Published online Aug 28, 2013. doi: 10.5319/wjo.v3.i3.42
Revised: June 14, 2013
Accepted: July 23, 2013
Published online: August 28, 2013
Processing time: 138 Days and 22.8 Hours
The stress response theory is a relatively new concept about the cause of idiopathic sudden sensorineural hearing loss (ISHL). A number of possible etiologies have been proposed in the literature, as discussed in this paper, but each proposed etiology has been both supported and refuted in the literature. However, the stress response theory can integrate hypotheses that have been advocated so far. The word “stress” refers to a constellation of physical and psychological stimuli including systemic viral and bacterial illness, systemic inflammatory disorders, and physical, mental or metabolic stress. Numerous studies have demonstrated adverse effects of systemic stress on health. Stress causes changes in the immune system and cytokine network through activation of the hypothalamus-pituitary-adrenal axis and the sympathetic nervous system. Several types of catecholamine and cytokine receptors are in the cochlea cells other than capillary cells, and then they can respond to systemic stressors. However, there are few studies examining how systemic stress is associated with cochlear dysfunction. The stress response theory addresses this question. In the theory, a variety of stressors and risk factors contribute to the onset of ISHL in varying degrees. The lateral wall of the cochlea has very unique responses to systemic stressors. It plays a critical role in causing ISHL. Systemic stressors converge at the lateral wall and trigger pathological activation of nuclear factor κ-light-chain-enhancer of activated B cells, a transcriptional factor known as a stress sensor. This activation enhances local expression of genes associated with immune and inflammatory system, resulting in cochlear dysfunction. We review the original stress response theory advocated by Adams et al and the integrative stress response theory that integrates our knowledge about the etiologies of ISHL so far.
Core tip: The present review focuses on the following four points. First, it summarizes etiologies proposed in the last decade to confirm what we know about the cause of idiopathic sudden sensorineural hearing loss (ISHL). Second, it reviews how systemic stressors affect the human body and the cochlea. Third, it reviews the characteristics of the lateral wall that show unique responses to systemic stressors. Finally, it reviews a relatively new concept about the cause of ISHL, the stress response theory, which integrates our knowledge of the cause of ISHL.
- Citation: Masuda M, Kanzaki J. Cause of idiopathic sudden sensorineural hearing loss: The stress response theory. World J Otorhinolaryngol 2013; 3(3): 42-57
- URL: https://www.wjgnet.com/2218-6247/full/v3/i3/42.htm
- DOI: https://dx.doi.org/10.5319/wjo.v3.i3.42
Idiopathic sudden sensorineural hearing loss (ISHL) is a moderately common otologic disorder characterized by new onset of unilateral reversible or irreversible sensorineural hearing loss, which generally develops over minutes or several hours. However, the etiology remains unknown. During the past decade, a number of papers on proposed etiologies have been published, including those on vascular disturbance, viral infection, and immune-mediated mechanisms. Table 1 summarizes papers about the cause of ISHL published during this decade[1-73]. Papers for and against vascular etiologies with analysis of genetic polymorphism are increasing. Yet, there is no conclusive evidence, and many different treatments exist for ISHL[74]. In fact, Nosrati-Zarenoe et al[75] reported no significant difference in outcomes between treated and non-treated patients (300 patients in total).
| Main category | Subcategory | Significantly associated factors | NOT significantly associated factor | Ref. |
| Vascular impairment | MTHFR poly., homocysteine | FV poly., PT poly., AT, LAC, protein S, protein C | [1,2] | |
| Medical history | MTHFR poly., FV Leiden poly., folate, cardioV risk factors | [3] | ||
| Medical history | Platelet GlyIa poly. | Platelet GlyIIIa poly., Framingham cardioV risk factors, FV Leiden poly., PT poly., history of cardioV events, brain stroke, antiphospholipid syndrome | [4,5] | |
| PAI-1 poly. | [6] | |||
| Medical history | CFH poly. with DM | HT, lipid | [7] | |
| Medical history | Low FMD of the brachial artery | Low C-IMTl, LDL, cardioV risk factors | [8] | |
| Vertebrobasilar junction angulation | [9] | |||
| High global oxidative stress index | [10] | |||
| FV Leiden poly., PT poly. | [11] | |||
| Medical history | SBP, personal/family history cardioV events | FV poly., PT poly., HT, DM, lipid, smoking, personal/family history or in the presence of thrombotic factors | [12] | |
| PKCH poly., MTHFR poly. | [13,14] | |||
| Cho, LDL | [15] | |||
| Cho, LDL, unsaturated fatty acid, coenzyme Q10, folate, homocysteine | MTHFR poly., FV poly., PT poly., antithrombin III, protein C and S, D-dimer, FG, activated protein C resistance | [16-18] | ||
| MTHFR poly., FV Leiden poly., PT poly., platelet GlyIIIaA1/A2 poly., homocysteine, Cho, FG, folate | [19,20] | |||
| MTHFR poly. | FV, PT, EPCR, PAI-1 | [21] | ||
| Medical history | ICAM-1, VCAM-1 | Cho, triglyceride, FG, ESR, smoking, DM | [22] | |
| MTHFR poly. with MTR poly., MTR poly. | MTHFR poly. alone | [23] | ||
| FV Leiden poly. | PT poly. | [24] | ||
| Auto-immunity | Cho, homocysteine, PAI-1, anticardiolipin antibodies | FV Leiden poly., FII poly., antithrombin, protein C and S, lupus anticoagulant, lipoprotein(a) | [25] | |
| eNOS poly. | [26] | |||
| FMD | [27] | |||
| Whole blood viscosity, erythrocyte deformability index, activated clotting time, clot rate, PAI-1 antigen, factor VIII:C | Plasma viscosity, FG | [28] | ||
| Auto-immunity | Antiendothelial cell antibody | [29,30] | ||
| Cytokine | IL-1B poly., TNF-β poly. | [31,32] | ||
| TNF-α | IL-10, IL-12 | [33] | ||
| IL-6 poly. | IL-4R poly., IL-10 poly., TNF-α poly., TNFRSF1B poly., VEGF poly. | [34] | ||
| IL-1A poly. | IL-1B poly. | [35] | ||
| Vascular impairment | IL-6, IL-8, ICAM-1, VCAM-1, E-selectin, MCP-1, lipid, FG | [36] | ||
| TNF-α, sCD40, sCD40L, T lymphocyte, CD40, cyclooxygenase 2, CD38 positive T or B lymphocyte | Monocyte, macrophage | [37] | ||
| Cellular stress | HSP70 poly. | [38] | ||
| GPX1 poli., PON1 poli., PON2 poli., SOD2 poli. | [39] | |||
| GST poly., CYP poly. | [40] | |||
| Auto-immunity | Anti-HSP70 antibody, TNF-α, ESR, ANA, antiphospholipid antibody | [41] | ||
| Auto-immunity | Anti-HSP70 antibody, anti-phospholipids antibody | [42] | ||
| HSP70 | [43] | |||
| GST poly. | [44] | |||
| Infection | IgA to HSV1 | IgG and IgM to CMV, VZV, HSV1, and HSV2. IgA to CMV, VZV, and HSV2 | [45] | |
| Borrelia | [46] | |||
| Herpes zoster | [47] | |||
| Recent subclinical viral infection (cytomegalovirus, herpes simplex, Epstein-Barr virus), toxoplasmosis infections | [48] | |||
| Enterovirus, cytomegalovirus, Epstein-Barr virus | [49] | |||
| Auto-immunity | Anti-double stranded DNA, RF, antiphospholipid IgG and M, antinuclear antibody, complements C3 and C4 | [50] | ||
| T cell responding to cochlin | [51] | |||
| Stress response theory | Neutrophil, NKCA, IL-6 | TNF, hCRP | [52] | |
| Histological evidence of severe osmotic stress of the organ of Corti | [53] | |||
| Medical history | HIV | [54] | ||
| Vascular impairment | SLE | [55] | ||
| Vascular impairment | AMI | [56] | ||
| Migrane with HT | [57] | |||
| Vascular impairment | ED | [58] | ||
| Vascular impairment | DM | [59] | ||
| Chronic kidney disease with and without DM | [60] | |||
| Allergy | [61] | |||
| Male with OSA | Female with OSA | [62] | ||
| Vascular impairment | CardioV risk factors, DM, Cho | [63] | ||
| Family history of ISHL | [64] | |||
| Vascular impairment | CerebroV stroke | [65] | ||
| Other aetiologies | Aquaporin 4 and 5 poly., estrogen receptor α poly. | [66] | ||
| Round window membrane rupture | [67] | |||
| Endolymphatic hydrops | [68] | |||
| Eustachian tube dysfunction | [69] | |||
| General anaesthesia | [70] | |||
| Month, weather | [71] | |||
| HLA-DQB1 and -DRB1 | [72] | |||
| Season, weather | [73] |
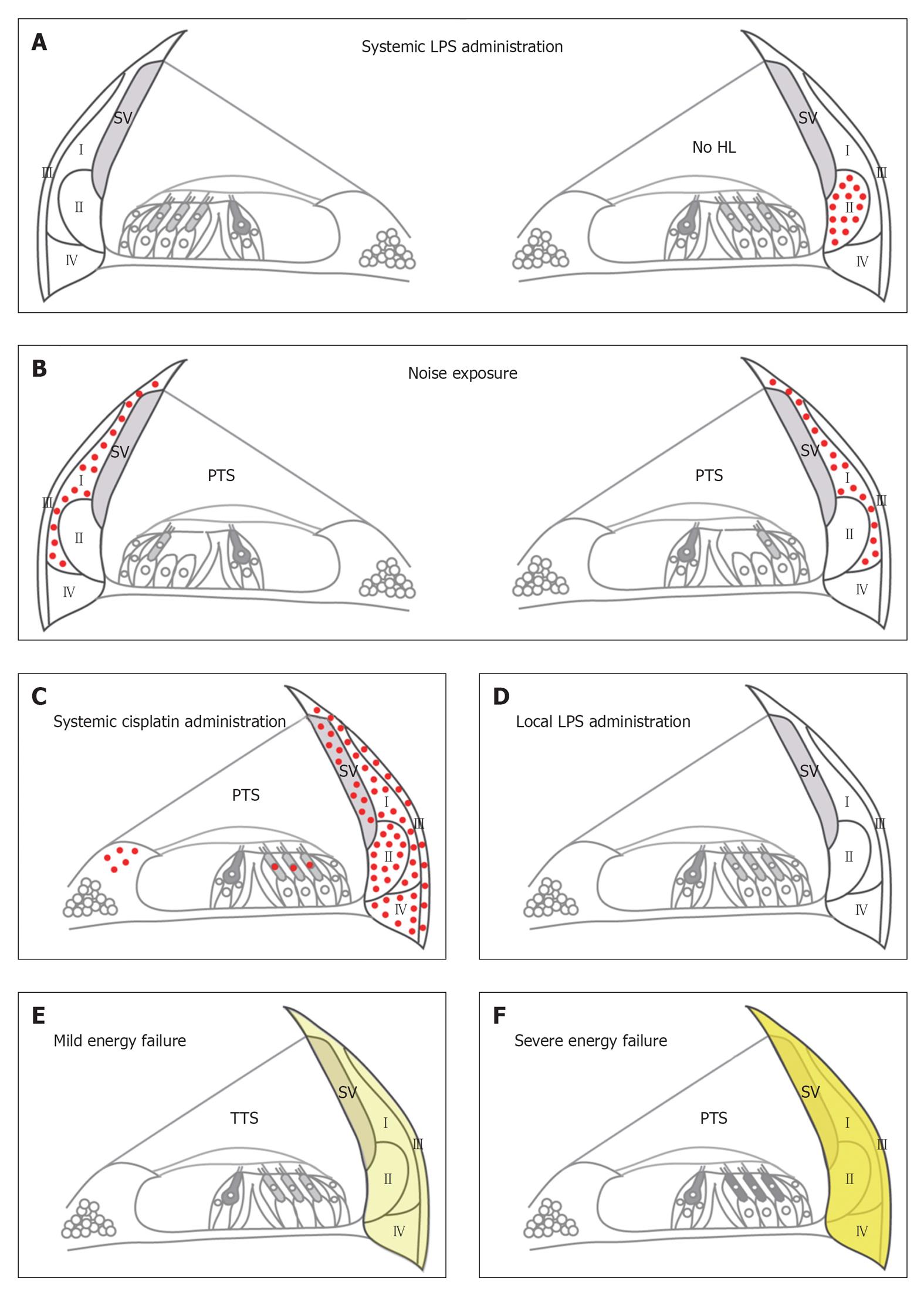
To develop and apply the best treatment for ISHL, we must reveal the pathophysiology. Most papers focus on one cause of the disease, and each proposed etiology has papers that support and refute it, as shown in Table 1. On the other hand, the stress response theory can integrate the various hypotheses proposed up to this point, and can explain the clinical characteristics of ISHL. Originally, the theory was advocated by Merchant et al[53] and Adams[76]. They proposed that ISHL might be a result of pathologic activation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) in the cochlear lateral wall. NF-κB is activated by various stressors, acting as a “stress sensor”. It plays a pivotal role in regulating expression of genes associated with immune system and inflammatory responses. For example, interleukin (IL)-6, tumor necrosis factor-α (TNF-α), inducible nitric oxide (iNOS), and intercellular adhesion molecule 1 (ICAM-1) are NF-κB responsive genes[77]. The authors demonstrated that NF-κB was activated in the unilateral cochlear lateral wall by the systemic stressor, i.e., intraperitoneal bacterial endotoxin lipopolysaccharide (LPS) injection, but not by the local stressor, i.e., intratympanic LPS injection (Figure 1A and D). Then, they speculated that ISHL would be the result of pathologic NF-κB activation responding to the systemic stressor.
In the present review, we describe the association of stress and the onset of ISHL, extending the original concept of the stress response theory. To begin, we will quickly review the influence of the chronic psychosocial and physiological stressors on the human body.
Selye et al[78,79] defined stress response as the body’s nonspecific response when a human being is subjected to stressors, including psychosocial, physical, and biological stimuli. More than half a century ago, Selye et al[80] showed that stress caused damage to organs like the heart and the kidney. In recent years, there is accumulating evidence that chronic stress results in many diseases including dermatitis, depression, cardiovascular disease, osteopenia/osteoporosis, immune suppression, and insulin resistance through the activation of the hypothalamus-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS)[81-83].
Briefly, stressors induce release of corticotropin-releasing hormone (CRH) from the hypothalamus, CRH induces adrenocorticotropic hormone (ACTH) release from the anterior pituitary, ACTH induces glucocorticoid (GC) secretion from the adrenal cortex, and GC induces norepinephrine (NEP) and epinephrine (EP) release from the adrenal medulla. Stressors and CRH also activate the locus coeruleus of the brainstem, eliciting an SNS response and resulting in NEP and EP release. Furthermore, chronic psychological stress results in a decrease in the sensitivity of immune cells to GC that normally terminates the inflammatory response, which then increases a variety of disease risks[84].
It is well known that the cardiovascular system is directly regulated by the NEP and EP from the SNS, and acetylcholine from the parasympathetic nervous system. In addition, converging evidence from animal and human studies indicates that there is an association between stress and cardiovascular disease[81]. However, the effect of these systemic stress-induced catecholamines on the cochlea remains unknown.
Several types of adrenergic and muscarinic acetylcholine receptors are located in the cochlea and in the endolymphatic sac, as well as in blood vessels like the spiral modiolar artery (Table 2)[85-94]. Their exact functions and synapse formation with autonomic nerve fibers are not clear. However, the stress-induced circulating EP and NEP increase will relay the SNS activity to the inner ear. Parasympathetic nervous system activity will also affect the inner ear, because the activity can be relayed to the whole body by the circulating acetylcholine-synthesizing T cells[95], even if the parasympathetic nerve and the cochlea cells do not have synaptic formation.
| Location | Adrenergic receptor | Cholinergic receptor | |||||||
| α1 | α2 | β1 | β2 | 1β | M1 | M2 | M3 | M5 | |
| IHC | ○ | ○ | ○ | ○ | ○ | ||||
| OHC | ○ | ○ | ○ | ○ | |||||
| Deiters’ cells | ○ | ○ | ○ | ○ | ○ | ○ | |||
| Hensen’s cells | ○ | ○ | |||||||
| Outer sulcus | ○ | ||||||||
| Stria vascularis2 | ○ | ||||||||
| Strial marginal cell | ○ | ○ | |||||||
| Capillaries in the stria vascularis | ○ | ||||||||
| Spiral ligament2 | ○ | ○ | ○ | ○ | ○ | ○ | |||
| Rissener’s membrane | ○ | ||||||||
| Spiral ganglion | ○ | ○ | ○ | ○ | ○ | ○ | |||
| Nerve fibers approaching HCs | ○ | ○ | ○ | ||||||
| Efferent fibers of the intraganglionic spiral bundle | ○ | ○ | |||||||
| Spiral modiolar artery | ○ | ○ | |||||||
| Endolymphatic sac | ○ | ||||||||
Considering that these receptors are on the vessels of the cochlea, the lateral wall, and the endolymphatic sac, they probably contribute to the following functions: (1) regulation of the blood flow; (2) maintenance of the inner ear lymphatic ion homeostasis; and (3) enhancement of the stress response in the lateral wall. We will describe these again later in this review.
Chronic stress disrupts immune system homeostasis and induces susceptibility to infectious and autoimmune diseases[96,97]. Furthermore, there are frequent associations between infectious diseases and autoimmune diseases[98]. The natural killer (NK) cell has critical roles in resistance against both viral and bacterial infections[99,100], and in regulating autoimmunity[101]. However, NK cell activity (NKCA) is reduced by chronic stress like fatigue, stressful life events, inability to cope with stress, and shortness of sleep[102-106]. Therefore, such chronic stress has the potential to put the host in danger of infectious and autoimmune disease.
IL-1, IL-6, and TNF are well known pro-inflammatory cytokines. They are pleiotropic and work as both effectors and regulators of stress response composed of the HPA axis and the SNS[107-109]. Nitric oxide (NO) is also involved in the HPA axix response[110].
IL-1 is a potent pro-inflammatory cytokine[111], and is produced centrally and periphery following exposure to immunological and psychological stressors[108]. It directly activates the HPA axis and central nervous system, and can even cause depressive symptoms. IL-1 is also known to induce IL-6 strongly[107].
IL-6 is induced by stress as well as by IL-1. Stress-induced increases in IL-6 are a robust finding, and increases are typically higher in adverse psychological conditions[107]. Work stress is associated with an enhancement of IL-6 production by leukocytes before and after infectious stressor and with a lower capacity of GC to suppress IL-6 production[112].
Local and circulating IL-6 can influence the whole body via classical- and trans-signaling, respectively. In classical signaling, IL-6 works in cells that express the membrane-bound IL-6 receptor, but only few cells express it, so this signaling works locally. In trans-signaling, a complex of circulating IL-6 bound to the IL-6 receptor, which occurs naturally or by cleavage from apoptotic neutrophil, can control inflammatory response through binding with glycoprotein (gp130), displayed by all cells[113].
The underlying mechanism of IL-6 increase under stress is associated with activation of NF-κB[114,115]. Cortisol and catecholamines in the HPA axis and the SNS induce and enhance NF-κB activation under psychosocial stress[114,116]. Then, NF-κB induces IL-6 expression. IL-6 is not only a transcriptional target of NF-κB, but also an activator of NF-κB[117,118]. Therefore, a positive feedback loop can be made between the two.
Chronic stress also disturbs the circadian rhythm of serum IL-6 levels. Although serum levels of IL-6 reflect circadian cycle, IL-6 decreases less during the night in individuals experiencing negative mood or fatigue than those experiencing uplift events[119]. Conversely, IL-6 elevation per se generates fatigue, fever, and sleep-related symptoms such as daytime somnolence[107].
Additionally, TNF-α also increases under chronic psychosocial stress[106,120-123]. There is in fact a personality type associated with high TNF-α, distressed personality[124]. It may be a possible reason why final health status is different among individuals under the same stress.
NO is generally identified as a molecule involved in neurotransmission, neuromodulation, controlling arterial diameter, and protecting blood vessels from deleterious consequences of platelet aggregation and activation of inflammatory responses[125,126]. It is also involved in the LPS-induced HPA axis response under basal conditions and during its adaptation to chronic social stress circumstances[110]. Excess NO induced by increased iNOS expression leads to the formation of a powerful oxidant, peroxynitrite. It results in cell death by many mechanisms, including lipid peroxidation, protein nitration, DNA damage, or the irreversible inhibition of respiration[127].
The description above concerns the stress response of the whole body. Next, we will review the characteristics of the lateral wall that play a leading role in the stress response theory.
The lateral wall consists of the stria vascularis and the spiral ligament, in which there are four types of fibrocytes (Figure 1). The fibrocytes are classified based on general location, and localization of sodium-potassium- adenosine- triphosphatase (Na+/K+-ATPase) and the gap junction protein connexin 26[128-131]. They have a critical role in the potassium ion recycling mechanism[132], and could also be implicated in the mechanisms of glucose transport in the cochlea[133]. Type III fibrocytes have even contractility and regulate tension of the basilar membrane, thereby determining auditory sensitivity[134].
In the stria vascularis, there are three types of cells that express multiple ion-transport apparatuses[135]. Therefore, the lateral wall is essential for maintaining cochlear homeostasis, and thus for normal hearing[128,135]. Degeneration of the lateral wall may be implicated in the survival of sensory cells[136].
Hoya et al[137], Mizutari et al[138] and Okamoto et al[139] demonstrated that different degrees of acute energy failure in the cochlear lateral wall cause different degrees of degeneration of the lateral wall fibrocytes, resulting in different degrees of hearing loss (Figure 1E and F). They administered the mitochondrial toxin 3-nitropropionic acid (3-NP) in the rat cochlea through the round window. Five hundred mmol/L 3-NP caused a permanent threshold shift of more than 80 dB at 8-20 kHz 3 h after 3-NP administration[139]. Marked degeneration of type II fibrocytes, type IV fibrocytes, and cells in the stria vascularis were detected at the same time. Lateral wall degeneration was progressive for at least 14 d. In the organ of Corti, mitochondrial translocation in outer hair cells and mild degeneration of Deiters cells were observed 7 and 14 d after the administration, respectively.
On the other hand, 300 mmol/L 3-NP caused a completely reversible threshold shift at 8 kHz and degeneration of the lateral wall was not observed 3 wk after the 300 mmol/L 3-NP administration[138]. These suggest that mild energy failure of the lateral wall causes temporal and mild disturbance of cochlear homeostasis and temporal hearing loss without loss of inner ear cells. However, severe energy failure causes loss of the lateral wall cells, and then induces structural changes in the organ of Corti resulting in permanent hearing loss.
Wang et al[140] demonstrated that different intensities of an octave band noise (8-16 kHz) resulted in degeneration of different kinds of fibrocytes in the lateral wall and different degrees of hearing loss. In the lateral wall, 94 dB SPL noise caused loss of type IV fibrocytes alone, and resulted in only about 10 dB threshold shift at most. However, more than 112 dB SPL noise caused loss of type I, II, and IV fibrocytes, and resulted in more than 60 dB threshold shift.
These findings suggest that degree of the energy failure and the extent of the dysfunctional region in the lateral wall are the critical indicators of the degree of acute hearing loss.
NF-κB is activated in the lateral wall 24 h or earlier after loading stressors (Table 3)[141-146]. Interestingly, the region where NF-κB is activated changes in the lateral wall depending on the kind of stressor, the degree of the stress intensity, and/or the genetic background of animal.
| Animal | Stressor | Time point | NF-κB | Other factors | Ref. | |||
| Response | Location | Factor | Response | Location | ||||
| CBA/CaJ mice | LPS, ip | 24 h | Activation | Unilateral, II >> I, Lim. | [141] | |||
| LPS, ip + dexamethasone, ip | No activation | |||||||
| Anti-CD3, ip | Activation | I | ||||||
| Taxol, ip | Activation | I | ||||||
| 100 dB SPL | Activation | Bilateral, I >> II, Lim. | ||||||
| CBA mice | 117 dB SPL | 4 h | Transcription | LW | [142] | |||
| 2-12 h1 (4 h) | iNOS | Transcription | LW | |||||
| 0-12 h (6 h) | ICAM-1 | Transcription | LW | |||||
| 2-12 h (6 h) | VCAM-1 | Transcription | LW | |||||
| 14 h | ICAM-1 | Expression | SV | |||||
| 14 h | VCAM-1 | Expression | SV | |||||
| Swiss-Webster mice | Ag | 7 d | Leukocytes | Expression2 | SL3 | [143] | ||
| 90 or 100 dB SPL | 7 d | No expression | ||||||
| 90 or 100 dB SPL + Ag | 7 d | No expression | ||||||
| 118 dB SPL | 4 h | Activation | I, II, IV | |||||
| 7 d | No activation | Leukocytes | Expression2 | LW | ||||
| ICAM-1 | Expression | II | ||||||
| 118 dB SPL + Ag | 4 h | Activation | I, II, IV, HC, SC | |||||
| 7 d | Activation | HC, SC | Leukocytes | Expression | LW4 | |||
| Activation | ICAM-1 | Expression | II, III >> I4 | |||||
| C57/Bl6J mice | 124 dB SPL | 2 h | Activation | I, II, III, IV, SV | iNOS | Most of NF-κB activated cells | [144] | |
| 72 h | Activation | I, II, III, IV, SV5 | iNOS | Most of NF-κB activated cells5 | ||||
| Sprague-Dawley rats | Cisplatin, ip | 24 h | Activation | I, II, III, IV, SV, OHC, Lim. | IL-1β | Expression | II, IV >> I, III, SMV | [145] |
| IL-6 | Expression | SMV | ||||||
| TNF-α | Expression | I, II, III, IV, SV, Lim., SMV, HC | ||||||
| Cisplatin + TNF-α inhibitor | No activation | IL-1β | No expression, no transcription | |||||
| IL-6 | No expression, no transcription | |||||||
| TNF-α | No expression, no transcription | |||||||
| Sprague-Dawley rats | 124 dB SPL | 3 h | IL-6 | Expression | III, IV | [146] | ||
| 6 h | Expression | I, II, III, IV | ||||||
| 12 h | Expression | I, II, III, IV, SV, SG | ||||||
| 24 h | Expression | I, II, III, IV, SG6 | ||||||
Adams et al[141] demonstrated that NF-κB of type I fibrocytes was mainly activated by an octave-band noise (90-112 dB SPL) exposure using CBA/J (Figure 1B). Masuda et al[144] applied noise more than two orders of magnitude greater (124 dB SPL), and demonstrated that NF-κB of the whole lateral wall was activated using C57/Bl6J mice. Miyao et al[143] demonstrated that NF-κB of type I, II, and IV fibrocytes was activated by octave-band noise (118 dB SPL) exposure using Swiss-Webster mice. These results suggest that the same kind of stressor at different intensities or with different genetic backgrounds activates NF-κB of different regions in the lateral wall.
Different kinds of stressors also cause the different regional activation of NF-κB. As mentioned above, noise first induces NF-κB activation of type I fibrocytes in the CBA/Bl6J mice. However, systemic inflammatory stress by peritoneal injection of LPS, a Gram-negative bacterial component, induces the activation in type II fibrocytes with little activation in type I fibrocytes in mice of the same genetic background (Figure 1A)[76,141]. Systemic TNF secretion by intraperitoneal anti-CD3 or taxol injection induces the same NF-κB activation as that by LPS[141]. In another report, intraperitoneal administration of cisplatin induces NF-κB activation in the whole lateral wall (Figure 1C), and this activation was inhibited by TNF-α inhibitor[145]. These suggest that LPS and cisplatin induces NF-κB activation through TNF and/or other factors that remain to be determined.
NF-κB activation in the lateral wall is quick after loading a stressor. For example, activation was confirmed 2 h after noise exposure in the whole lateral wall of C57/Bl6J mice, and less but still significant activation was observed after 72 h (Table 3)[144]. Using Swiss-Webster mice, the activation was observed 4 h after noise exposure but not 7 d after[142]. With intraperitoneal LPS, taxol, or anti-CD3 injection, it was observed in the type I fibrocytes of CBA/CaJ mice after 24 h[141].
The promptly activated NF-κB regulates expression of several inflammatory factors like IL-1β, IL-6, TNF-α, iNOS, ICAM-1, and vascular cell adhesion molecule 1 (VCAM-1). IL-1β, IL-6, and TNF-α are pro-inflammatory cytokines and they are effectors and regulators of the HPA axis and the SNS, and excess NO induced by iNOS increase results in cell death, as discussed previously. ICAM-1 and VCAM-1 are critical in mediating adhesion of leukocytes to vascular endothelial cells and transendothelial migration in a variety of acute and chronic inflammatory diseases[147,148]. They also play an essential role in regulating microvascular permeability[149].
It is noteworthy that multiple stressors enhance and prolong the NF-κB activation and the target gene expression, as compared with a single stressor. Miyao et al[143] demonstrated that noise-exposure plus intrathecal antigen injection induced longer NF-κB activation, much more intense and wider regional ICAM-1 expression, and more leukocytes induction in the lateral wall than noise-exposure alone or antigen injection alone. The NF-κB activation was observed 4 h after but not 7 d after noise-exposure alone. On the other hand, with noise-exposure plus antigen challenge, the activation was observed even 7 d after.
There is an anecdotal hypothesis about the onset of ISHL, in which so-called “stress” (i.e., psychological and physical stressors) may be associated with the onset of ISHL. It is reported that fatigue, stressful life events, inability to cope with stress, and shortness of sleep are involved in the onset of ISHL[150-152]. However, this hypothesis has a contradictory survey, as the other hypotheses do (Table 1 and see Merchant et al[153]). According to a survey by Japanese Ministry of Health, Labor and Welfare in 1975, rates of ISHL patients complaining of psychological and physical stress were unexpectedly low, 13.7% and 22.5%, respectively. This may suggest that a subjective scale of stress is different among individuals and it is difficult to analyze individual stress just by questionnaires.
Concerning the viral hypothesis, many reports could not show histopathological and biomolecular evidences of viral invasion or infection of the inner ear. With respect to the vascular hypothesis, it alone is not enough to explain the clinical characteristics of ISHL. For example, ISHL is not necessarily more prevalent in the elderly, does not accompany other vascular disease, and does not generally recur, making it very different from the cerebral ischemia. Furthermore, only two of 29 ears with ISHL examined showed histopathological evidence of vascular insult to the cochlea, consisting of deposition of connective tissue and new bone within the cochlea.
Finally, Merchant et al[53] and Adams et al[76,141] proposed that the stress response of the lateral wall to systemic stress is the cause of ISHL. They observed the inner ear of a patient who died 9 d after the onset of ISHL. In the affected cochlea, the organ of Corti showed marked swelling with edema, vacuole formation within the cytoplasm, and blurring of cell boundaries. They interpreted this as evidence that the cells in the organ of Corti were under severe osmotic stress, which must have resulted from lymphatic homeostasis disruption in the cochlea. In their paper published in 2005, they speculated that osmotic stress-induced NF-κB activation within the supporting cells may be an important mechanism causing ISHL in addition to the activation in the lateral wall[53]. However, using a sophisticated animal model in 2009, they demonstrated that cells of the organ of Corti and spiral ganglion were remarkable for the lack of NF-κB activation by systemic inflammatory stress[141]. On the other hand, type II fibrocytes in the lateral wall predominantly showed the activation. The lateral wall plays an essential role in maintaining the cochlear homeostasis. In addition, NF-κB is a well-known transcription factor that directly leads to inflammatory cytokine production, and it was observed in animal and human lateral walls, but not in the organ of Corti. Conclusively, the original hypothesis by Adams et al[141] is that ISHL is the result of the stress response of the cochlear lateral wall through NF-κB activation responding to the systemic stress and dysfunction of the lateral wall, and the changes of the organ of Corti cells are the secondary phenomenon to the lateral wall dysfunction.
They demonstrated that intraperitoneal LPS injection, i.e., systemic stress, consistently resulted in NF-κB activation in the lateral wall unilaterally but not bilaterally, and the intratympanic LPS injection, i.e., local stress, did not induce the lateral wall NF-κB activation of the mouse cochlea (Figure 1A and D). This seems to reflect the clinical characteristics of the onset of ISHL: acute onset is consistent with the prompt activation profile of NF-κB, most of cases with ISHL are unilateral, and it is not accompanied with the middle ear inflammation. They speculated that systemic cytokines like TNF-α induced by intraperitoneal LPS injection activate the lateral wall NF-κB.
However, intraperitoneal LPS injection alone activates NF-κB in the type II fibrocytes alone and did not cause hearing loss in mice[141]. Additionally, intraperitoneal injection of anti-CD3 and taxol, which are known to induce TNF secretion, activate NF-κB in the type II cells alone. These observations shed light on the two points: (1) a wider range of NF-κB activation in the lateral wall is needed to cause hearing loss; and (2) systemic stress by infection followed by cytokine increase alone is not enough to induce such a wide range of NF-κB activation. Therefore, the synergistic effect of multiple stressors must be necessary to induce the wide range of lateral wall NF-κB activation resulting in hearing loss.
Next, we will discuss and review how a variety of stressors including psychological and physical stressors converge in lateral wall NF-κB activation and cause ISHL.
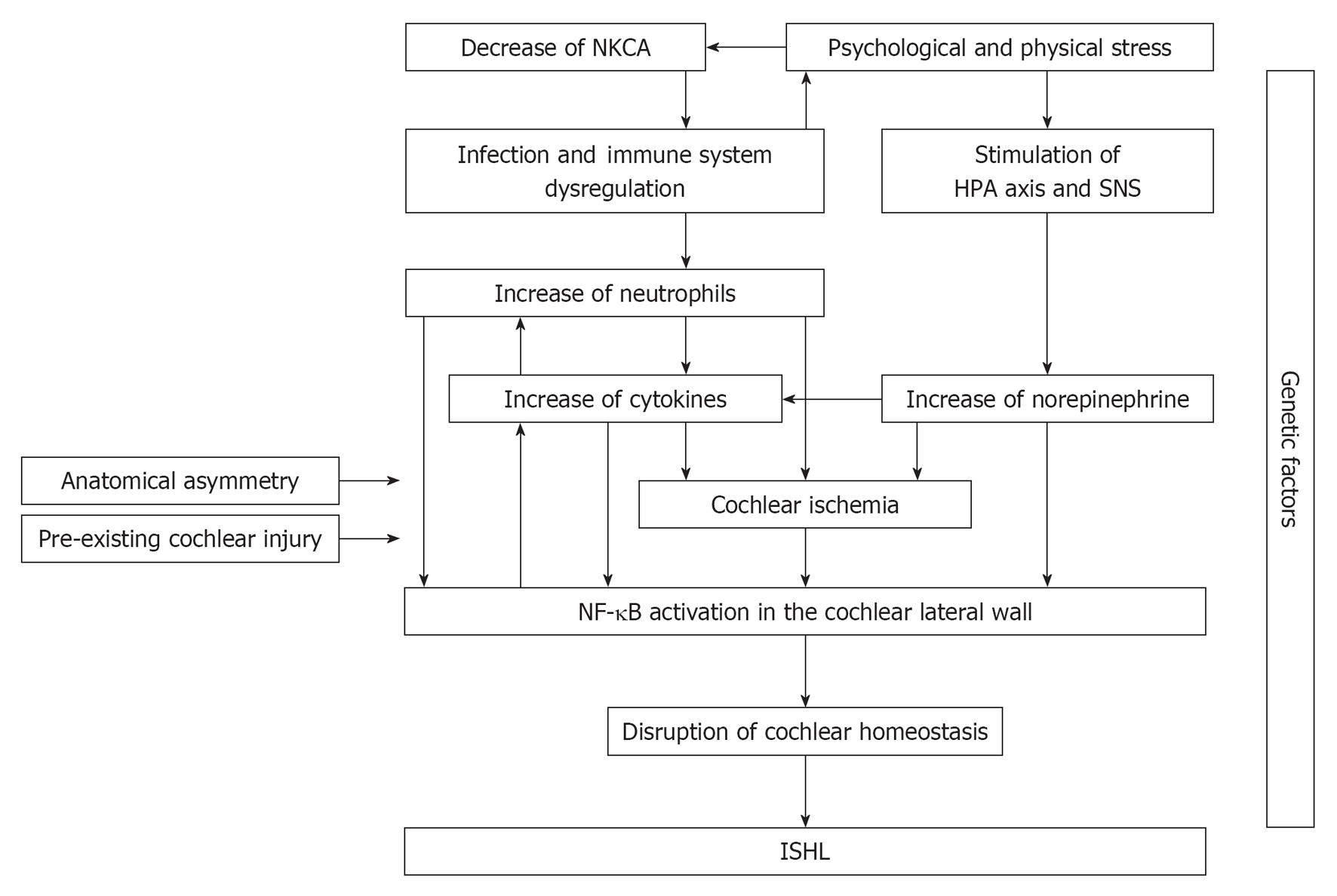
We have reviewed how psychosocial and physical stress affect the HPA axis, the SNS, the immune system, inflammatory factors, and a cytokine network. These systemic stress responses can synergistically induce and enhance lateral wall NF-κB activation (Figure 2). Although it is still impossible to demonstrate the live NF-κB activation in the human cochlea, Masuda et al[52] recently found evidence for the stress response theory using ISHL patients’ blood sample analysis.
So-called “stress,” as in chronic psychosocial and physical stress, results in EP and NEP increases through the HPA axis and SNS activation, and the cochlear lateral wall expresses these receptors (Table 2). Therefore, the stress-induced catecholamines can induce and enhance NEP-dependent NF-κB activation. Therefore, the stress-induced catecholamines can induce and enhance NEP-dependent NF-κB activation[114,154], and induce the target gene expression including pro-inflammatory cytokine, adhesion molecules, and iNOS (Table 3).
Stress decreases NKCA, resulting in dysregulation of the immune system and subclinical infections. This immune system disturbance is involved in the stress response theory. In fact, some authors have suggested that subclinical infection is associated with onset in some ISHL patients after detecting the elevated erythrocyte sedimentation rate or specific antibodies against viruses[45,48,155].
Infection also enhances immune system dysregulation, cytokine production, and psychological stress[156]. Note that bacteria and virus do not attack the inner ear directly in the “infection” we describe here, but they change the whole immune system and have influence on the inner ear homeostasis afterward. These will lead to an increase of circulating neutrophil and cytokines like IL-6; there is a positive feedback loop between neutrophil and IL-6[157-159]. Stress also results in an abnormal immune state. Furthermore, the neutrophil increase induces cochlear energy shortage by impeding the blood flow, because neutrophils have a thrombogenic profile and are known to have association with the risk and prognosis of myocardial infarction and stroke[160-162]. Such an energy shortage induces stress response of the cochlear lateral wall.
Increase of circulating cytokines can also activate lateral wall NF-κB. IL-6 is a target and a regulator of NF-κB, and can have an impact on the NF-κB activation through classic and trans-signaling because the IL-6 receptor and gp130 are expressed in the lateral wall[163]. Circulating TNF-α activates NF-κB of the lateral wall. It also enhances microvascular tone and reduces blood flow in the cochlea[164], resulting in the lateral wall energy shortage.
The whole systemic stressors mentioned above converge synergistically to the NF-κB activation in the lateral wall. The NF-κB activation initiates inflammatory responses in the lateral wall locally. The NF-κB-induced inflammatory cytokines will affect the lateral wall cell function that maintains cochlear homeostasis. The cytokines will also exacerbate inflammatory responses of the lateral wall through enhancing vascular permeability and recruitment of leukocytes[165,166], because the blood supply to the lateral wall is abundant. In rabbits, for example, the lateral wall contains more than 80% of total cochlear blood[167]. The disruption of cochlear homeostasis ultimately causes ISHL. In fact, an ISHL-affected ear has high concentration of proteins in the inner ear fluid space using fluid-attenuated inversion recovery MRI[168-170], suggesting the disruption of cochlear homeostasis.
To explain clinical characteristics of ISHL, the integrative stress response theory should be integrated with other possible factors. At first, ISHL usually affects the unilateral ear, and the prevalence is much lower in childhood than in adulthood. Minor pre-existing subclinical damage in the inner ear or asymmetry of terminal vascular structure (for example, stenotic or not stenotic, straight or torturous) could be a potential explanation for the clinical characteristics of ISHL.
All persons under stress do not suffer from ISHL. Therefore, there must be innate factors for ISHL onset, probably including polymorphisms of genes encoding coagulation factors, vascular tone, and cytokines, among others (Table 1). Even individual personality is likely be involved in differential stress response.
A quest for a single definitive cause of ISHL does not seem to be reasonable after reviewing the literature. The basic and critical concept of the stress response theory is that ISHL must not result from a specific single and local cause in the inner ear. Moreover, ISHL should encompass several causes contributing to different degrees of severity and prognosis. Synchronism of different types of factors and different degrees of contribution of each factor could result in the individual ISHL case. Some of these factors must occur rarely, and each factor must occur in a temporally appropriate order to trigger pathological NF-κB activation in the cochlear lateral wall. Therefore, ISHL does not recur frequently, even in the same individual.
We have described the possibility that psychosocial and physical stress increase the likelihood of disruption of cochlear homeostasis. Long-term stress should be detected objectively, as in HbA1c for analyzing blood sugar level over periods of 1 or 2 mo in diabetic patients. Monocyte chemotactic protein-1, epidermal growth factor, and vascular endothelial growth factor have been expected to be prolonged psychosocial stress markers[171], but the validity is still controversial[172]. There are controversies about the association of pro-inflammatory cytokines and ISHL as well[31-36,41,52] (Table 1). It may not be enough to measure and analyze the value of each biomarker separately. A new method that analyzes a complicated network consisting of multiple factors will be needed. Broderick et al[173] focused on the network of cytokines in which cytokine-cytokine associations are demonstrated topologically, and they demonstrated that the network of subjects with chronic fatigue syndrome deferred in topology significantly compared with healthy subjects.
Therefore, it is vital to integrate of our knowledge and comprehensive analysis of possible etiologies to reveal the pathophysiology of ISHL.
P- Reviewers Ciuman R, Gross M, Nakashima T S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Fusconi M, Chistolini A, Angelosanto N, Pignoloni P, Tombolini M, De Virgilio A, Pagliarella M, de Vincentiis M. Role of genetic and acquired prothrombotic risk factors in genesis of sudden sensorineural hearing loss. Audiol Neurootol. 2011;16:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Fusconi M, Chistolini A, de Virgilio A, Greco A, Massaro F, Turchetta R, Benincasa AT, Tombolini M, de Vincentiis M. Sudden sensorineural hearing loss: a vascular cause Analysis of prothrombotic risk factors in head and neck. Int J Audiol. 2012;51:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Lin RJ, Krall R, Westerberg BD, Chadha NK, Chau JK. Systematic review and meta-analysis of the risk factors for sudden sensorineural hearing loss in adults. Laryngoscope. 2012;122:624-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Ballesteros F, Alobid I, Tassies D, Reverter JC, Scharf RE, Guilemany JM, Bernal-Sprekelsen M. Is there an overlap between sudden neurosensorial hearing loss and cardiovascular risk factors. Audiol Neurootol. 2009;14:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Ballesteros F, Tassies D, Reverter JC, Alobid I, Bernal-Sprekelsen M. Idiopathic sudden sensorineural hearing loss: classic cardiovascular and new genetic risk factors. Audiol Neurootol. 2012;17:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Cho SH, Chen H, Kim IS, Yokose C, Kang J, Cho D, Cai C, Palma S, Busi M, Martini A. Association of the 4 g/5 g polymorphism of plasminogen activator inhibitor-1 gene with sudden sensorineural hearing loss. A case control study. BMC Ear Nose Throat Disord. 2012;12:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Nishio N, Teranishi M, Uchida Y, Sugiura S, Ando F, Shimokata H, Sone M, Otake H, Kato K, Yoshida T. Contribution of complement factor H Y402H polymorphism to sudden sensorineural hearing loss risk and possible interaction with diabetes. Gene. 2012;499:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Ciccone MM, Cortese F, Pinto M, Di Teo C, Fornarelli F, Gesualdo M, Mezzina A, Sabatelli E, Scicchitano P, Quaranta N. Endothelial function and cardiovascular risk in patients with idiopathic sudden sensorineural hearing loss. Atherosclerosis. 2012;225:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Kim C, Sohn JH, Choi HC. Vertebrobasilar angulation and its association with sudden sensorineural hearing loss. Med Hypotheses. 2012;79:202-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Capaccio P, Pignataro L, Gaini LM, Sigismund PE, Novembrino C, De Giuseppe R, Uva V, Tripodi A, Bamonti F. Unbalanced oxidative status in idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2012;269:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Lan MY, Shiao JY, Hsu YB, Lin FY, Lin JC. A preliminary study on the role of inherited prothrombotic risk factors in Taiwanese patients with sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2011;268:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Mosnier I, Stepanian A, Baron G, Bodenez C, Robier A, Meyer B, Fraysse B, Bertholon P, Defay F, Ameziane N. Cardiovascular and thromboembolic risk factors in idiopathic sudden sensorineural hearing loss: a case-control study. Audiol Neurootol. 2011;16:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Uchida Y, Sugiura S, Ando F, Shimokata H, Nakashima T. Association of the C677T polymorphism in the methylenetetrahydrofolate reductase gene with sudden sensorineural hearing loss. Laryngoscope. 2010;120:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Uchida Y, Sugiura S, Nakashima T, Ando F, Shimokata H. Contribution of 1425G/A polymorphism in protein kinase C-Eta (PRKCH) gene and brain white matter lesions to the risk of sudden sensorineural hearing loss in a Japanese nested case-control study. J Neurogenet. 2011;25:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Oreskovic Z, Shejbal D, Bicanic G, Kekic B. Influence of lipoproteins and fibrinogen on pathogenesis of sudden sensorineural hearing loss. J Laryngol Otol. 2011;125:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Cadoni G, Agostino S, Scipione S, Galli J. Low serum folate levels: a risk factor for sudden sensorineural hearing loss. Acta Otolaryngol. 2004;124:608-611. [PubMed] |
| 17. | Cadoni G, Scipione S, Rocca B, Agostino S, La Greca C, Bonvissuto D, Paludetti G. Lack of association between inherited thrombophilic risk factors and idiopathic sudden sensorineural hearing loss in Italian patients. Ann Otol Rhinol Laryngol. 2006;115:195-200. [PubMed] |
| 18. | Cadoni G, Scorpecci A, Cianfrone F, Giannantonio S, Paludetti G, Lippa S. Serum fatty acids and cardiovascular risk factors in sudden sensorineural hearing loss: a case-control study. Ann Otol Rhinol Laryngol. 2010;119:82-88. [PubMed] |
| 19. | Capaccio P, Cuccarini V, Ottaviani F, Fracchiolla NS, Bossi A, Pignataro L. Prothrombotic gene mutations in patients with sudden sensorineural hearing loss and cardiovascular thrombotic disease. Ann Otol Rhinol Laryngol. 2009;118:205-210. [PubMed] |
| 20. | Capaccio P, Ottaviani F, Cuccarini V, Bottero A, Schindler A, Cesana BM, Censuales S, Pignataro L. Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope. 2007;117:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Yildiz Z, Ulu A, Incesulu A, Ozkaptan Y, Akar N. The importance of thrombotic risk factors in the development of idiopathic sudden hearing loss. Clin Appl Thromb Hemost. 2008;14:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Quaranta N, Ramunni A, Brescia P, D’Elia A, Vacca A, Ria R. Soluble intercellular adhesion molecule 1 and soluble vascular cell adhesion molecule 1 in sudden hearing loss. Otol Neurotol. 2008;29:470-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Gross M, Friedman G, Eliashar R, Koren-Morag N, Goldschmidt N, Atta IA, Ben-Yehuda A. Impact of methionine synthase gene and methylenetetrahydrofolate reductase gene polymorphisms on the risk of sudden sensorineural hearing loss. Audiol Neurootol. 2006;11:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Görür K, Tuncer U, Eskandari G, Ozcan C, Unal M, Ozsahinoglu C. The role of factor V Leiden and prothrombin G20210A mutations in sudden sensorineural hearing loss. Otol Neurotol. 2005;26:599-601. [PubMed] |
| 25. | Marcucci R, Alessandrello Liotta A, Cellai AP, Rogolino A, Berloco P, Leprini E, Pagnini P, Abbate R, Prisco D. Cardiovascular and thrombophilic risk factors for idiopathic sudden sensorineural hearing loss. J Thromb Haemost. 2005;3:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Fatini C, Mannini L, Sticchi E, Cecchi E, Bruschettini A, Leprini E, Pagnini P, Gensini GF, Prisco D, Abbate R. eNOS gene affects red cell deformability: role of T-786C, G894T, and 4a/4b polymorphisms. Clin Appl Thromb Hemost. 2005;11:481-488. [PubMed] |
| 27. | Balletshofer BM, Stock J, Rittig K, Lehn-Stefan A, Braun N, Burkart F, Plontke S, Klingel R, Häring HU. Acute effect of rheopheresis on peripheral endothelial dysfunction in patients suffering from sudden hearing loss. Ther Apher Dial. 2005;9:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Mannini L, Paniccia R, Cecchi E, Alessandrello Liotta A, Leprini E, Berloco P, Pagnini P, Abbate R, Franco Gensini G, Prisco D. Reduced erythrocyte deformability and hypercoagulability in idiopathic sudden sensorineural hearing loss. Clin Hemorheol Microcirc. 2005;33:47-55. [PubMed] |
| 29. | Cadoni G, Agostino S, Manna R, De Santis A, Fetoni AR, Vulpiani P, Ottaviani F. Clinical associations of serum antiendothelial cell antibodies in patients with sudden sensorineural hearing loss. Laryngoscope. 2003;113:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Cadoni G, Fetoni AR, Agostino S, De Santis A, Manna R, Ottaviani F, Paludetti G. Autoimmunity in sudden sensorineural hearing loss: possible role of anti-endothelial cell autoantibodies. Acta Otolaryngol Suppl. 2002;122:30-33. [PubMed] |
| 31. | Um JY, Jang CH, Kim HL, Cho YB, Park J, Lee SJ, Kim YB, Kim HJ, Ahn KS, Jang HJ. Proinflammatory cytokine IL-1 β polymorphisms in sudden sensorineural hearing loss. Immunopharmacol Immunotoxicol. 2013;35:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Um JY, Jang CH, Kim KY, Kim SJ, Kim NH, Moon PD, Choi IY, Myung NY, Jeong HJ, Hong SH. Candidate genes of cerebrovascular disease and sudden sensorineural hearing loss. Clin Appl Thromb Hemost. 2010;16:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Demirhan E, Eskut NP, Zorlu Y, Cukurova I, Tuna G, Kirkali FG. Blood levels of TNF-α, IL-10, and IL-12 in idiopathic sudden sensorineural hearing loss. Laryngoscope. 2013;123:1778-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Hiramatsu M, Teranishi M, Uchida Y, Nishio N, Suzuki H, Kato K, Otake H, Yoshida T, Tagaya M, Suzuki H. Polymorphisms in genes involved in inflammatory pathways in patients with sudden sensorineural hearing loss. J Neurogenet. 2012;26:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Furuta T, Teranishi M, Uchida Y, Nishio N, Kato K, Otake H, Yoshida T, Tagaya M, Suzuki H, Sugiura M. Association of interleukin-1 gene polymorphisms with sudden sensorineural hearing loss and Ménière’s disease. Int J Immunogenet. 2011;38:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Haubner F, Martin L, Steffens T, Strutz J, Kleinjung T. The role of soluble adhesion molecules and cytokines in sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2011;144:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Kassner SS, Schöttler S, Bonaterra GA, Stern-Sträter J, Sommer U, Hormann K, Kinscherf R, Gössler UR. Proinflammatory and proadhesive activation of lymphocytes and macrophages in sudden sensorineural hearing loss. Audiol Neurootol. 2011;16:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Chien CY, Chang NC, Tai SY, Wang LF, Wu MT, Ho KY. Heat shock protein 70 gene polymorphisms in sudden sensorineural hearing loss. Audiol Neurootol. 2012;17:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Teranishi M, Uchida Y, Nishio N, Kato K, Otake H, Yoshida T, Suzuki H, Sone M, Sugiura S, Ando F. Polymorphisms in genes involved in oxidative stress response in patients with sudden sensorineural hearing loss and Ménière’s disease in a Japanese population. DNA Cell Biol. 2012;31:1555-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Um JY, Jang CH, Kim SJ, Kim HL, Kim SY, Cho YB, Hong SH. Steroid combination therapy and detoxification enzyme gene polymorphisms in sudden sensorineural hearing loss patients. Otol Neurotol. 2011;32:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Süslü N, Yilmaz T, Gürsel B. Utility of anti-HSP 70, TNF-alpha, ESR, antinuclear antibody, and antiphospholipid antibodies in the diagnosis and treatment of sudden sensorineural hearing loss. Laryngoscope. 2009;119:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Gross M, Eliashar R, Ben-Yaakov A, Ulmansky R, Elidan J. Prevalence and clinical significance of anticardiolipin, anti-beta2-glycoprotein-1, and anti-heat shock protein-70 autoantibodies in sudden sensorineural hearing loss. Audiol Neurootol. 2008;13:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Park SN, Yeo SW, Park KH. Serum heat shock protein 70 and its correlation with clinical characteristics in patients with sudden sensorineural hearing loss. Laryngoscope. 2006;116:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Cadoni G, Boccia S, Scipione S, Arzani D, Cianfagna F, Ricciardi G, Paludetti G, Agostino S. Glutathione s-transferase gene polymorphisms in Italian patients with sudden sensorineural hearing loss. Otol Neurotol. 2006;27:1166-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Scalia G, Palermo CI, Maiolino L, Costanzo CM, Zappal D, Grillo C, Martines AM, Cocuzza S, Russo R, Serra A. Detection of serum IgA to HSV1 and its diagnostic role in sudden hearing loss. New Microbiol. 2013;36:41-47. [PubMed] |
| 46. | Bakker R, Aarts MC, van der Heijden GJ, Rovers MM. No evidence for the diagnostic value of Borrelia serology in patients with sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Sheu JJ, Keller JJ, Chen YH, Wu CS, Lin HC. No increased risk of sudden sensorineural hearing loss following recent herpes zoster: a nationwide population-based study. Acta Otolaryngol. 2012;132:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Kikidis D, Nikolopoulos TP, Kampessis G, Stamatiou G, Chrysovergis A. Sudden sensorineural hearing loss: subclinical viral and toxoplasmosis infections as aetiology and how they alter the clinical course. ORL J Otorhinolaryngol Relat Spec. 2011;73:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Gross M, Wolf DG, Elidan J, Eliashar R. Enterovirus, cytomegalovirus, and Epstein-Barr virus infection screening in idiopathic sudden sensorineural hearing loss. Audiol Neurootol. 2007;12:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Cho CH, Jung BS, Jung JH, Lee JH, Lee JH. Expression of autoantibodies in patients with sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 2013;122:131-134. [PubMed] |
| 51. | Baek MJ, Park HM, Johnson JM, Altuntas CZ, Jane-Wit D, Jaini R, Solares CA, Thomas DM, Ball EJ, Robertson NG. Increased frequencies of cochlin-specific T cells in patients with autoimmune sensorineural hearing loss. J Immunol. 2006;177:4203-4210. [PubMed] |
| 52. | Masuda M, Kanzaki S, Minami S, Kikuchi J, Kanzaki J, Sato H, Ogawa K. Correlations of inflammatory biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2012;33:1142-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 53. | Merchant SN, Adams JC, Nadol JB. Pathology and pathophysiology of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2005;26:151-160. [PubMed] |
| 54. | Lin C, Lin SW, Weng SF, Lin YS. Increased risk of sudden sensorineural hearing loss in patients with human immunodeficiency virus aged 18 to 35 years: a population-based cohort study. JAMA Otolaryngol Head Neck Surg. 2013;139:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Lin C, Lin SW, Weng SF, Lin YS. Risk of sudden sensorineural hearing loss in patients with systemic lupus erythematosus: a population-based cohort study. Audiol Neurootol. 2013;18:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Keller JJ, Wu CS, Kang JH, Lin HC. Association of acute myocardial infarction with sudden sensorineural hearing loss: a population-based case-control study. Audiol Neurootol. 2013;18:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Chu CH, Liu CJ, Fuh JL, Shiao AS, Chen TJ, Wang SJ. Migraine is a risk factor for sudden sensorineural hearing loss: a nationwide population-based study. Cephalalgia. 2013;33:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Keller JJ, Chen YK, Lin HC. A case-control analysis on the association between erectile dysfunction and sudden sensorineural hearing loss in Taiwan. J Sex Med. 2012;9:1411-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Lin SW, Lin YS, Weng SF, Chou CW. Risk of developing sudden sensorineural hearing loss in diabetic patients: a population-based cohort study. Otol Neurotol. 2012;33:1482-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 60. | Lin C, Hsu HT, Lin YS, Weng SF. Increased risk of getting sudden sensorineural hearing loss in patients with chronic kidney disease: a population-based cohort study. Laryngoscope. 2013;123:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Keleş E, Sapmaz E, Gödekmerdan A. The role of allergy in the etiopathogenesis of idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2013;270:1795-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Sheu JJ, Wu CS, Lin HC. Association between obstructive sleep apnea and sudden sensorineural hearing loss: a population-based case-control study. Arch Otolaryngol Head Neck Surg. 2012;138:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Aimoni C, Bianchini C, Borin M, Ciorba A, Fellin R, Martini A, Scanelli G, Volpato S. Diabetes, cardiovascular risk factors and idiopathic sudden sensorineural hearing loss: a case-control study. Audiol Neurootol. 2010;15:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 64. | Gäckler A, Eickelmann AK, Brors D, Dazert S, Epplen JT, Kunstmann E. Positive family history of idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2010;267:1843-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Lin HC, Chao PZ, Lee HC. Sudden sensorineural hearing loss increases the risk of stroke: a 5-year follow-up study. Stroke. 2008;39:2744-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 66. | Nishio N, Teranishi M, Uchida Y, Sugiura S, Ando F, Shimokata H, Sone M, Otake H, Kato K, Yoshida T. Polymorphisms in genes encoding aquaporins 4 and 5 and estrogen receptor α in patients with Ménière’s disease and sudden sensorineural hearing loss. Life Sci. 2013;92:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Haubner F, Rohrmeier C, Koch C, Vielsmeier V, Strutz J, Kleinjung T. Occurence of a round window membrane rupture in patients with sudden sensorineural hearing loss. BMC Ear Nose Throat Disord. 2012;12:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Chen X, Zhang XD, Gu X, Fang ZM, Zhang R. Endolymphatic space imaging in idiopathic sudden sensorineural hearing loss with vertigo. Laryngoscope. 2012;122:2265-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Park JJ, Luedeke I, Luecke K, Emmerling O, Westhofen M. Eustachian tube function in patients with inner ear disorders. Eur Arch Otorhinolaryngol. 2013;270:1615-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Punj J, Pandey R, Darlong V. Sensorineural hearing loss after general anaesthesia: 52 cases reported until now! Anaesthesia. 2009;64:226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Lin HC, Lee HC, Chao PZ, Wu CS. The effects of weather on the incidence of sudden sensorineural hearing loss: a 5-year population-based study. Audiol Neurootol. 2006;11:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Amor-Dorado JC, Paco L, Martin J, Lopez-Nevot MA, Gonzalez-Gay MA. Human leukocyte antigen-DQB1 and -DRB1 associations in patients with idiopathic sudden sensorineural hearing loss from a defined population of Northwest Spain. Acta Otolaryngol. 2005;125:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Danielides V, Nousia CS, Bartzokas A, Lolis CJ, Kateri M, Skevas A. Weather conditions and sudden sensorineural hearing loss. BMC Ear Nose Throat Disord. 2002;2:2. [PubMed] |
| 74. | Finger RP, Gostian AO. Idiopathic sudden hearing loss: contradictory clinical evidence, placebo effects and high spontaneous recovery rate--where do we stand in assessing treatment outcomes. Acta Otolaryngol. 2006;126:1124-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Nosrati-Zarenoe R, Arlinger S, Hultcrantz E. Idiopathic sudden sensorineural hearing loss: results drawn from the Swedish national database. Acta Otolaryngol. 2007;127:1168-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 76. | Adams JC. Clinical implications of inflammatory cytokines in the cochlea: a technical note. Otol Neurotol. 2002;23:316-322. [PubMed] |
| 77. | Mattson MP, Culmsee C, Yu Z, Camandola S. Roles of nuclear factor kappaB in neuronal survival and plasticity. J Neurochem. 2000;74:443-456. [PubMed] |
| 78. | Selye H, Fortier C. Adaptive reactions to stress. Res Publ Assoc Res Nerv Ment Dis. 1949;29:3-18. [PubMed] |
| 79. | Selye H, McKeown T. Studies on the physiology of the maternal placenta in the rat. Proc Roy Soc Lond. 1935;119:1-35. |
| 80. | Selye H, Bajusz E. Sensitization by potassium deficiency for the production of myocardial necrosis by stress. Am J Pathol. 1959;35:525-535. [PubMed] |
| 81. | Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 82. | Hall JM, Cruser D, Podawiltz A, Mummert DI, Jones H, Mummert ME. Psychological Stress and the Cutaneous Immune Response: Roles of the HPA Axis and the Sympathetic Nervous System in Atopic Dermatitis and Psoriasis. Dermatol Res Pract. 2012;2012:403908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 83. | Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 894] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 84. | Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA. 2012;109:5995-5999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 845] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 85. | Khan KM, Drescher MJ, Hatfield JS, Ramakrishnan NA, Drescher DG. Immunohistochemical localization of adrenergic receptors in the rat organ of corti and spiral ganglion. J Neurosci Res. 2007;85:3000-3012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Khan KM, Sarfaraz N, Siddiqui S, Malik ZA, Salim Z. Expression of G protein alpha subunits in the lateral wall of the rat cochlea. J Anat. 2003;202:293-301. [PubMed] |
| 87. | Gruber DD, Dang H, Shimozono M, Scofield MA, Wangemann P. Alpha1A-adrenergic receptors mediate vasoconstriction of the isolated spiral modiolar artery in vitro. Hear Res. 1998;119:113-124. [PubMed] |
| 88. | Ross MD. Glycogen accumulation in Reissner’s membrane following chemical sympathectomy with 6-hydroxydopamine. Acta Otolaryngol. 1978;86:314-330. [PubMed] |
| 89. | Wangemann P, Liu J, Shimozono M, Schimanski S, Scofield MA. K+ secretion in strial marginal cells is stimulated via beta 1-adrenergic receptors but not via beta 2-adrenergic or vasopressin receptors. J Membr Biol. 2000;175:191-202. [PubMed] |
| 90. | Fauser C, Schimanski S, Wangemann P. Localization of beta1-adrenergic receptors in the cochlea and the vestibular labyrinth. J Membr Biol. 2004;201:25-32. [PubMed] |
| 91. | Mori N, Uozumi N. Evidence that beta 2-receptors mediate action of catecholamines on endolymphatic sac DC potential. Am J Physiol. 1991;260:R911-R915. [PubMed] |
| 92. | Schimanski S, Scofield MA, Wangemann P. Functional beta2-adrenergic receptors are present in nonstrial tissues of the lateral wall in the gerbil cochlea. Audiol Neurootol. 2001;6:124-131. [PubMed] |
| 93. | Khan KM, Drescher MJ, Hatfield JS, Khan AM, Drescher DG. Muscarinic receptor subtypes are differentially distributed in the rat cochlea. Neuroscience. 2002;111:291-302. [PubMed] |
| 94. | Kanzaki J, Masuda M. Correlation between stress and acute sensorineural hearing loss: stress and sudden deafness (in Japanese). Audiology Japan. 2013;56:137-152. |
| 95. | Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1130] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 96. | Dimitrijevic M, Stanojevic S, Kustrimovic N, Leposavic G. End-point effector stress mediators in neuroimmune interactions: their role in immune system homeostasis and autoimmune pathology. Immunol Res. 2012;52:64-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Dragoş D, Tănăsescu MD. The effect of stress on the defense systems. J Med Life. 2010;3:10-18. [PubMed] |
| 98. | Shi FD, Ljunggren HG, Sarvetnick N. Innate immunity and autoimmunity: from self-protection to self-destruction. Trends Immunol. 2001;22:97-101. [PubMed] |
| 99. | Costantini C, Micheletti A, Calzetti F, Perbellini O, Pizzolo G, Cassatella MA. Neutrophil activation and survival are modulated by interaction with NK cells. Int Immunol. 2010;22:827-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 100. | King PT, Ngui J, Farmer MW, Hutchinson P, Holmes PW, Holdsworth SR. Cytotoxic T lymphocyte and natural killer cell responses to non-typeable Haemophilus influenzae. Clin Exp Immunol. 2008;152:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 101. | Shi FD, Zhou Q. Natural killer cells as indispensable players and therapeutic targets in autoimmunity. Autoimmunity. 2011;44:3-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 102. | Cohen F, Kemeny ME, Zegans LS, Johnson P, Kearney KA, Stites DP. Immune function declines with unemployment and recovers after stressor termination. Psychosom Med. 2007;69:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Borella P, Bargellini A, Rovesti S, Pinelli M, Vivoli R, Solfrini V, Vivoli G. Emotional stability, anxiety, and natural killer activity under examination stress. Psychoneuroendocrinology. 1999;24:613-627. [PubMed] |
| 104. | Fletcher MA, Zeng XR, Maher K, Levis S, Hurwitz B, Antoni M, Broderick G, Klimas NG. Biomarkers in chronic fatigue syndrome: evaluation of natural killer cell function and dipeptidyl peptidase IV/CD26. PLoS One. 2010;5:e10817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 105. | Fondell E, Axelsson J, Franck K, Ploner A, Lekander M, Bälter K, Gaines H. Short natural sleep is associated with higher T cell and lower NK cell activities. Brain Behav Immun. 2011;25:1367-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 106. | Bellingrath S, Rohleder N, Kudielka BM. Healthy working school teachers with high effort-reward-imbalance and overcommitment show increased pro-inflammatory immune activity and a dampened innate immune defence. Brain Behav Immun. 2010;24:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 107. | Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. 2012;1261:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 108. | Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 109. | Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595-638. [PubMed] |
| 110. | Gadek-Michalska A, Spyrka J, Bugajski J. Psychosocial stress affects the involvement of prostaglandins and nitric oxide in the lipopolysaccharide-induced hypothalamic-pituitary-adrenal response. J Physiol Pharmacol. 2005;56:287-298. [PubMed] |
| 111. | Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1316] [Cited by in RCA: 1362] [Article Influence: 104.8] [Reference Citation Analysis (0)] |
| 112. | Bellingrath S, Rohleder N, Kudielka BM. Effort-reward-imbalance in healthy teachers is associated with higher LPS-stimulated production and lower glucocorticoid sensitivity of interleukin-6 in vitro. Biol Psychol. 2013;92:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 113. | Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1902] [Cited by in RCA: 2299] [Article Influence: 164.2] [Reference Citation Analysis (0)] |
| 114. | Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 624] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 115. | Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 116. | Richlin VA, Arevalo JM, Zack JA, Cole SW. Stress-induced enhancement of NF-kappaB DNA-binding in the peripheral blood leukocyte pool: effects of lymphocyte redistribution. Brain Behav Immun. 2004;18:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 713] [Cited by in RCA: 714] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 118. | Jeong HJ, Hong SH, Park RK, Shin T, An NH, Kim HM. Hypoxia-induced IL-6 production is associated with activation of MAP kinase, HIF-1, and NF-kappaB on HEI-OC1 cells. Hear Res. 2005;207:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 119. | Rief W, Mills PJ, Ancoli-Israel S, Ziegler MG, Pung MA, Dimsdale JE. Overnight changes of immune parameters and catecholamines are associated with mood and stress. Psychosom Med. 2010;72:755-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 120. | Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, Gourby B, Bourrilhon C, Florence G, Gomez-Merino D. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine. 2011;56:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 121. | von Känel R, Bellingrath S, Kudielka BM. Association between burnout and circulating levels of pro- and anti-inflammatory cytokines in schoolteachers. J Psychosom Res. 2008;65:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Lalive PH, Burkhard PR, Chofflon M. TNF-alpha and psychologically stressful events in healthy subjects: potential relevance for multiple sclerosis relapse. Behav Neurosci. 2002;116:1093-1097. [PubMed] |
| 123. | Weber C, Arck P, Mazurek B, Klapp BF. Impact of a relaxation training on psychometric and immunologic parameters in tinnitus sufferers. J Psychosom Res. 2002;52:29-33. [PubMed] |
| 124. | Mommersteeg PM, Pelle AJ, Ramakers C, Szabó BM, Denollet J, Kupper N. Type D personality and course of health status over 18 months in outpatients with heart failure: multiple mediating inflammatory biomarkers. Brain Behav Immun. 2012;26:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 125. | Heinrich UR, Helling K. Nitric oxide--a versatile key player in cochlear function and hearing disorders. Nitric Oxide. 2012;27:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 126. | Kerr PM, Tam R, Narang D, Potts K, McMillan D, McMillan K, Plane F. Endothelial calcium-activated potassium channels as therapeutic targets to enhance availability of nitric oxide. Can J Physiol Pharmacol. 2012;90:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 127. | Keynes RG, Garthwaite J. Nitric oxide and its role in ischaemic brain injury. Curr Mol Med. 2004;4:179-191. [PubMed] |
| 128. | Zhao HB, Kikuchi T, Ngezahayo A, White TW. Gap junctions and cochlear homeostasis. J Membr Biol. 2006;209:177-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 129. | Kikuchi T, Kimura RS, Paul DL, Adams JC. Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol (Berl). 1995;191:101-118. [PubMed] |
| 130. | Spicer SS, Schulte BA. Differentiation of inner ear fibrocytes according to their ion transport related activity. Hear Res. 1991;56:53-64. [PubMed] |
| 131. | Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4:339-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 230] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 132. | Kelly JJ, Forge A, Jagger DJ. Development of gap junctional intercellular communication within the lateral wall of the rat cochlea. Neuroscience. 2011;180:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 133. | Suzuki T, Matsunami T, Hisa Y, Takata K, Takamatsu T, Oyamada M. Roles of gap junctions in glucose transport from glucose transporter 1-positive to -negative cells in the lateral wall of the rat cochlea. Histochem Cell Biol. 2009;131:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Kelly JJ, Forge A, Jagger DJ. Contractility in type III cochlear fibrocytes is dependent on non-muscle myosin II and intercellular gap junctional coupling. J Assoc Res Otolaryngol. 2012;13:473-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 135. | Hibino H, Nin F, Tsuzuki C, Kurachi Y. How is the highly positive endocochlear potential formed The specific architecture of the stria vascularis and the roles of the ion-transport apparatus. Pflugers Arch. 2010;459:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 136. | Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J Assoc Res Otolaryngol. 2001;2:118-129. [PubMed] |
| 137. | Hoya N, Okamoto Y, Kamiya K, Fujii M, Matsunaga T. A novel animal model of acute cochlear mitochondrial dysfunction. Neuroreport. 2004;15:1597-1600. [PubMed] |
| 138. | Mizutari K, Matsunaga T, Kamiya K, Fujinami Y, Fujii M, Ogawa K. Caspase inhibitor facilitates recovery of hearing by protecting the cochlear lateral wall from acute cochlear mitochondrial dysfunction. J Neurosci Res. 2008;86:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 139. | Okamoto Y, Hoya N, Kamiya K, Fujii M, Ogawa K, Matsunaga T. Permanent threshold shift caused by acute cochlear mitochondrial dysfunction is primarily mediated by degeneration of the lateral wall of the cochlea. Audiol Neurootol. 2005;10:220-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 140. | Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 428] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 141. | Adams JC, Seed B, Lu N, Landry A, Xavier RJ. Selective activation of nuclear factor kappa B in the cochlea by sensory and inflammatory stress. Neuroscience. 2009;160:530-539. [PubMed] |
| 142. | Yamamoto H, Omelchenko I, Shi X, Nuttall AL. The influence of NF-kappaB signal-transduction pathways on the murine inner ear by acoustic overstimulation. J Neurosci Res. 2009;87:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 143. | Miyao M, Firestein GS, Keithley EM. Acoustic trauma augments the cochlear immune response to antigen. Laryngoscope. 2008;118:1801-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 144. | Masuda M, Nagashima R, Kanzaki S, Fujioka M, Ogita K, Ogawa K. Nuclear factor-kappa B nuclear translocation in the cochlea of mice following acoustic overstimulation. Brain Res. 2006;1068:237-247. [PubMed] |
| 145. | So H, Kim H, Lee JH, Park C, Kim Y, Kim E, Kim JK, Yun KJ, Lee KM, Lee HY. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J Assoc Res Otolaryngol. 2007;8:338-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 146. | Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res. 2006;83:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 147. | Rahman A, Fazal F. Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal. 2009;11:823-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 148. | Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111:5271-5281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 149. | Sumagin R, Kuebel JM, Sarelius IH. Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1. Am J Physiol Cell Physiol. 2011;301:C804-C813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 150. | Neuser J, Knoop T. Sudden idiopathic hearing loss: psychopathology and antecedent stressful life-events. Br J Med Psychol. 1986;59:245-251. [PubMed] |
| 151. | Nakamura M, Aoki N, Nakashima T, Hoshino T, Yokoyama T, Morioka S, Kawamura T, Tanaka H, Hashimoto T, Ohno Y. Smoking, alcohol, sleep and risk of idiopathic sudden deafness: a case-control study using pooled controls. J Epidemiol. 2001;11:81-86. [PubMed] |
| 152. | Nakashima T, Tanabe T, Yanagita N, Wakai K, Ohno Y. Risk factors for sudden deafness: a case-control study. Auris Nasus Larynx. 1997;24:265-270. [PubMed] |
| 153. | Merchant SN, Durand ML, Adams JC. Sudden deafness: is it viral. ORL J Otorhinolaryngol Relat Spec. 2008;70:52-60; discussion 60-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 154. | Mazzeo RS, Donovan D, Fleshner M, Butterfield GE, Zamudio S, Wolfel EE, Moore LG. Interleukin-6 response to exercise and high-altitude exposure: influence of alpha-adrenergic blockade. J Appl Physiol. 2001;91:2143-2149. [PubMed] |
| 155. | Mattox DE, Simmons FB. Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1977;86:463-480. [PubMed] |
| 156. | Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 157. | Kimbrell MR, Warshakoon H, Cromer JR, Malladi S, Hood JD, Balakrishna R, Scholdberg TA, David SA. Comparison of the immunostimulatory and proinflammatory activities of candidate Gram-positive endotoxins, lipoteichoic acid, peptidoglycan, and lipopeptides, in murine and human cells. Immunol Lett. 2008;118:132-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 158. | Suwa T, Hogg JC, English D, Van Eeden SF. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol. 2000;279:H2954-H2960. [PubMed] |
| 159. | Wolters PJ, Wray C, Sutherland RE, Kim SS, Koff J, Mao Y, Frank JA. Neutrophil-derived IL-6 limits alveolar barrier disruption in experimental ventilator-induced lung injury. J Immunol. 2009;182:8056-8062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 160. | Nijm J, Wikby A, Tompa A, Olsson AG, Jonasson L. Circulating levels of proinflammatory cytokines and neutrophil-platelet aggregates in patients with coronary artery disease. Am J Cardiol. 2005;95:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 161. | Khuseyinova N, Koenig W. Biomarkers of outcome from cardiovascular disease. Curr Opin Crit Care. 2006;12:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 162. | Maugeri N, Manfredi AA, Maseri A. Clinical and experimental evidences on the prothrombotic properties of neutrophils. Srp Arh Celok Lek. 2010;138 Suppl 1:50-52. [PubMed] |
| 163. | Wakabayashi K, Fujioka M, Kanzaki S, Okano HJ, Shibata S, Yamashita D, Masuda M, Mihara M, Ohsugi Y, Ogawa K. Blockade of interleukin-6 signaling suppressed cochlear inflammatory response and improved hearing impairment in noise-damaged mice cochlea. Neurosci Res. 2010;66:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 164. | Scherer EQ, Yang J, Canis M, Reimann K, Ivanov K, Diehl CD, Backx PH, Wier WG, Strieth S, Wangemann P. Tumor necrosis factor-α enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke. 2010;41:2618-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 165. | Wang Y, Yu C, Pan Y, Li J, Zhang Y, Ye F, Yang S, Zhang H, Li X, Liang G. A novel compound C12 inhibits inflammatory cytokine production and protects from inflammatory injury in vivo. PLoS One. 2011;6:e24377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 166. | Maruo N, Morita I, Shirao M, Murota S. IL-6 increases endothelial permeability in vitro. Endocrinology. 1992;131:710-714. [PubMed] |
| 167. | Gyo K. Experimental study of transient cochlear ischemia as a cause of sudden deafness. World J Otorhinolaryngol. 2013;3:1-15. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (2)] |
| 168. | Sugiura M, Naganawa S, Teranishi M, Nakashima T. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging findings in patients with sudden sensorineural hearing loss. Laryngoscope. 2006;116:1451-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 169. | Yoshida T, Sugiura M, Naganawa S, Teranishi M, Nakata S, Nakashima T. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging findings and prognosis in sudden sensorineural hearing loss. Laryngoscope. 2008;118:1433-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 170. | Ryu IS, Yoon TH, Ahn JH, Kang WS, Choi BS, Lee JH, Shim MJ. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging in sudden sensorineural hearing loss: correlations with audiologic and vestibular testing. Otol Neurotol. 2011;32:1205-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 171. | Asberg M, Nygren A, Leopardi R, Rylander G, Peterson U, Wilczek L, Källmén H, Ekstedt M, Akerstedt T, Lekander M. Novel biochemical markers of psychosocial stress in women. PLoS One. 2009;4:e3590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 172. | Jonsdottir IH, Hägg DA, Glise K, Ekman R. Monocyte chemotactic protein-1 (MCP-1) and growth factors called into question as markers of prolonged psychosocial stress. PLoS One. 2009;4:e7659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 173. | Broderick G, Fuite J, Kreitz A, Vernon SD, Klimas N, Fletcher MA. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav Immun. 2010;24:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 174. | Kang HS, Park JJ, Ahn SK, Hur DG, Kim HY. Effect of high dose intravenous vitamin C on idiopathic sudden sensorineural hearing loss: a prospective single-blind randomized controlled trial. Eur Arch Otorhinolaryngol. 2013;270:2631-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |