GW. Formoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3-month trial. Eur Respir J. 2002;19:936-943. [PubMed] |
| 63. | Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 769] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 64. | Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22:912-919. [PubMed] |
| 65. | Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. [PubMed] |
| 66. | Dahl R, Greefhorst LA, Nowak D, Nonikov V, Byrne AM, Thomson MH, Till D, Della Cioppa G. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 67. | Rennard SI, Anderson W, ZuWallack R, Broughton J, Bailey W, Friedman M, Wisniewski M, Rickard K. Use of a long-acting inhaled beta2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 68. | Rossi A, Kristufek P, Levine BE, Thomson MH, Till D, Kottakis J, Della Cioppa G. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069. [PubMed] |
| 69. | Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, Peterson S, Olsson H. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81. [PubMed] |
| 70. | Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res. 2010;11:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 71. | Available from: http: //www.clinicaltrials.gov/. 2013. 14-6-2013. |
| 72. | NICE clinical guideline. Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care. Available from: http: //www.nice.org.uk/nicemedia/live/13029/49425/49425.pdf. |
| 73. | Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 501] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 74. | Pauwels RA, Löfdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, Ohlsson SV. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 648] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 75. | Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, Yates JC, Willits LR, Vestbo J. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009;10:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 76. | Jung KS, Park HY, Park SY, Kim SK, Kim YK, Shim JJ, Moon HS, Lee KH, Yoo JH, Lee SD. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respir Med. 2012;106:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | Guenette JA, Webb KA, O’Donnell DE. Effect of fluticasone/salmeterol combination on dyspnea and respiratory mechanics in mild-to-moderate COPD. Respir Med. 2013;107:708-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 79. | Tashkin DP, Murray HE, Skeans M, Murray RP. Skin manifestations of inhaled corticosteroids in COPD patients: results from Lung Health Study II. Chest. 2004;126:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 543] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 81. | Kim V, Han MK, Vance GB, Make BJ, Newell JD, Hokanson JE, Hersh CP, Stinson D, Silverman EK, Criner GJ. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140:626-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 82. | Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847-852. [PubMed] |
| 83. | Brightling CE, McKenna S, Hargadon B, Birring S, Green R, Siva R, Berry M, Parker D, Monteiro W, Pavord ID. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 267] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 84. | Leigh R, Pizzichini MM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27:964-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 85. | Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, Monteiro W, Berry M, Parker D, Wardlaw AJ. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 333] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 86. | Miravitlles M, Soler-Cataluña JJ, Calle M, Soriano JB. Treatment of COPD by clinical phenotypes: putting old evidence into clinical practice. Eur Respir J. 2013;41:1252-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 87. | Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 469] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 88. | Andreassen H, Vestbo J. Chronic obstructive pulmonary disease as a systemic disease: an epidemiological perspective. Eur Respir J Suppl. 2003;46:2s-4s. [PubMed] |
| 89. | Fabbri LM, Luppi F, Beghé B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 397] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 90. | Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med. 2006;100:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 91. | McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 356] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 92. | Maclay JD, McAllister DA, Macnee W. Cardiovascular risk in chronic obstructive pulmonary disease. Respirology. 2007;12:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 93. | Sevenoaks MJ, Stockley RA. Chronic Obstructive Pulmonary Disease, inflammation and co-morbidity--a common inflammatory phenotype? Respir Res. 2006;7:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 94. | Decramer M, Rennard S, Troosters T, Mapel DW, Giardino N, Mannino D, Wouters E, Sethi S, Cooper CB. COPD as a lung disease with systemic consequences--clinical impact, mechanisms, and potential for early intervention. COPD. 2008;5:235-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 95. | Loukides S, Bartziokas K, Vestbo J, Singh D. Novel anti-inflammatory agents in COPD: targeting lung and systemic inflammation. Curr Drug Targets. 2013;14:235-245. [PubMed] |
| 96. | Sveger T, Thelin T, McNeil TF. Young adults with alpha 1-antitrypsin deficiency identified neonatally: their health, knowledge about and adaptation to the high-risk condition. Acta Paediatr. 1997;86:37-40. [PubMed] |