Copyright
©2013 Baishideng.
World J Otorhinolaryngol. Feb 28, 2013; 3(1): 1-15
Published online Feb 28, 2013. doi: 10.5319/wjo.v3.i1.1
Published online Feb 28, 2013. doi: 10.5319/wjo.v3.i1.1
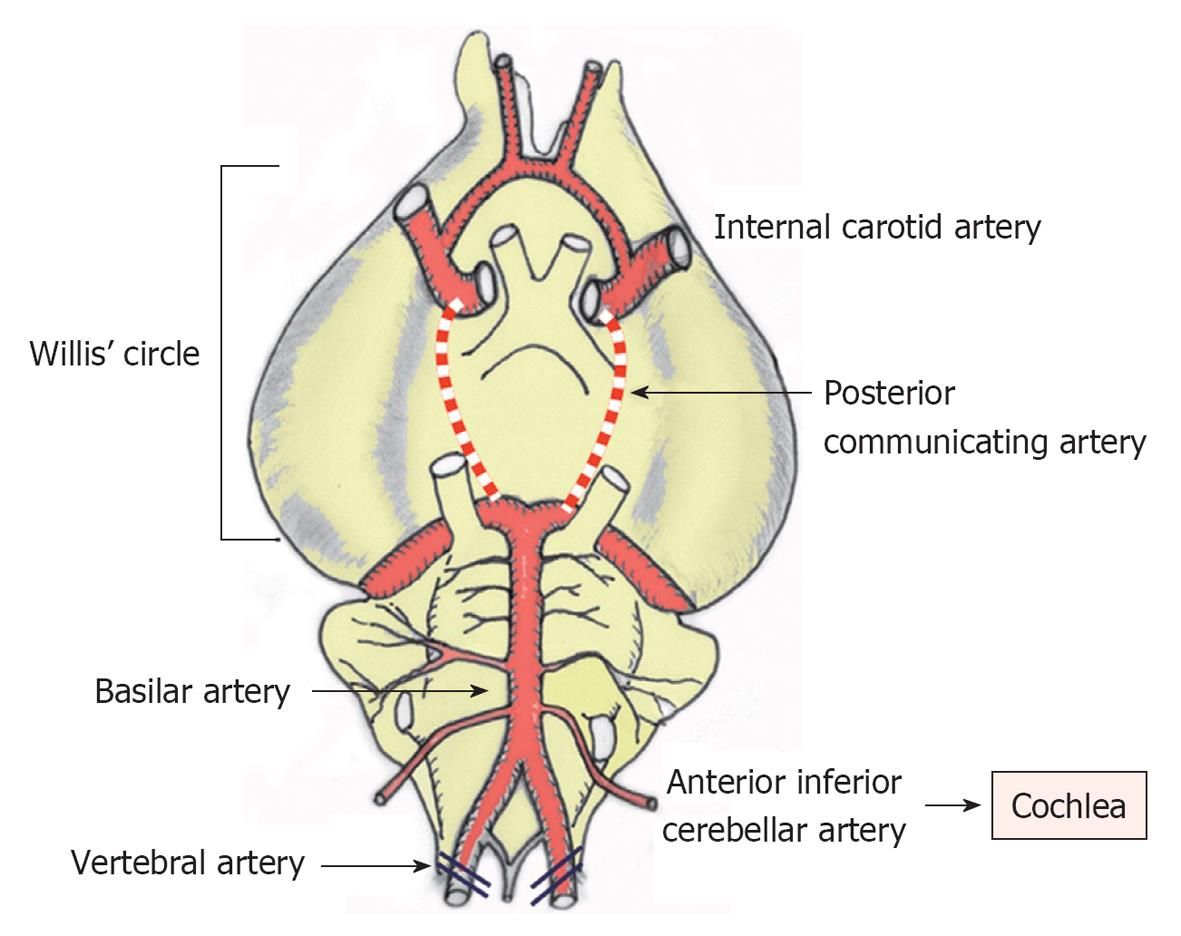
Figure 1 Brain arteries in the gerbil.
The posterior communicating arteries of the Circle of Willis close at around 1 mo after birth. Thus, cochlear ischemia can be induced by occluding the bilateral vertebral arteries at the neck in adult animals.
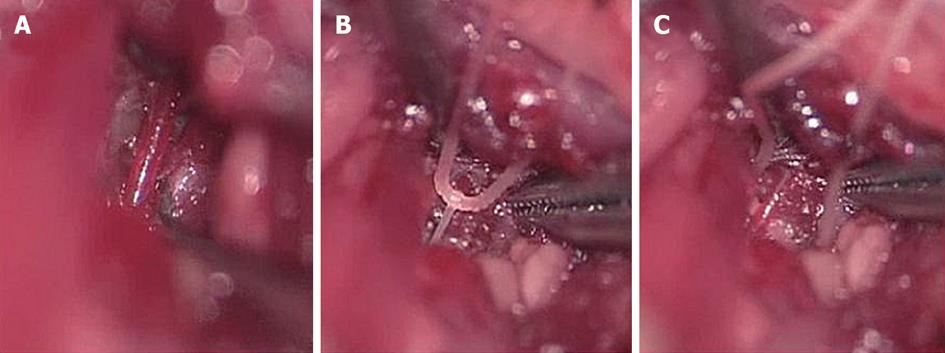
Figure 2 Transient interruption of cochlear blood flow.
A: Exposure of the vertebral artery; B: Interruption of cochlear blood flow by pulling the silk thread looped around the artery; C: After inducing transient ischemia, the thread was released and removed to allow recirculation. Originated from[33], with permission.
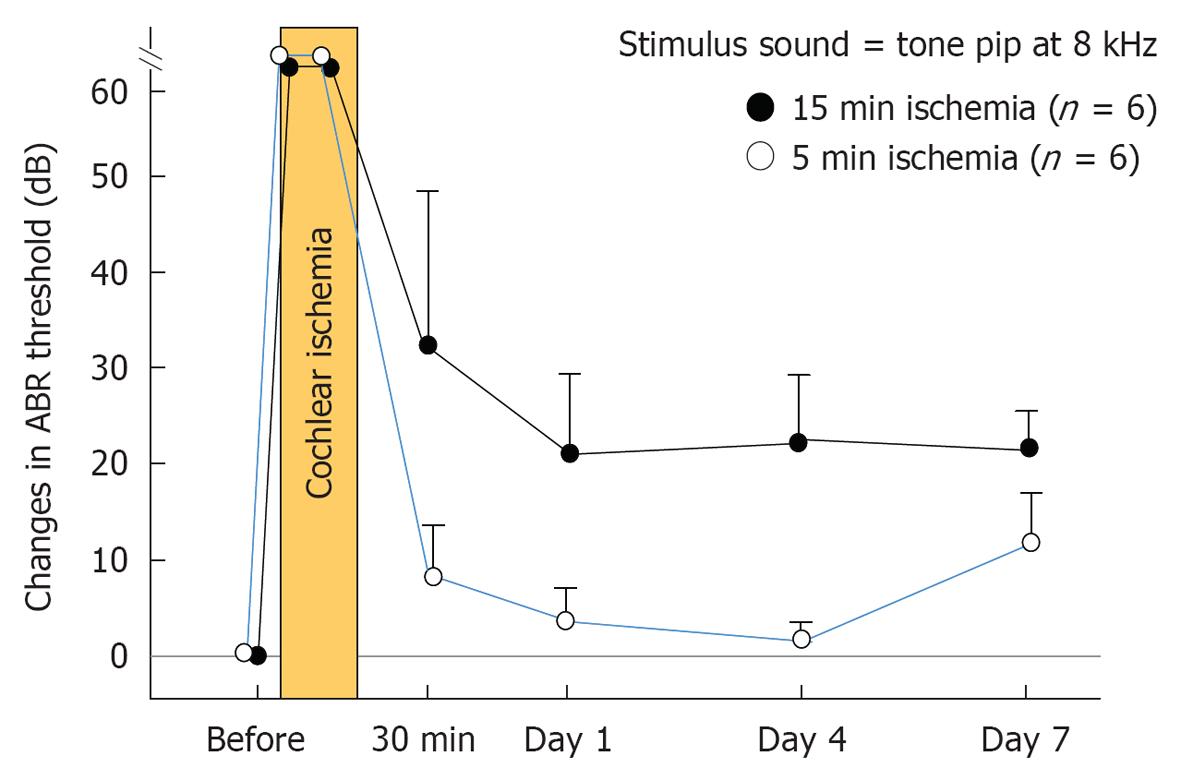
Figure 3 Changes in auditory brainstem response threshold after transient cochlear ischemia.
Auditory brainstem response (ABR) threshold before vertebral artery occlusion was defined as 0 dB. With 5 min ischemia, the ABR threshold at 8 kHz recovered almost completely on days 1 and 4, but became slightly worse on day 7, likely due to delayed neural cell death. With 15 min ischemia, the ABR threshold was almost constant after day 1. It remained constant in the range of 20-30 dB. The vertical scale indicates grade of hearing loss, expressed as change in ABR threshold.
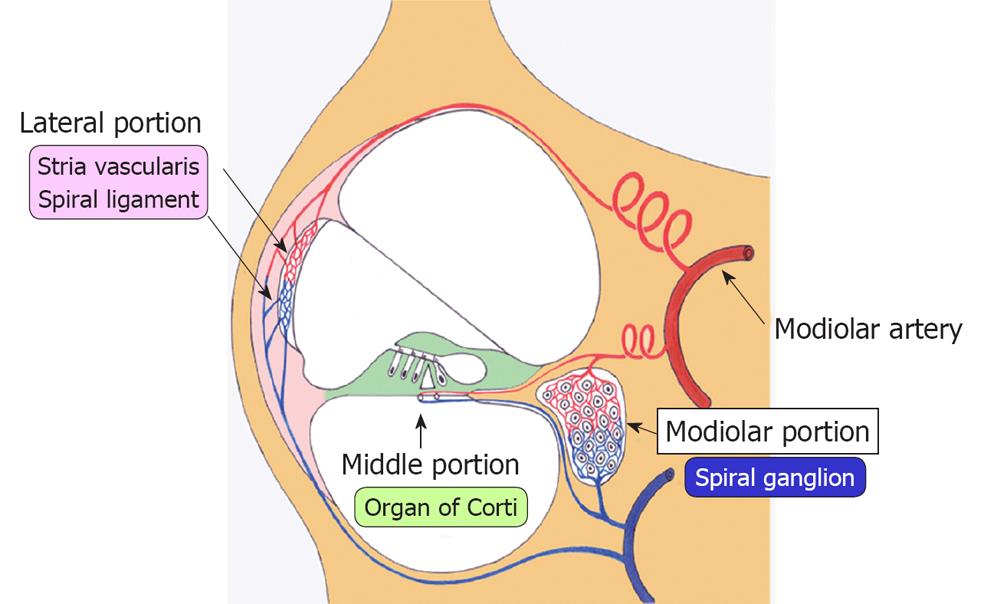
Figure 4 Blood supply to the cochlea.
Blood supply to the cochlea via modiolar artery can be divided into three regions: the lateral, middle, and modiolar regions. Blood supply to the lateral region, consisting of the stria vascularis and spiral ligament, was the largest among the three regions, supplying more than 80% of total cochlear blood in the rabbit.
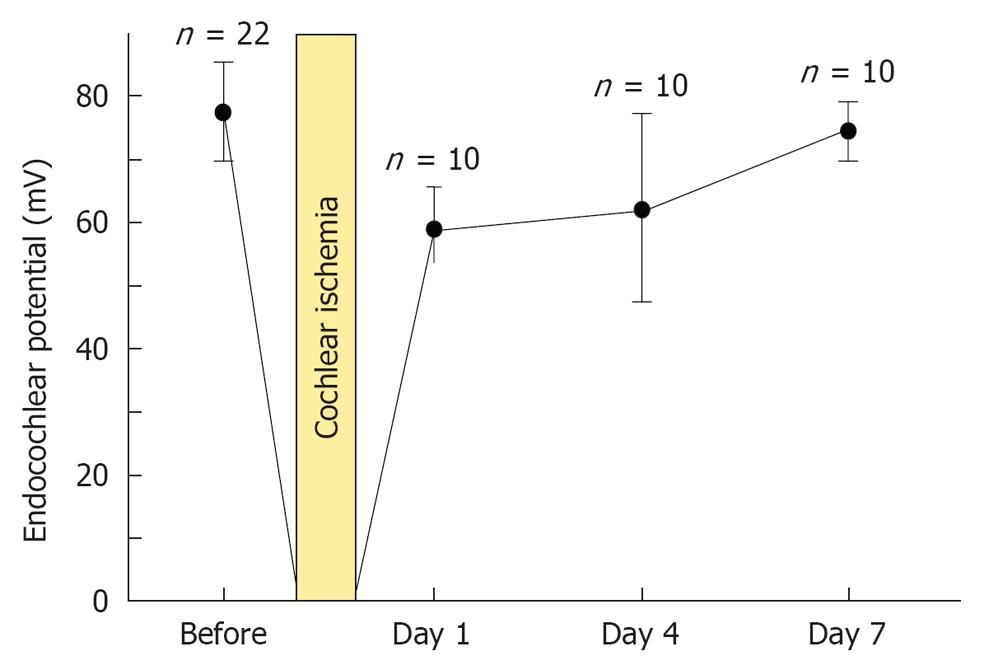
Figure 5 Sequential changes in endocochlear potential after transient cochlear ischemia.
Each point indicates the mean voltage of the endocochlear potential. Vertical bars show one standard deviation. Originated from[16], with permission.
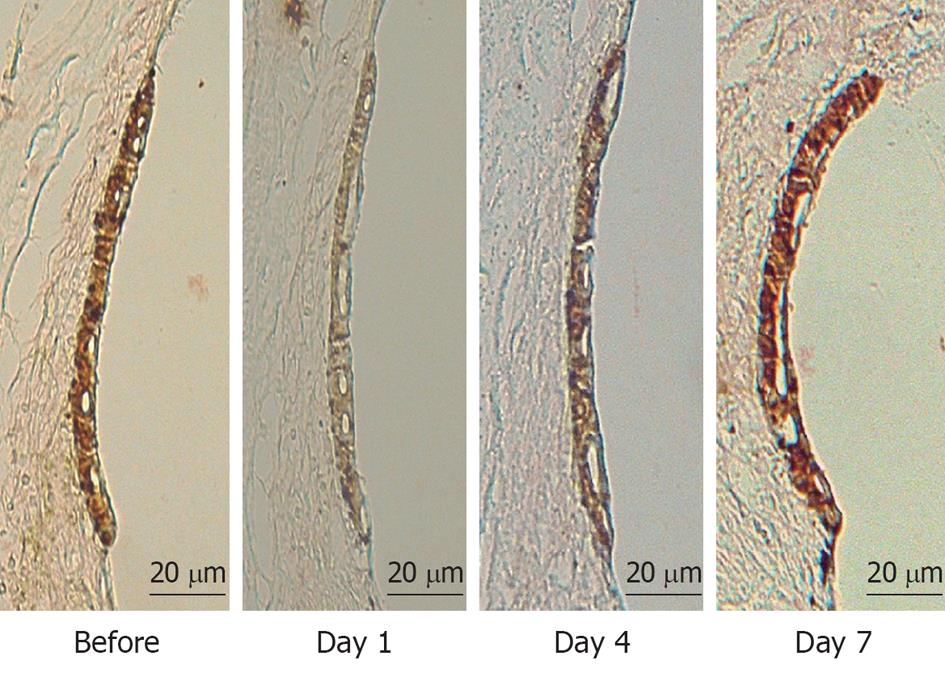
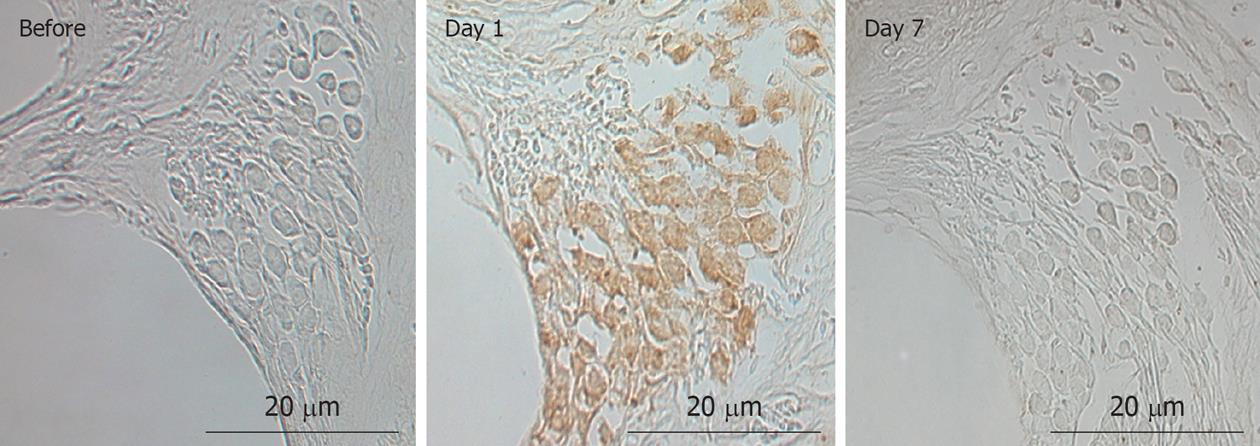
Figure 6 Immunostaining of Na+-K+ ATPase in the stria vascularis before and 1, 4, and 7 d after ischemic insult.
Immunostaining of Na+-K+ ATPase decreased markedly on day 1, and improved slightly on day 4. It recovered to preischemic levels on day 7.
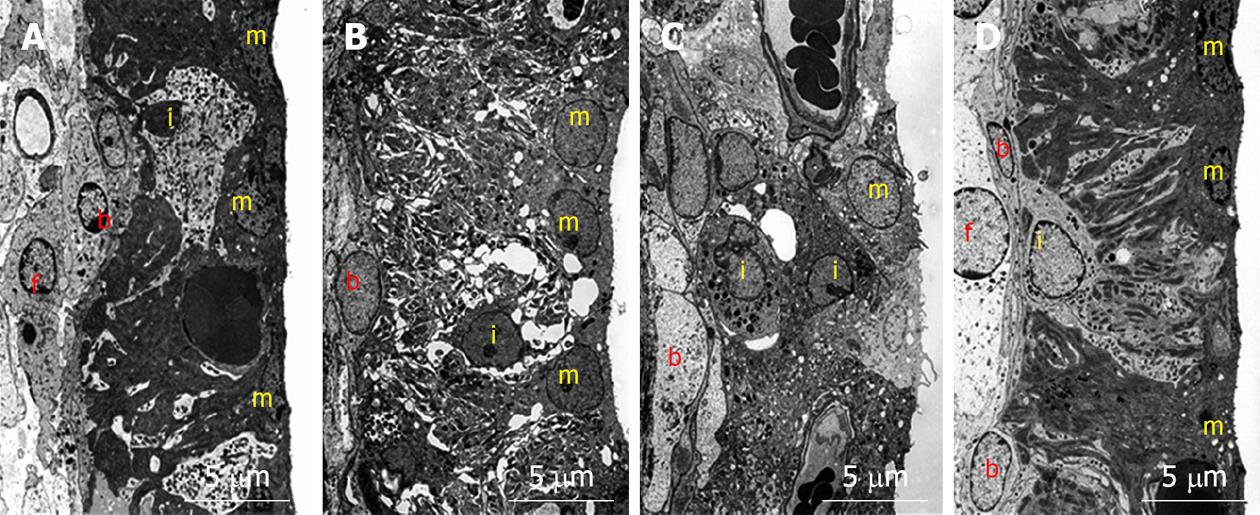
Figure 7 Transmission electron microscopy findings in the stria vascularis before and 1, 4, and 7 d after ischemic insult.
A: Marginal cells on the medial aspect of the stria vascularis showed extensive branching processes with intermediate cells. The basal cells, located on the lateral aspect of the stria vascularis, connected to the intermediate cells and type 1 fibrocytes of the spiral ligament by means of gap junctions; B: Vacuoles were seen in marginal cells. The intercellular space increased and intermediate cells seemed to have shrunk; C: Vacuoles persisted in marginal cells. The intermediate cells were still shrunken, although the intercellular space was smaller than that on day 1; D: The intercellular space was no longer enlarged, although a few small vacuoles were found in marginal cells. The extensive branching processes appeared to have a normal shape. m: Marginal cell; i: Intermediate cell; b: Basal cell; f: Type 1 fibrocyte.
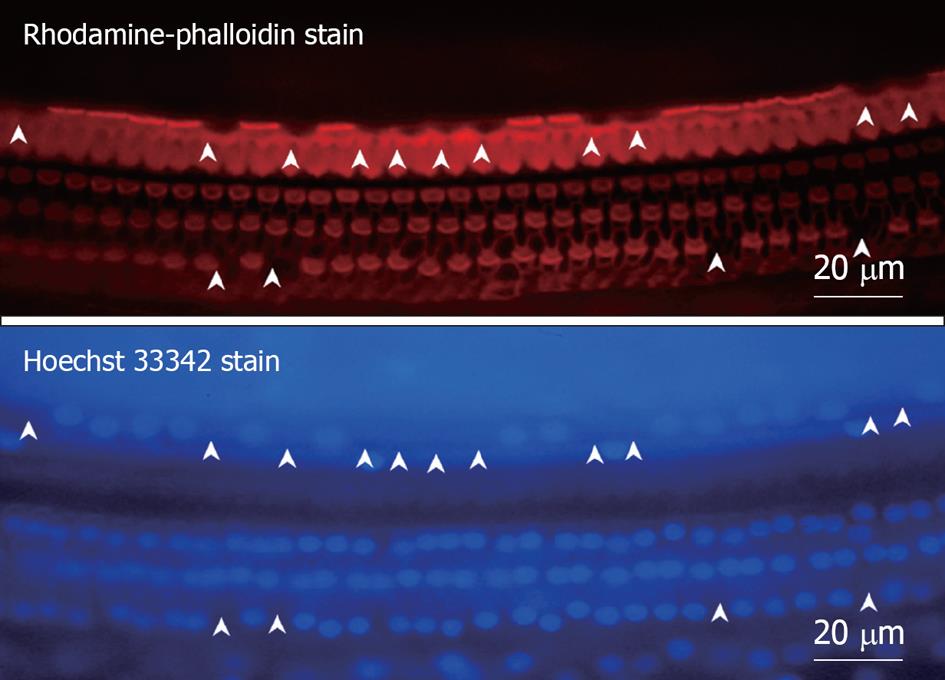
Figure 8 Fluorescence microscopic findings of the organ of Corti 7 d after ischemic insult.
The specimen was stained with rhodamine-phalloidin and Hoechst 33342. Three rows of outer hair cells (OHC) and a single row of inner hair cells (IHC) could be observed, and the cell loss was more severe in IHC than OHC. Arrowheads indicate loss of hair cells.
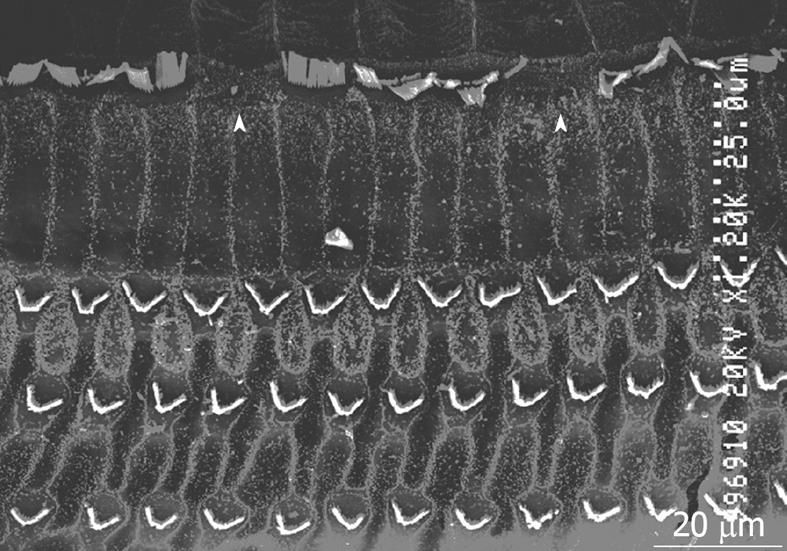
Figure 9 Scanning electron microscopy findings of the organ of Corti 7 d after ischemic insult.
Arrowheads indicate damaged inner hair cells.
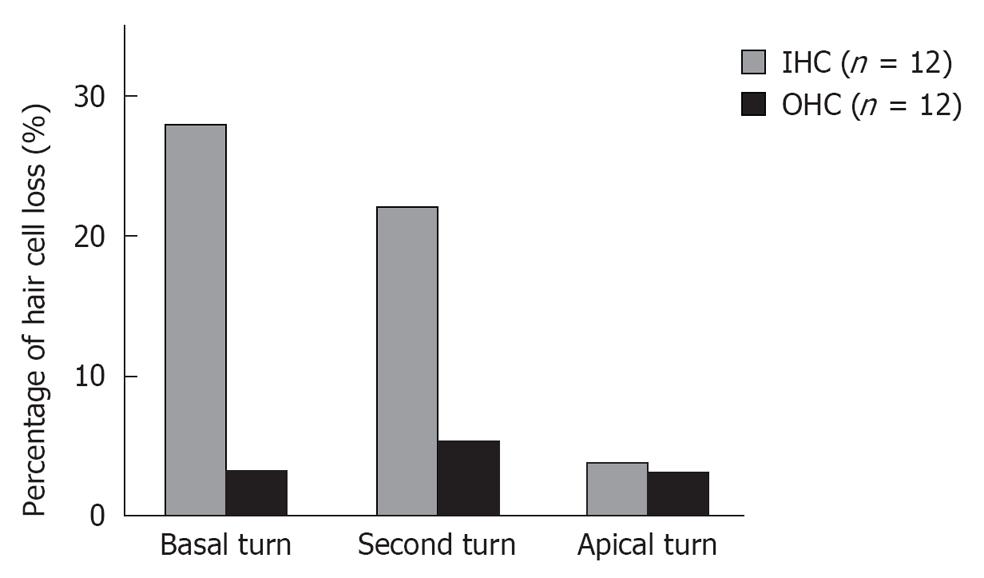
Figure 10 Percentages of hair cell loss at three turns 7 d after ischemic insult.
Loss of inner hair cells (IHC) was more severe at the basal and second turn than at the apical turn. No such difference by turn was recognized in the loss of outer hair cell (OHC).
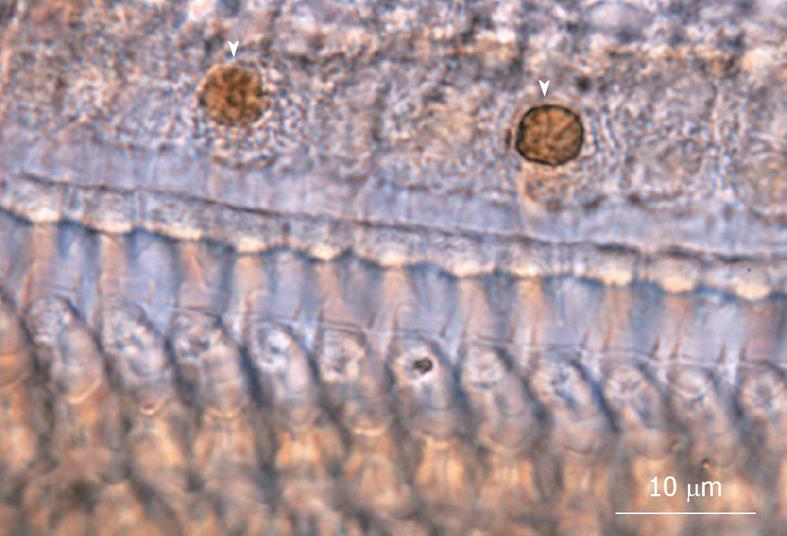
Figure 11 TUNEL staining of inner hair cells.
TUNEL staining was positive in inner hair cells (IHC), suggesting cell death due to apoptosis. The arrowhead indicates TUNEL-positive IHC. Originated from[18], with permission.
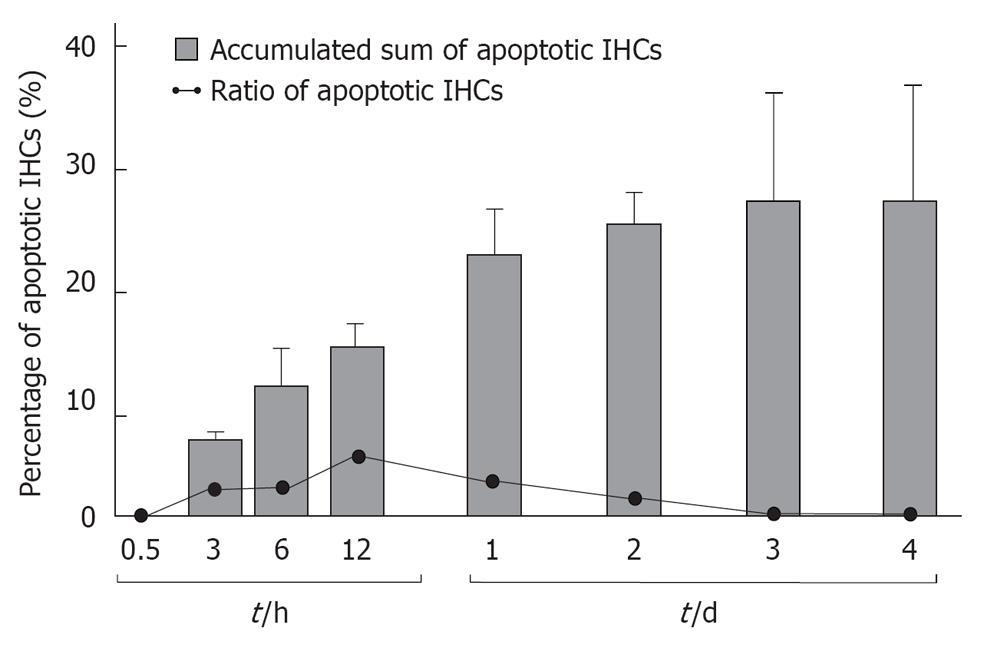
Figure 12 Daily incidence of inner hair cell apoptosis and its cumulative sum after ischemic insult.
Incidence of inner hair cells (IHCs) apoptosis was maximal 12 h after ischemic insult. It did not occur after day 3. Vertical bars indicate one standard deviation. Originated from[18], with permission.
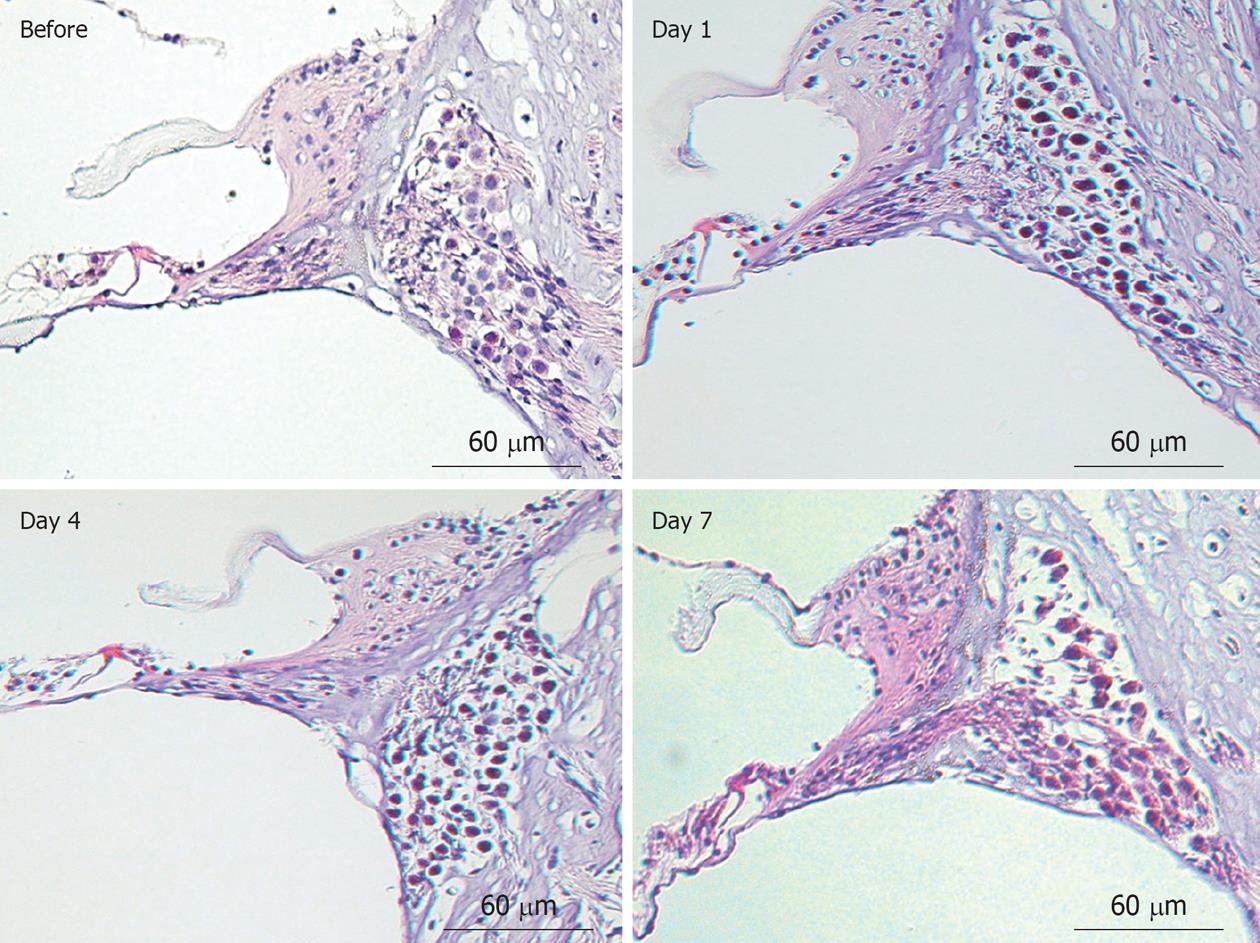
Figure 13 Histological findings of the organ of Corti and the spiral ganglion before and 1, 4, and 7 d after ischemic insult (hematoxylin and eosin staining).
Originated from[19], with permission.
Figure 14 Immunostaining of Bax at the spiral ganglion before and after ischemic insult.
Bax was expressed on day 1, but disappeared by day 7. Originated from[19], with permission.
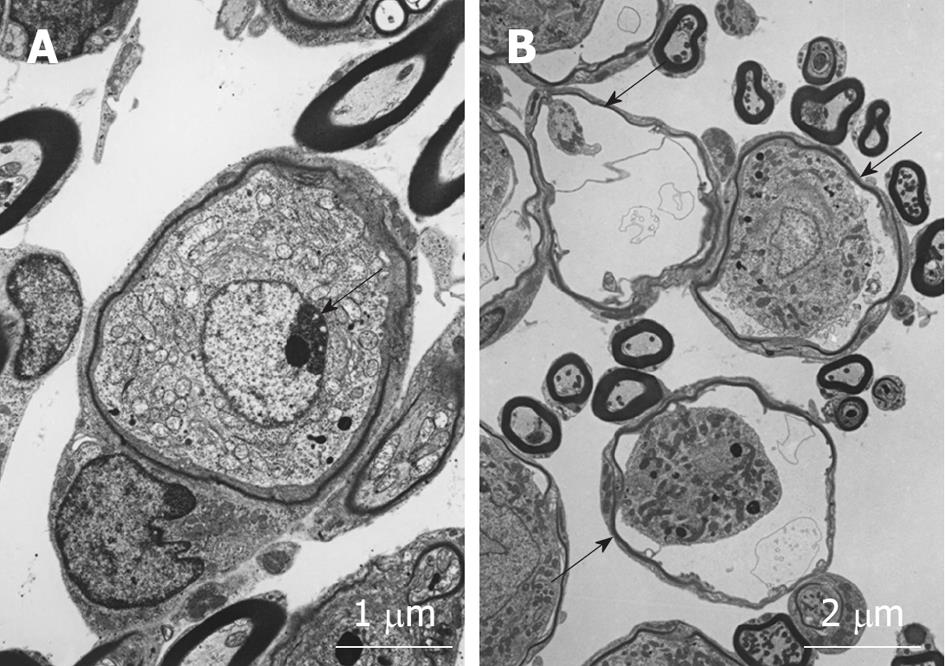
Figure 15 Transmission electron microscopy findings of spiral ganglion cells 1 dafter ischemic insult.
A: Nucleus of the spiral ganglion cell underwent chromatin condensation (arrow) and segmentation, suggesting cell death due to an apoptotic process; B: The spiral ganglion cells appeared shrunken (arrows).
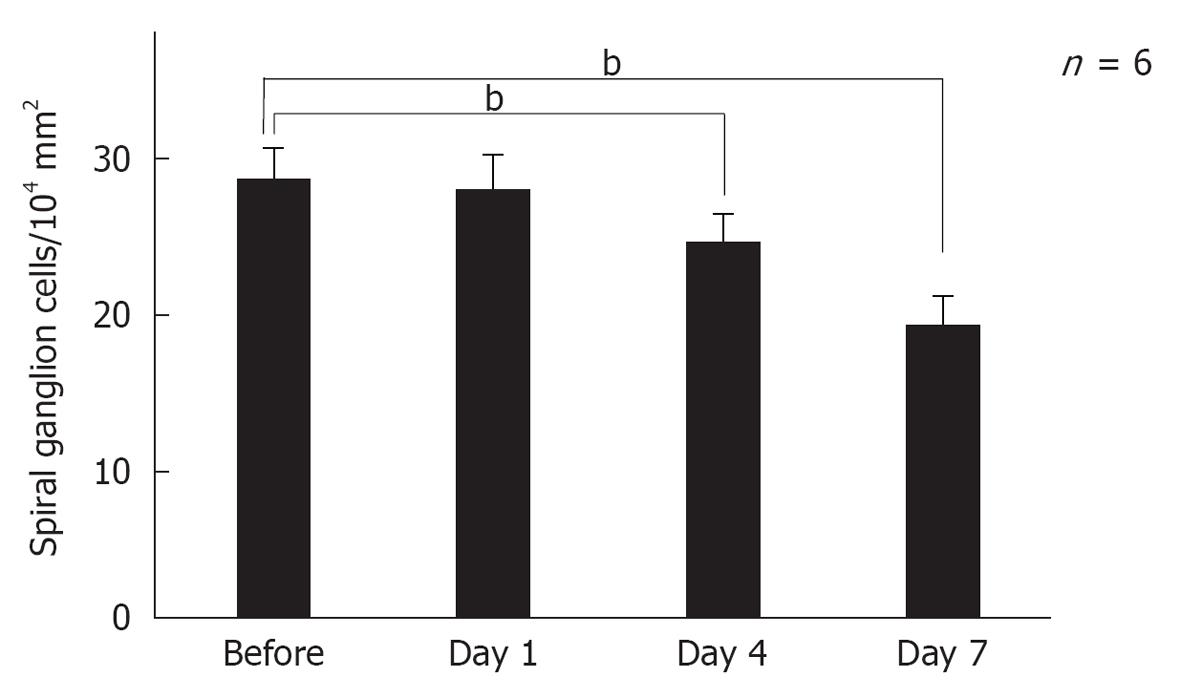
Figure 16 Sequential changes in the number of spiral ganglion neurons after ischemic insult.
The number of spiral ganglion cells decreased gradually after ischemic insult. It did not change on day 1, but decreased on days 4 and 7. Each point indicates the mean number of the spiral ganglion neurons per 104 mm2 before and 1, 4, and 7 d after ischemia. Vertical bars indicate one standard deviation. bP < 0.01 vs the control group.
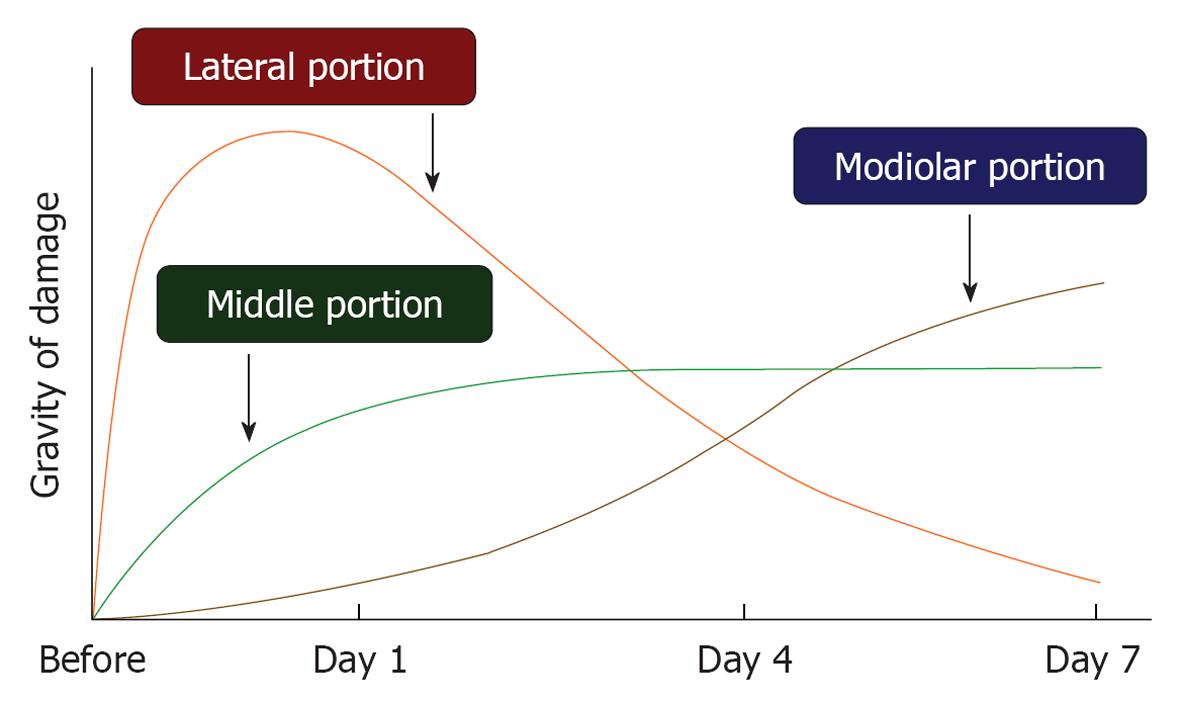
Figure 17 Sequential progression of ischemic damage in three regions of the cochlea.
In the lateral region, including the stria vascularis and spiral ligament, ischemic damage occurred immediately after the insult, which recovered gradually to be almost normal on day 7. In the middle region, loss of inner hair cells progressed for the first 2-3 d; thereafter, no further deterioration occurred. In the modiolar region, damage to the spiral ganglion cell was minor for a few days, but became prominent thereafter. The vertical scale indicates grade of damage.
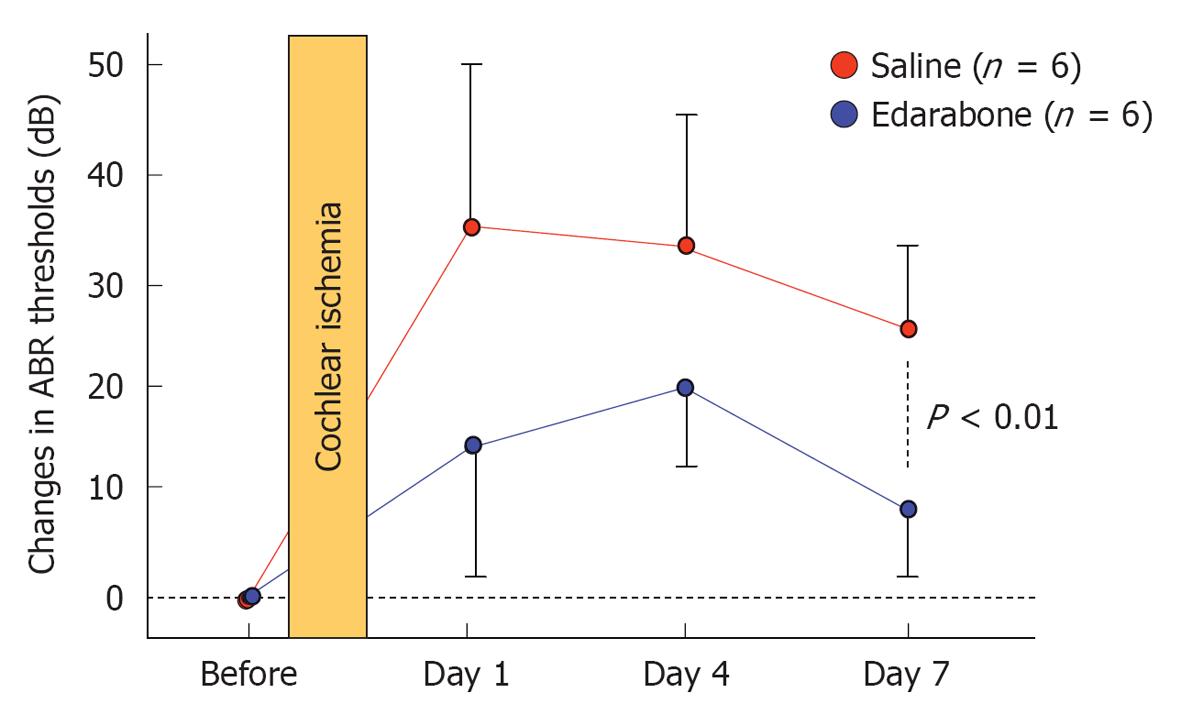
Figure 18 Edaravone ameliorates elevation of auditory brainstem responses threshold after ischemic insult.
Originated from[21], with permission.
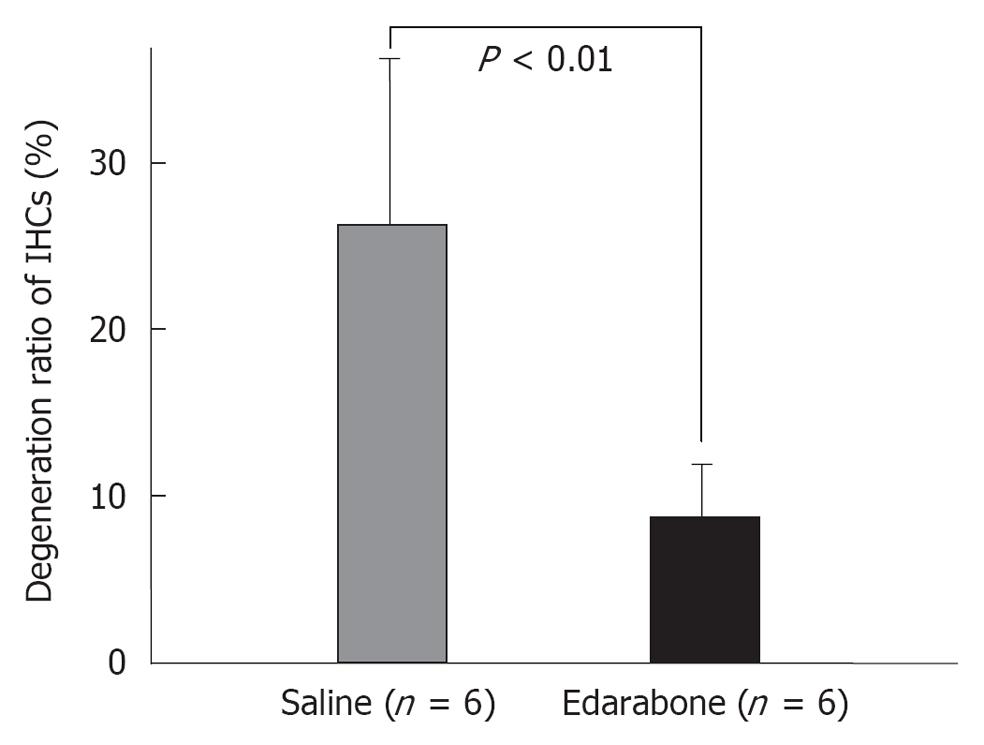
Figure 19 Edaravone prevents inner hair cell loss after ischemic insult.
IHCs: Inner hair cells. Originated from[21], with permission.
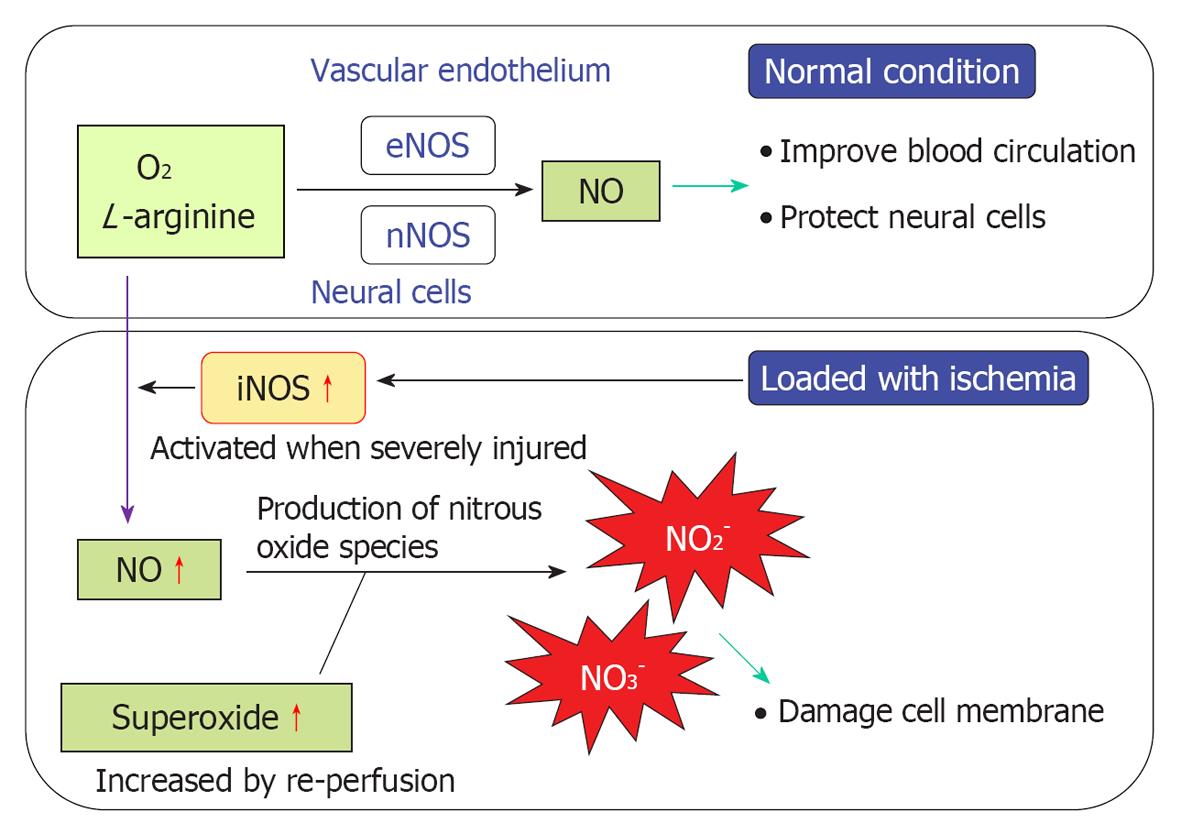
Figure 20 Mechanisms of nitrous oxide and free radical production.
In a normal environment, nitrous oxide (NO) not only improves blood circulation by dilating blood vessels, but also protects neural cells against ischemic damage. Production of NO is regulated by enzyme activities of endothelial NO synthase (eNOS) and neuronal NO synthase (nNOS). When transient ischemia occurs, production of NO is facilitated by the expression of inducible NO synthase (iNOS). As superoxide species increase in response to reperfusion, the excessive amounts of NO react with superoxide, resulting in the production of nitrite (NO2-) and peroxynitrite (NO3-). Free radicals are strong toxins that cause destruction of the cell membrane, resulting in cell death.
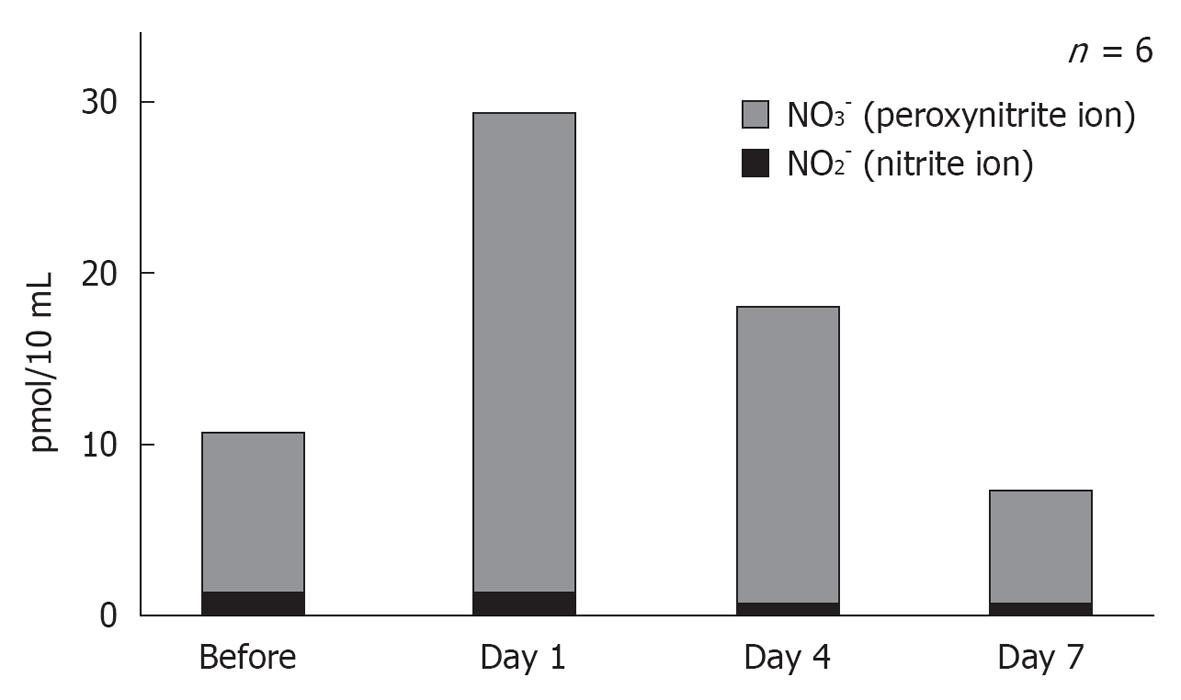
Figure 21 Concentration of nitride and peroxynitride in the scala tympani after ischemic insult.
The levels of nitrogen oxides (NO2-), especially peroxynitride (NO3-), increased significantly on day 1 after ischemia, and then decreased gradually thereafter. Originated from[17], with permission.
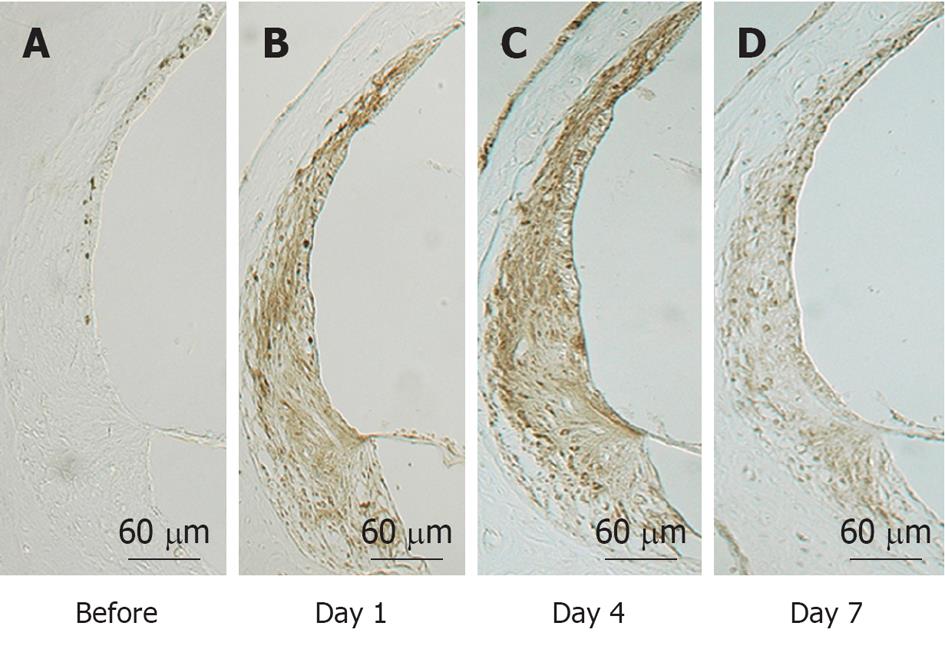
Figure 22 Immunostaining of the lateral wall of the cochlea for inducible nitrous oxide synthase before (A) and 1 (B), 4 (C), and 7 d (D) after ischemia.
The stria vascularis and the spiral ligament showed marked immunostaining of inducible nitrous oxide synthase on days 1 and 4, which decreased by day 7.
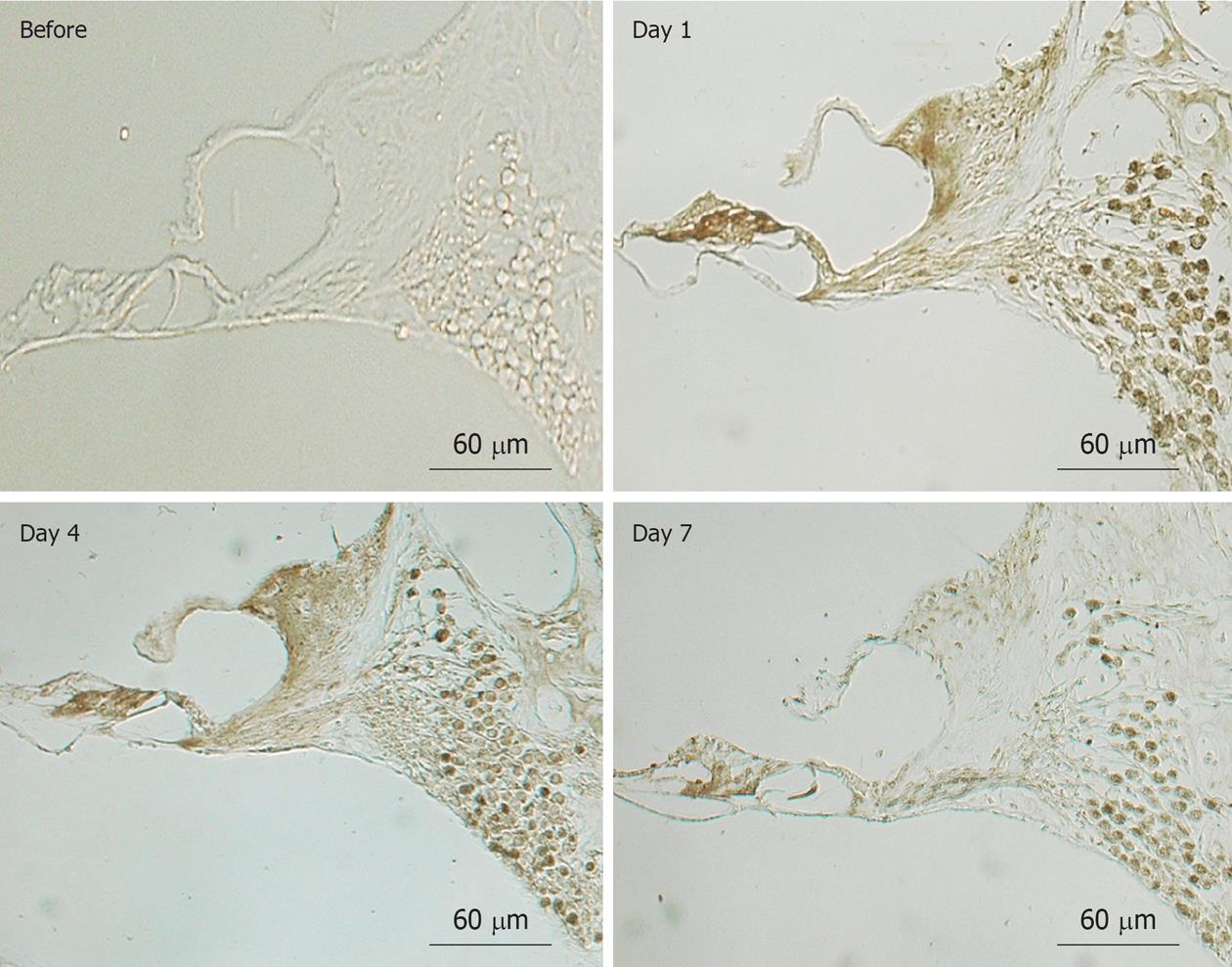
Figure 23 Immunostaining of the organ of Corti and the spiral ganglion for inducible nitrous oxide synthase before and 1, 4, and 7 d after ischemia.
Immunostaining for inducible nitrous oxide synthase was observed in the inner hair cells, spiral ganglion cells, and spiral limbus on days 1 and 4. It was obviously decreased on day 7, but the synaptic area underneath the outer hair cells and the spiral ganglion cells was still positively stained. Originated from[17], with permission.
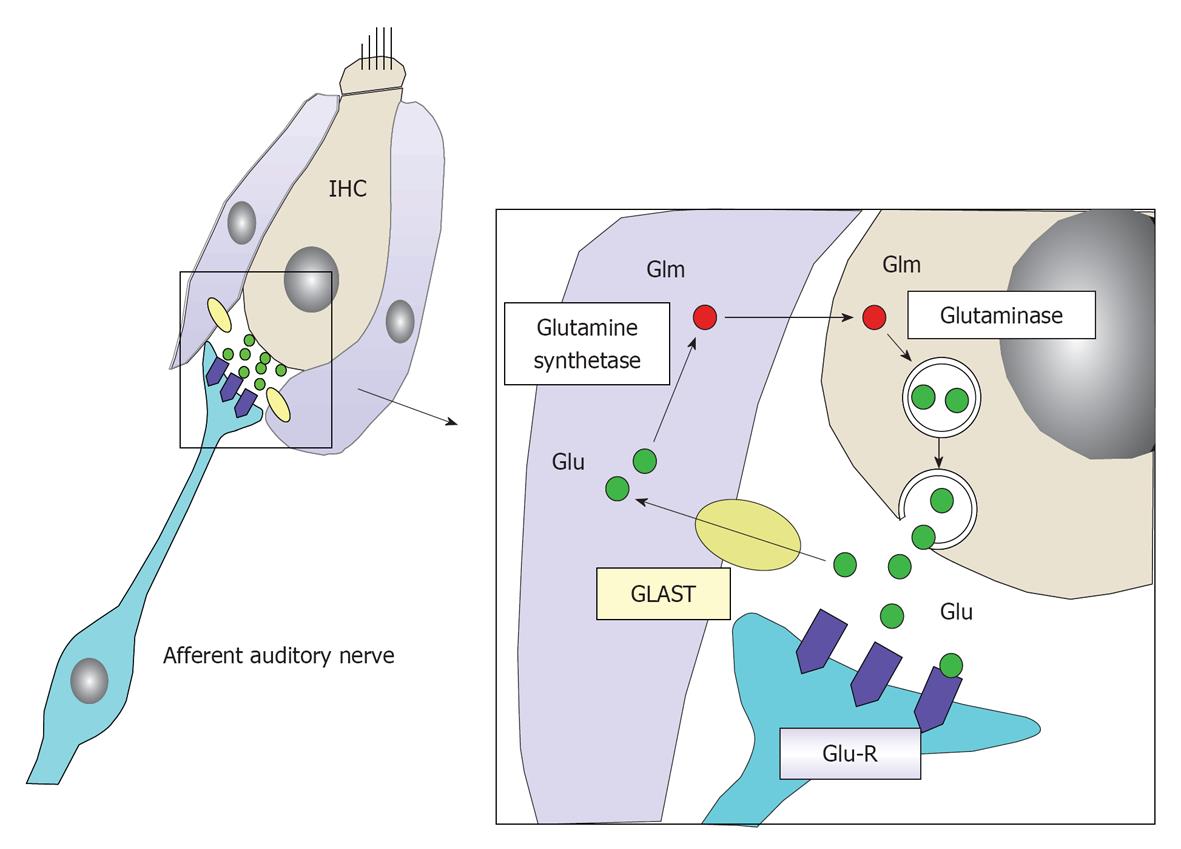
Figure 24 Schematic drawing of glutamate recycle system as a neurotransmitter between inner hair cells and the primary auditory neuron.
Glutamate is an excitatory neurotransmitter between inner hair cells (IHCs) and primary auditory neurons. It is released into the synaptic cleft in response to depolarization of IHCs. After stimulating a primary auditory neuron by binding to a glutamate receptor (Glu-R), it is absorbed by the surrounding supporting cells (inner phalangeal cell and inner pillar cells) by means of glutamate-aspartate transporter (GLAST). It is transformed into glutamine, and then transported to IHCs and stored in a vesicle until the next depolarization of the IHC. In this way, the glutamate is recycled.
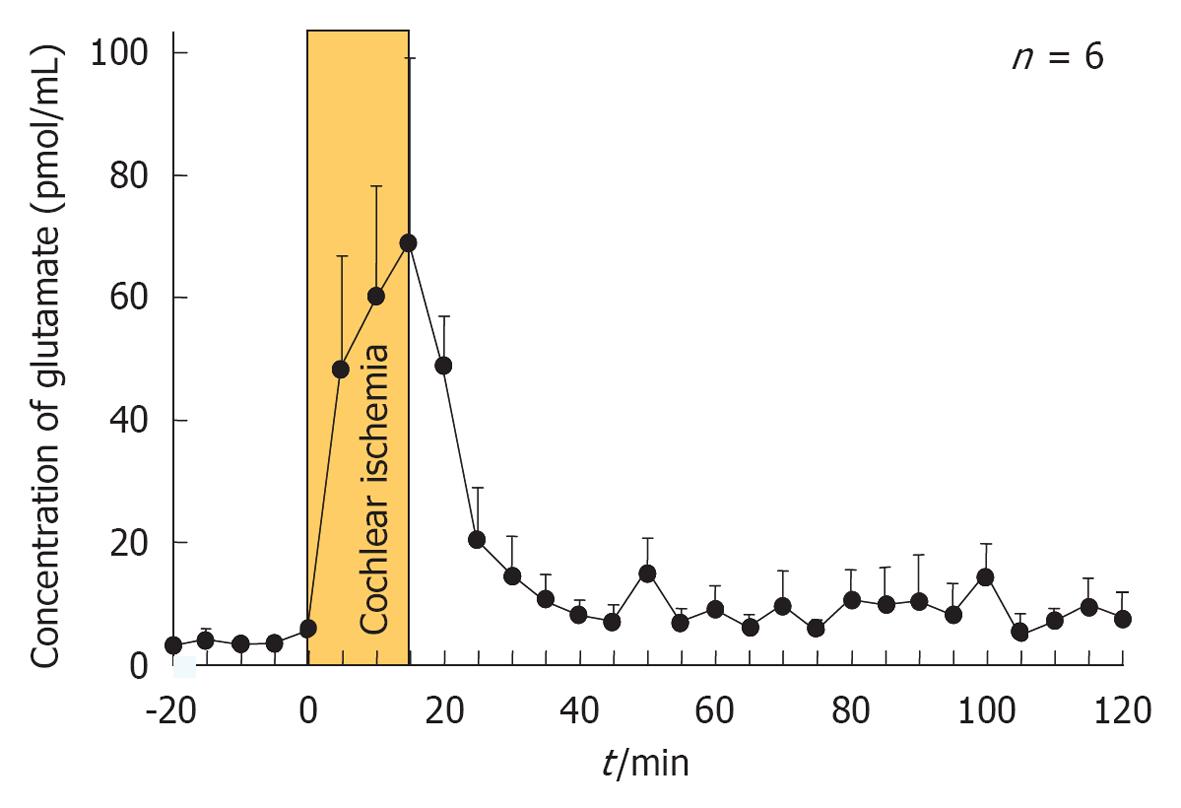
Figure 25 Glutamate concentration in the scala tympani after transient cochlear ischemia.
Originated from[30], with permission.
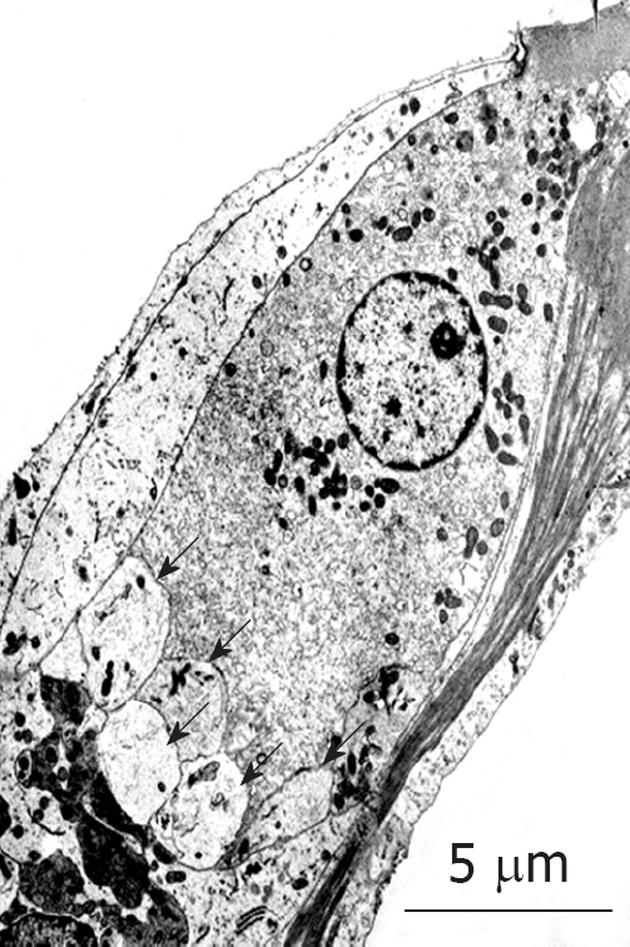
Figure 26 Transmission electron microscopy findings for inner hair cells after exposure to ischemia.
Many vesicles formed at the synaptic cleft (arrows). Originated from[30], with permission.
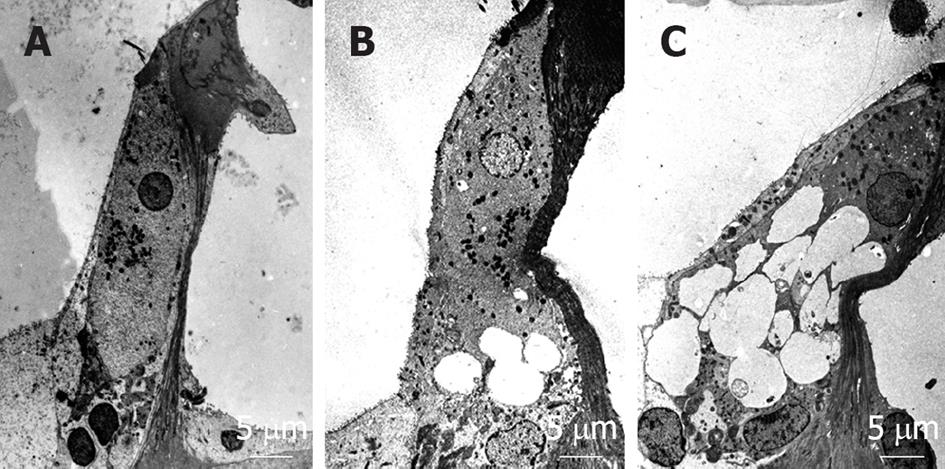
Figure 27 Dendritic terminals in synaptic contact with inner hair cells at the basal turn following administration of (A) artificial perilymph, (B) 50 mmol/L α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate, or (C) 200 mmol/L α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate.
Swelling of the dendritic terminals was not observed after injection of artificial perilymph. Although swelling of the dendritic terminals was observed at both concentrations of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), the extent of swelling was more pronounced with 200 μmol/L than with 50 μmol/L AMPA. Originated from[25], with permission.
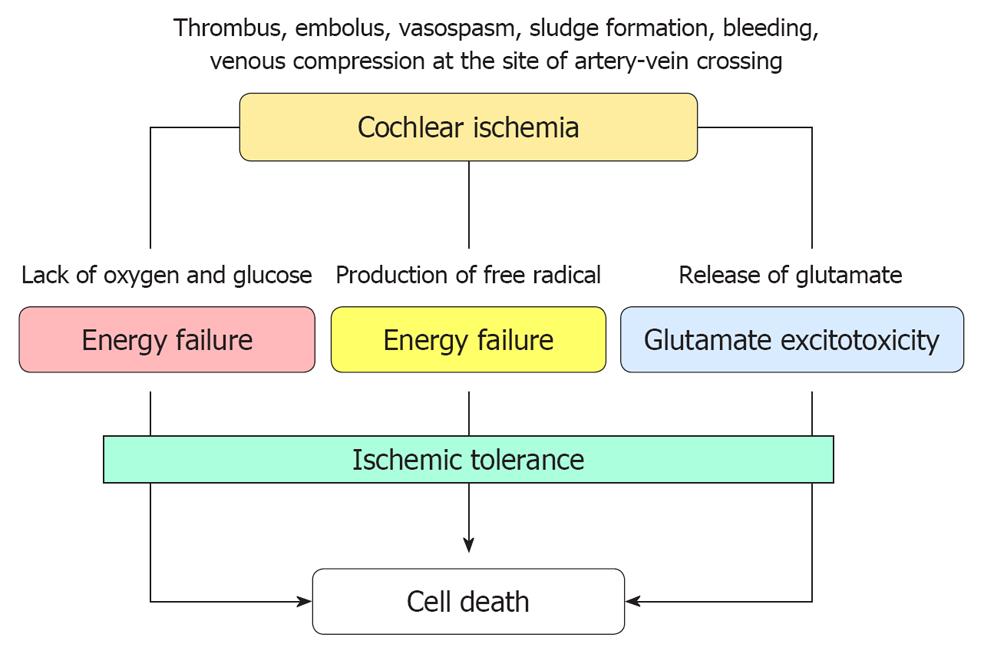
Figure 28 Suggested mechanism of cell death after transient cochlear ischemia.
Cell death is considered to result from energy failure, production of free radical species, and glutamate excitotoxicity. Cell death is prevented, to some extent, by the mechanism of ischemic tolerance.
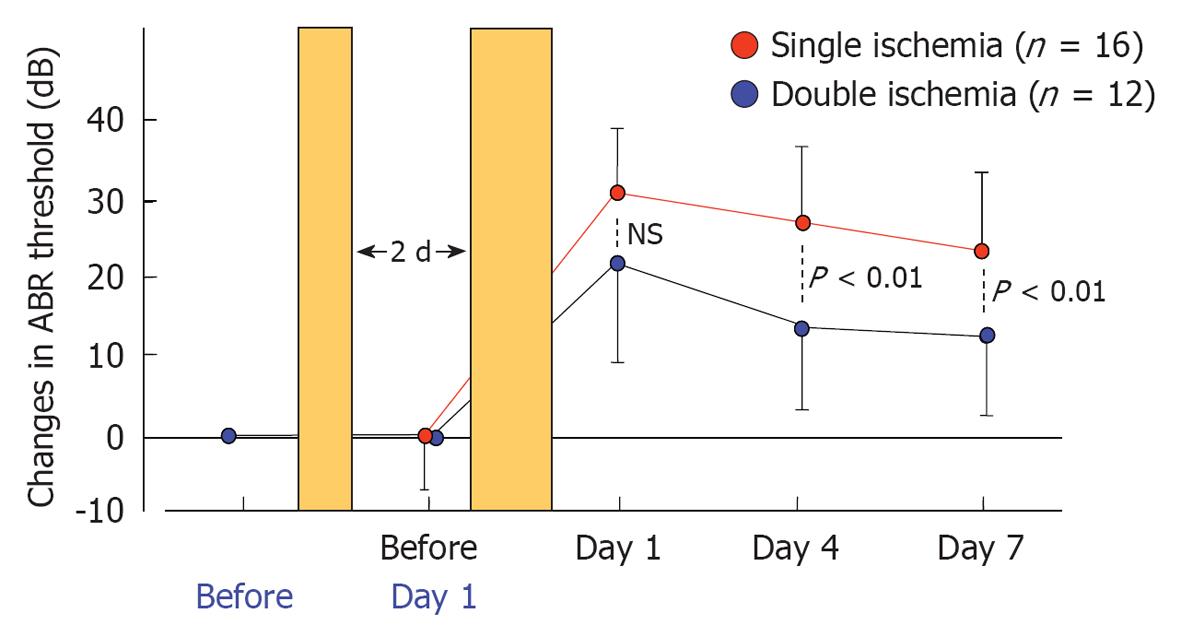
Figure 29 Auditory brainstem responses threshold shifts after single and double cochlear ischemia.
Temporal profiles of the shift in the mean auditory brainstem responses (ABR) threshold in the single ischemia group (n = 16) and the double ischemia group (n = 12). All values are presented as mean ± SD. Preconditioning sublethal ischemia for 2 min significantly suppressed the elevation of the ABR threshold with subsequent lethal ischemia for 15 min. Originated from[28], with permission.
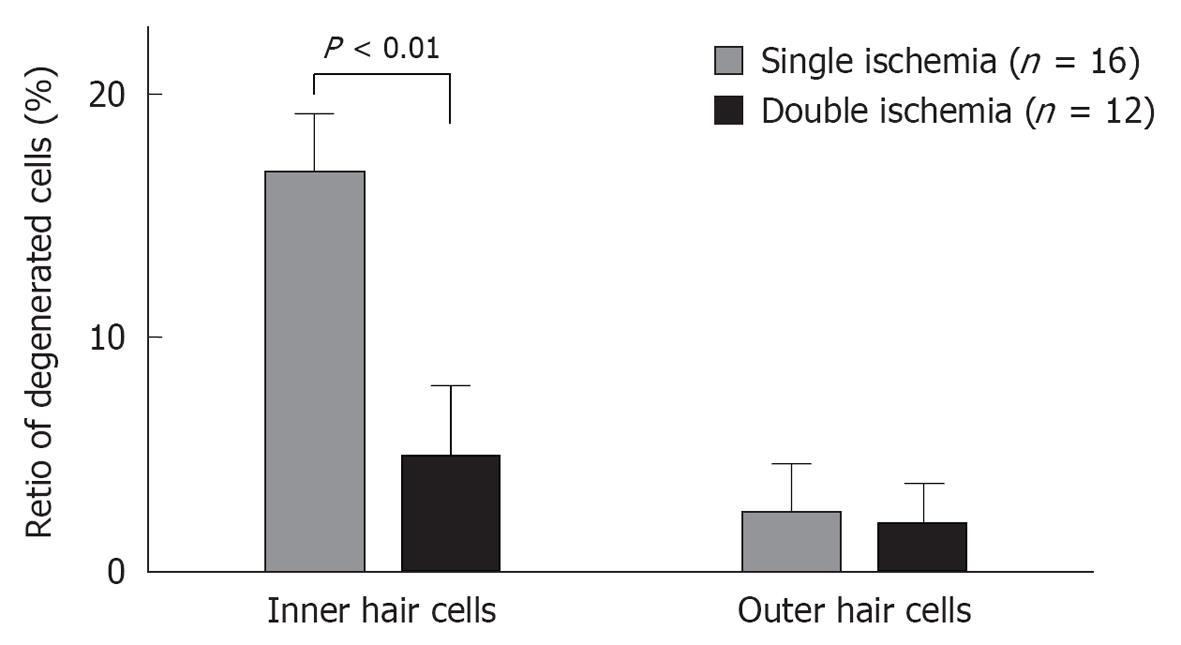
Figure 30 Loss of inner and outer hair cells after single and double cochlear ischemia.
Pretreatment with sublethal cochlear ischemia reduced inner hair cell (IHC) damage 7 d after lethal cochlear ischemia. In the double ischemia group (n = 12), the proregion of defects in IHCs was significantly lower than that in the single ischemia group (n = 16). On the other hand, there was no statistically significant difference in the amount of outer hair cell loss between the two groups.
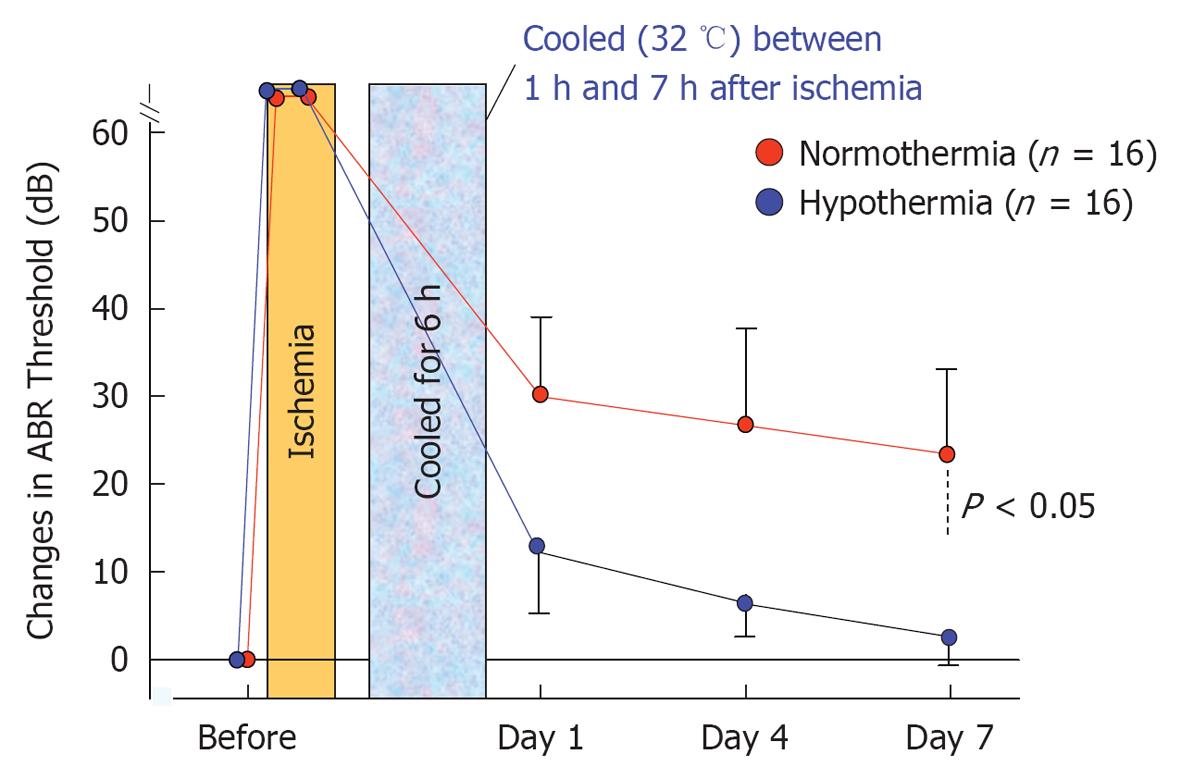
Figure 31 Sequential changes in auditory brainstem responses thresholds after ischemic insult in normothermic and mildly hypothermic animals.
Mild hypothermia (32 °C) from 1 to 7 h after ischemic insult prevented elevations in auditory brainstem responses threshold. The differences between the two groups were statistically significant (P < 0.05).
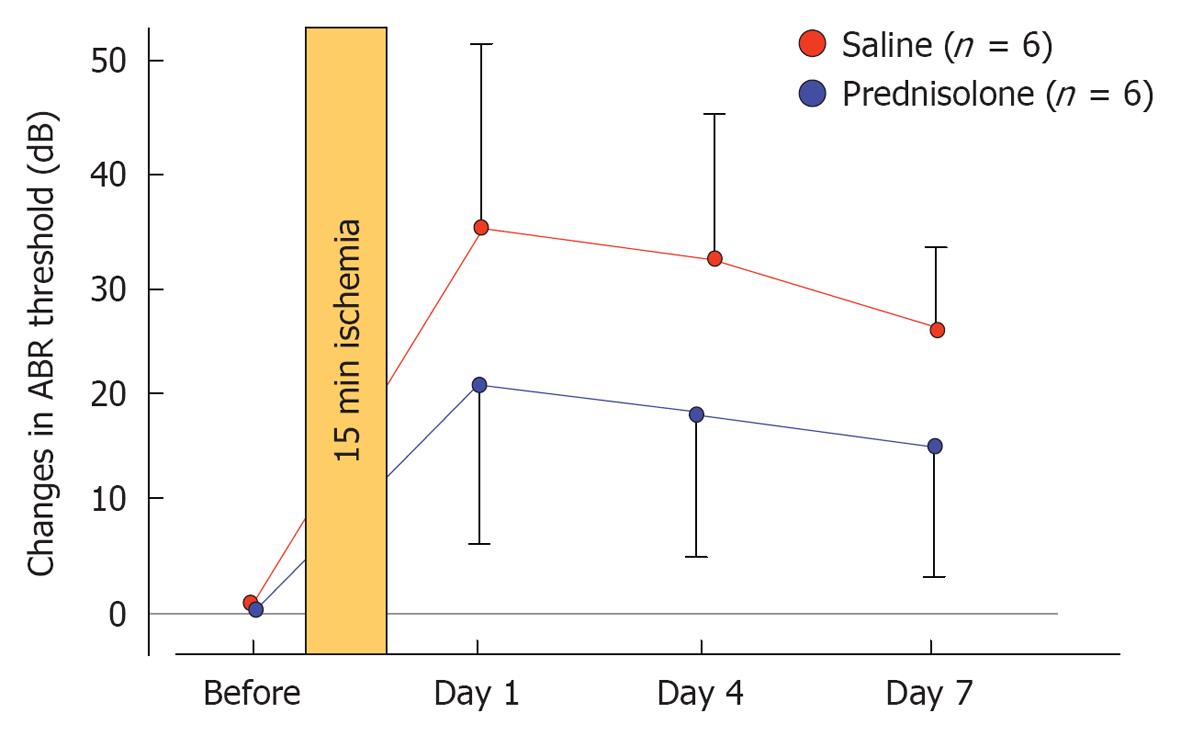
Figure 32 Effects of prednisolone on the shift of auditory brainstem responses threshold after transient cochlear ischemia.
Hearing was assessed before and 1, 4, and 7 d after ischemic injury. An increase in auditory brainstem responses threshold that was observed on day 7 was lower in the prednisolone-treated group than in the control group, although the difference was not statistically significant. Originated from[34], with permission.
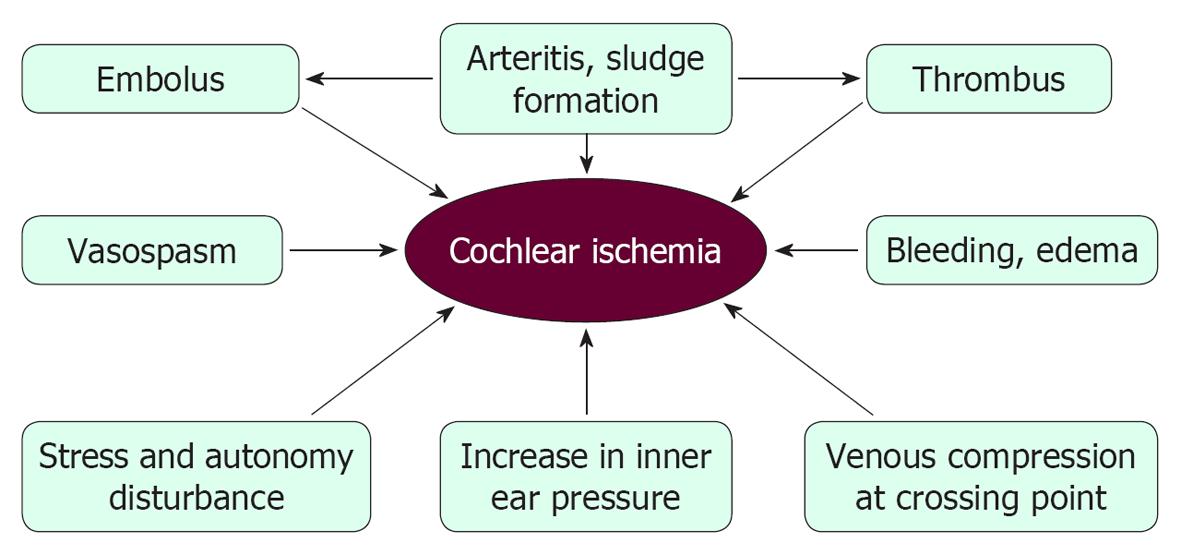
Figure 33 Possible mechanisms of cochlear ischemia that might result in idiopathic sudden sensorineural hearing loss.
- Citation: Gyo K. Experimental study of transient cochlear ischemia as a cause of sudden deafness. World J Otorhinolaryngol 2013; 3(1): 1-15
- URL: https://www.wjgnet.com/2218-6247/full/v3/i1/1.htm
- DOI: https://dx.doi.org/10.5319/wjo.v3.i1.1