Published online Nov 28, 2013. doi: 10.5320/wjr.v3.i3.104
Revised: July 12, 2013
Accepted: July 17, 2013
Published online: November 28, 2013
Processing time: 159 Days and 20.7 Hours
Non-small cell lung cancer (NSCLC) is the major cause of cancer-related deaths worldwide. Recent advances in molecular biology have resulted in the clinical use of several molecularly targeted drugs, which usually exhibit cytostatic antitumor activity, to improve the survival of NSCLC patients. The epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) gefitinib and erlotinib have been approved for the treatment of NSCLC, and several phase III trials have demonstrated that sensitizing EGFR mutations are biomarkers for predicting a favorable clinical outcome of NSCLC patients treated with the EGFR-TKIs. The Response Evaluation Criteria in Solid Tumors is generally used to assess the therapeutic response to antitumor drugs based on the morphological changes in tumor size as evaluated by computed tomography or magnetic resonance imaging. However, such assessment may not always reflect the treatment efficacy of cytostatic drugs, such as EGFR-TKIs. In this regard, functional imaging methods, including 18F-fluorodeoxyglucose measured by positron emission tomography (FDG-PET), are potentially beneficial. An increasing body of evidence indicates the usefulness of FDG-PET to predict treatment efficacy for NSCLC patients treated with EGFR-TKIs. In this review, we summarize the current understanding of the potential role of FDG-PET in the clinical use of EGFR-TKIs for NSCLC.
Core tip: Molecularly targeted drugs including epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), which usually exhibit cytostatic antitumor activity, have emerged for the treatment of non-small cell lung cancer. The Response Evaluation Criteria in Solid Tumors is generally used to assess the therapeutic response based on the morphological changes in tumor size as evaluated by computed tomography or magnetic resonance imaging. However, such assessment may not always reflect the clinical outcome of cytostatic drugs, such as EGFR-TKIs. In this regard, 18F-fluorodeoxyglucose measured by positron emission tomography (FDG-PET) is potentially beneficial. Here we summarize the role of FDG-PET to predict the treatment efficacy in NSCLC treated with EGFR-TKIs.
- Citation: Sunaga N, Kaira K, Hisada T, Yamada M. FDG-PET for predicting efficacy of EGFR-tyrosine kinase inhibitors in lung cancer. World J Respirol 2013; 3(3): 104-109
- URL: https://www.wjgnet.com/2218-6255/full/v3/i3/104.htm
- DOI: https://dx.doi.org/10.5320/wjr.v3.i3.104
Lung cancer is the major cause of cancer-related deaths worldwide[1]. Lung cancer comprises two major histological types: small cell lung cancer and non-small cell lung cancer (NSCLC), and the latter represents 80%-85% of all lung cancers[2]. The majority of patients with NSCLC have locally advanced or metastatic disease at the time of diagnosis, and chemotherapy with cytotoxic agents remains marginally effective[3,4]. In recent years, molecularly targeted drugs, which usually exhibit cytostatic antitumor activity, have emerged for the treatment of NSCLC. The effective use of molecularly targeted drugs requires the identification of biomarkers to predict treatment response and clinical outcomes in NSCLC patients[5]. Recent advances in basic and translational research have identified epidermal growth factor receptor (EGFR) mutation as the most promising biomarker for predicting the treatment efficacy of the EGFR-tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib for NSCLC patients[6-11]. However, the clinical benefit of EGFR-TKI treatment is not confined to patients whose tumors harbor EGFR mutations, and some EGFR-mutated NSCLC patients do not respond to EGFR-TKIs[12]. Furthermore, sufficient tumor samples to test EGFR mutation status are not always available, and alternative methods to predict the efficacy of EGFR-TKI therapy are therefore warranted.
The therapeutic response to antitumor drugs is generally evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST)[13]. On the RECIST evaluation, target lesions are measured before and after chemotherapy by morphological imaging technologies, including computed tomography (CT) and magnetic resonance imaging (MRI). However, morphological changes in tumors usually take several weeks to occur after chemotherapy, and targeted tumor lesions contain noncancerous cell components such as necrotic, cystic and fibrotic lesions. Thus, RECIST evaluation based on size-measurements of total tumor volume may not always reflect the treatment efficacy, especially when patients are treated with cytostatic drugs[14].
In a phase II trial of gefitinib monotherapy, NSCLC patients who had achieved stable disease (SD) had a favorable prognosis compared to those with progressive disease (PD)[15]. Similarly, of the NSCLC patients treated with gefitinib, the overall survival (OS) of patients with SD was significantly longer compared to those with PD[16]. In addition, erlotinib significantly prolonged OS despite a response rate of less than 10% in a large phase III trial, possibly due to a high proportion of patients with SD[17]. These findings highlight the limitations of the RECIST criteria regarding the assessment of treatment efficacy for cytostatic drugs such as EGFR-TKIs. In this regard, molecular imaging methods, such as 18F-fluorodeoxyglucose measured by positron emission tomography (FDG-PET), are advantageous because of their ability to detect changes in glucose metabolism, proliferative activities and the vascularization of tumors, which occur earlier than morphological changes[18,19]. Currently, FDG-PET has been the most wide-spreading imaging technique used as a diagnostic tool in various cancers, including NSCLC[20].
Several lines of evidence have indicated the value of FDG-PET to predict the therapeutic response and clinical outcome of EGFR-TKI therapy in NSCLC patients as shown in Table 1[21-26]. In 2008, we reported a preliminary study that assessed the roles of FDG-PET in predicting the treatment efficacy of gefitinib in five NSCLC patients[27]. The patients underwent FDG-PET 2 d (early phase) and 4 wk (late phase) after administration of gefitinib, and FDG uptake was evaluated as the maximum standardized uptake value (SUVmax) of the target lesions, which were evaluable by conventional CT. Of the four patients with sensitizing EGFR mutations, two patients exhibited a partial response (PR), and others had SD with decreased tumor size but did not achieve PR as evaluated by RECIST. In all of these patients, FDG uptake markedly decreased at the earlier phase from baseline as assessed by a mean ± SD SUVmax% of 60% ± 14% (60% ± 18% and 59% ± 12% for PR and SD groups, respectively). Notably, the two patients with SD had a long-term progression-free survival (PFS) of ≥ 12.5 mo and an OS of ≥ 16.9 mo. While these results were obtained from a small sample size, our findings suggest that FDG-PET can potentially assess the treatment efficacy of gefitinib for NSCLC patients more accurately than morphological evaluation.
| Ref. | EGFR-TKIs | Patient no. | Evaluation timing | EGFR mutation status | Significant association | |||
| Mutant | Wild-type | Unknown | PFS or TTP1 | OS | ||||
| Takahashi et al[25] | Gefitinib | 20 | 2 d | 12 | 3 | 5 | Yes | NA |
| Soto Parra et al[24] | Erlotinib | 23 | 2 d | NA | NA | NA | Yes | No |
| Mileshkin et al[23] | Erlotinib | 51 | 2 wk | 4 | 30 | 17 | Yes | Yes |
| Zander et al[26] | Erlotinib | 34 | 1 wk | 4 | 24 | 6 | Yes | Yes |
| Benz et al[22] | Erlotinib | 22 | 2 wk | 4 | 1 | 17 | Yes1 | Yes |
| Bengtsson et al[21] | Erlotinib | 125 | 2 wk | 10 | 90 | 25 | NA | Yes |
Recently, Takahashi et al[25] reported a similar study of 20 lung adenocarcinoma patients receiving gefitinib monotherapy. In their study, changes in tumor FDG uptake 2 d after gefitinib initiation were positively correlated with changes in tumor size assessed by CT 1 mo after the treatment. In addition, metabolic responders defined as ∆SUVmax% < -20% had significantly longer PFS than metabolic non-responders (∆SUVmax% ≥ -20%) when SUVmax changes were evaluated after 2 d of treatment. This study demonstrates that the earlier metabolic response at 2 d could predict the prognosis of geftinib-treated NSCLC patients. These findings indicate that early FDG-PET assessment is useful to predict the treatment efficacy of gefitinib monotherapy compared to the conventional morphological assessment by CT or MRI.
Several studies have assessed the role of FDG-PET to predict the treatment efficacy of the EGFR-TKI erlotinib[21-24,26,28-30]. Aukema et al[28] assessed NSCLC patients who received neoadjuvant erlotinib by FDG-PET 1 wk after treatment. Of the resected tumors, 70% of metabolic responders defined as ∆SUVmax% ≤ -25% [interquartile ranges (IQRs), 30%-91%] and 40% of non-responders (IQRs, 20%-50%) were necrotic. The metabolic and pathologic responses correlated significantly, suggesting that a change in FDG uptake is closely associated with the pathologic response to erlotinib. Other clinical studies have prospectively investigated whether an early FDG-PET assessment could predict the tumor response to erlotinib and survival in NSCLC patients. Soto Parra et al[24] reported that the metabolic response evaluated by FDG-PET 2 d after erlotinib initiation was significantly associated with a longer PFS in NSCLC patients. In a study by Mileshkin et al[23], the metabolic response (∆SUVmax% ≤ -15%) at 2 wk after erlotinib initiation was significantly associated with both improved PFS and OS in NSCLC patients receiving 2nd/3rd-line erlotinib monotherapy. Of note, in a subset of patients with wild-type EGFR, early metabolic responders tended to have a longer PFS compared to the metabolic non-responders[23]. Similar findings were also observed in another study, in which the metabolic response (∆SUVpeak% ≤ -30%) 1 wk after erlotinib initiation was significantly associated with both improved PFS and OS in NSCLC patients, irrespective of EGFR mutation status[26]. The same group subsequently investigated the predictive values of changes in FDG uptake using different SUV parameters (SUVmax, SUV2Dpeak, SUV3Dpeak, SUV50, SUVA50, SUVA41, SUV70, SUVA70 and SUVRTL) and found that SUVmax best assesses the early metabolic response[30]. They also found that a lower residual FDG uptake measured by SUVmax and SUV2Dpeak (but not other SUV parameters) at the early phase of treatment was associated with a significantly longer PFS[29]. Furthermore, another group reported that the patients with progressive metabolic disease (∆SUVpeak% ≥ 30%) 2 wk after erlotinib initiation had a significantly shorter time to progression and OS compared to those with stable metabolic disease or a metabolic response of ∆SUVpeak% ≤ -30%[22]. In a recent study that assessed FDG-PET in 2nd/3rd-line erlotinib monotherapy for NSCLC patients, the absence of new lesions by FDG-PET 2 wk after erlotinib initiation was the most predictive marker for OS as opposed to changes in FDG uptake (∆SUVmax% ≤ -35%)[21]. However, FDG changes were a predictor of OS only when EGFR mutation status was not accounted for[21].
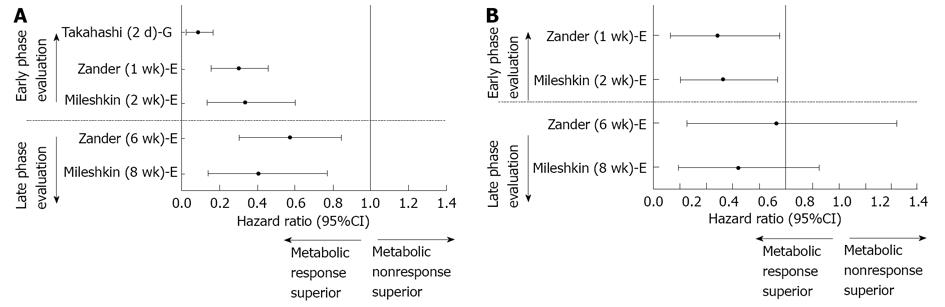
The predictive value of the late phase FDG-PET assessment on clinical outcome of EGFR-TKI-treated NSCLC patients has also been evaluated. In a recent study, of 38 NSCLC patients who underwent FDG-PET scan at 6 wk after erlotinib initiation, the metabolic responders with ∆SUVpeak% ≤ -25% survived longer than the non-responders[31]. Importantly, two studies evaluated the predictive value of both early and late FDG-PET assessments on survival of NSCLC patients treated with erlotinib[23,26]. In both studies, FDG-PET response at the late phase (6 or 8 wk) was not significantly associated with improved overall survival. The hazard ratios for PFS and OS in these studies are summarized in Figure 1. Overall, these findings indicate that FDG-PET assessment at the early phases of EGFR-TKI therapy could predict the clinical outcome of NSCLC patients better than assessment at a later phase, irrespective of EGFR mutation status.
Previous studies have investigated the molecular mechanisms of FDG-PET to predict the tumor response to EGFR-TKIs at an early stage[32,33]. Treatment with gefitinib reduced FDG uptake in the H3255 and HCC4006 NSCLC cell lines harboring sensitizing EGFR mutations within 2 h and reduced FDG uptake in the xenografts of these tumor cells after 2 d of treatment[33]. The gefitinib-mediated decrease in FDG uptake preceded the inhibition of cell proliferation and induction of apoptosis, and this phenomenon was accompanied with the translocation of glucose transporters from the cell membrane to the cytoplasm. This finding suggests that FDG-PET could detect the antitumor effects of EGFR-TKIs at a molecular level before phenotypic changes occur in tumors[33]. In contrast, Ullrich et al[32] failed to demonstrate a significant decrease in FDG uptake after 2 and 4 d of erlotinib treatment in tumor xenografts of the EGFR-mutated NSCLC cell lines PC9 and HCC827. These inconsistent results may be due to the differences in cellular context, such as differences in the expression levels of glucose transporters and the sensitivity to EGFR-TKIs.
Clinical studies have also revealed the diverse effects of EGFR-TKIs on the early metabolic response of FDG-PET in EGFR-mutant NSCLC patients[23,25,26]. For instance, in a study by Takahashi et al[25], 4 of 12 (33%) patients with EGFR mutations were metabolic non-responders. Three of these patients had stable metabolic disease and one patient had progressive metabolic disease 2 d after gefitinib initiation. Taken together, these findings suggest that FDG-PET assessment could identify the patients who benefit from EGFR-TKI therapies in a population of EGFR-mutated NSCLC patients. FDG metabolism is also likely closely linked to the sensitivity of NSCLC to EGFR-TKIs.
In summary, an increasing body of evidence indicates the potential of FDG-PET to predict the treatment efficacy and clinical outcome of NSCLC patients who are treated with EGFR-TKIs. The metabolic response has previously been assessed by different criteria, including EORTC[34] and PERCIST [14]. Thus, standardizing the PET response assessment is essential to establish the predictive values of FDG-PET. Moreover, further studies with larger patient numbers will be needed to evaluate the predictive values of FDG-PET to assess the clinical efficacy of EGFR-TKIs in NSCLC patients.
The authors apologize to other investigators for the omission of any references.
P- Reviewer: Ou WB S- Editor: Song XX L- Editor: A E- Editor: Wang CH
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25540] [Article Influence: 1824.3] [Reference Citation Analysis (7)] |
| 2. | Larsen JE, Minna JD. Molecular biology of lung cancer: clinical implications. Clin Chest Med. 2011;32:703-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Breathnach OS, Freidlin B, Conley B, Green MR, Johnson DH, Gandara DR, O’Connell M, Shepherd FA, Johnson BE. Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J Clin Oncol. 2001;19:1734-1742. [PubMed] |
| 4. | Goffin J, Lacchetti C, Ellis PM, Ung YC, Evans WK. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol. 2010;5:260-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Coate LE, John T, Tsao MS, Shepherd FA. Molecular predictive and prognostic markers in non-small-cell lung cancer. Lancet Oncol. 2009;10:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3961] [Cited by in RCA: 4389] [Article Influence: 292.6] [Reference Citation Analysis (0)] |
| 7. | Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2837] [Cited by in RCA: 3282] [Article Influence: 218.8] [Reference Citation Analysis (0)] |
| 8. | Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5906] [Cited by in RCA: 6475] [Article Influence: 404.7] [Reference Citation Analysis (0)] |
| 9. | Morita S, Okamoto I, Kobayashi K, Yamazaki K, Asahina H, Inoue A, Hagiwara K, Sunaga N, Yanagitani N, Hida T. Combined survival analysis of prospective clinical trials of gefitinib for non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2009;15:4493-4498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2700] [Cited by in RCA: 3261] [Article Influence: 232.9] [Reference Citation Analysis (0)] |
| 11. | Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4228] [Cited by in RCA: 4356] [Article Influence: 335.1] [Reference Citation Analysis (0)] |
| 12. | John T, Liu G, Tsao MS. Overview of molecular testing in non-small-cell lung cancer: mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene. 2009;28 Suppl 1:S14-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13076] [Article Influence: 523.0] [Reference Citation Analysis (0)] |
| 14. | Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S-150S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2916] [Cited by in RCA: 2798] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 15. | Kris MG, Natale RB, Herbst RS, Lynch TJ, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2064] [Cited by in RCA: 2004] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 16. | Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Harita S, Gemba K, Yonei T, Bessho A, Tanimoto M. Continued gefitinib treatment after disease stabilisation prolongs survival of Japanese patients with non-small-cell lung cancer: Okayama Lung Cancer Study Group experience. Ann Oncol. 2005;16:1817-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 4215] [Article Influence: 210.8] [Reference Citation Analysis (0)] |
| 18. | Minn H, Joensuu H, Ahonen A, Klemi P. Fluorodeoxyglucose imaging: a method to assess the proliferative activity of human cancer in vivo. Comparison with DNA flow cytometry in head and neck tumors. Cancer. 1988;61:1776-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallires E, Wood DE. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000;6:3837-3844. [PubMed] |
| 20. | Oriuchi N, Higuchi T, Ishikita T, Miyakubo M, Hanaoka H, Iida Y, Endo K. Present role and future prospects of positron emission tomography in clinical oncology. Cancer Sci. 2006;97:1291-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Bengtsson T, Hicks RJ, Peterson A, Port RE. 18F-FDG PET as a surrogate biomarker in non-small cell lung cancer treated with erlotinib: newly identified lesions are more informative than standardized uptake value. J Nucl Med. 2012;53:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Benz MR, Herrmann K, Walter F, Garon EB, Reckamp KL, Figlin R, Phelps ME, Weber WA, Czernin J, Allen-Auerbach MS. (18)F-FDG PET/CT for monitoring treatment responses to the epidermal growth factor receptor inhibitor erlotinib. J Nucl Med. 2011;52:1684-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Mileshkin L, Hicks RJ, Hughes BG, Mitchell PL, Charu V, Gitlitz BJ, Macfarlane D, Solomon B, Amler LC, Yu W. Changes in 18F-fluorodeoxyglucose and 18F-fluorodeoxythymidine positron emission tomography imaging in patients with non-small cell lung cancer treated with erlotinib. Clin Cancer Res. 2011;17:3304-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Soto Parra HJ, Tiseo M, Cosentino S, Ardizzoni A, Latteri F, Pumo V, Cordio S, Bordonaro R, Spadaro P. Usefulness of 18FDG-positron emission tomography for early prediction of erlotinib treatment outcome in non-small cell lung cancer patients: Results of a pilot study. J Clin Oncol. 2009;27:abstr 7568. |
| 25. | Takahashi R, Hirata H, Tachibana I, Shimosegawa E, Inoue A, Nagatomo I, Takeda Y, Kida H, Goya S, Kijima T. Early [18F]fluorodeoxyglucose positron emission tomography at two days of gefitinib treatment predicts clinical outcome in patients with adenocarcinoma of the lung. Clin Cancer Res. 2012;18:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Zander T, Scheffler M, Nogova L, Kobe C, Engel-Riedel W, Hellmich M, Papachristou I, Toepelt K, Draube A, Heukamp L. Early prediction of nonprogression in advanced non-small-cell lung cancer treated with erlotinib by using (18)F fluorodeoxyglucose and (18)F fluorothymidine positron emission tomography. J Clin Oncol. 2011;29:1701-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Sunaga N, Oriuchi N, Kaira K, Yanagitani N, Tomizawa Y, Hisada T, Ishizuka T, Endo K, Mori M. Usefulness of FDG-PET for early prediction of the response to gefitinib in non-small cell lung cancer. Lung Cancer. 2008;59:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Aukema TS, Kappers I, Olmos RA, Codrington HE, van Tinteren H, van Pel R, Klomp HM. Is 18F-FDG PET/CT useful for the early prediction of histopathologic response to neoadjuvant erlotinib in patients with non-small cell lung cancer? J Nucl Med. 2010;51:1344-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Kobe C, Scheffler M, Holstein A, Zander T, Nogova L, Lammertsma AA, Boellaard R, Neumaier B, Ullrich RT, Dietlein M. Predictive value of early and late residual 18F-fluorodeoxyglucose and 18F-fluorothymidine uptake using different SUV measurements in patients with non-small-cell lung cancer treated with erlotinib. Eur J Nucl Med Mol Imaging. 2012;39:1117-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Kahraman D, Scheffler M, Zander T, Nogova L, Lammertsma AA, Boellaard R, Neumaier B, Ullrich RT, Holstein A, Dietlein M. Quantitative analysis of response to treatment with erlotinib in advanced non-small cell lung cancer using 18F-FDG and 3’-deoxy-3’-18F-fluorothymidine PET. J Nucl Med. 2011;52:1871-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | O’Brien ME, Myerson JS, Coward JI, Puglisi M, Trani L, Wotherspoon A, Sharma B, Cook G, Ashley S, Gunapala R. A phase II study of 18F-fluorodeoxyglucose PET-CT in non-small cell lung cancer patients receiving erlotinib (Tarceva); objective and symptomatic responses at 6 and 12 weeks. Eur J Cancer. 2012;48:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Ullrich RT, Zander T, Neumaier B, Koker M, Shimamura T, Waerzeggers Y, Borgman CL, Tawadros S, Li H, Sos ML. Early detection of erlotinib treatment response in NSCLC by 3’-deoxy-3’-[F]-fluoro-L-thymidine ([F]FLT) positron emission tomography (PET). PLoS One. 2008;3:e3908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Su H, Bodenstein C, Dumont RA, Seimbille Y, Dubinett S, Phelps ME, Herschman H, Czernin J, Weber W. Monitoring tumor glucose utilization by positron emission tomography for the prediction of treatment response to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2006;12:5659-5667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 34. | Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1315] [Article Influence: 50.6] [Reference Citation Analysis (0)] |