Published online Feb 28, 2013. doi: 10.5319/wjo.v3.i1.1
Revised: January 28, 2013
Accepted: February 5, 2013
Published online: February 28, 2013
The etiology of sudden deafness or idiopathic sudden sensorineural hearing loss (ISSHL) remains unclear. Over the past 15 years, we have investigated the mechanisms of ischemic-induced hearing loss using a gerbil model of transient cochlear ischemia. In the gerbil, cochlear ischemia can be induced by occluding the bilateral vertebral arteries simultaneously at the neck, because the posterior communicating arteries of the Circle of Willis close spontaneously around 1 mo after birth. When 15 min ischemia was loaded on this animal, permanent hearing loss of about 25 dB and the death of hair cells, especially inner hair cells were induced. These pathological changes were mainly due to lack of an energy source, glutamate excitotoxicity, and the production of free radicals, especially superoxide and nitrous oxide species. Ischemic damage could be prevented by various procedures, such as cooling the cochlea, intratympanic administration of insulin-like growth factor 1 or AM-111 (an anti-apoptotic agent), and systemic administration of prednisolone (steroid), edarabone (free radical scavenger), ginsenoside Rb1 (Kanpo), hematopoietic stem cells, glia-cell derived neurotrophic factor, and liposome-encapsulated hemoglobin (artificial red blood cells). We also found that the cochlea was protected by the ischemic tolerance, indicating that minor cochlear ischemia alleviates or prevents inner ear damage in subsequent severe cochlear ischemia. As ISSHL usually occurs suddenly, with no preceding sign or symptom, we suggest that most ISSHL cases are caused by circulatory disturbance, probably at the stria vascularis.
- Citation: Gyo K. Experimental study of transient cochlear ischemia as a cause of sudden deafness. World J Otorhinolaryngol 2013; 3(1): 1-15
- URL: https://www.wjgnet.com/2218-6247/full/v3/i1/1.htm
- DOI: https://dx.doi.org/10.5319/wjo.v3.i1.1
Sudden deafness, also called idiopathic sudden sensorineural hearing loss (ISSHL), is a disease of inner ear causing acute hearing loss of unknown etiology. It occurs in approximately 30 cases per 100 000 people a year in Japan, most frequently involving those 50-60 years of age. Presently, ISSHL is considered a symptom of various diseases, including circulatory disturbances, viral infection, endolymphatic hydrops/labyrinthine membrane rupture, and disruption of endolymphatic homeostasis triggered by stress hormones and other hormones as well. As such hearing loss usually occurs suddenly, with no preceding sign or symptom, many investigators have suggested that ISSHL is caused by acute interruption of cochlear blood flow, and steroids and vasodilator agents are often prescribed for the treatment of this disease. Recently, many scientific papers have been published supporting this vascular theory, including circulatory disturbances, as a cause of ISSHL. Suckfüll[1] reported that plasma fibrinogen was raised in patients with ISSHL, suggesting increased blood coagulation. Fortunately, their hearing impairment improved following low-density lipoprotein apheresis. De Felice et al[2] reported a strong correlation between a non-functioning posterior communicating artery (PCA) of the Circle of Willis and the incidence of ISSHL. Because the inner ear is nourished solely by the labyrinthine artery, a branch of the basilar artery, a non-functioning PCA may increase the risk of disturbing the continuous blood supply to the cochlea. Large-scale statistical analyses have demonstrated that ISSHL is a strong risk factor for stroke[3] and cardiovascular disease[4]. Due to recent advances in gene analysis technology, various single nucleotide polymorphisms have been found to be closely associated with ISSHL incidence[5-8]. In Japan, Hato[9] showed that the PRKCH gene, an expression gene of protein kinase C, was associated with the incidence of ISSHL as well as stroke[10]. The allele ratio of G→A in the RPKCH gene is 2.0 in ISSHL and 1.7 in lacunar stroke. Using three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging, Yoshida et al[11] reported high signal areas in the cochlea of 31 of 48 patients with ISSHL, suggesting a high concentration of protein or hemorrhage in the cochlea. They noted that hearing prognoses of such patients were poor compared to those without a high signal. These findings are all consistent with the vascular theory of the etiology of ISSHL.
Over the past 15 years, we have investigated the mechanism(s) of ischemia-induced hearing loss using a gerbil model of transient cochlear ischemia. Because this animal can live long after the induction of transient ischemia, experimental studies were undertaken to assess cochlear histopathology, the mechanism(s) of ischemic cochlear damage, and responses to various treatment modalities. We also found that the cochlea was protected by the mechanism known as ischemic tolerance. In this phenomenon, minor cochlear ischemia alleviates or prevents inner ear damage in subsequent severe cochlear ischemia. Here, we present our experimental data concerning transient cochlear ischemia, report the findings of ischemic tolerance in the cochlea, and demonstrate the therapeutic effects of various treatment modalities.
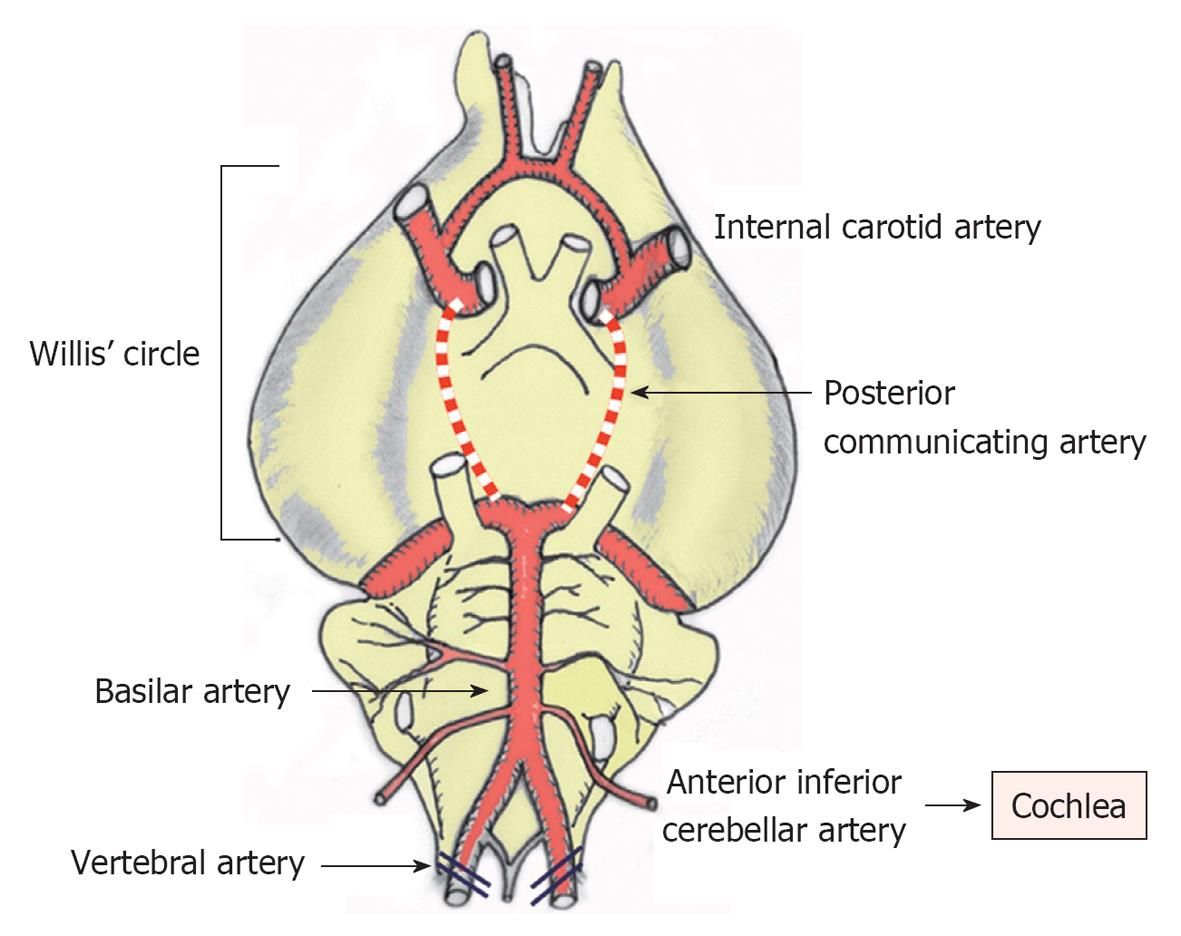
Because the nourishing artery of the cochlea comes from the basilar artery, transient cochlea ischemia is difficult to induce without damaging the brain and other neuronal tissues. Indeed, such experiments were previously considered not feasible in small animals because of technical difficulties. Using a technique called experimental hindbrain ischemia[12], we succeeded in making a chronic animal model of transient cochlear ischemia using the Mongolian gerbil. In this animal, the posterior communicating arteries of the Circle of Willis characteristically close spontaneously around 1 mo after birth. As the cochleae receive their blood supply solely from the vertebral arteries in the adult, transient cochlear ischemia is readily induced by obstructing the bilateral vertebral arteries at the neck[13] (Figure 1).
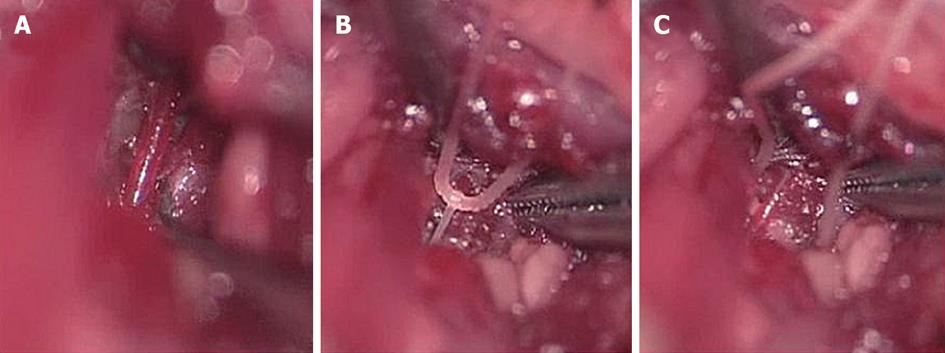
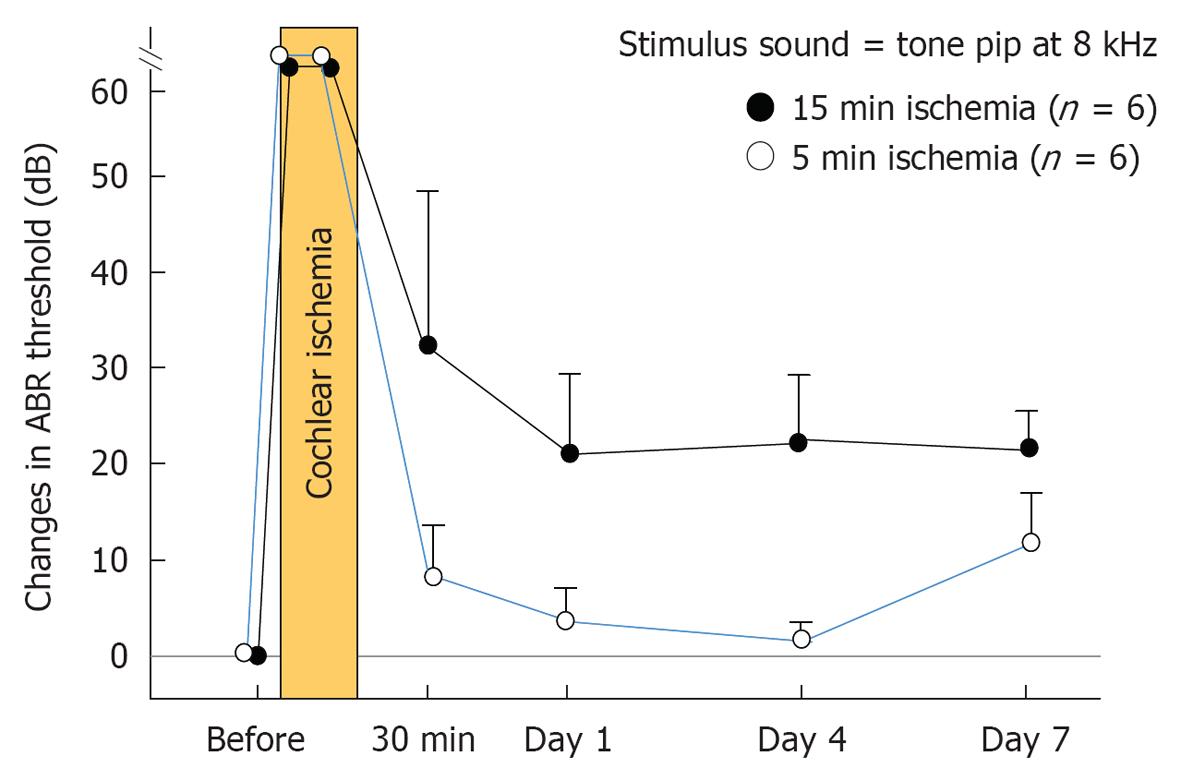
Under general anesthesia, the vertebral arteries were exposed bilaterally and dissected free from the surrounding connective tissue through a ventral transverse incision of the neck. Silk threads (4-0) were loosely looped around each artery, and ischemia was induced in the bilateral cochleae by pulling the ligatures with weights of 5 g for 5 or 15 min. The threads were subsequently removed to allow reperfusion, which was confirmed by observation using an operating microscope (Figure 2). As cochlear damage was minor with 5 min ischemia[14], we used 15 min ischemia in the following studies (Figure 3). The hearing of the animal was assessed by recording electrocochleogram, auditory brainstem responses (ABR), or distortion product oto-acoustic emission, depending on the purpose of the study.
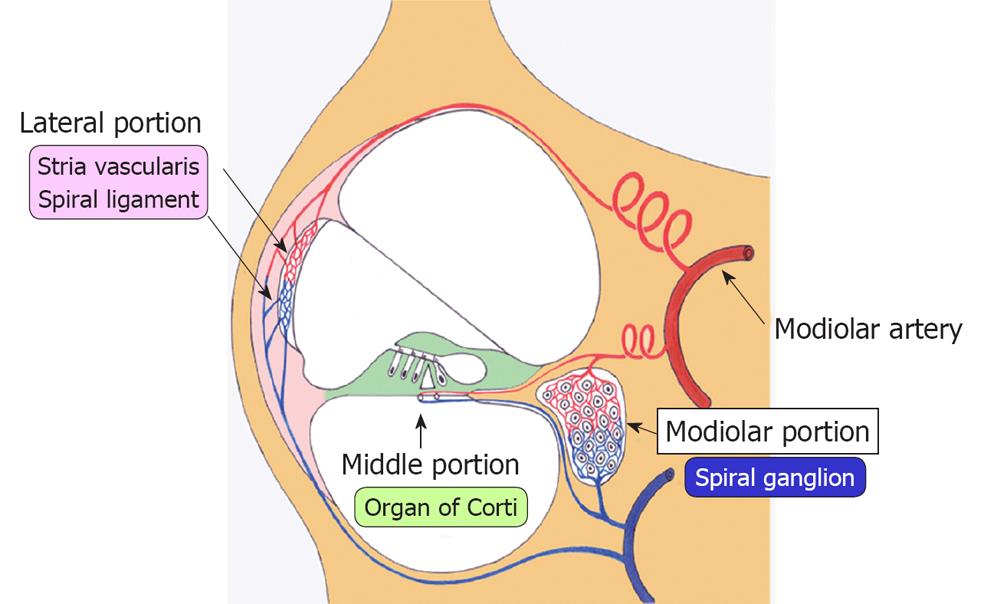
Ischemic damage of the cochlea can be divided into three regions: the lateral, middle, and modiolar regions (Figure 4). The lateral region includes the stria vascularis and spiral ligament, while the middle region is composed of the hair cells and supporting cells in the organ of Corti. The modiolar region is located in the center of the cochlea, and includes the spiral ganglion. The three regions of the inner ear receive arterial blood supply from the labyrinthine artery, via the spiral modiolar artery. According to rabbit experiments using microspheres, 82% of cochlear blood flow distributes to the lateral region, 9% to the middle region, and 9% to the modiolar region. In rats, the distribution is 57%, 19%, and 24%, respectively[15]. These findings suggest that blood supply to the lateral region is the largest among the three regions, although the distribution ratio differs by animal species.
Lateral region (stria vascularis and spiral ligament): The main function of the stria vascularis is to constantly supply K+ into the scala media through an ion channel that consumes ATP as an energy source. According to the K+ recycling theory, K+ in the scala media is absorbed by hair cells through mechanical ion channels on the surface of stereocilia called tip links or side links. The channels open and close corresponding to the vibration of the basilar membrane. Once absorbed, K+ facilitates Ca2+ release from stores into the cytoplasm and causes depolarization of the hair cell. Following firing of the hair cells, K+ released to the outside is then absorbed by the surrounding support cells. It flows laterally, through a gap junction between the supporting cells in the direction of the stria vascularis, where it is again released to the scala media via ATP. Because the ion channel at the stria vascularis needs a large amount of ATP, interruption of the blood supply to this area impairs ATP production, resulting in failure of K+ transport into the scala media. In this way, transient ischemia causes an energy shortage at the stria vascularis and induces decreased endocochlear potential (EP).
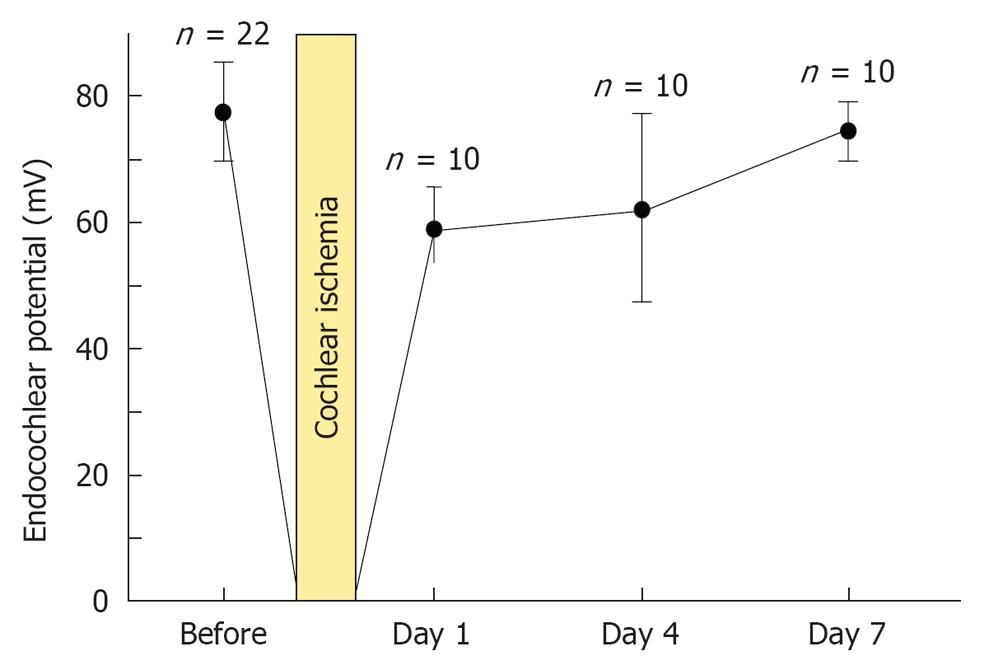
As shown in Figure 5, the decrease in EP following 15 min ischemia was reversible. In normal conditions, the EP value was 80.0 mV (n = 22). With ischemia, EP was markedly decreased, to around -20 mV. On the following day, it recovered to about 60 mV. It returned to normal by day 7. This indicates that disturbed function of the lateral region returns to normal by day 7[16].
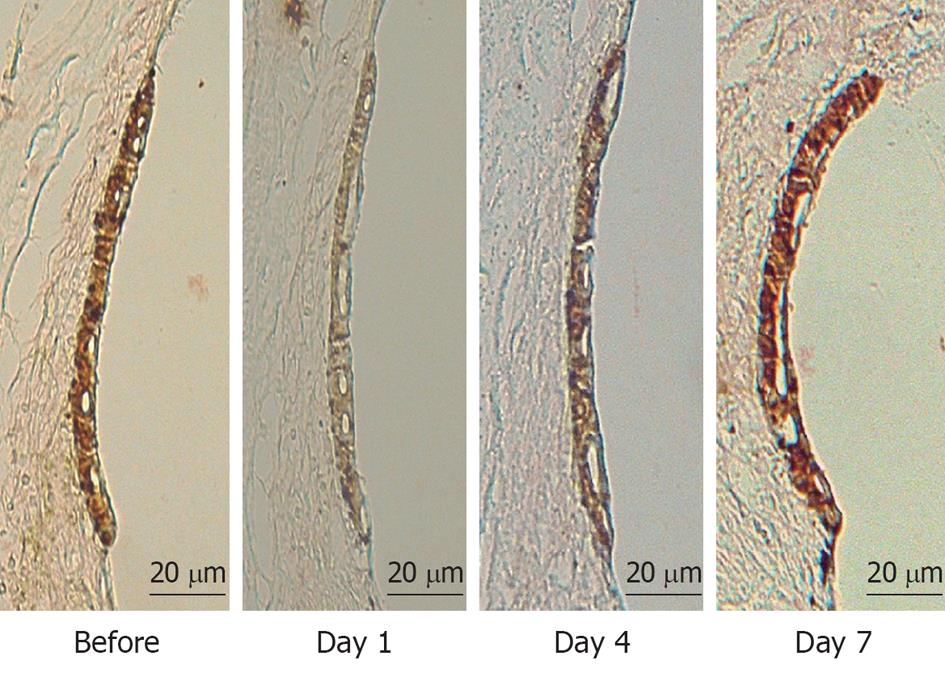
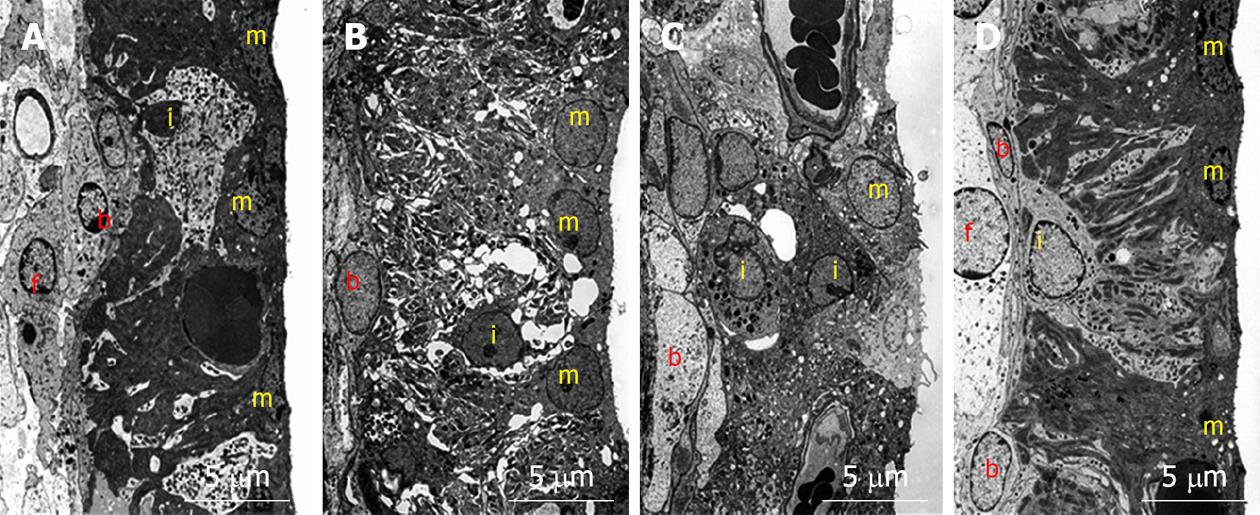
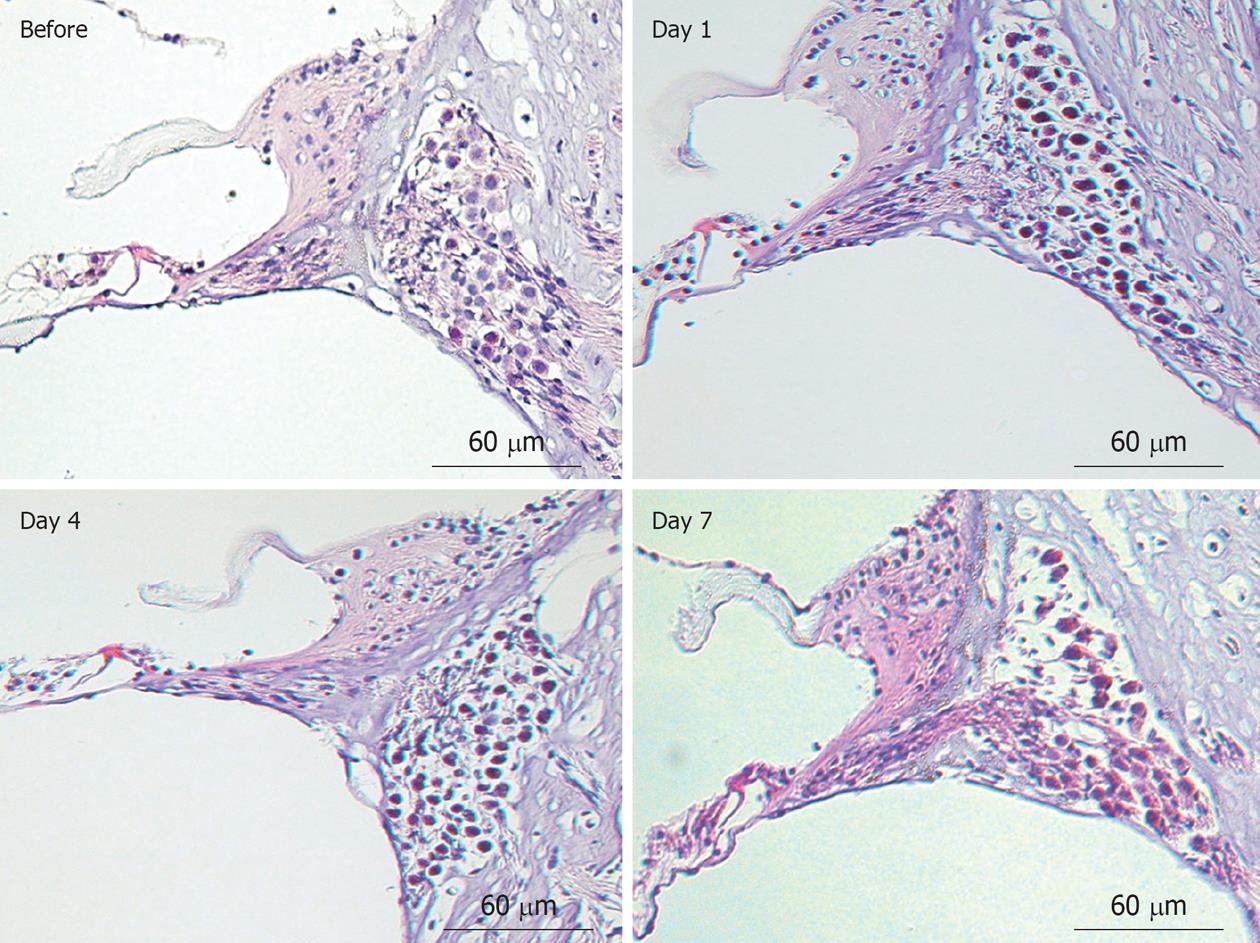
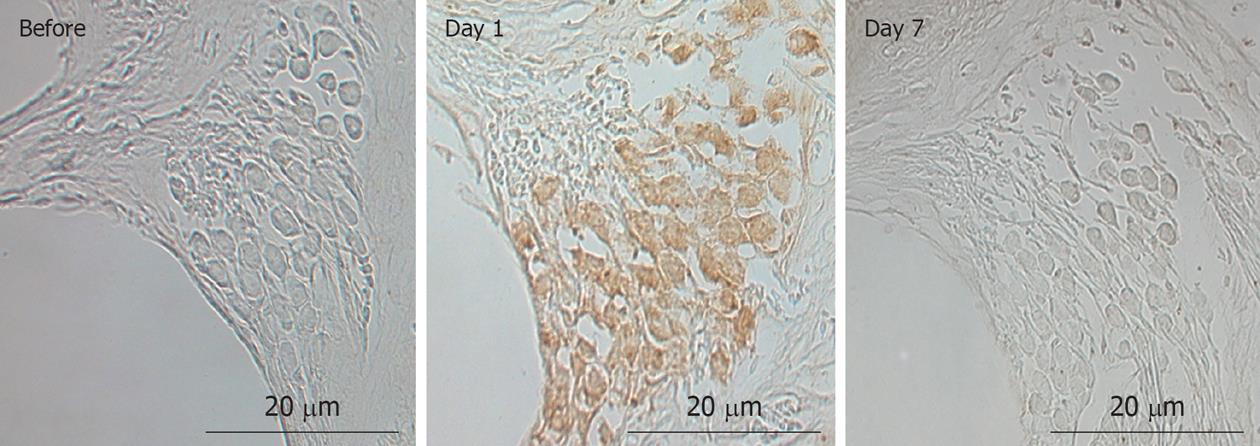
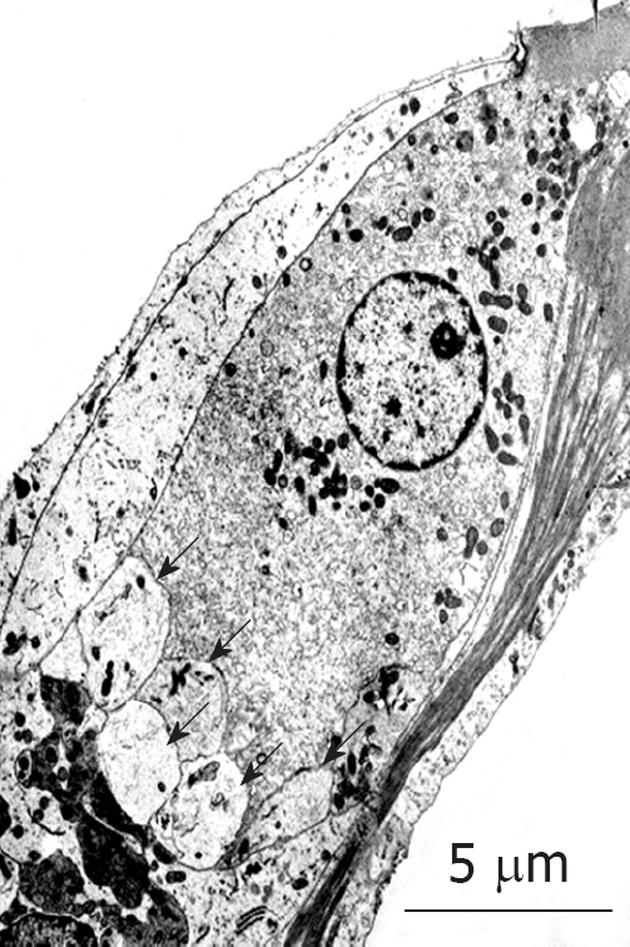
To investigate what happened in and around the stria vascularis, we performed histological staining with hematoxylin and eosin, which revealed no apparent change during the course of recovery. However, immunostaining of Na+-K+ ATPase, a marker of the Na/K-pump, and of connexin 26, a marker of gap junctions, showed that the levels of these markers were reduced on days 1 and 4, but recovered to preischemic levels on day 7 (Figure 6). Transmission electron microscope (TEM) studies demonstrated that water retention was prominent in the interstitial layer of the scala tympani on day 1, which became milder on day 4, and almost disappeared on day 7[17] (Figure 7). These histological findings were consistent with the sequential changes in the EP value that recovered on day 7.
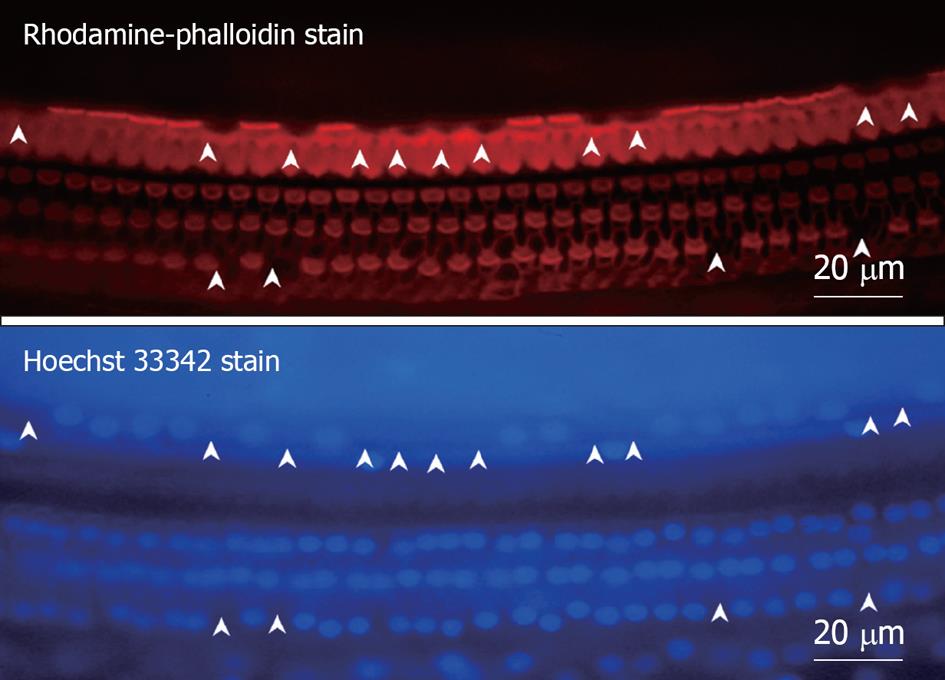
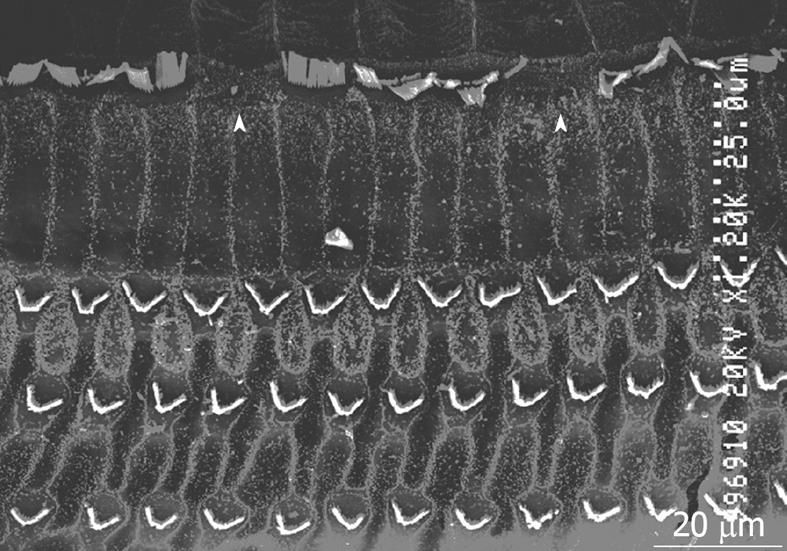
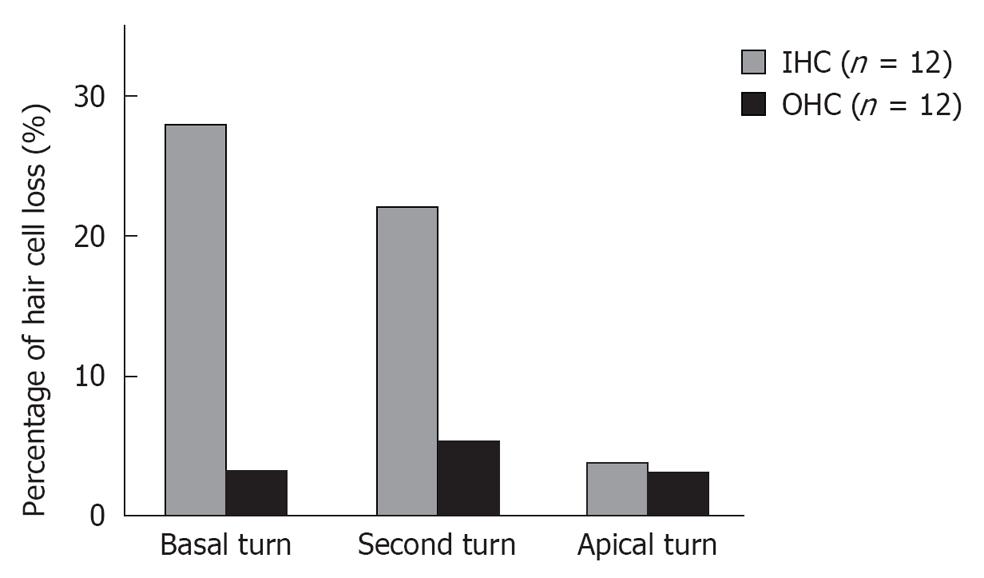
Middle region (organ of Corti): In the organ of Corti, ischemic damage was more severe in the inner hair cells (IHCs) than in the outer hair cells (OHCs): the cochlear pathology differed from that of other inner ear diseases, such as acoustic trauma and aminoglycoside ototoxicity, that cause severe damage mainly to the OHCs. Figure 8 shows a typical finding at the basal turn 7 d after ischemia, stained with rhodamine-phalloidin and Hoechst 33342. IHC-predominant damage was also seen by scanning electron microscopy as shown in Figure 9. Percentages of IHC and OHC losses at the three turns of the cochlea are summarized in Figure 10. Loss of IHC was extensive at the basal and second turns, but not at the apical turn. In contrast, no such difference, by turn, was observed in OHCs. This indicates that the underlying mechanisms of ischemic damage apparently differ somewhat between IHCs and OHCs.
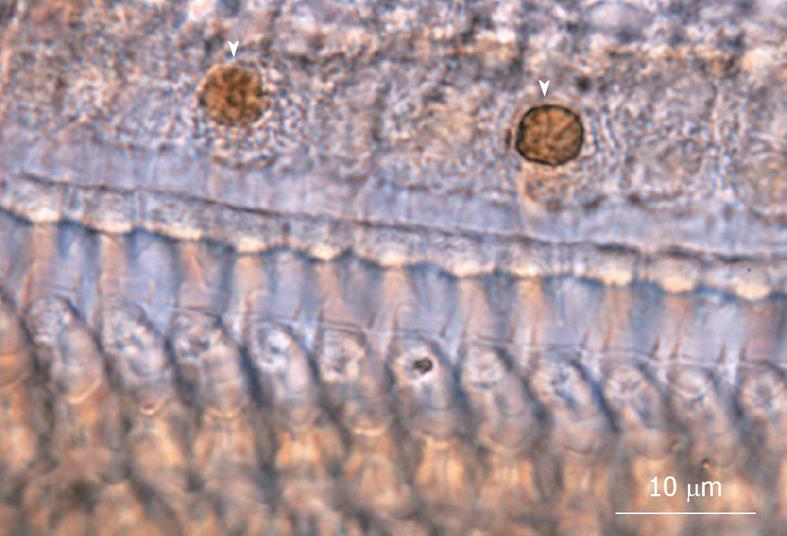
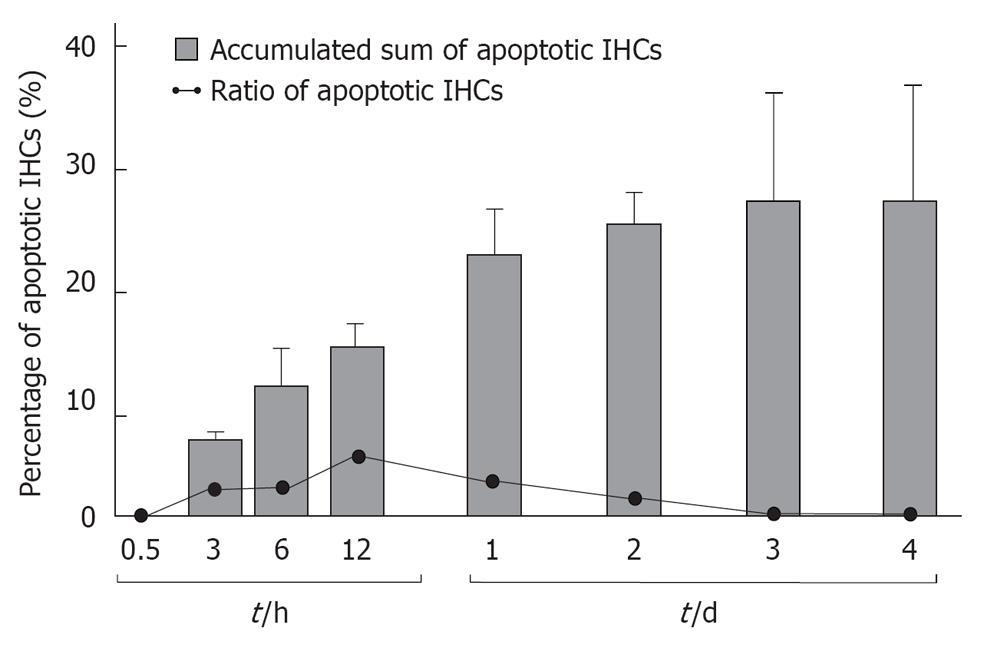
TUNEL staining (Figure 11) and TEM studies showed that the loss of IHCs was due to apoptosis, triggered by ischemic insult. Sequential counting of IHCs showed that the rate of IHC loss increased gradually until day 3. Then it remained constant[18] (Figure 12).
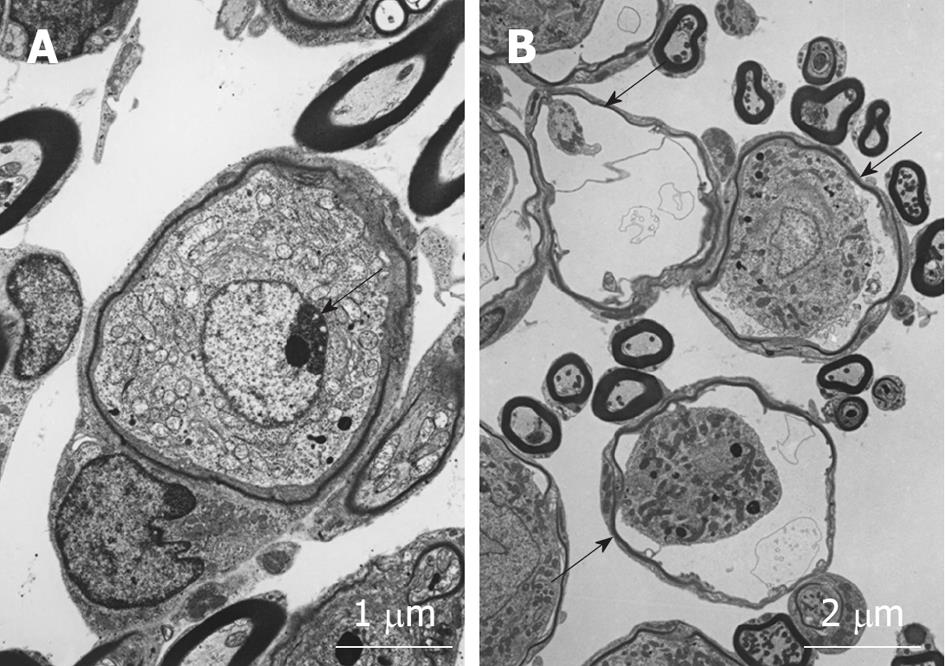
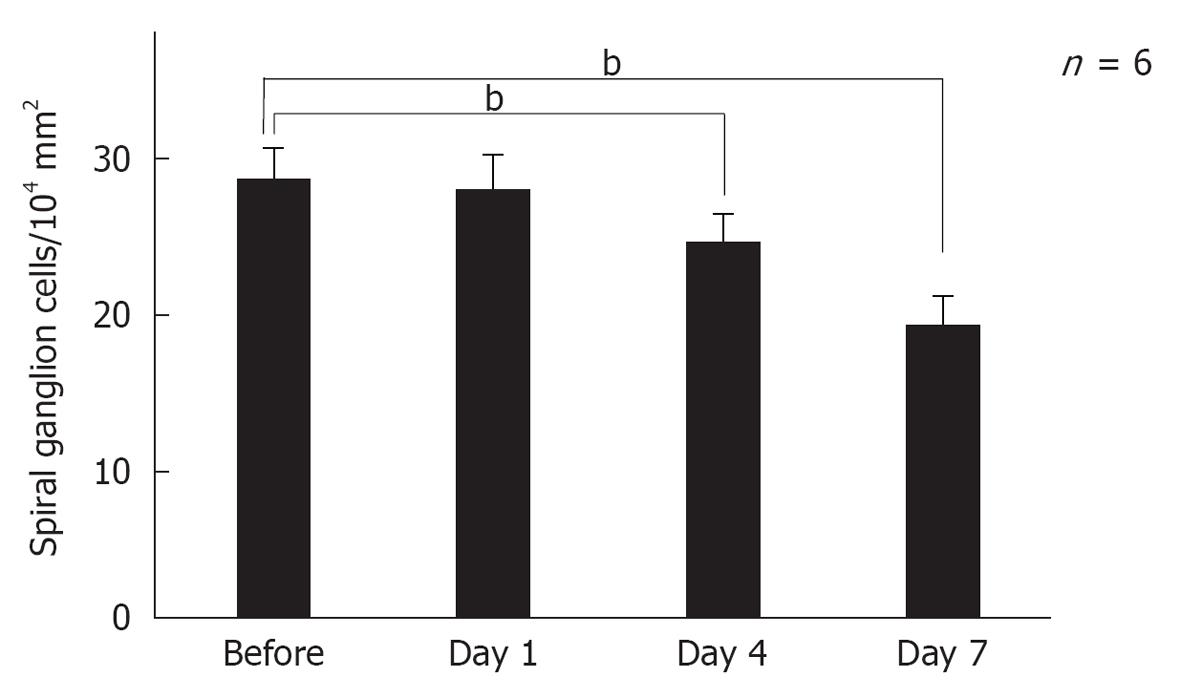
Modiolar region (spiral ganglion): HE findings in the spiral ganglion are shown in Figure 13. Loss of the ganglion neurons started on day 4 and progressed until day 7. Figure 14 shows immunofluorescent findings for Bax, an apoptosis-promoting protein, in the spiral ganglion. It was expressed on day 1, but not on day 7. TEM observations indicated that nuclei of the spiral ganglion cells (SGCs) underwent condensation or segmentation, suggesting cell death by apoptosis (Figure 15). The number of SGCs after transient ischemia is shown in Figure 16. A cell decrease was prominent between day 4 and day 7, suggesting delayed cell death in the spiral ganglion[14,19].
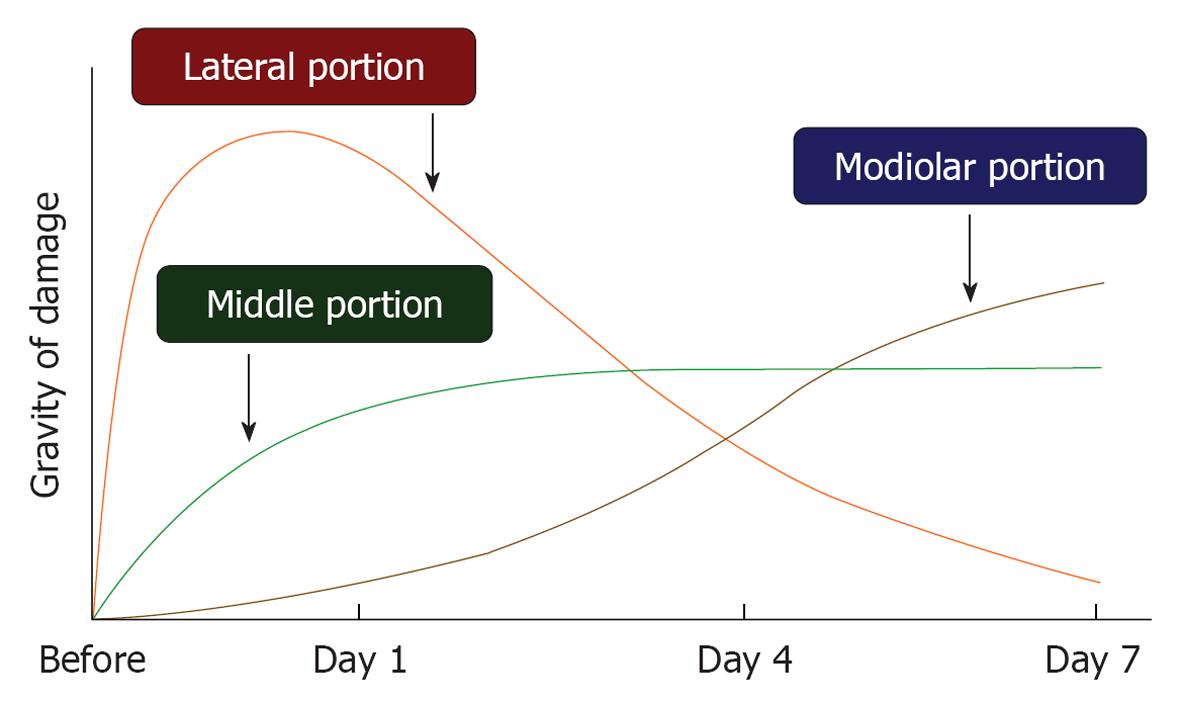
Time course of ischemic damage in the three regions of the cochlea: As shown in Figure 3, the ABR threshold at 8 kHz increased 20-30 dB on the next day after 15 min ischemia. The increase remained unchanged or changed a little thereafter. This indicated that hearing loss of an experimental animal became stable after 1 d of ischemia. Meanwhile, time course of ischemic damage was somewhat different among cochlear regions (Figure 17). In the lateral region, severe ischemic damage occurred immediately after the insult, which recovered gradually to a preischemic level within a week. In the middle region, loss of hair cells progressed slowly until day 3, then the decrease stopped. Ischemic damage was more severe in IHCs than in OHCs. Death of IHCs was due to activation of the apoptotic process; it was maximal 12 h after the insult. In the modiolar region, neuronal damage progressed more slowly and steadily. The number of SGCs decreased most prominently between days 4 and 7. Degeneration of the SGCs occurred initially from the ischemic insult, but later by secondary degeneration, corresponding to IHC death.
These findings suggest that when hearing loss is unchanged or slightly recovered after ischemic insult, the degenerating site gradually shifts to other regions.
Although the blood supply to the cochlea was stopped only for 15 min, the effects were much larger than expected. At least three mechanisms were thought to be related to the cochlear damage.
Energy supply deletion: The energy source of the cochlea is ATP, produced from glucose and oxygen via the process of glycolysis. Thus, transient cochlear ischemia causes depletion of the energy supply, which induces cochlear damage. According to Thalmann et al[20], the ATP concentration decreases rapidly in the stria vascularis and the spiral ganglion, but the decrease is gradual in the organ of Corti. This is probably because glucose and oxygen dissolved in the endolymph are used slowly by hair cells and supporting cells of the organ of Corti. The time needed to decrease the ATP concentration from normal (16 mmol/kg) to a low level (below 2 mmol/kg) after death takes 3 min in the stria vascularis, 15 min in the spiral ganglion, and 60 min in the Corti organ.
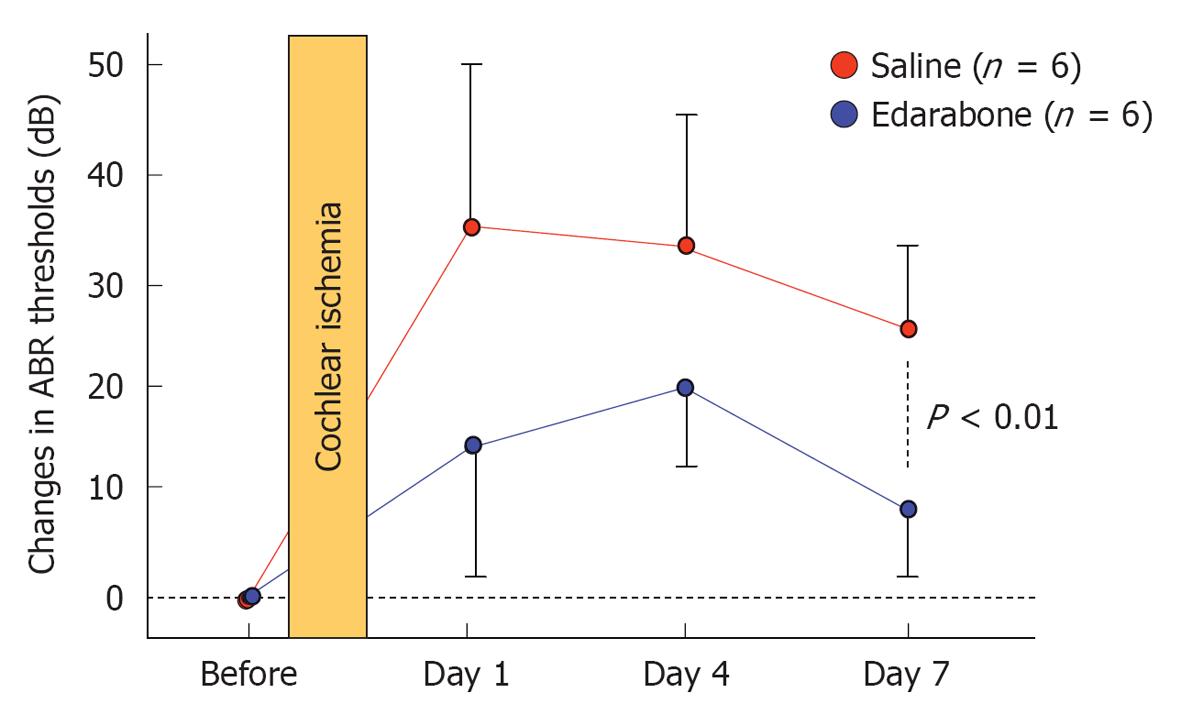
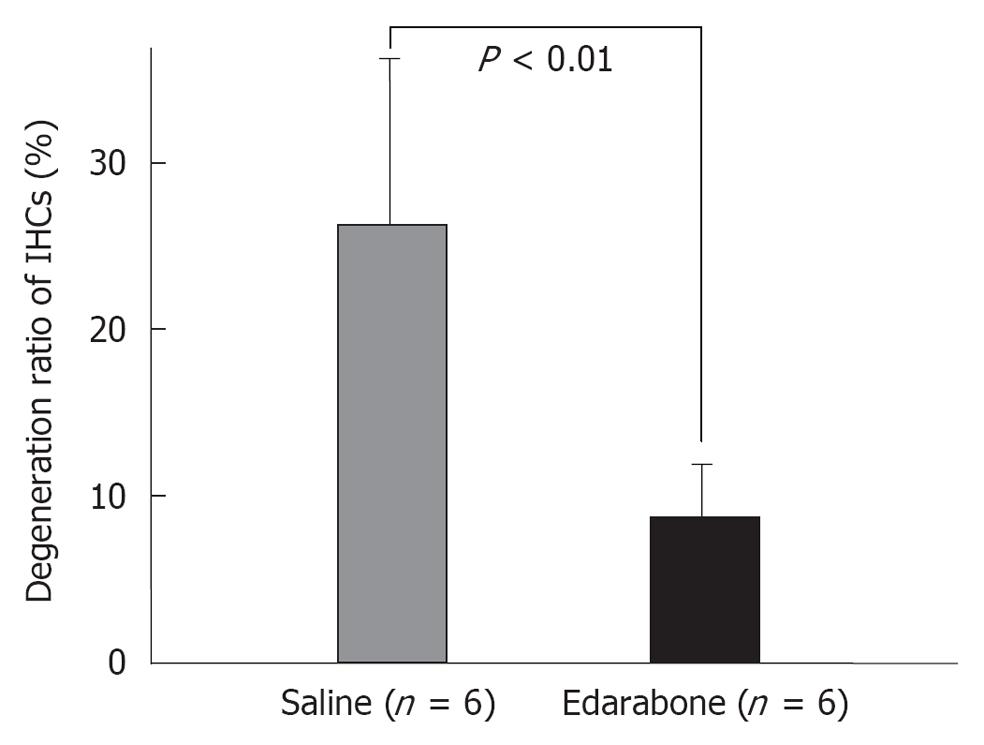
Free radicals: Free radicals such as superoxide and nitrous oxide (NO) species are produced in the course of ischemia/reperfusion processes. They induce destruction of cell membranes. As superoxide disappears so quickly after production, direct measurement of its concentration in the inner ear is not feasible. We investigated the production of superoxide and oxygen free radicals using edarabone, a free radical scavenger, originally developed as an anti-stroke agent. If free radicals are present, administration of edarabone alleviates their toxic effects dose-dependently. According to our previous study[21], administration of edarabone 1 h after ischemia significantly prevented the increase in the ABR threshold (Figure 18); hair cell loss was also prevented (Figure 19). These findings suggest that free radicals were produced after ischemic insult, and administration of edaravone prevented the toxic effects on the cochlea. Edaravone inhibits activation of the lipoxygenase pathway in the arachidonic acid cascade, which in turn prevents overproduction of superoxide anions. In addition, it scavenges NO directly in a dose-dependent manner.
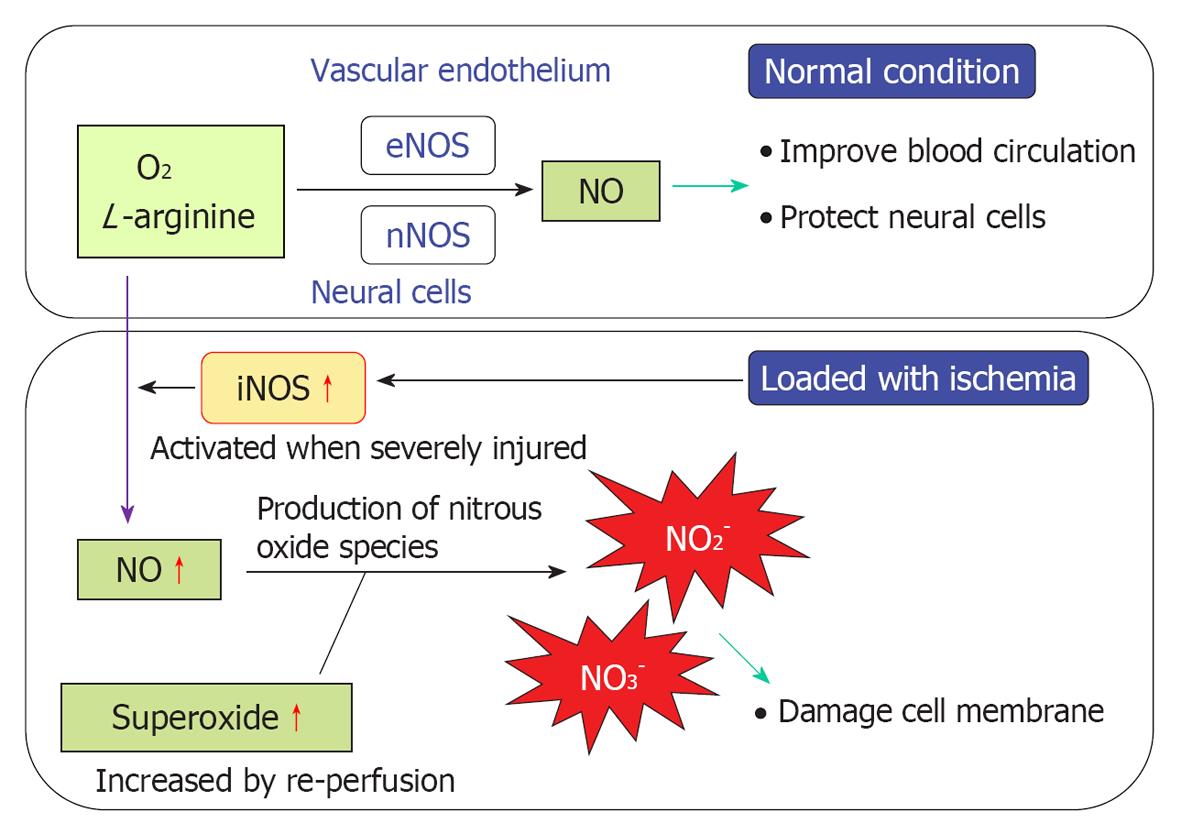
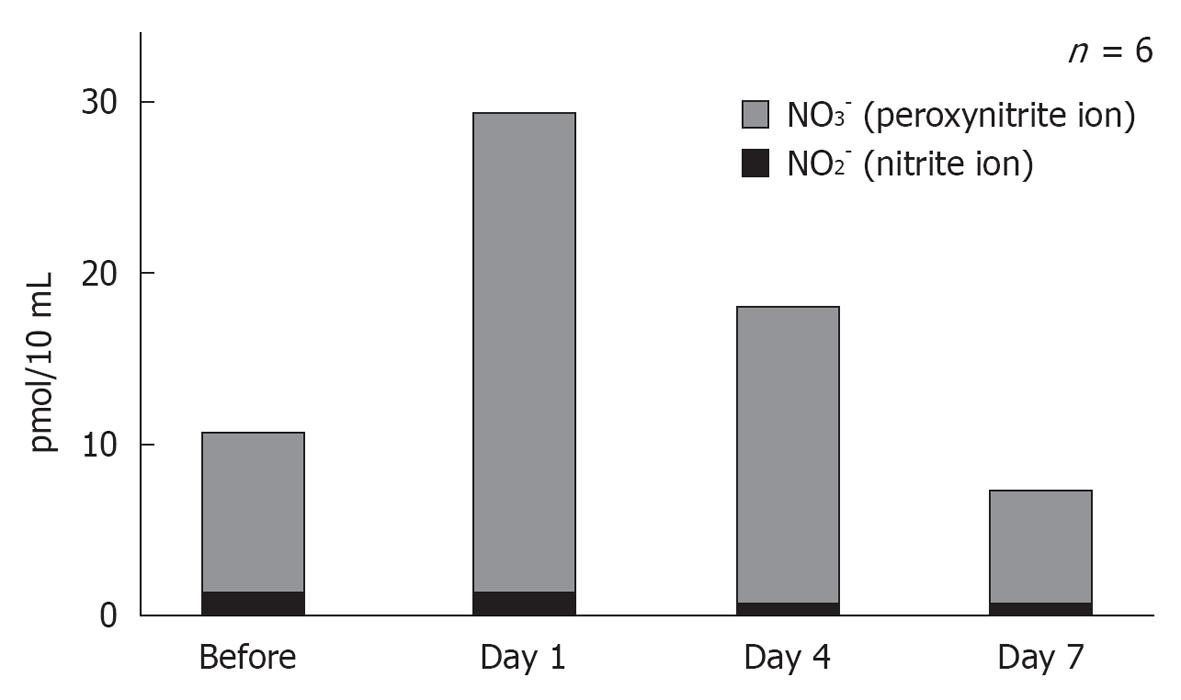
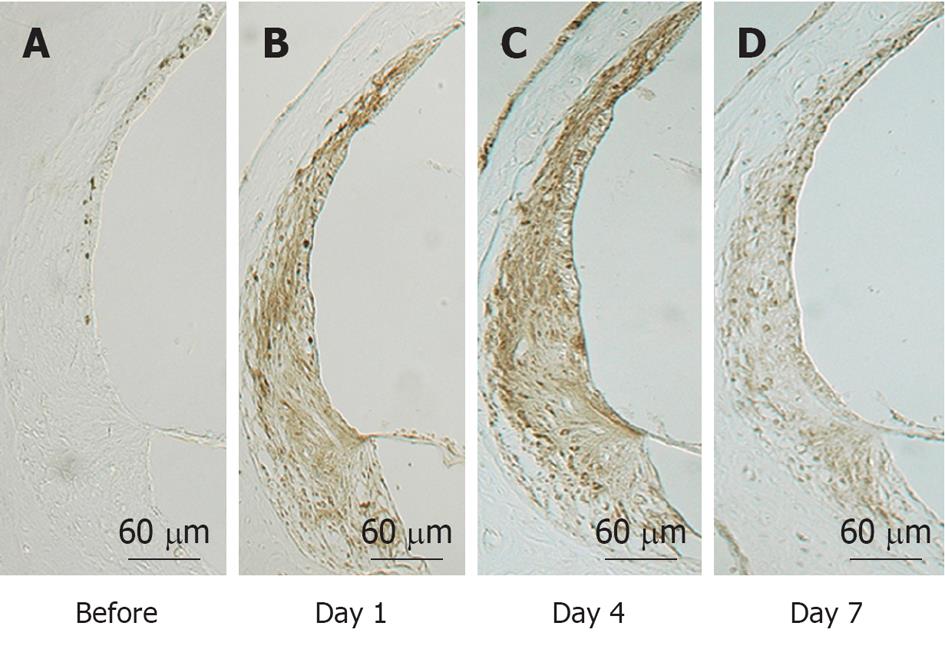
NO plays an important role regulating vasodilatation and protecting neuronal tissues (Figure 20). When physical stress such as transient ischemia occurs, NO is produced in excess, due to the enzymatic activity of inducible NO synthase (iNOS). On the other hand, large amounts of superoxide are produced in the process of reperfusion after transient arterial occlusion. When NO reacts with superoxide, harmful free radicals such as nitrite (NO2-) and peroxynitrite (NO3-) are produced. Peroxynitrite causes cell membrane disintegration through lipid peroxidation. Figure 21 shows NO2- and NO3- concentrations in the perilymph at the scala tympani of the basal turn. Concentrations increased markedly on days 1 and 4, and returned to normal levels on day 7. Immunostaining showed that iNOS expression was prominent at the stria vascularis, spiral ligament, organ of Corti, and the spiral ganglion. The iNOS expression decreased gradually after ischemic insult but was still evident on day 7 (Figures 22 and 23).
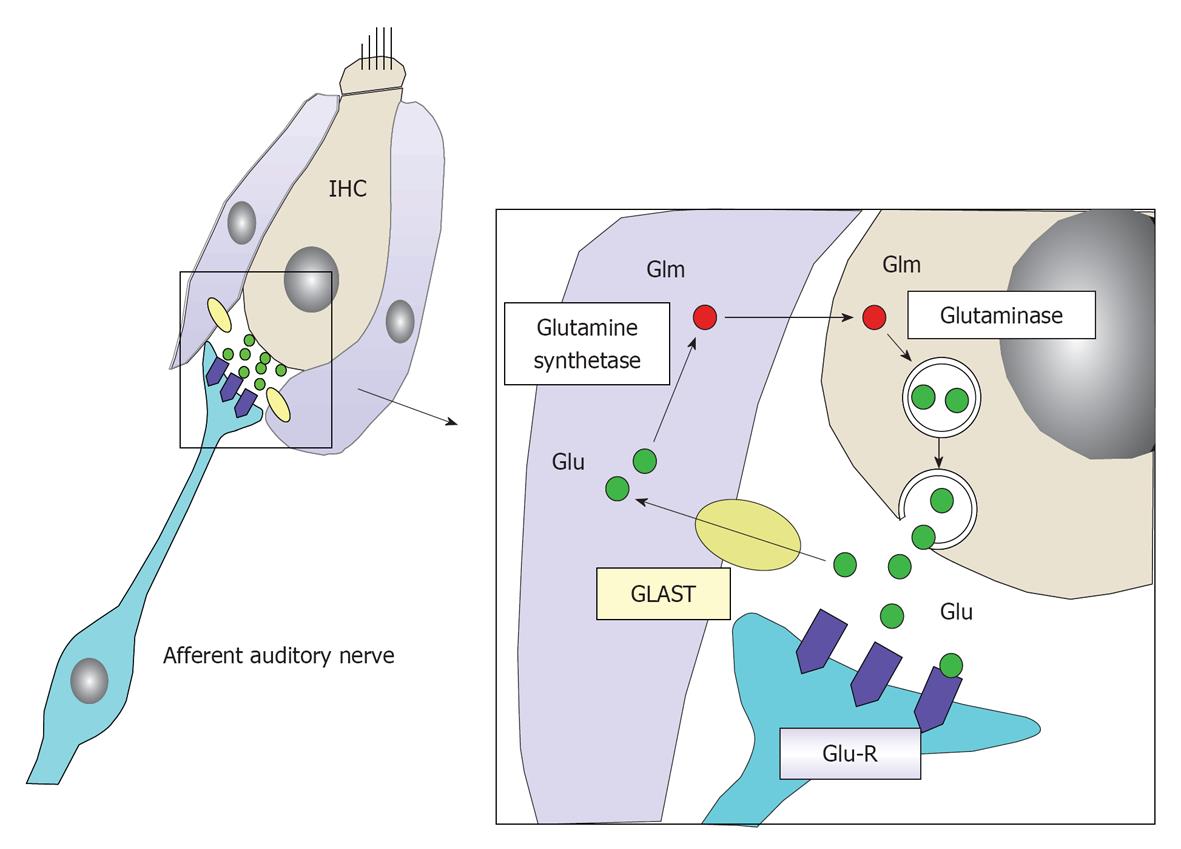
Glutamate: Glutamate is a neurotransmitter at the synapse between IHCs and the primary afferent auditory nerve. When sound comes into the inner ear, K+ enters and accumulates in IHCs, causing depolarization of the IHCs. Glutamate is released into the synaptic cleft in response to the firing of IHCs. After depolarization of IHCs, glutamate is absorbed by the surrounding supporting cells and IHCs, and is transformed into glutamine by enzymatic activity of the glutamate-aspartate transporter. Then glutamine is transferred to IHCs and encapsulated by vesicles, where it is transformed again into glutamate (Figure 24). In this way, glutamate is recycled around the synapses of IHCs[22,23].
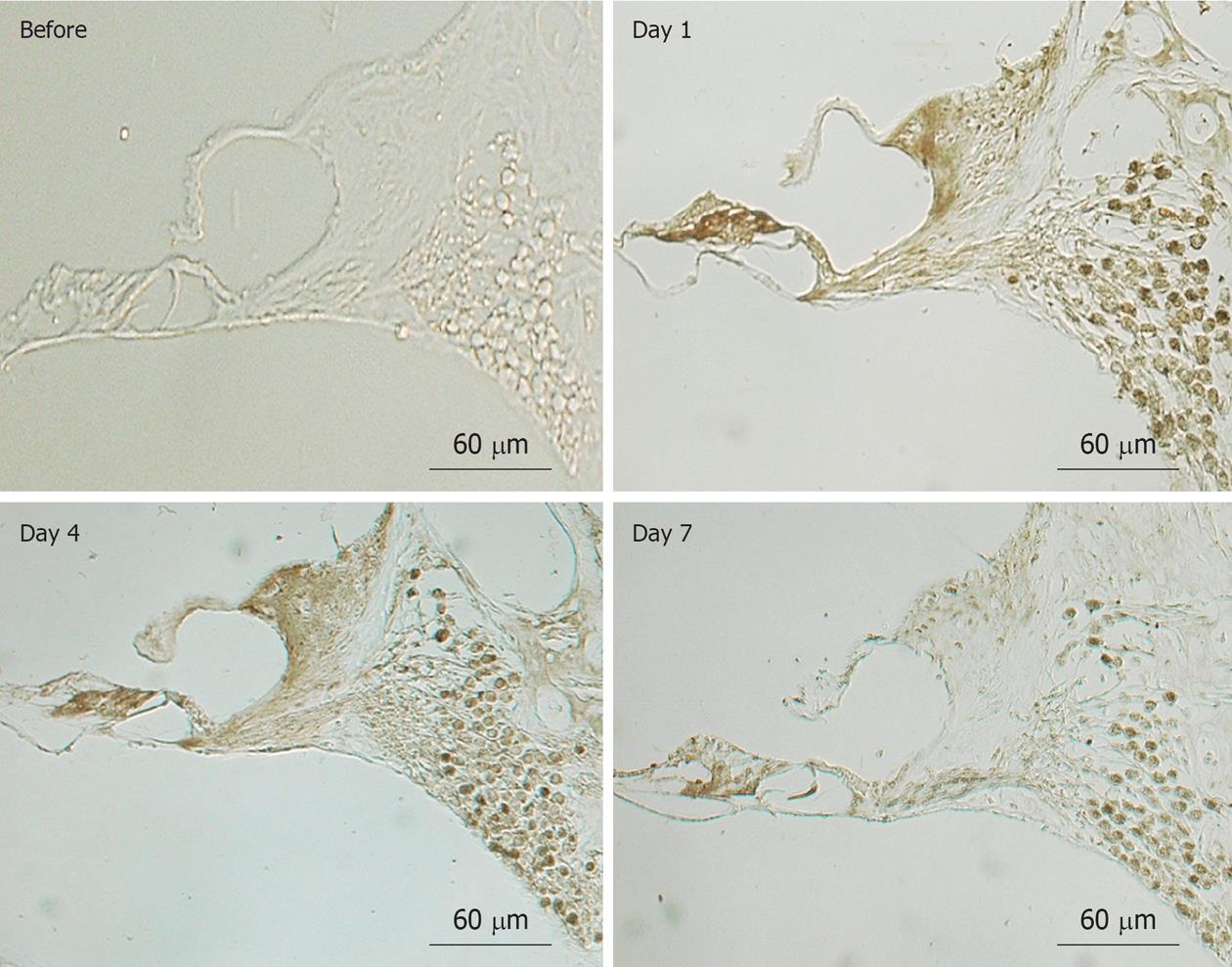
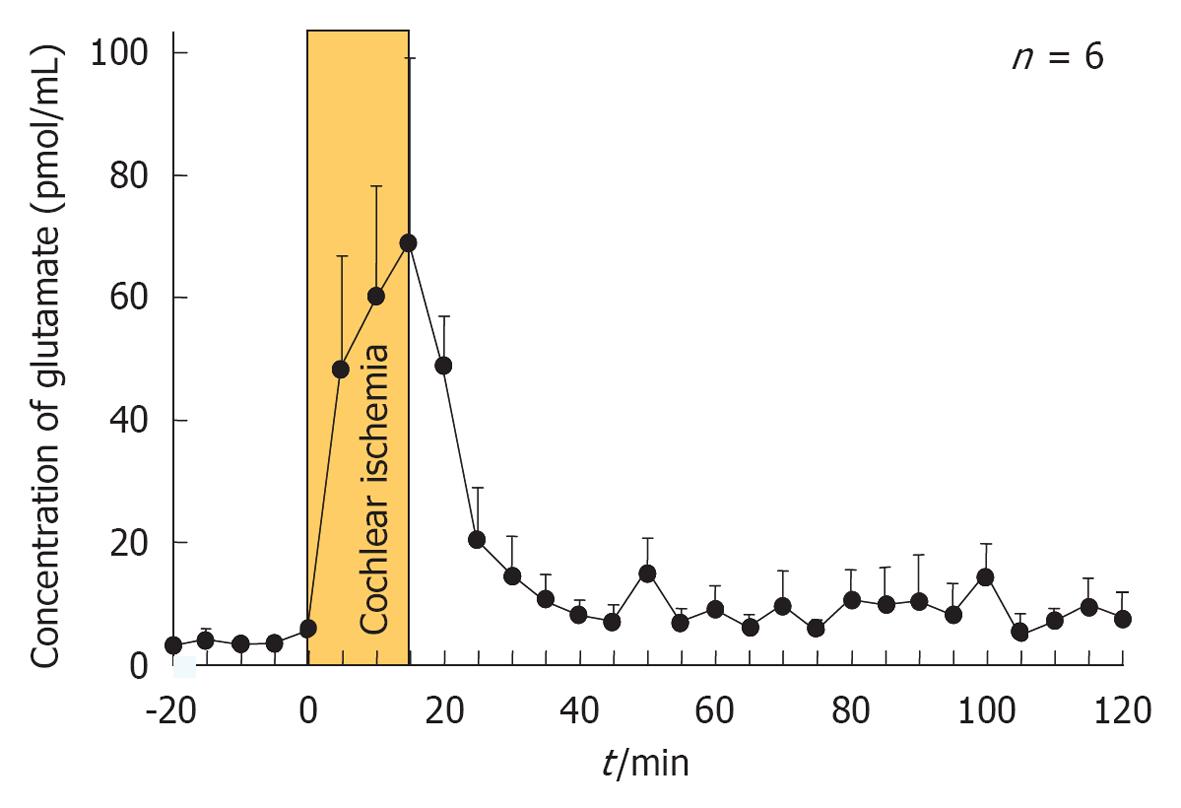
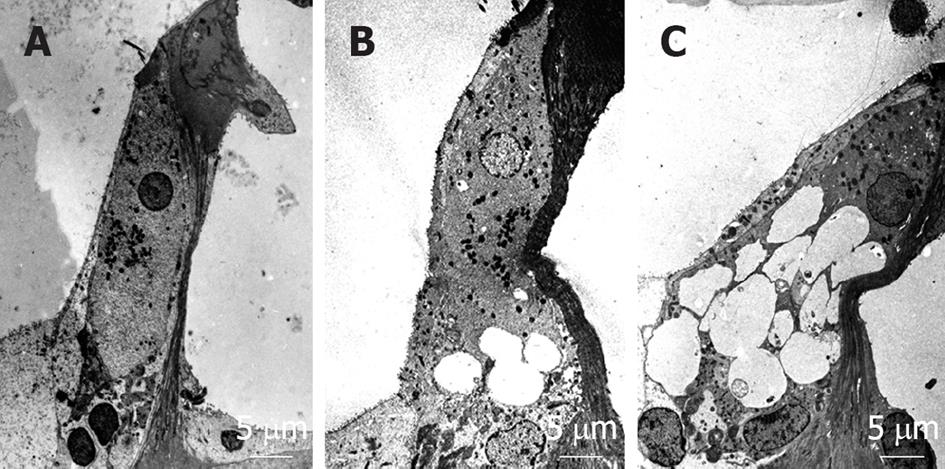
When transient cochlear ischemia is induced, the glutamate recycling system is disturbed because ATP is not produced. In that situation, glutamate in the synaptic cleft is not absorbed, but flows out to the extracellular space. Figure 25 shows sequential changes in the glutamate concentration in the scala tympani after ischemic insult. The increase in glutamate concentration was marked but soon subsided after reperfusion. Histological findings revealed vacuolar formation in the synaptic cleft of IHCs as a result of the transient cochlear ischemia[24] (Figure 26). Furthermore, administration of AMPA (glutamate agonist) caused vesicle formation around synapses of IHCs, resembling the histological findings for transient cochlear ischemia[25]. Vesicle formation was more prominent when higher concentrations of AMPA were administered (Figure 27). A glutamate antagonist, DNQX, prevented IHC damage induced by cochlear ischemia. Such ischemic damage was not seen in OHCs.
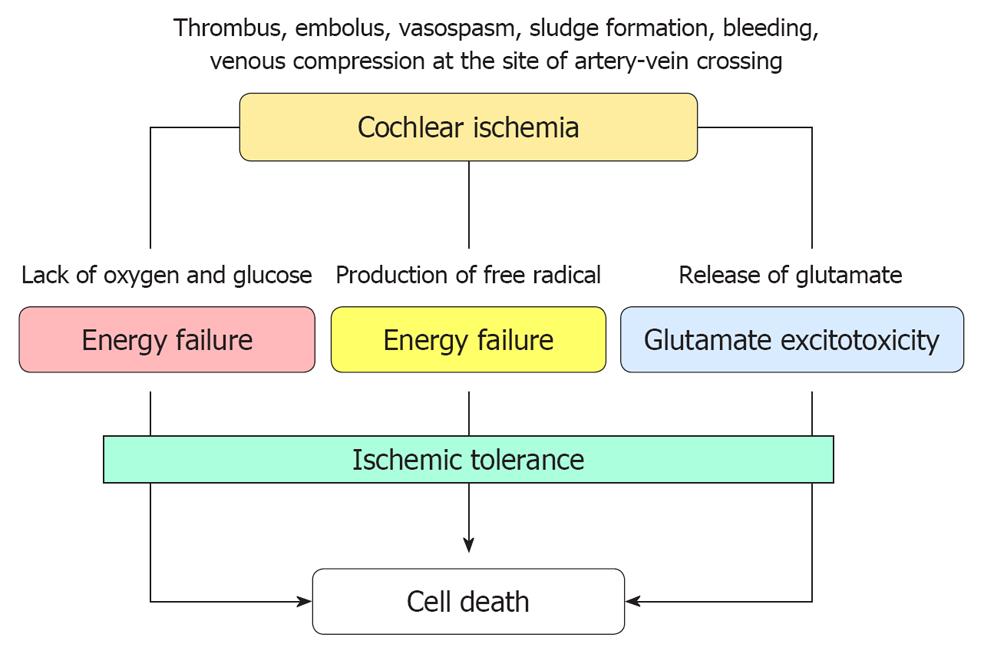
Interactions of various mechanisms in ischemic cochlear damage: When blood supply to the cochlea is stopped completely, all structures in the inner ear are destined to die as a result of energy depletion. If the ischemia is transient, superoxide is produced in excess following recovery of the blood supply. Furthermore, iNOS is induced at the site of the lesion and facilitates excessive production of NO. By reacting with superoxide, NO is transformed into NO2- or NO3-. These are strongly toxic and damage cell membranes. Ischemic insult also induces glutamate ototoxicity, which is considered a major cause of IHC death. Figure 28 shows the proposed interacting mechanisms of ischemic cochlear damage.
Ischemic tolerance is a preconditioning phenomenon that is activated by mild stressors, and allows survival when exposed to subsequent potentially lethal stressors. After first being reported in the brain[26], this cytoprotection phenomenon has been found in other organs, such as the heart, liver, and spinal cord. It became widely recognized as a pertinent and important process in understanding how the brain protects itself against ischemia. At present, the underlying mechanism(s) of ischemic tolerance remain(s) unclear. According to Kirino[27], there are two main mechanisms of ischemic tolerance. First, there is a cellular defense mechanism that arises by posttranslational modification of proteins or by expression of new proteins via a signal transduction system. These cascades of events may strengthen the influence of survival factors or may inhibit apoptosis. Second, there is a cellular stress response and the synthesis of stress proteins, leading to an increased capacity for health maintenance inside the cell. These proteins work as cellular chaperones by unfolding misfolded cellular proteins.
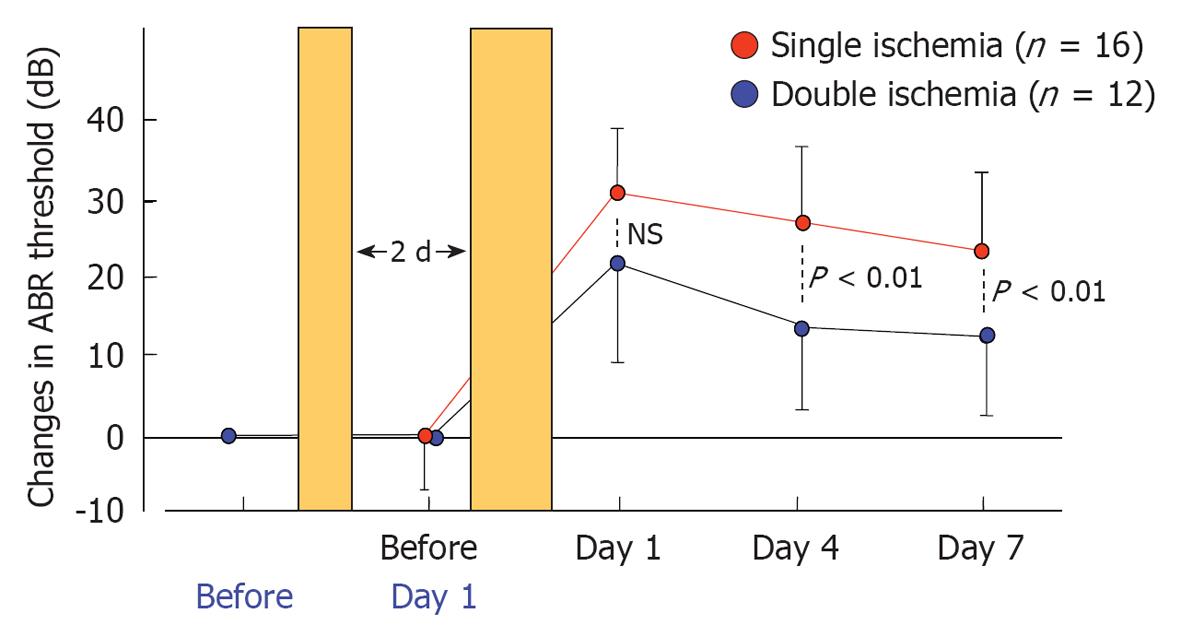
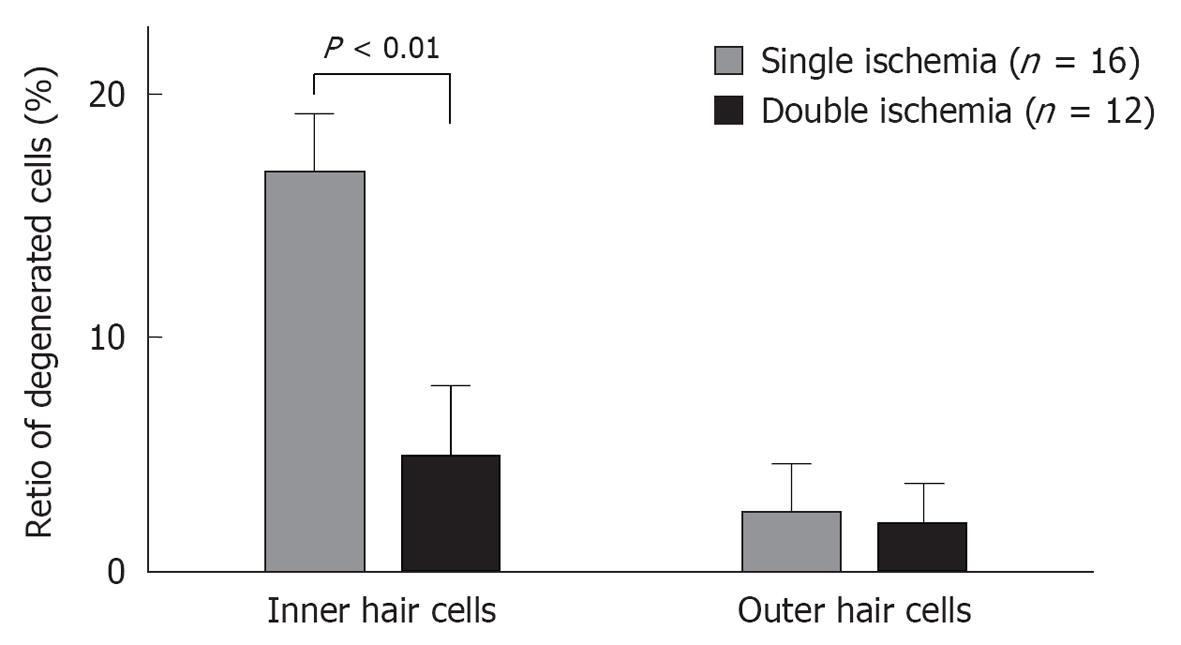
Presently, the greatest drawback of the vascular theory as an etiology of ISSHL is that it does not explain why recurrence and bilateral incidence of ISSHL are very rare. This issue may be resolved by studying ischemic tolerance in the cochlea. Using an animal model of transient cochlear ischemia, we investigated whether ischemic tolerance existed in the cochlea[28]. The animals were divided into two groups: the single ischemia group and the double ischemia group. In the single ischemia group, animals were subjected to 15 min ischemia. In the double ischemia group, animals were subjected to 2 min ischemia 2 d before 15 min ischemia. Figure 29 shows the sequential changes in ABR thresholds in the two groups. There was no change in ABR threshold on day 1 in the single ischemia group. On day 3, 15 min ischemia was induced in both groups. As shown in this figure, hearing loss on days 4 and 7 was more severe in the single ischemia group than in the double ischemia group (P < 0.05). Figure 30 summarizes the ratios of degenerated IHCs in the single and double ischemia groups. IHC loss was more severe in the single ischemia group, whereas fewer cells died in the double ischemia group. These findings suggest that ischemic preconditioning ameliorated ischemia-induced hearing impairment and loss of IHCs.
These results suggest that the rare recurrence of ISSHL might be due to ischemic tolerance in the cochlea. Unlike the immune system generally, this phenomenon does not work for long; the effect persists at most 7 d in brain ischemia. To assess whether this effect could be extended for longer periods, repeated minor ischemia would be necessary. If long-persisting ischemic tolerance can be induced by repeated minor ischemia, this phenomenon might be useful as a method for protecting the cochlea from ischemic damage.
We have investigated various treatment modalities using the animal model, including hypothermia and topical or general administration of test agents. Therapeutic hypothermia is already used as a medical treatment for ischemic brain injuries. Topical administration of test agents by placing them on the round window membrane is an effective way to deliver a medicine into the inner ear. As the amount of agent absorbed from the middle ear is limited, the incidence of possible side effects may also be minimized. We have investigated two agents, insulin-like growth factor 1 (IGF-1) and AM-111, using this method. Systemic administration was performed by administering the various test agents intravenously or intraperitoneally to investigate their protective effects in ischemic damage. Agents tested were prednisolone (steroid hormone), edarabone (antioxidant), prosaposine-derived synthetic peptide (saposine), ginsenoside Rb1 (gRb1) (Kanpo), liposome-encapsulated hemoglobin (LEH), glial cell-derived neurotrophic factor (GDNF), and hematopoietic and neural stem cells.
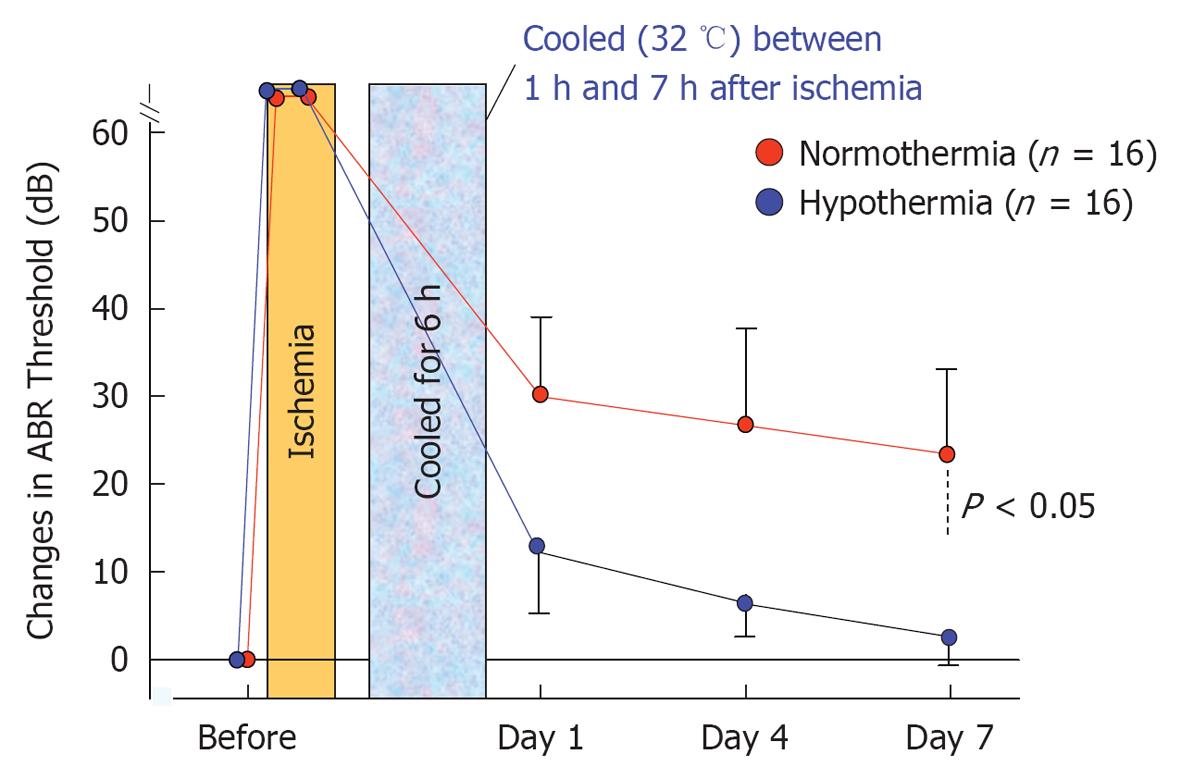
The effects of post-ischemic mild hypothermia on ischemic cochlear damage were investigated by changing the timing and duration of cooling[29,30]. Animals were subjected to mild hypothermia (32 °C) following transient cochlear ischemia. They were divided into six groups, based on the start and end of hypothermia after reperfusion (n = 16 for each group). As shown in Figure 31, post-ischemic mild hypothermia effectively alleviated hearing impairment and hair cell loss when it was applied 1-7 h after reperfusion[31]. The protective effects were more prominent with earlier and longer application of hypothermia. Mild hypothermia 6-9 h after reperfusion did not prevent ischemic damage to the cochlea.
IGF-1: We tested the protective effects of recombinant human IGF-1, applied locally with a gelatin-hydrogel, against ischemic cochlear damage in gerbils. IGF-1 or distilled water (control) immersed in gelatin-hydrogel was applied to the round window membrane through the otic bulla 30 min after ischemic insult (n = 6 for each group). Local administration of IGF-1 significantly reduced the elevation of ABR threshold at 8 kHz on days 1, 4, and 7 after ischemic insult. A histological study also showed that the survival rate of IHCs 7 d after ischemia increased after administration of IGF-1 with the hydrogel. As the gelatin hydrogel dissolved slowly in the body, IGF-1 was released continuously and transported into the inner ear. These findings suggest that local application of IGF-1, in gelatin hydrogel, may prevent ischemic damage to the cochlea[32].
AM-111 (anti-apoptotic agent): AM-111, a cell-permeable peptide inhibitor of c-Jun N-terminal kinase, was investigated for its protective effects against ischemic damage of the cochlea. After induction of transient cochlear ischemia, 10 μL AM-111 at a concentration of l, 10, or 100 μmol/L in a hyaluronic acid gel formulation was applied to the round window 30 min after the insult (n = 6 for each group). Treatment effects were evaluated by ABR and histology of the inner ear. In controls, transient cochlear ischemia caused a 25.0 ± 5.0 dB increase in the ABR threshold at 8 kHz, and a decrease of 13.3% ± 2.3% in IHCs at the basal turn on day 7. Ischemic damage was mild at 2 and 4 kHz. When the animals were treated with AM-111 at 100 μmol/L, cochlear damage was significantly reduced: the increase in ABR threshold was 3.3 ± 2.4 dB at 8 kHz, and the IHC loss was 3.1% ± 0.6% at the basal turn on day 7. The effects of AM-111 were concentration-dependent: 100 μmol/L was more effective than 1 μmol/L or 10 μmol/L. Direct application of AM-111 in a gel formulation to the round window effectively prevented acute hearing loss due to transient cochlear ischemia[33].
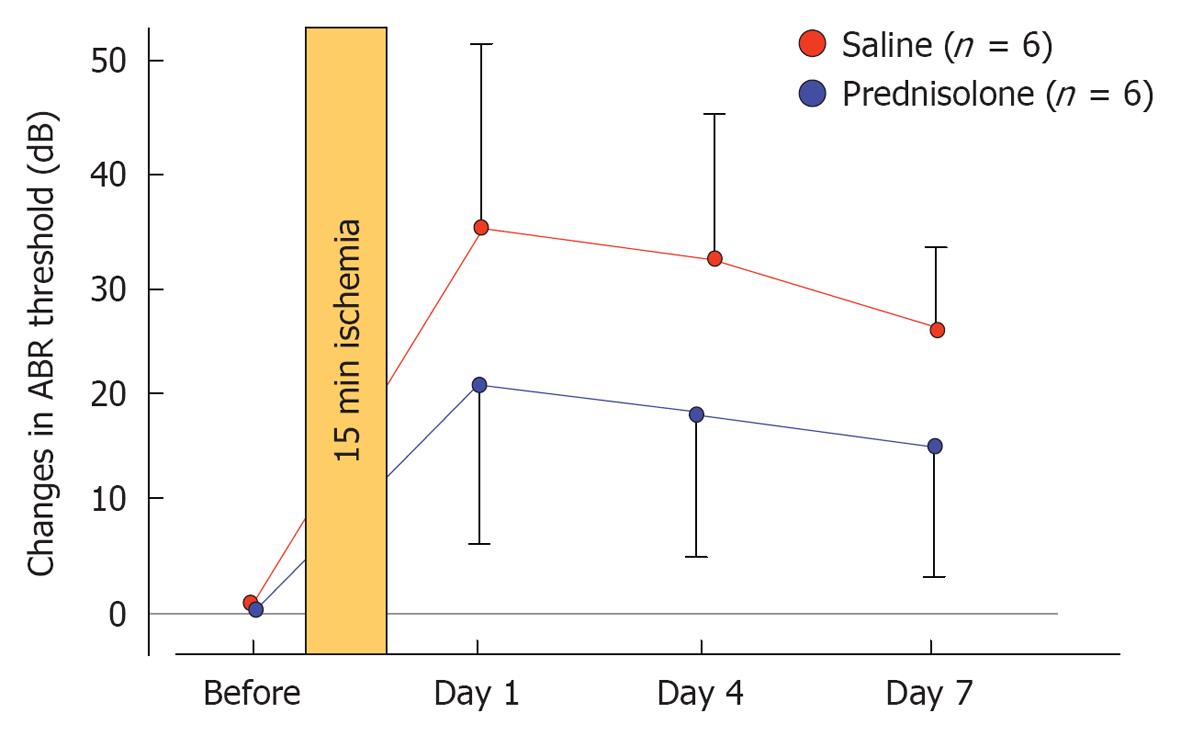
Prednisolone (steroid): The effects of prednisolone on ischemia-induced cochlear damage were investigated. After inducing 15 min ischemia, animals were treated by intraperitoneal injection of prednisolone (1 mg/kg) or physiological saline (control) (n = 6 for each group). Sequential changes in hearing were evaluated by recording ABR before and 1, 4, and 7 d after treatment. The increase in ABR threshold on day 7 was 24.2 ± 8.6 dB in control animals but 14.2 ± 9.2 dB in prednisolone-treated animals (Figure 32). Histological staining for hair cells using rhodamine-phalloidin and Hoechst 33342 showed that the percentage of IHC loss at the basal turn was 26.5% ± 11.4% in the control and 5.3% ± 3.0% in the prednisolone-treated group. These results indicate that prednisolone protects against inner ear damage caused by ischemic insult, even when administered after the insult[34].
Edarabone (antioxidant agent): Edaravone, a free radical scavenger, has potent protective effects on ischemic damage. Edaravone (1 mg/kg, iv) or saline was administered 1 h after ischemia (n = 6 for each group). In animals treated with saline, the ABR threshold shift was 24.1 ± 4.2 dB and there was a 26.5% ± 11.4% decrease in the number of IHCs. In contrast, in animals treated with edaravone, the threshold shift was 7.5 ± 4.2 dB and only 8.8% ± 3.5% of IHCs were lost. These results suggest that edaravone protects against ischemic damage of the inner ear following transient ischemia[21].
Prosaposin-derived synthetic peptide: A peptide resembling the neurotrophic region of prosaposin (18-mer peptide, PS-pep) was synthesized artificially and administered subcutaneously four times: immediately and 1, 2 and 3 d after induction of transient cochlear ischemia (n = 6 for each group). On day 7, the ABR threshold shift was 33.3 ± 16.3 dB in animals treated with saline, while it was 12.5 ± 8.2 dB in animals treated with 2.0 mg/kg PS-pep. This alleviation was not seen in animals treated with 0.2 mg/kg PS-pep or saline. Histological examinations conducted on day 7 showed that higher doses of PS-pep significantly alleviated IHC loss, whereas a low dose did not. In addition, an increase in the anti-apoptotic factor bcl-2 was also noted in the IHCs of animals treated with higher doses of PS-pep. These findings suggest that PS-pep prevents hearing loss and cochlear damage due to transient cochlear ischemia by activating an anti-apoptotic pathway[35].
gRb1 (Kanpo): gRb1 is a Kanpo medicine that has protective effects on ischemic brain damage, in addition to other various effects such as regeneration of blood vessels, activation of plasmins, and release of corticosteroids. Using this agent, on day 7 after ischemia, the percentage of SGCs decreased to 67.5% from the preischemic baseline in the basal turn in the control group, whereas it was 90.2% in the gRb1-treated group. Immunohistochemical staining showed TUNEL-positive reactions in the SGCs, with fragmented nuclei. We also investigated the protective effects of gRb1 against ischemic injury in the cochlea. On day 7, the ABR threshold shift in the gRb1-treated group was 14.2 ± 3.8 dB and that in the control group was 22.5 ± 2.9 dB. Furthermore, loss of IHCs in the gRb1-treated group was 8.6% ± 2.6% and that in the control group was 26.5% ± 11.4%. These differences were statistically significant. These findings indicate that gRb1 prevents hearing loss caused by ischemic insult[19].
LEH: LEH was originally developed as an artificial red blood cell (RBC). The experimental animals were randomly assigned to receive 2 mL/kg of low-affinity LEH (l-LEH, P50 = 40 mmHg), high-affinity LEH (h-LEH, P50 = 10 mmHg), homologous RBCs, or saline 30 min before transient cochlear ischemia (n = 6 for each group). Sequential changes in hearing were assessed by recording ABR at 8, 16, and 32 kHz 1, 4, and 7 d after ischemic insult, and then the animals were sacrificed for histological studies. The ABR study showed that h-LEH was more protective than l-LEH in suppressing hearing loss, in contrast to RBCs or saline treatment. In the morphological study, loss of IHCs was most effectively protected against by h-LEH. These findings suggest that pretreatment with h-LEH is significantly more protective than l-LEH in mitigating hearing loss and underlying pathological damage, in contrast to transfusion or saline infusion 7 d after transient cochlear ischemia[36].
GDNF: GDNF promotes the survival and differentiation of dopaminergic neurons, and is able to prevent apoptosis of motor neurons induced by axotomy. We assessed the utility of an adenoviral vector expressing GDNF (Ad-GDNF) in ischemia-reperfusion injury of the gerbil cochlea. The vector was injected through the round window 4 d before ischemic insult. The distribution of a reporter transgene was confirmed throughout the cochlea, from the basal to the apical turn, and Western blot analysis indicated significant upregulation of GDNF protein 11 d following virus inoculation. Hearing ability was assessed by sequentially recording electrocochleogram, and the degree of hair cell loss was evaluated in specimens stained with rhodamine-phalloidin and Hoechst 33342. On day 7 after ischemia, the shift in compound action potentials threshold in electrocochleogram and IHC loss were markedly suppressed in the Ad-GDNF group, compared to the control group. These results suggest that adenovirus-mediated overexpression of GDNF may be useful for protection against hair cell damage, which otherwise eventually occurs after transient ischemia in the cochlea[37].
Hematopoietic stem cells: Transplantation of hematopoietic stem cells (HSCs) is considered a potential approach for promoting the repair of damaged organs. We investigated the influence of HSCs on progressive hair cell degeneration after transient cochlear ischemia. After induction of ischemia, animals were treated with an intramuscular injection of HSCs. This procedure prevented degeneration of IHCs and ameliorated hearing impairment. In addition, the protein level of GDNF in the organ of Corti was upregulated after cochlear ischemia and treatment with HSCs augmented this upregulation of GDNF. Furthermore, HSCs injected into the cochlea remained in the perilymphatic space, although they did not transdifferentiate into cochlear cell types or fuse with injured hair cells after ischemia. This suggests that HSCs have therapeutic potential, possibly through paracrine effects. Based on these findings, we concluded that intramuscular injection of HSCs may be a potential new therapeutic strategy for hearing loss[38].
Neural stem cells: Neural stem cells are multipotent progenitor cells that show self-renewal activity. We assessed the use of neural stem cells for ameliorating ischemia-reperfusion injury in the gerbil cochlea. Neural stem cells were injected into the inner ear through the round window 1 d after ischemic insult. Immunostaining for nestin showed that the distribution of neural stem cells was concentrated within the organ of Corti. Seven days after ischemia, the injury-induced shift in ABR and progressive IHC damage were markedly reduced on the neural stem cell-transplanted side. These results suggest that the transplantation of neural stem cells is therapeutically useful for preventing the damage to hair cells that occurs after transient ischemia of the cochlea[39].
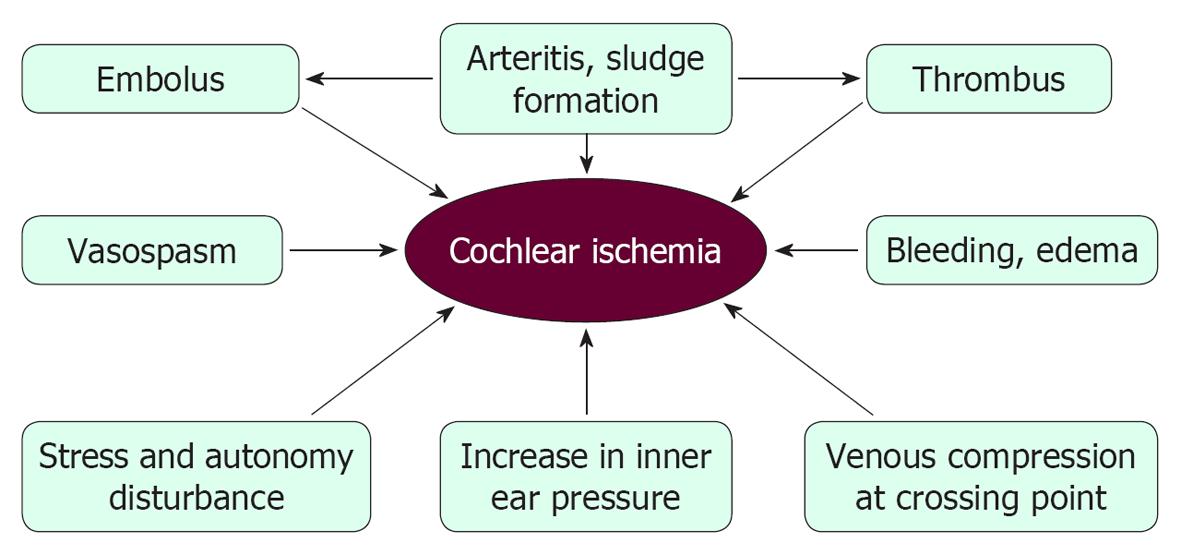
Presently, vascular theory as an etiology of ISSHL remains speculative, because cochlear pathology is difficult to assess in live humans. Figure 33 illustrates the possible causes of ischemic damage in the cochlea. Many risk factors, such as hyperlipoproteinemia, hyperglycemia, obesity, smoking, and stress, have been correlated with the incidence of ISSHL. However, the real cause(s) of this disease remain(s) unknown, primarily because of technical difficulties. As 30%-40% of ISSHL patients heal spontaneously, permanent occlusion of the major nourishing artery would not seem to be a major cause of ISSHL. Instead, we believe that ISSHL is caused by transient or local ischemia, caused by microcirculatory disturbances in the stria vascularis.
Arterial-venous compression is a known cause of branch retinal vein occlusion, which is the second most common cause of blindness in America. ISSHL has similar clinical characteristics to BRVC, as shown in Table 1. If arterial-venous compression is a cause of ISSHL, the capillary network in the stria vascularis would be the most likely site of the lesion. As ATP production occurs mainly in the lateral region, even a minor circulatory disturbance could cause ischemic damage in the cochlea.
| ISSHL | Branch retinal vein occlusion | |
| Annual incidence in Japan | 35 000 | 30 000-50 000 |
| Age preponderance (yr) | 50-60 | 60 |
| Sex difference | No | No |
| Incidence | Sudden | Sudden |
| Background disease | Unknown | Arteriosclerosis, diabetes mellitus |
| Involved site | Stria vascularis? | Retinal vein |
| Bilateral incidence | Rare | 2%-4% |
| Recurrence | Rare | Rare |
| Spontaneous healing | 30%-50% | 30%-50% |
| Effects of steroid | Effective | Effective |
Using adult gerbils, we induced transient cochlear ischemia without a craniotomy. Ischemic insult caused mild hearing loss and sporadic loss of hair cells, especially IHCs. Mechanisms of induced cochlear damage included depletion of the energy supply, production of free radicals, and glutamate ototoxicity. We found that the cochlea shows ischemic tolerance, which may explain why recurrence is rare in ISSHL. We also reported the results of various treatment modalities, such as hypothermia and the topical and general administration of test agents.
P- Reviewer Ciuman R S- Editor Wen LL L- Editor A E- Editor Zheng XM
| 1. | Suckfüll M. Fibrinogen and LDL apheresis in treatment of sudden hearing loss: a randomised multicentre trial. Lancet. 2002;360:1811-1817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | De Felice C, De Capua B, Tassi R, Mencattini G, Passàli D. Non-functioning posterior communicating arteries of circle of Willis in idiopathic sudden hearing loss. Lancet. 2000;356:1237-1238. [PubMed] [Cited in This Article: ] |
| 3. | Lin HC, Chao PZ, Lee HC. Sudden sensorineural hearing loss increases the risk of stroke: a 5-year follow-up study. Stroke. 2008;39:2744-2748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Ballesteros F, Alobid I, Tassies D, Reverter JC, Scharf RE, Guilemany JM, Bernal-Sprekelsen M. Is there an overlap between sudden neurosensorial hearing loss and cardiovascular risk factors. Audiol Neurootol. 2009;14:139-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Görür K, Tuncer U, Eskandari G, Ozcan C, Unal M, Ozsahinoglu C. The role of factor V Leiden and prothrombin G20210A mutations in sudden sensorineural hearing loss. Otol Neurotol. 2005;26:599-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Capaccio P, Ottaviani F, Cuccarini V, Ambrosetti U, Fagnani E, Bottero A, Cenzuales S, Cesana BM, Pignataro L. Sudden hearing loss and MTHFR 677C>T/1298A>C gene polymorphisms. Genet Med. 2005;7:206-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Rudack C, Langer C, Stoll W, Rust S, Walter M. Vascular risk factors in sudden hearing loss. Thromb Haemost. 2006;95:454-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Capaccio P, Cuccarini V, Ottaviani F, Fracchiolla NS, Bossi A, Pignataro L. Prothrombotic gene mutations in patients with sudden sensorineural hearing loss and cardiovascular thrombotic disease. Ann Otol Rhinol Laryngol. 2009;118:205-210. [PubMed] [Cited in This Article: ] |
| 9. | Hato N. SNP and sudden deafness. Clinical features and mechanics of ischemia-induced hearing loss. Matsuyama: Sagawa Press 2007; 113-114 (in Japanese). [Cited in This Article: ] |
| 10. | Kubo M, Hata J, Ninomiya T, Matsuda K, Yonemoto K, Nakano T, Matsushita T, Yamazaki K, Ohnishi Y, Saito S. A nonsynonymous SNP in PRKCH (protein kinase C eta) increases the risk of cerebral infarction. Nat Genet. 2007;39:212-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Yoshida T, Sugiura M, Naganawa S, Teranishi M, Nakata S, Nakashima T. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging findings and prognosis in sudden sensorineural hearing loss. Laryngoscope. 2008;118:1433-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Hata R, Matsumoto M, Hatakeyama T, Ohtsuki T, Handa N, Niinobe M, Mikoshiba K, Sakaki S, Nishimura T, Yanagihara T. Differential vulnerability in the hindbrain neurons and local cerebral blood flow during bilateral vertebral occlusion in gerbils. Neuroscience. 1993;56:423-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Hakuba N, Koga K, Shudou M, Watanabe F, Mitani A, Gyo K. Hearing loss and glutamate efflux in the perilymph following transient hindbrain ischemia in gerbils. J Comp Neurol. 2000;418:217-226. [PubMed] [Cited in This Article: ] |
| 14. | Koga K, Hakuba N, Watanabe F, Shudou M, Nakagawa T, Gyo K. Transient cochlear ischemia causes delayed cell death in the organ of Corti: an experimental study in gerbils. J Comp Neurol. 2003;456:105-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Nakashima T, Naganawa S, Sone M, Tominaga M, Hayashi H, Yamamoto H, Liu X, Nuttall AL. Disorders of cochlear blood flow. Brain Res Brain Res Rev. 2003;43:17-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Morizane I, Hakuba N, Shimizu Y, Shinomori Y, Fujita K, Yoshida T, Shudou M, Gyo K. Transient cochlear ischemia and its effects on the stria vascularis. Neuroreport. 2005;16:799-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Morizane I, Hakuba N, Hyodo J, Shimizu Y, Fujita K, Yoshida T, Gyo K. Ischemic damage increases nitric oxide production via inducible nitric oxide synthase in the cochlea. Neurosci Lett. 2005;391:62-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Taniguchi M, Hakuba N, Koga K, Watanabe F, Hyodo J, Gyo K. Apoptotic hair cell death after transient cochlear ischemia in gerbils. Neuroreport. 2002;13:2459-2462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Fujita K, Hakuba N, Hata R, Morizane I, Yoshida T, Shudou M, Sakanaka M, Gyo K. Ginsenoside Rb1 protects against damage to the spiral ganglion cells after cochlear ischemia. Neurosci Lett. 2007;415:113-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Thalmann R, Kusakari J, Miyoshi T. Dysfunctions of energy releasing and consuming processes of the cochlea. Laryngoscope. 1973;83:1690-1712. [PubMed] [Cited in This Article: ] |
| 21. | Maetani T, Hakuba N, Taniguchi M, Hyodo J, Shimizu Y, Gyo K. Free radical scavenger protects against inner hair cell loss after cochlear ischemia. Neuroreport. 2003;14:1881-1884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Hakuba N, Gyo K, Yanagihara N, Mitani A, Kataoka K. Efflux of glutamate into the perilymph of the cochlea following transient ischemia in the gerbil. Neurosci Lett. 1997;230:69-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Hakuba N, Koga K, Gyo K, Usami SI, Tanaka K. Exacerbation of noise-induced hearing loss in mice lacking the glutamate transporter GLAST. J Neurosci. 2000;20:8750-8753. [PubMed] [Cited in This Article: ] |
| 24. | Hakuba N, Matsubara A, Hyodo J, Taniguchi M, Maetani T, Shimizu Y, Tsujiuchi Y, Shudou M, Gyo K. AMPA/kainate-type glutamate receptor antagonist reduces progressive inner hair cell loss after transient cochlear ischemia. Brain Res. 2003;979:194-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Hyodo J, Hakuba N, Hato N, Takeda S, Okada M, Omotehara Y, Gyo K. Glutamate agonist causes irreversible degeneration of inner hair cells. Neuroreport. 2009;20:1255-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 893] [Cited by in F6Publishing: 840] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 27. | Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283-1296. [PubMed] [Cited in This Article: ] |
| 28. | Takeda S, Hata R, Cao F, Yoshida T, Hakuba N, Hato N, Gyo K. Ischemic tolerance in the cochlea. Neurosci Lett. 2009;462:263-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Watanabe F, Koga K, Hakuba N, Gyo K. Hypothermia prevents hearing loss and progressive hair cell loss after transient cochlear ischemia in gerbils. Neuroscience. 2001;102:639-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Hyodo J, Hakuba N, Koga K, Watanabe F, Shudou M, Taniguchi M, Gyo K. Hypothermia reduces glutamate efflux in perilymph following transient cochlear ischemia. Neuroreport. 2001;12:1983-1987. [PubMed] [Cited in This Article: ] |
| 31. | Takeda S, Hakuba N, Yoshida T, Fujita K, Hato N, Hata R, Hyodo J, Gyo K. Postischemic mild hypothermia alleviates hearing loss because of transient ischemia. Neuroreport. 2008;19:1325-1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Fujiwara T, Hato N, Nakagawa T, Tabata Y, Yoshida T, Komobuchi H, Takeda S, Hyodo J, Hakuba N, Gyo K. Insulin-like growth factor 1 treatment via hydrogels rescues cochlear hair cells from ischemic injury. Neuroreport. 2008;19:1585-1588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Omotehara Y, Hakuba N, Hato N, Okada M, Gyo K. Protection against ischemic cochlear damage by intratympanic administration of AM-111. Otol Neurotol. 2011;32:1422-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Maetani T, Hyodo J, Takeda S, Hakuba N, Kiyofumi G. Prednisolone prevents transient ischemia-induced cochlear damage in gerbils. Acta Otolaryngol Suppl. 2009;24-27. [PubMed] [Cited in This Article: ] |
| 35. | Terashita T, Saito S, Miyawaki K, Hyodo M, Kobayashi N, Shimokawa T, Saito K, Matsuda S, Gyo K. Localization of prosaposin in rat cochlea. Neurosci Res. 2007;57:372-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Okada M, Kawaguchi AT, Hakuba N, Takeda S, Hyodo J, Imai K, Hato N, Gyo K. Liposome-encapsulated hemoglobin alleviates hearing loss after transient cochlear ischemia and reperfusion in the gerbil. Artif Organs. 2012;36:178-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 37. | Hakuba N, Watabe K, Hyodo J, Ohashi T, Eto Y, Taniguchi M, Yang L, Tanaka J, Hata R, Gyo K. Adenovirus-mediated overexpression of a gene prevents hearing loss and progressive inner hair cell loss after transient cochlear ischemia in gerbils. Gene Ther. 2003;10:426-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Yoshida T, Hakuba N, Morizane I, Fujita K, Cao F, Zhu P, Uchida N, Kameda K, Sakanaka M, Gyo K. Hematopoietic stem cells prevent hair cell death after transient cochlear ischemia through paracrine effects. Neuroscience. 2007;145:923-930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Hakuba N, Hata R, Morizane I, Feng G, Shimizu Y, Fujita K, Yoshida T, Sakanaka M, Gyo K. Neural stem cells suppress the hearing threshold shift caused by cochlear ischemia. Neuroreport. 2005;16:1545-1549. [PubMed] [Cited in This Article: ] |