Peer-review started: February 3, 2023
First decision: February 21, 2023
Revised: March 3, 2023
Accepted: May 8, 2023
Article in press: May 8, 2023
Published online: May 26, 2023
Processing time: 112 Days and 5.7 Hours
The coronavirus disease 2019 (COVID-19) pandemic has had a tremendous adverse impact on the global health system, public sector, and social aspects. It is unarguably the worst pandemic of the century. However, COVID-19 management is a mystery in front of us, and an authentic treatment is urgently needed. Various repurposed drugs, like ivermectin, remdesivir, tocilizumab, baricitinib, etc., have been used to treat COVID-19, but none are promising. Antibody therapy and their combinations are emerging modalities for treating moderate COVID-19, and they have shown the potential to reduce hospitalisations. One antibody monotherapy, bamlanivimab, and two cocktails, casirivimab/imdevimab and bamlanivimab/ esterivimab, have received authorization for emergency use by the United States Food and Drug Administration for the treatment of mild COVID-19 in high risk individuals. The European Emergency has made similar recommendations for use of the drug in COVID-19 patients without oxygen therapy. This brief review will focus on monoclonal antibodies and their combination cocktail therapy in managing COVID-19 infection.
Core Tip: The coronavirus disease 2019 (COVID-19) pandemic is a severe public health emergency that necessitated the rapid development of novel medicines and viral detection technologies. Monoclonal antibodies against the receptor-binding domain of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein have become an important target for the creation of therapeutic antibodies. The use of antibody cocktails is anticipated to be a key component of an efficient COVID-19 treatment plan because SARS-CoV-2 has a high mutation rate, particularly when subjected to the selection pressure of aggressively applied preventive vaccinations and neutralising antibodies.
- Citation: Bajpai J, Kant S, Verma AK, Pradhan A. Monoclonal antibody for COVID-19: Unveiling the recipe of a new cocktail. World J Respirol 2023; 12(1): 1-9
- URL: https://www.wjgnet.com/2218-6255/full/v12/i1/1.htm
- DOI: https://dx.doi.org/10.5320/wjr.v12.i1.1
The coronavirus disease 2019 (COVID-19) pandemic has placed a high burden on healthcare systems globally[1]. The first case of COVID-19 was reported on January 30, 2020. As of July 20, 2021, India had the highest number of COVID-19 instances, with more than 30 million[2]. The second wave of COVID-19 was more severe than the first. There was a reported shortage of drugs, oxygen, hospital beds, and vaccines. Some patients with COVID-19 will develop acute disease and multiorgan complications, but there are currently no proven therapeutics to prevent or reduce COVID-19 related hospitalisations, complications, or mortality.
Various drugs are approved for patients hospitalised with severe COVID-19 infection, but only a few drugs for mild COVID-19 patients who are not sick enough to be hospitalised are available[3]. Monoclonal antibodies (mAbs) are a new treatment for mild COVID-19 outpatients with a high risk. Recently, cocktail therapy has been approved for mild to moderate COVID-19 patients. Immunity to viral infection is a multipronged response, comprising the innate response-which restricts viral replication and creates an antiviral state in the local tissue environment, the adaptive response- in which virus-specific CD4+ T cells, CD8+ T cells, and the antibodies produced by B cells to control and clear the infection and generate immune memory. COVID-19 appears to evade or delay the innate immune response. If adaptive immunity is delayed for too long (because of efficient viral evasion, diminished innate immunity in the patient, or both), then the inability to control infection puts patients at an increased risk for severe or even fatal COVID-19 disease[4].
Despite recent studies demonstrating immune responses to COVID-19 as far as up to 8 mo after symptom onset, much remains to be learned about post-infection immunity to COVID-19[5,6]. Immunity to seasonal human coronaviruses is usually of short duration, and reinfection has been documented in patients who have already been infected with COVID-19[7]. Moreover, some individuals might not benefit from vaccination, as the vaccine trials published to date have not shown 100% efficacy, and real-world experience has demonstrated breakthrough events[8,9]. Furthermore, large parts of the population are still not vaccinated primarily because of supply issues and in part because of vaccine hesitancy. Thus, there is a pressing need for alternative therapies for COVID-19 patients. This article reviewes the currently approved cocktail therapy(-ies) in the management of COVID-19 disease.
An antibody is a protein molecule naturally developed by the immune response to infection. Antibodies are an essential factor in immunity against most viral diseases. Monoclonal antibodies (mAbs) are a single isotype with a defined specificity targeting with high potency a particular antigen via the antigen-binding fragment. As such, mAbs against COVID-19 have been derived from plasma donated by patients who recovered from COVID-19[10]. Polyclonal antibodies are usually defined as a mixture of diverse antibodies with mixed affinities for their targets. However, in the world of COVID-19 therapeutics, the term “polyclonal antibodies” is more descriptive of convalescent plasma with several antibody components[11].
mAbs are designed in a laboratory and mimic the natural immune system in response to infection. They are created for a specific target of infectious particles. A mAb is produced by exposing white blood cells to a particular viral protein cloned to produce antibodies to treat several infections and cancers[12]. mAbs that bind to the spike (S) protein of the COVID-19 virus stop the virus from binding the angiotensin-converting enzyme II (ACE2) receptor of human cells and prevent its invasion and replication[13]. mAbs have been effective against new COVID-19 variants B.1.1.7.
Even though more than 75 mAbs have been licensed by the United States Food and Drug Administration (FDA), only three are used to treat or prevent infectious diseases like anthrax, respiratory syncytial virus, and Clostridium difficile, and two are used to treat Ebola virus diseases. mAbs are intended for patients recently diagnosed with COVID-19 who are not very sick and have risk factors for severe infection[14-16].
This article focuses on mAbs with neutralising activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which work by targeting the receptor binding domain (RBD) of the viral S protein, thereby preventing viral attachment to the ACE2 receptor and preventing a critical step in viral entry and infection. Bamlanivimab is a recombinant, neutralising human immunoglobulin G1 (IgG1) mAb effective against the S protein of COVID-19. Etesevimab is a recombinant, fully human monoclonal neutralising antibody that binds to the surface S protein receptor-binding domain with high affinity and blocks viral binding to the ACE2 receptor of the host cell surface. Imdevimab and Casirivimab are IgG1 that act against SARS-CoV-2 S protein. Thus, the antibody cocktail thwarts the attachment of the virus and its entry into the human cell.
Casirivimab (REGN10933) and imdevimab (REGN 10987) were developed by Regeneron F and Hoffmann-La Roche Ltd pharmaceuticals, respectively, and bamlanivimab and the cocktail of bamlanivimab and esterivimab were developed by Eli Lilly and AbCellera, respectively. The following studies or their scientific data reveal how these mAbs are helpful in treating COVID-19 (Table 1).
| Ref. | Study type | Dose and duration | Primary outcome | Secondary outcomes | Primary result | Additional characteristics | Adverse effects |
| Chen et al[17], BLAZE-1 | Randomised, double-blind, placebo-controlled, single-dose trial | Total 452 patients; 309 in the bamlanivimab (LY-CoV555) group and 143 in the placebo group. mAb at doses of 700 mg, 2800 mg, and 7000 mg and placebo administered within 3 d after positive SARS-CoV-2 results | The change from baseline to day 11 (4 d) in SARS-CoV-2 viral load | COVID-19 related inpatient hospitalisation, a visit to the emergency department, death, safety, symptom severity, and time points for viral clearance | The viral load at day 11 was lower in patients who received 2800 mg drug compared to the placebo group | High-risk subgroups (an age of ≥ 65 yr or a BMI of ≥ 35), the percentage of hospitalisation was 4.2% in the LY-CoV555 group and 14.6% in the placebo group | Serious adverse events occurred in none of the patient treatment groups, diarrhoea was reported in 3.2% of the patients |
| Weinreich et al[23], REGN-COV2 | Double-blind, phase 1-3 trial, 275 (1:1:1) non-hospitalised patients with COVID-19 | REGN-COV2 is a combination of casirivimab (REGN10933) and imdevimab (REGN10987). Among the 275 patients, 90 were assigned to receive high-dose (8.0 g), 92 to receive low-dose (2.4 g), and 93 to receive placebo | The time-weighted average change in viral load from baseline (day 1) through day 7 | The percentage of patients with at least one COVID-19 related medically attended visit through day 29 | REGN-COV2 enhanced clearance of virus, particularly in patients in whom an endogenous immune response had not yet been initiated | The median age was 44.0 yr, 49% were male, 13% identified as Black or African American, and 56% as Hispanic or Latino | In this interim analysis, both REGN-COV2 doses (2.4 g and 8.0 g) were associated with few and low-grade toxic effects (1%) in the combined REGN-COV2 dose groups |
| Gottlieb et al[20], BLAZE 1 | Multipart, 49 United States centres including phase 2/3, randomised, double-blind, placebo-controlled, single-infusion study (BLAZE-1) ambulatory patients (n = 613) and had one or more mild to moderate symptom | Patients were randomised to receive a single infusion of bamlanivimab [700 mg (n = 101), 2800 mg (n = 107), or 7000 mg (n = 101)], the combination treatment (2800 mg of bamlanivimab and 2800 mg of etesevimab (n = 112), or placebo (n = 156) | Change in SARS-CoV-2 log viral load at day 11 (± 4 d) | A total of nine prespecified secondary outcome measures were evaluated. Three focused on viral load (time to viral clearance; the proportion of patients with viral support at days 7, 11, 15, and 22; time to symptom improvement; time to symptom resolution; and the balance of patients showing symptom improvement or resolution at days 7, 11, 15, and 22), and 1 focused on clinical outcomes (the proportion of patients with a COVID-19 related hospitalisation, emergency department visit, or death) at day 29 | Among the 577 patients who were randomised and received an infusion, 533 (92.4%) completed the efficacy evaluation period (day 29). The change in log viral load from baseline at day 11 was –3.72 for 700 mg, –4.08 for 2800 mg, –3.49 for 7000 mg, –4.37 for combination treatment, and –3.80 for placebo | The mean age of patients was 44.7 ± 15.7 yr. A total of 315 patients (54.6%) were female, 245 patients (42.5%) identified as Hispanic, and 387 patients (67.1%) had at least one risk factor for severe COVID-19 (aged ≥ 55 yr, BMI ≥ 30, or ≥ 1 relevant comorbidity such as hypertension) | Immediate hypersensitivity reactions were reported in 9 patients (6 bamlanivimab, 2 combination treatment, and 1 placebo). No deaths occurred during the study treatment |
| BLAZE-2[21] | Randomised, double-blind, single-dose, phase 3 placebo-controlled trial, 966 participants (300 residents and 666 staff) who tested negative for SARS-CoV-2 at baseline | Bamlanivimab 4200 mg or placebo only if a nursing home recorded at least one confirmed case of SARS-CoV-2 infection among residents or facility staff from a sample collected within the last 7 d | To find incidence of COVID-19, defined as the detection of SARS-CoV-2 by reverse transcriptase-PCR and mild or worse disease severity within 21 d of detection, within 8 wk of randomisation | To find incidence of moderate or worse COVID-19 severity and incidence of SARS-CoV-2 infection | Bamlanivimab significantly reduced the incidence of COVID-19 in the prevention population compared with placebo [8.5% vs 15.2%; odds ratio: 0.43 (95%CI: 0.28-0.68); P < 0.001]; absolute risk difference, −6.6 (95%CI: −10.7 to −2.6 percentage points) | Significantly reduced the incidence of moderate or worse COVID-19 compared with placebo (8.3% vs 14.1%) | The rate of participants with adverse events was 20.1% in the bamlanivimab group and 18.9% in the placebo group. The most common adverse events were urinary tract infection (2.0%) in bamlanivimab and (2.4%) placebo and hypertension (1.2%) in bamlanivimab and (1.7%) placebo |
| Lundgren et al[18], ACTIVE-3 | Randomised, double-blind, placebo-controlled trial | Hospitalised COVID-19 patients (n = 314) without end organ failure, single infusion of the neutralising mAb antibody LY-CoV555 (at a dose of 7000 mg) | A sustained recovery, as assessed in a time-to-event analysis, through day 90 as well as two ordinal outcomes that were measured at day 5 | Death from any cause | Hospitalised patients with COVID-19 who received mAb did not have better clinical outcomes at day 5 than those who received placebo | The majority of patients had hypoxemia and tested the effect of LY-CoV555 on a background of remdesivir and substantial glucocorticoid therapy | Serious adverse events (19%) in the LY-CoV555 group and (14%) in the placebo |
| REGN-COV206724 | Phase (I-III) adaptive randomised placebo control double-blind | COVID-19 in infected non-hospitalised patients (n = 4567; REGN-COV2067) | 1200 mg cocktail (n = 736), placebo (n = 748), and another group cocktail dose 2400 mg IV (n = 1355), placebo (n = 1341) | Clinically significant effect on risk of COVID-19 hospitalisation or all-cause death in high-risk non-hospitalised patients and confirm safety | Cocktail of casirivimab and imdevimab significantly reduced the risk of hospitalisation or death by 70% (1200 mg IV) and 71% (2400 mg IV) compared to placebo | Cocktail therapy reduced symptom duration from 14 d to 10 d (median numbers) | Not mentioned |
The BLAZE 1 trial was a phase 2/3 trial that enrolled 452 ambulatory COVID-19 patients and was given bamlanivimab as one of three doses [bamlanivimab (LYCoV555) -700 mg, 2800 mg, or 7000 mg in intravenous (IV) infusion] or placebo. The quantitative virologic endpoints and clinical outcomes were assessed[17]. The immediate result was the change in the viral load by day 11. For patients who received a 2800 mg (middle) antibody dose, viral load decreased by a factor of 3.4. The patients who received the 700 mg (lower) dose or the 7000 mg (higher) amount showed a more negligible difference from the placebo in the viral load change from baseline. In addition, bamlanivimab antibody therapy resulted in fewer hospitalisations and/or emergency room visits (1.9% in 2800 mg treatment group compared to 6.3% in the placebo group).
The ACTIVE -3 trial enrolled 314 (163 drug group and 151 placebo group) hospitalised COVID-19 patients without end organ failure[18]. All the patients were also on supportive care as background therapy, including an antiviral drug and when indicated, supplemental oxygen and glucocorticoids. Bamlanivimab at a 7000 mg or placebo dose was administered as a single IV infusion over 1 h. The results revealed that mAbs when administered with remdesivir did not show efficacy among hospitalised COVID-19 patients without end organ failure.
This randomised phase 3 clinical trial enrolled 966 residents and staff at a United States nursing facility with at least one confirmed COVID-19 index case and who were negative at baseline for COVID-19 infection and serology. The incidence of COVID-19 disease among those treated with the antibody bamlanivimab vs placebo (8.5% vs 15.2% respectively) was lower[19]. Bamlanivimab monotherapy compared with a placebo reduced the risk of COVID-19 in residents and staff of nursing facilities.
The BLAZE-1 phase 3 trial showed that the cocktail of bamlanivimab and etesevimab was associated with a significant reduction in viral load compared to the placebo. In contrast, bamlanivimab monotherapy did not result in a substantial reduction. The cocktail was also shown to reduce the number of hospitalisations. The trial included 518 patients in the treatment arm who received a single infusion of bamlanivimab 2800 mg and etesevimab 2800 mg together, and 517 patients received a placebo[20]. The primary endpoint was COVID-19 related hospitalisations or death by any cause during the 29-d follow-up. Hospitalisation or death occurred in 36 (7%) patients who received a placebo compared to 11 (2%) patients treated with bamlanivimab and etesevimab together (a 70% reduction). Ten deaths (2%) deaths occurred in the placebo group. Thus, all-cause death was significantly lower in the bamlanivimab and etesevimab group compared to the placebo group. The United States FDA granted Emergency Use Authorisation (EUA) for the 700 mg dose of bamlanivimab for ambulatory COVID-19 patients at high risk[21,22].
The EUA advised the population on the benefits of monotherapy despite uncertainties[21,22]. The authorised dosage of 700 mg bamlanivimab and 1400 mg etesevimab administered together was based on analyses of available preclinical, clinical, and virologic data as well as pharmacokinetic and pharmacodynamic modelling, which supported that the authorised dosage was expected to have a similar clinical and virologic effect to 2800 mg bamlanivimab and 2800 mg etesevimab administered together.
REGN-COV-2: The mAbs casirivimab and imdevimab bind to the non-overlapping portion of the RBD. A phase 1/2/3 trial (NCT04425629) is taking place across several countries. The phase 3 trial results have been reported. The trial enrolled 4576 patients with one risk factor for severe COVID-19, and an IV infusion of 1200 mg or 2400 mg caserivimab/imdevimab vs placebo was given. The trial reached its primary outcome and depicted that the casirivimab and imdevimab cocktail significantly reduced the risk of hospitalisation or death by 70% in the 1200 mg dose arm and 71% in the 2400 mg dose arm; both were significant compared with placebo[23]. In addition, benefits in the secondary outcomes were also found, including a 4-d reduction in the median duration of symptoms vs placebo.
Interim data from the first 275 patients (phase 1/2 portion) revealed that the cocktail showed virological efficacy resulting in an overall reduction in viral load of 0.25 log10 RNA copies/mL (95%CI: 0.60, 0.10) for the 2400 mg dose and a reduction of 0.56 log10 RNA copies/mL (95%CI: 0.91, 0.21) for an 8000 mg dose (combined dose reduction was 0.41 log10 RNA copies/mL, 95%CI: 0.71, 0.10) vs placebo at day 7.
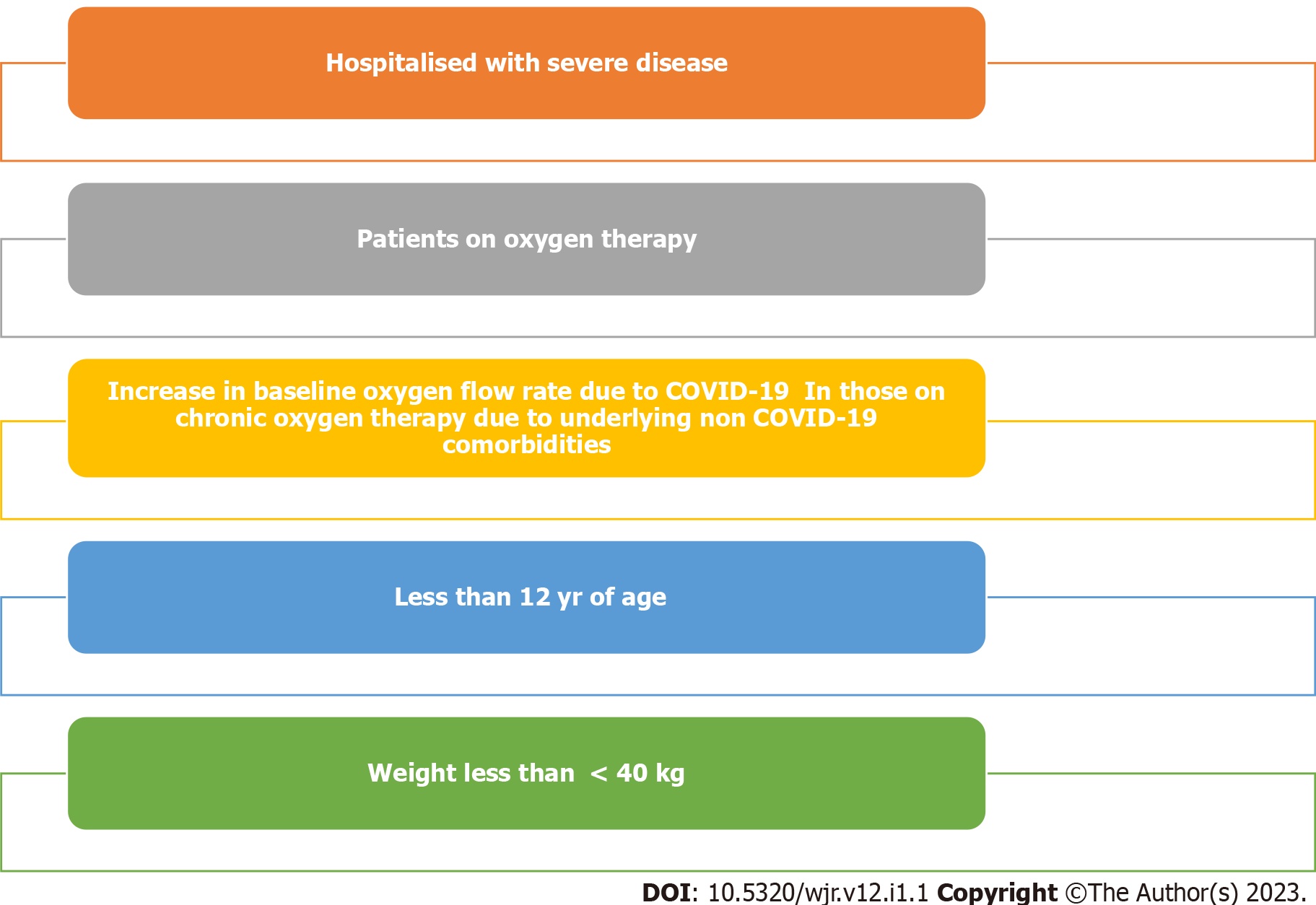
No data on infectious virus titres or time to the cessation of viral shedding endpoints have been reported, similar to the situation with bamlanivimab or other mAb studies. An ongoing dose-ranging phase 2 companion trial in low-risk symptomatic or asymptomatic non-hospitalised patients with COVID-19 (NCT04666441) showed significant and comparable viral load reductions in casirivimab/imdevimab doses ranging from 300 mg to 2400 mg delivered via IV or subcutaneously (SC). The casirivimab/imdevimab cocktail has received EUA by the United States FDA for the treatment of ambulatory patients with mild to moderate COVID-19 and a high risk of hospitalisation, and the EUA has similarly recommended casirivimab/imdevimab for use in COVID-19 patients who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID-19 (Figure 1).
A trial showed that SC injection of the antibody cocktail casirivimab and imdevimab reduced the risk of symptomatic COVID-19 infection by 81% in household contact with an infected person without COVID-19 antibodies. The trial was conducted by the National Institute of Allergy and Infectious Disease. The individuals treated with the cocktail therapy with symptoms were relieved in 1 wk compared to 3 wk in placebo. The FDA has given EUA for the use of casirivimab and imdevimab antibody combination for the treatment of mild to moderate COVID-19 in adults and children over 12 years and weigh more than 40 kg who are at high risk for progressing to severe disease/hospitalisation. The trial showed that the cocktail antibodies casirivimab and imdevimab were more effective when given as early as possible[24]. The Indian drug regulatory body, the Central Drugs Standards Control Organisation, has recently approved the cocktail regimen for use in the country in the fight against COVID-19. The drug, marketed by Cipla Inc. in India, is currently in vogue for clinical use[25].
Another contemporary mAb has been evaluated and is primarily targeted against the COVID-19 S protein. Sotrovimab, a mAb, also blocks the attachment and viral entry into the host cell. A phase 1/2/3 double-blind placebo-controlled trial enrolled 583 non-hospitalised mild to moderate COVID-19 adult patients. Of these, 291 received sotrovimab, and the rest received a placebo within 5 d of symptoms. The primary endpoint was hospitalisation or death through day 29. The result showed 21 (7%) patients were hospitalised or died in the placebo arm compared to 3 (1%) patients in the sotrovimab group. An 85% reduction in hospitalisation or death in the treatment group was observed[26]. Sotrovimab showed activity against the current variants reported in the United Kingdom, South Africa, Brazil, California, New York, and India. The EUA recommends a 500-mg single IV dose of sotrovimab for non-hospitalised mild to moderate COVID-19 patients[27].
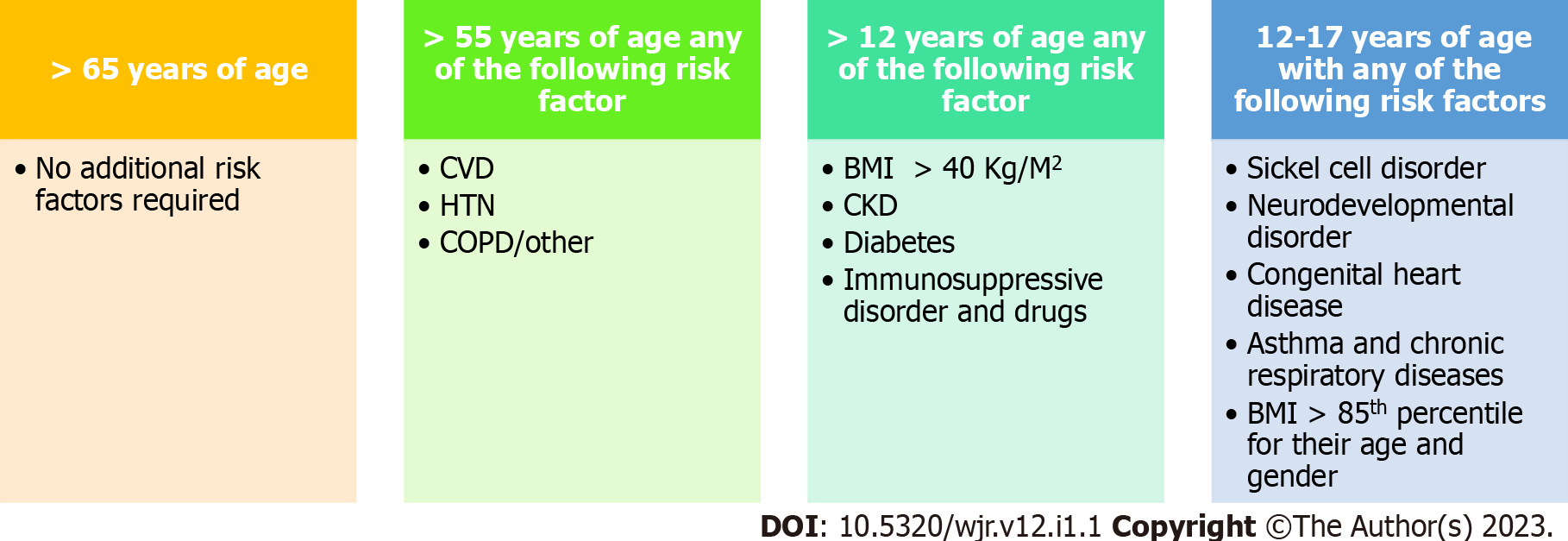
mAb therapies have shown promise for treating non-hospitalised patients with mild to moderate COVID-19. The EUA recommends that mAb treatment be given within 10 d of symptom onset or as early as 72 h of positive COVID-19 result. However, treatment should begin as early as possible to mitigate viral proliferation. In the REGN-COV2 study, the effect of REGN-COV2 on viral load was most pronounced among patients with a negative serum antibody test result at baseline. Furthermore, most trials administered mAb treatment within 3 d of a positive COVID-19 test result and a median of 3- 4 d after symptom onset. Altogether, these studies suggest that early mAb treatment is more efficacious than the later treatment for COVID-19 patients. Indeed, by the time a patient reaches the lung injury phase of infection, it is too late for mAb treatment to be effective, as suggested by the results from the ACTIV-3 study (Figure 2).
A 600 mg of each or a combined 1200 mg of the caserivimab and imdevimab cocktail has been approved for administration. This can be given either IV or SC. The administration of a total dose of cocktail antibody takes around 30 min. The patient should be kept on observation for 1 h to check for any adverse effects. The price for a dose of 1200 mg cocktail (600 mg of caserivimab and 600 mg of imdevimab) is INR 59750 (700 USD approx). This drug should be stored at 2-8 ºC.
The clinical efficacy and safety profiles do not differ between mAb monotherapy and cocktails. Yet, monotherapy vs combination therapy is particularly relevant given the emergence of variant strains from the United Kingdom, South Africa, Brazil, California, New York, and India. The results from one study suggested that a mAb cocktail, particularly one combining antibodies that bind distinct and non-overlapping regions, can minimise mutational escape[28]. More importantly, viral mutations can reduce the effectiveness of mAb monotherapy. A recent preprint publication reported that bamlanivimab and casirivimab are effective against the South African variant. Several variants have been labelled by the Centers for Disease Control and Prevention as “variants of concern” because the mutations they carry increase transmission, increase disease severity, and reduce the efficacy of mAb therapy and vaccinations.
There is growing evidence that mAb treatment is effective, safe, and well-tolerated. Patients should know that mAb treatment is available to all patients at a high cost in India and that mAb treatment should be started within 72 h of a positive COVID-19 test result to affect the clinical course of COVID-19. Further studies on mAb efficacy and safety in different patient populations (e.g., young children and pregnant women) are needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Arteaga-Livias K, Peru; Rotondo JC, Italy S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhao S
| 1. | Bezemer GFG, Garssen J. TLR9 and COVID-19: A Multidisciplinary Theory of a Multifaceted Therapeutic Target. Front Pharmacol. 2020;11:601685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Corona virus update (Live). [cited 21 March 2021]. In: Worldometer [Internet]. Available from http://www.worldometer.info/coronavirus/. |
| 3. | Rotondo JC, Martini F, Maritati M, Mazziotta C, Di Mauro G, Lanzillotti C, Barp N, Gallerani A, Tognon M, Contini C. SARS-CoV-2 Infection: New Molecular, Phylogenetic, and Pathogenetic Insights. Efficacy of Current Vaccines and the Potential Risk of Variants. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1199] [Cited by in RCA: 1299] [Article Influence: 324.8] [Reference Citation Analysis (0)] |
| 5. | Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C, Rayaprolu V, Rawlings SA, Peters B, Krammer F, Simon V, Saphire EO, Smith DM, Weiskopf D, Sette A, Crotty S. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2187] [Cited by in RCA: 1928] [Article Influence: 482.0] [Reference Citation Analysis (0)] |
| 6. | Rotondo JC, Martini F, Maritati M, Caselli E, Gallenga CE, Guarino M, De Giorgio R, Mazziotta C, Tramarin ML, Badiale G, Tognon M, Contini C. Advanced Molecular and Immunological Diagnostic Methods to Detect SARS-CoV-2 Infection. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 7. | Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 437] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 8. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10666] [Article Influence: 2133.2] [Reference Citation Analysis (1)] |
| 9. | Lin DY, Baden LR, El Sahly HM, Essink B, Neuzil KM, Corey L, Miller J; COVE Study Group. Durability of Protection Against Symptomatic COVID-19 Among Participants of the mRNA-1273 SARS-CoV-2 Vaccine Trial. JAMA Netw Open. 2022;5:e2215984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 218] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 11. | Marovich M, Mascola JR, Cohen MS. Monoclonal Antibodies for Prevention and Treatment of COVID-19. JAMA. 2020;324:131-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 12. | Pantaleo G, Correia B, Fenwick C, Joo VS, Perez L. Antibodies to combat viral infections: development strategies and progress. Nat Rev Drug Discov. 2022;21:676-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 143] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 13. | Ali MG, Zhang Z, Gao Q, Pan M, Rowan EG, Zhang J. Recent advances in therapeutic applications of neutralizing antibodies for virus infections: an overview. Immunol Res. 2020;68:325-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Romero JR. Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: results from four years of palivizumab usage. Pediatr Infect Dis J. 2003;22:S46-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | FDA Approves Treatment for Ebola Virus. 2020. [cited 30 March 2021]. In: US Food and Drug Administration [Internet]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-treatment-ebola-virus. |
| 16. | Mulangu S, Dodd LE, Davey RT Jr, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, Ali R, Coulibaly S, Levine AC, Grais R, Diaz J, Lane HC, Muyembe-Tamfum JJ; PALM Writing Group, Sivahera B, Camara M, Kojan R, Walker R, Dighero-Kemp B, Cao H, Mukumbayi P, Mbala-Kingebeni P, Ahuka S, Albert S, Bonnett T, Crozier I, Duvenhage M, Proffitt C, Teitelbaum M, Moench T, Aboulhab J, Barrett K, Cahill K, Cone K, Eckes R, Hensley L, Herpin B, Higgs E, Ledgerwood J, Pierson J, Smolskis M, Sow Y, Tierney J, Sivapalasingam S, Holman W, Gettinger N, Vallée D, Nordwall J; PALM Consortium Study Team. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med. 2019;381:2293-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1089] [Article Influence: 181.5] [Reference Citation Analysis (0)] |
| 17. | Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL, Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P, Skovronsky DM; BLAZE-1 Investigators. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med. 2021;384:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1009] [Cited by in RCA: 1009] [Article Influence: 252.3] [Reference Citation Analysis (0)] |
| 18. | ACTIV-3/TICO LY-CoV555 Study Group; Lundgren JD, Grund B, Barkauskas CE, Holland TL, Gottlieb RL, Sandkovsky U, Brown SM, Knowlton KU, Self WH, Files DC, Jain MK, Benfield T, Bowdish ME, Leshnower BG, Baker JV, Jensen JU, Gardner EM, Ginde AA, Harris ES, Johansen IS, Markowitz N, Matthay MA, Østergaard L, Chang CC, Davey VJ, Goodman A, Higgs ES, Murray DD, Murray TA, Paredes R, Parmar MKB, Phillips AN, Reilly C, Sharma S, Dewar RL, Teitelbaum M, Wentworth D, Cao H, Klekotka P, Babiker AG, Gelijns AC, Kan VL, Polizzotto MN, Thompson BT, Lane HC, Neaton JD. A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:905-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 326] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 19. | Cohen MS, Nirula A, Mulligan MJ, Novak RM, Marovich M, Yen C, Stemer A, Mayer SM, Wohl D, Brengle B, Montague BT, Frank I, McCulloh RJ, Fichtenbaum CJ, Lipson B, Gabra N, Ramirez JA, Thai C, Chege W, Gomez Lorenzo MM, Sista N, Farrior J, Clement ME, Brown ER, Custer KL, Van Naarden J, Adams AC, Schade AE, Dabora MC, Knorr J, Price KL, Sabo J, Tuttle JL, Klekotka P, Shen L, Skovronsky DM; BLAZE-2 Investigators. Effect of Bamlanivimab vs Placebo on Incidence of COVID-19 Among Residents and Staff of Skilled Nursing and Assisted Living Facilities: A Randomized Clinical Trial. JAMA. 2021;326:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 20. | Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Klekotka P, Shen L, Skovronsky DM. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:632-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 722] [Article Influence: 180.5] [Reference Citation Analysis (0)] |
| 21. | Fact sheet for health care providers: Emergency Use Authorization (EUA) of bamlanivimab. [cited 21 March 2021]. In: US Food and Drug Administration [Internet]. Available from: https://www.fda.gov/media/143603/download. |
| 22. | Fact sheet for health care providers: Emergency Use Authorization (EUA) of bamlanivimab and etesevimab. [cited 21 March 2021]. In: US Food and Drug Administration [Internet]. Available from: https://www.fda.gov/media/145802/download. |
| 23. | Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD, Turner KC, Hooper AT, Hamilton JD, Baum A, Kyratsous CA, Kim Y, Cook A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T, Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD; Trial Investigators. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med. 2021;384:238-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1293] [Article Influence: 323.3] [Reference Citation Analysis (0)] |
| 24. | Fact sheet for health care providers: Emergency Use Authorization (EUA) of REGEN-COV™ (casirivimab with imdevimab). [cited 21 March 2021]. In: US Food and Drug Administration [Internet]. Available from: https://www.fda.gov/media/145611/download. |
| 25. | CDSCO approves antibody cocktail drug for restricted emergency use to treat mild Covid-19 cases. [cited 21 March 2021]. In: India Today [Internet]. Available from: https://www.indiatoday.in/coronavirus-outbreak/story/cdsco-approves-antibody-cocktail-drug-for-restricted-emergency-use-to-treat-mild-covid-19-cases-1804859-2021-05-20. |
| 26. | Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, Sarkis E, Solis J, Zheng H, Scott N, Cathcart AL, Hebner CM, Sager J, Mogalian E, Tipple C, Peppercorn A, Alexander E, Pang PS, Free A, Brinson C, Aldinger M, Shapiro AE; COMET-ICE Investigators. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med. 2021; 385: 1941-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 771] [Article Influence: 192.8] [Reference Citation Analysis (0)] |
| 27. | Fact sheet for healthcare providers: emergency use authorization (EUA) of sotrovimab. [cited 21 March 2021]. In: US Food and Drug Administration [Internet]. Available from: https://www.fda.gov/media/149534/download. |
| 28. | Baum A, Fulton BO, Wloga E, Copin R, Pascal KE, Russo V, Giordano S, Lanza K, Negron N, Ni M, Wei Y, Atwal GS, Murphy AJ, Stahl N, Yancopoulos GD, Kyratsous CA. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014-1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1116] [Cited by in RCA: 1016] [Article Influence: 203.2] [Reference Citation Analysis (0)] |