Published online May 12, 2016. doi: 10.5318/wjo.v6.i2.10
Peer-review started: December 12, 2014
First decision: February 7, 2015
Revised: February 19, 2016
Accepted: April 14, 2016
Article in press: April 18, 2016
Published online: May 12, 2016
Processing time: 511 Days and 7.4 Hours
Imaging in ophthalmology is playing an increasingly important role not only in screening, but also in monitoring and assessing response to treatment in an objective manner. Technical advances in various modes of imaging acquisition provide more detailed images. These can be combined and reviewed on one screen in the place of acquisition or sent for a remote assessment. Moreover, the machines are more user-friendly, which reduces the need for highly skilled technicians. In this article the authors describe currently available and experimental ophthalmic imaging modalities and their impact on clinical practice.
Core tip: This article summarizes currently available and emerging imaging modalities in ophthalmology with respect to image acquisition, review options (i.e., software and file formats) and clinical application of the results.
- Citation: Saeed MU, Oleszczuk JD. Advances in retinal imaging modalities: Challenges and opportunities. World J Ophthalmol 2016; 6(2): 10-19
- URL: https://www.wjgnet.com/2218-6239/full/v6/i2/10.htm
- DOI: https://dx.doi.org/10.5318/wjo.v6.i2.10
Diseases of the posterior segment of the eye such as diabetic retinopathy, age-related macular degeneration and glaucoma are the most common causes of blindness worldwide. Modern imaging modalities have given us a better understanding of these conditions as well as many rare retinal and optic nerve diseases that were previously poorly understood. Numerous emerging techniques enable screening, monitoring and provide objective measures against which treatment effectiveness can be gauged.
Examination of the live human retina has evolved from an indirect ophthalmoscope, direct ophthalmoscope, then with a slit lamp and various lenses, through fundus fluorescein angiography (FFA), fundus photography, three-dimensional photographs, confocal scanning laser ophthalmoscope (cSLO) and optical coherent tomography (OCT) to new portable instruments capable of multimodal imaging. In this article the authors set out to summarize currently available and emerging techniques with respect to image acquisition, review and their clinical application.
Film based retinal photography has become almost completely obsolete. Conventional retinal photography is now undertaken with digital systems and portable file formats. Infrared focusing, autocues and non-mydriatic imaging systems with small pupil setting improve the image acquisition. Autofocusing systems significantly reduce the need for manual focusing, aperture and exposure settings. This minimizes the need for highly skilled retinal photographers and enables other health professionals such as nurses, health care assistants, ophthalmologists and dedicated retinal photographers to take adequate pictures of the retina and the posterior segment. Digital images can be acquired by a technician and electronically transferred to a review station via system networks for analysis and treatment decisions. The results are instantly ready for viewing and make more efficient use of physician’s time.
The use of cameras in telemedicine is now common, i.e., for the purposes of diabetic retinopathy screening services[1]. Examples include the NHS diabetic eye screening programme (DESP) based on approved retinal camera systems such as Topcon NW8, Zeiss Cirrus 600 and Optovue Icam. An updated and full list of the approved cameras can be found on the DESP website[2].
In addition, there are automated systems available on the market, such as the DRS imaging system that senses the patient’s eye, self-aligns, focuses on the pupil and the retina and takes a retinal image. It then moves on to take the retinal image of the other eye. The operator or technician input is therefore minimised significantly[3].
Conventional cameras with traditional retinal imaging suite are capable of capturing 30-50 degree view of the retina and provide an upright image at the review station. Depending on the camera, its filters and software the following modalities are available.
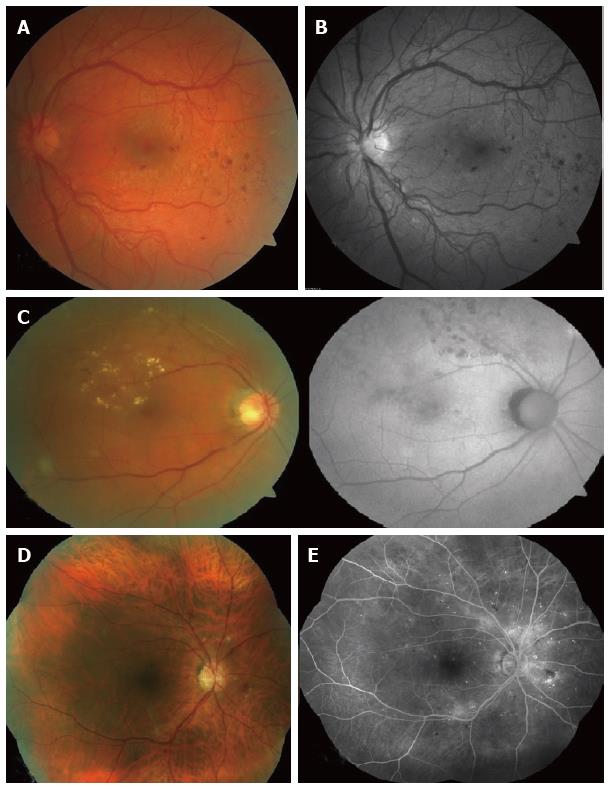
Colour fundus photos are taken with use of white light and usually require mydriasis. Non mydriatic cameras are also available. However, the authors experience is that the quality of image acquired without pupil dilatation is inferior to image with full pharmacologic pupillary dilation. The retinal vessels, optic disc, macula, vascular abnormalities, retinal pigment epithelium (RPE) changes and deposits can be depicted. It can be repeated on consecutive visits and thus allow an objective comparison of the retina and optic nerve appearance, which is particularly useful in diabetic retinopathy (Figure 1A) and glaucoma patients[4,5]. Colour fundus photography is also useful in the assessment of systemic conditions such as bacterial endocarditis, leukemia (Roth spots), hypertension (i.e., silver wiring), raised intracranial pressure (papilloedema), etc.[6-8].
Red-free photos can be obtained using a red-free filter to improve contrast of blood vessels and nerve fiber layer (NFL). This is useful in detecting NFL defects (glaucoma)[9]. Digital red-free photography may also have a higher level of detection ability for all retinal lesions of diabetic retinopathy in a diabetic screening setting (Figure 1B)[10].
Green-free retinal images are taken using green filters that allow visualization of retinal changes such as angioid streaks in pseudoxanthoma elasticum. However, the yield of clinically useful information is much less than that of colour photographs, red-free photos or autofluorescence imaging[11].
Fundus autofluorescence (FAF) is an underutilized mode of retinal imaging in clinical practice. This technique relies on the property of naturally occurring fluorescence in retinal tissue that can be physiological or pathological. The optic nerve, retinal blood vessels, and the fovea normally appear dark against a variable background of fluorescence from the RPE (Figure 1C). Alteration of physiological autofluorescence may be a manifestation of retinal disease. Examples of abnormal autofluorescence include optic nerve drusen, astrocytic hamartoma, and lipofuscin[12]. Loss of autofluorescence may be observed in macular conditions such as macular degeneration, geographic atrophy, macular telangiectasia, retinitis pigmentosa, central serous chorioretinopathy, macular dystrophies, and pseudoxanthoma elasticum[13,14]. Longitudinal FAF imaging allows monitoring of geographic atrophy progression. Increased deposition of lipofuscin occurs in Stargardt disease, adult onset vitelliform and Best’s disease[15].
Previously retinal images were limited by tedious software and difficulties with merging images acquired separately. The inclusion of software allowing auto-construction of a larger mosaic image of the retina (2-field, 3-field, 5-field or 7-field) was a major step ahead in retinal photography (Figure 1D and E). Data that is achieved in such a fashion may be far superior to current standards. For example, DESP pictures include a colour retinal photograph centered on the optic nerve and another retinal photograph centered on the macula. Reconstructed wide field images may yield more information about the retina, especially with the respect to retinal mid-periphery. This might be also useful in documenting pigmented lesions, i.e., choroidal naevi in the retinal periphery, etc.
Optometrists in the United Kingdom are increasingly using retinal imaging as a part of their routine examination in addition to refraction and fundoscopy. This has probably improved the detection of retinal pathologies and has led to increased referrals to the hospital eye services.
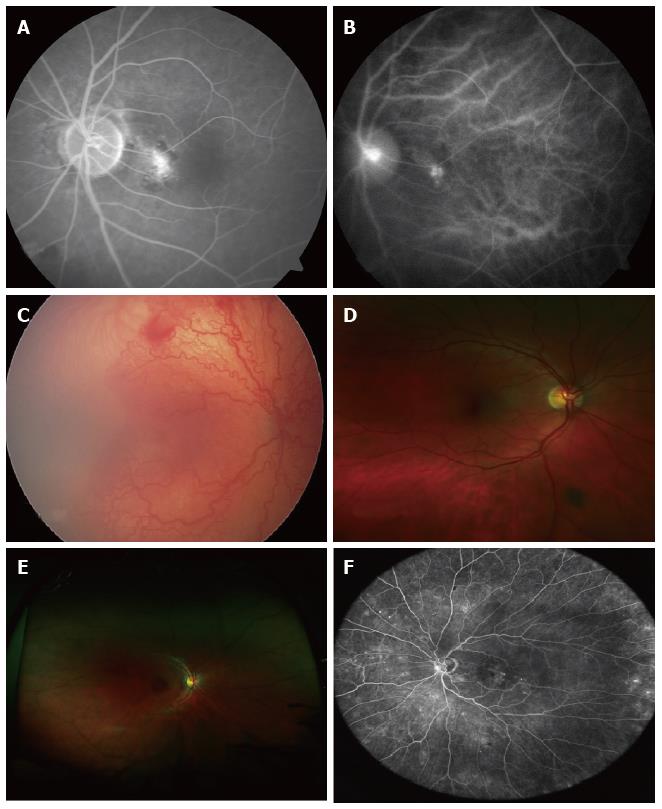
Angiography highlights the retinal vessels following intravenous injection of a fluorescent dye, as opposed to autofluorescence that does not require any dye. Sodium fluorescein angiography (FFA) is a widely used technique for retinal imaging (Figure 1E, Figure 2A), whereas indocyanine green (ICG) angiography is used to assess choroidal pathology (i.e., idiopathic polypoidal choroidal vasculopathy) (Figure 2B). Angiography can be performed using digital fundus camera or SLO. In some cases, FFA and ICG angiography may be performed simultaneously, for example in age-related macular degeneration (AMD) patients in order to exclude or diagnose idiopathic IPCV or other AMD variants. This may be a departmental or local policy depending upon disease prevalence.
Serial photographs taken against a digital timed counter show vascular perfusion. Images are interpreted in terms of hypo- and hyperfluorescence. In correlation with colour photographs as well as OCT they may reveal possible underlying mechanisms of the disease.
Subtle lateral displacement of the camera during colour retinal photography or angiography provides a stereo image of the retina when viewing two pictures together. This delivers a three-dimensional image seen by a special viewer. The observer may therefore appreciate any elevation or depression of tissues in the area of interest[16]. An example is optic nerve cupping or pigment epithelial detachment.
The cSLO can be used for several retinal imaging modalities including FFA, ICG and FAF. Monochromatic laser illumination combined with a confocal optical system produce high-contrast, finely detailed images. A laser beam scans across the fundus to illuminate the retina point-by-point. The light reflected from the retinal tissues is captured by a photomultiplier. The output of the photomultiplier is recorded and displayed in a digital video format. High acquisition speed and eye-tracking software enables image sampling of several frames to produce a mean image (digital reformatted version). The standard format of view varies by instrument. The standard cSLO displays red-free retinal images and does not convey images in a colour format. The field of view is usually 30°. Auxiliary lenses (15°, 20°, 35° and 55°) are available for cSLO systems such as Heidelberg Spectralis HRA. The Staurenghi contact lens provides an angle of 150° with the Heidelberg HRA. The wide-angle lens is primarily used for peripheral imaging in either diabetic retinopathy or venous occlusive disease. This may be used as a red free ultra-wide image or in conjunction with fluorescein or indocyanine green angiography[17]. Obtained pictures are of excellent quality and provide good insight into the retinal periphery. However this modality involves a skilled retinal photographer, one assistant and another person for intravenous dye injection.
Wide field contact imaging systems such as RetCam by Clarity Medical Systems, CA, United States are capable of capturing retinal images in colour mode as well as fluorescein angiography mode. This mode of photography requires topical anaesthesia, a coupling agent (clear gel) and a speculum. It is extensively used in paediatric ophthalmology for management of retinopathy of prematurity[18] (Figure 2C) and as an objective documentation of other conditions, such as retinal haemorrhages in non-accidental injuries. The latest version, RetCam3 can record short (up to 2 min) high-resolution videos of retinal status. Pictures can be saved in EMR compatible files (i.e., DICOM format).
Wide and ultra-wide field imaging systems utilize SLO technology combined with an ellipsoidal mirror. These are capable of capturing up to 200° of the retina in a single image[19]. It has been shown that changes in peripheral retina can be related with central retinal abnormalities[20]. This makes wide and ultra-wide field imaging particularly useful in detection and classification of diabetic retinopathy, especially when it comes to peripheral retinal changes[21,22] as well as in cases of retinopathy of prematurity (ROP)[23,24].
The Optos Optomap® is one of the retinal imaging instruments with capabilities of capturing an ultra-wide field format of 200° (Figure 2D). The machine is bulky and therefore difficult to move around in the clinic. However it is easy to use and has been utilized in adults, children and babies. It has been successfully utilized for ROP screening in prematurely-born infants[22-24]. Optos images can be used to guide laser treatment in patients with proliferative diabetic retinopathy[25]. The newer models (i.e., Optomap Daytona and California) are more compact.
The Optomap 200Tx is a device based on the cSLO principle. The image acquisition is based on red, green and blue scanning lasers and the picture of the retina is a colour-coded, digital reconstruction of the image acquired from these sources. The final image has different colours and hues when compared to retinal pictures taken on other conventional systems (Figure 2E, note eyelash artefact).
Heidelberg SPECTRALIS and HRA2 have detachable non-contact lenses which enable peripheral ultra-widefield imaging. These can capture images in colour, red-free, fluorescein and indocyanine green angiogram mode (Figure 2F).
Both Heidelberg HRA and Optomap systems have useful options, such as multicolour imaging obtained by digital subtraction techniques. Not only various layers of the retina, but also blood vessels, drusen, haemorrhages can be depicted and enhanced using different reflectance properties of the retina and choroid with blue, green and infra-red laser with the cSLO.
The Nidek F-10 utilizes a similar technology to produce colour, blue, green, infra-red, autofluorescence, fluorescein and indocyanine indocyanine green angiograms. The images are 40°-60° field of view.
Handheld retinal imaging devices like Versacam (Nidek) are useful in detecting retinal disorders, particularly when trying to obtain retinal photographs in children, bed bound adults or patients who may find slitlamp-based instruments difficult to cope with, for example those with ankylosing spondylitis or spine problems. The quality of the image obtained may be variable and is usually dependent on the cooperation of the patient, skill of the photographer, pupillary dilation and ocular media clarity. The photographs then usually need to be downloaded onto a more permanent media.
OCT provides and in vivo, cross-sectional image of ocular tissues. It relies on low coherence interferometry. High resolution of the OCT scans provides fine details of posterior segment, from the vitreo-retinal interface, through a cross-section of all the layers of the retina to the RPE-choroid complex. It allows establishing a diagnosis of vitreo-macular traction, epiretinal membranes, macular pseudoholes, lamellar and full-thickness holes, as well as retinal edema, RPE changes, PED detachments, etc. Repeatability of measurements enables quantitative analysis of disease progression and/or response to treatment. Rapid scanning of patient suspected of wet macular degeneration can be carried out without the need of invasive retinal imaging techniques like FFA. Therapeutic decisions can be based on the OCT scans in wet macular degeneration, diabetic maculopathy, macular edema, central serous chorioretinopathy and many others. It is particularly useful in conjunction with FFA.
There are different techniques available to acquire an OCT scan as described below.
Time-domain OCT: Time-domain OCT, where the reference mirror is moved mechanically to different positions, resulting in different flight time delays for the reference arm light[26]. Due to a very limited speed at which the mirror can be moved, data is acquired at approximately 400 axial scans per second and the resolution is up to 8-10 nm.
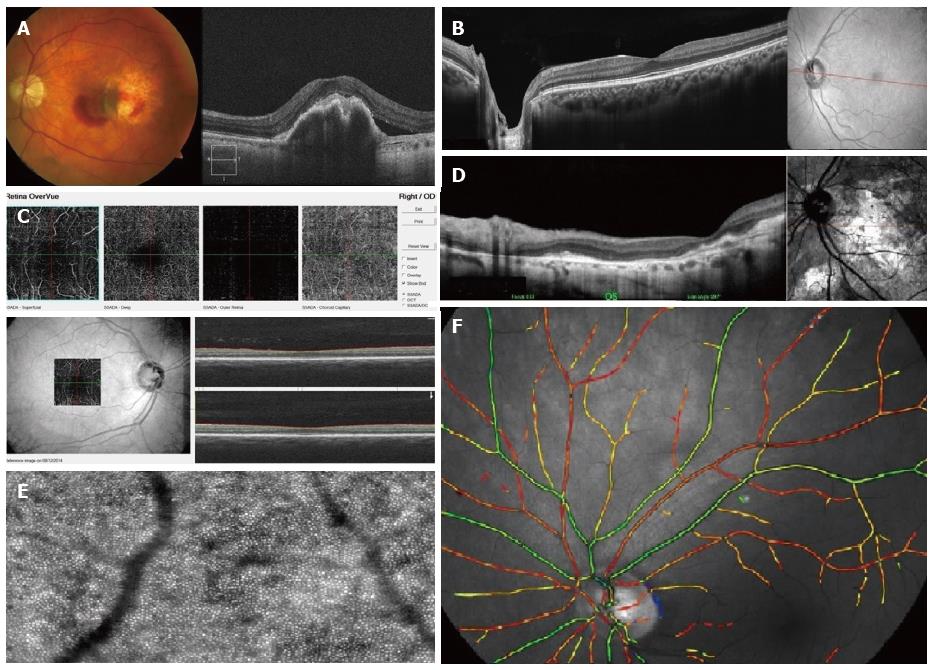
Spectral-domain OCT: Spectral-domain OCT (SD-OCT) uses much faster technology. It simultaneously measures multiple wavelengths of reflected light across a spectrum. This principle is also known as Spectral or Fourier domain and is 100 times faster than Time-domain. It acquires 40000 A-scans per second. Increased speed means faster acquisition of scans and a higher number of scans give a higher resolution (down to 6 nm) (Figure 3A).
Swept-source OCT: The scan speed in swept-source instruments is approximately 100000 A-scans per second. It also allows wide-field B-scans (12 mm vs 6-9 mm with conventional SD-OCT). Examples include Optovue RTVue-100 (Figure 3B) and Topcon DRI-OCT1. Wide scans make it possible to present the optic nerve and macula on the same scan. Simultaneous high-quality visualization of the vitreous, retina, and choroid is also possible because of wavelengths of 1000 to 1300 nm with higher penetration, as opposed to spectral domain OCTs using approximately 850 nm wavelength sources. This is also known as enhanced depth imaging/full depth imaging[27-29]. Clinical uses include, e.g., measurement of choroidal thickness in CSR and uveitis cases involving choroid, such as Vogt-Koyanagi-Harada, apart from the standard spectrum of diseases imaged with a spectral domain OCT. Another newly introduced capability is enhanced vitreous interface which can capture bursa premacularis, and other vitreous structures, i.e., Bermeister’s papilla, papillomacular hyaloid detachment, opacities within the vitreous[30].
Certain swept source OCTs can acquire images of retinal and choroidal blood vessels at various levels, examples include OptoVue (Figure 3C). This examination is non-invasive and does not require administration of any dye.
cSLO OCT: cSLO uses laser light instead of a white light to illuminate the retina. It scans the retina point by point by a focused laser beam and then captures the reflected light through a small aperture (confocal pinhole). The OCTs obtained by this method has a high resolution of up to 3 nm. Skilled operator input and longer time of acquisition is required though. Examples include the Heidelberg Spectralis and Optomap SLO (Figure 3D).
Intraoperative OCT: Intraoperative OCT captures live OCTs of both anterior and posterior segment. This can be particularly useful in epiretinal membrane peeling or partial thickness corneal grafts.
OCT angiography: It is the newest technology in retinal imaging. It is based on motion contrast generated by the blood flow. The advantage of OCT angiography over FFA is that no dye is required since the scans pick up the motion of the erythrocytes. It is thus a quick, safe and non-invasive method depicting the structure of blood vessels, particularly capillaries and providing volumetric data on the blood flow. Current limitations include a relatively small field of view and inability to show leakage[31].
The OCTs obtained using different techniques are not interchangeable. The comparisons between Time domain and Spectral domain OCTs show better measurement repeatability in favour of spectral domain technique[32,33]. The image quality also varies, time domain images having lower resolution[34]. The ease of acquisition of the OCT is also different from machine to machine with some instruments requiring negligible operator input whilst others requiring significant time and skill. Generally the quality and resolution of the OCT scans is the lowest with time domain OCT, improves with spectral domain OCT scans and is the highest with swept source OCT scans and cSLO scans.
Heidelberg Spectralis introduced a glaucoma module based on anatomic positioning system that uses the center of the fovea and the center of the Bruch’s membrane opening as reference points to perform the optic nerve head analysis. There are other OCTs such as Cirrus 5000 from Zeiss that also have incorporate glaucoma modules. They produce colour-coded maps of the optic nerve head as well as the retinal NFL (RNFL) thickness in all four quadrants. Serial imaging can reveal subtle differences in the glaucoma status.
Adaptive optics SLO: Adaptive optics SLO uses mechanically activated mirrors to correct the wavefront aberrations of the light reflected from the retina and thus has allowed individual photoreceptors to be imaged in vivo[35] (Figure 3E). This can be of use in certain retinopathies to provide quantitative measures of cone physiology, detection of microaneurysms and small vessel blood flow profiling and to capture nerve fiber layer defects in glaucomatous patients[36].
Scanning laser polarimetry is performed using SLO with an integrated polarimeter (GDx-VCC). It provides computer-based, real-time imaging of the peripapillary RNFL, particularly useful in glaucoma patients[37].
Hyperspectral imaging, oxymetry is a technique where multispectral reflectance is used to estimate the concentration of oxygenated and deoxygenated hemoglobin in the retinal tissue based on the fact that deoxygenated hemoglobin reflects longer wavelengths better than does oxygenated hemoglobin[38]. This is usually used in research settings (Figure 3F).
Multimodality imaging is becoming increasingly common in ophthalmology. However, for image information from multiple modalities to be usable in mutual context, images must be registered so that the independent information can be obtained and presented. Significant potential for clinical application of these imaging systems is reflected by numerous research projects that are being undertaken[39]. One example of such instrument is a swept source OCT utilizing vertical-cavity surface emitting laser technology that allows in vivo high speed retinal, anterior segment and full eye imaging. Operating modes of the device include: ultrahigh speed, high resolution retinal imaging (up to 580 kHz); high speed, long depth range anterior segment imaging (100 kHz) and ultralong range full eye imaging (50 kHz). High speed imaging enables wide-field retinal scanning, while increased light penetration at 1060 nm enables visualization of choroidal vasculature. Comprehensive volumetric data sets of the anterior segment from the cornea to posterior crystalline lens surface including axial eye length can be obtained.
Handheld SD-OCTs are commercially available (Bioptigen Envisu, Optovue iVue). They are of use in children, bedbound and wheelchair bound patients, intraoperatively as well as in developing countries[40].
Prototypes of ultra-high speed, handheld swept source optical coherence tomography are being developed. This technology should enable screening applications to identify early retinal disease, before irreversible vision impairment or loss occurs. Handheld OCT technology also promises to enable applications in a wide range of settings outside of the traditional ophthalmology or optometry clinics[41-43].
Different types of imaging devices enable various objective measurements, such as retinal thickness, optic disc diameters, drainage angle assessment, corneal thickness and curvature readings, axial length and IOL calculations (biometry). Vast majority of manufacturers have their own proprietary software to display raw images, scans, photographs, graphs and analyses in a given format. Examples include ibase (Topcon) and Visucam (Zeiss). It is also possible to export all the data to an archive database which can store images from various sources. Examples of such software include Forum (Zeiss), Synergy (Topcon) and Merge eye care PACS (merge healthcare systems). One database significantly simplifies the clinical data review process and improves efficiency by reducing time required to access every single examination performed on a different machine. Unfortunately, this is done at the expense of image quality, which is generally inferior to the display on manufacturer’s software.
Images can be presented in formats as diverse as .pdf, .JPEG, .Bit, .RAW, .Dicom, or .dcm to name a few. Currently no consensus exists between manufacturers regarding a uniform format. The data output is not standardized, which may become an issue if data from various platforms are imported into systems with non-compatible software. This makes comparison of data difficult when considering data acquired by different instruments (i.e., Zeiss OCT vs Topcon OCT).
Patient information including demographic data is usually stored in a database and the actual image is saved as a separate file which is then linked to the database by an internal code. If the link is broken, the patient data and imaging will become disconnected and thus untraceable. Data loss in this set up may become irrecoverable if both database and images are not separately backed up on secondary locations like servers or secondary disc drives. For this reason there appears to be a drift towards a DICOM file format, in which patient information is embedded within the image, enabling patient identification with Dicom imaging software.
All imaging should be secured and saved in a format which is readily available to clinicians. Many models of data sharing exist including secure server based intranet systems to internet cloud based versions. However, most clinical providers use protected systems (secure closed intranet systems) due to requirements of data protection and patient confidentiality. Software licensing of image reviewing systems has become a part of standard hard ware acquisition plans and deals offered by manufacturers.
Although slit-lamp examination remains a mainstay of clinical assessment, many conditions are diagnosed and treated on the basis of the imaging results. Examples include age-related macular degeneration, diabetic retinopathy and various forms of secondary choroidal neovascularization. For example in the United Kingdom, NICE guidelines for anti-VEGF treatment make it mandatory to assess central retinal thickness with OCT prior to application for diabetic macular edema therapy.
Retinal imaging has transformed the ophthalmic consultation. In many clinics, a retinal photograph and an OCT is standard practice. This may be performed before the patient sees the clinician. The consultation then proceeds with a history taking, examination and review of imaging before treatment decisions are made. This is a major change to a state where diagnosis and treatment planning is heavily reliant on imaging. Moreover, ophthalmic conditions can now be monitored in remote locations by having appropriate imaging done by technicians and the results being viewed by a physician. Examples include the English National Diabetic screening program with regular ophthalmic imaging of every diabetic patient on a yearly basis by a photographer and then screened by a trained grader. The results are conveyed to the patients by mail. The patient is only referred to an ophthalmologist whenever thresholds for referral are reached.
A compact, affordable instrument capable of multimodal imaging would be an ideal clinical aid. Even though there is a wide range of fine machines on the market, there are still hospitals and practitioners who cannot afford them.
Ease of use, capability to capture high quality retinal photographs with peripheral imaging and OCT should be present in every clinical system of the future. Transferability of images between multiple clinical systems and electronic patient records should be established as an international standard. Some manufacturers are already moving towards a DICOM format which allows embedded patient data in an image file.
A wide variety of new instruments allowing detailed examination of the retina has taken medical imaging to a whole different level. Many conditions that previously were not fully understood became more obvious when investigated with FFA, ICG and OCT, for example macular hole aetiology and vitreoretinal interface.
The role of imaging in ophthalmology has been recognized and well-established. Recent years have brought an enormous development in the image quality, speed and simplicity of image acquisition as well as the options to view, combine and compare the results from different machines. An increasing role of multi-modal imaging and telemedicine appears to be the future.
P- Reviewer: Issa SA, Nowak MS S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Williams GA, Scott IU, Haller JA, Maguire AM, Marcus D, McDonald HR. Single-field fundus photography for diabetic retinopathy screening: a report by the American Academy of Ophthalmology. Ophthalmology. 2004;111:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Public Health England. Diabetic eye screening: Commission and provide. Equipment guidance. Population screening programmes collection. Available from: https://www.gov.uk/government/collections/diabetic-eye-screening-commission-and-provide#des-equipment-guidance. |
| 3. | Williams GA; CenterVue. Digital Retinography System. Available from: http://centervue.com/articms/admin/upAllegati/932/1419261069.pdf. |
| 4. | Armaly MF. Optic cup in normal and glaucomatous eyes. Invest Ophthalmol. 1970;9:425-429. [PubMed] |
| 5. | Kinyoun JL, Martin DC, Fujimoto WY, Leonetti DL. Ophthalmoscopy versus fundus photographs for detecting and grading diabetic retinopathy. Invest Ophthalmol Vis Sci. 1992;33:1888-1893. [PubMed] |
| 6. | Patton N, Aslam TM, MacGillivray T, Deary IJ, Dhillon B, Eikelboom RH, Yogesan K, Constable IJ. Retinal image analysis: concepts, applications and potential. Prog Retin Eye Res. 2006;25:99-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 262] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 7. | Cheung N, Liew G, Lindley RI, Liu EY, Wang JJ, Hand P, Baker M, Mitchell P, Wong TY. Retinal fractals and acute lacunar stroke. Ann Neurol. 2010;68:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Wong TY, Cheung N, Islam FM, Klein R, Criqui MH, Cotch MF, Carr JJ, Klein BE, Sharrett AR. Relation of retinopathy to coronary artery calcification: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2008;167:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Kimura Y, Hangai M, Morooka S, Takayama K, Nakano N, Nukada M, Ikeda HO, Akagi T, Yoshimura N. Retinal nerve fiber layer defects in highly myopic eyes with early glaucoma. Invest Ophthalmol Vis Sci. 2012;53:6472-6478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Venkatesh P, Sharma R, Vashist N, Vohra R, Garg S. Detection of retinal lesions in diabetic retinopathy: comparative evaluation of 7-field digital color photography versus red-free photography. Int Ophthalmol. 2015;35:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Trélohan A, Martin L, Milea D, Bonicel P, Ebran JM. Retinal lesions in pseudoxanthoma elasticum: 51 patients. J Fr Ophtalmol. 2011;34:456-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Gili P, Flores-Rodríguez P, Yangüela J, Herreros Fernández ML. Using autofluorescence to detect optic nerve head drusen in children. J AAPOS. 2013;17:568-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Bird AC, Phillips RL, Hageman GS. Geographic atrophy: a histopathological assessment. JAMA Ophthalmol. 2014;132:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Balaskas K, Leung I, Sallo FB, Clemons TE, Bird AC, Peto T. Associations between autofluorescence abnormalities and visual acuity in idiopathic macular telangiectasia type 2: MacTel project report number 5. Retina. 2014;34:1630-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Bennett TJ. Fundus Autofluorescence. Ophthalmic Photographers’ Society. Available from: http://www.opsweb.org/page=Autofluorescence. |
| 16. | Bursell SE, Cavallerano JD, Cavallerano AA, Clermont AC, Birkmire-Peters D, Aiello LP, Aiello LM. Stereo nonmydriatic digital-video color retinal imaging compared with Early Treatment Diabetic Retinopathy Study seven standard field 35-mm stereo color photos for determining level of diabetic retinopathy. Ophthalmology. 2001;108:572-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Staurenghi G, Viola F, Mainster MA, Graham RD, Harrington PG. Scanning laser ophthalmoscopy and angiography with a wide-field contact lens system. Arch Ophthalmol. 2005;123:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Chiang MF, Melia M, Buffenn AN, Lambert SR, Recchia FM, Simpson JL, Yang MB. Detection of clinically significant retinopathy of prematurity using wide-angle digital retinal photography: a report by the American Academy of Ophthalmology. Ophthalmology. 2012;119:1272-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Soliman AZ, Silva PS, Aiello LP, Sun JK. Ultra-wide field retinal imaging in detection, classification, and management of diabetic retinopathy. Semin Ophthalmol. 2012;27:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Kernt M, Kampik A. Imaging of the peripheral retina. Oman J Ophthalmol. 2013;6:S32-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Neubauer AS, Kernt M, Haritoglou C, Priglinger SG, Kampik A, Ulbig MW. Nonmydriatic screening for diabetic retinopathy by ultra-widefield scanning laser ophthalmoscopy (Optomap). Graefes Arch Clin Exp Ophthalmol. 2008;246:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Kernt M, Hadi I, Pinter F, Seidensticker F, Hirneiss C, Haritoglou C, Kampik A, Ulbig MW, Neubauer AS. Assessment of diabetic retinopathy using nonmydriatic ultra-widefield scanning laser ophthalmoscopy (Optomap) compared with ETDRS 7-field stereo photography. Diabetes Care. 2012;35:2459-2463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 23. | Fung TH, Muqit MM, Mordant DJ, Smith LM, Patel CK. Noncontact high-resolution ultra-wide-field oral fluorescein angiography in premature infants with retinopathy of prematurity. JAMA Ophthalmol. 2014;132:108-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Theodoropoulou S, Ainsworth S, Blaikie A. Ultra-wide field imaging of retinopathy of prematurity (ROP) using Optomap-200TX. BMJ Case Rep. 2013;2013:pii: bcr2013200734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Muqit MM, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, Turner GS, Stanga PE. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol. 2013;91:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA. Optical coherence tomography. Science. 1991;254:1178-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9722] [Cited by in RCA: 7002] [Article Influence: 205.9] [Reference Citation Analysis (0)] |
| 27. | Wong IY, Koizumi H, Lai WW. Enhanced depth imaging optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2011;42 Suppl:S75-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Celik N, Pollithy S, Dithmar S. Full depth imaging: a new imaging technique using optical coherence tomography (OCT). Klin Monbl Augenheilkd. 2014;231:540-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | de Bruin DM, Burnes DL, Loewenstein J, Chen Y, Chang S, Chen TC, Esmaili DD, de Boer JF. In vivo three-dimensional imaging of neovascular age-related macular degeneration using optical frequency domain imaging at 1050 nm. Invest Ophthalmol Vis Sci. 2008;49:4545-4552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Liu JJ, Witkin AJ, Adhi M, Grulkowski I, Kraus MF, Dhalla AH, Lu CD, Hornegger J, Duker JS, Fujimoto JG. Enhanced vitreous imaging in healthy eyes using swept source optical coherence tomography. PLoS One. 2014;9:e102950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | De Carlo TE, Romano A, Waheed NK, Duker JS. Review of optical coherence tomography angiography. Int Journal of Retina and Vitreous. 2015;1:5. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 537] [Cited by in RCA: 703] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 32. | Leung CK, Chiu V, Weinreb RN, Liu S, Ye C, Yu M, Cheung CY, Lai G, Lam DS. Evaluation of retinal nerve fiber layer progression in glaucoma: a comparison between spectral-domain and time-domain optical coherence tomography. Ophthalmology. 2011;118:1558-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Leung CK, Cheung CY, Weinreb RN, Lee G, Lin D, Pang CP, Lam DS. Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49:4893-4897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Folgar FA, Jaffe GJ, Ying GS, Maguire MG, Toth CA. Comparison of optical coherence tomography assessments in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:1956-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Mujat M, Ferguson RD, Iftimia N, Hammer DX. Compact adaptive optics line scanning ophthalmoscope. Opt Express. 2009;17:10242-10258. [PubMed] |
| 36. | Takayama K, Ooto S, Hangai M, Ueda-Arakawa N, Yoshida S, Akagi T, Ikeda HO, Nonaka A, Hanebuchi M, Inoue T. High-resolution imaging of retinal nerve fiber bundles in glaucoma using adaptive optics scanning laser ophthalmoscopy. Am J Ophthalmol. 2013;155:870-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Da Pozzo S, Marchesan R, Ravalico G. Scanning laser polarimetry - a review. Clin Experiment Ophthalmol. 2009;37:68-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | van de Kraats J, van Norren D. Directional and nondirectional spectral reflection from the human fovea. J Biomed Opt. 2008;13:024010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Abràmoff MD, Garvin MK, Sonka M. Retinal imaging and image analysis. IEEE Rev Biomed Eng. 2010;3:169-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 551] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 40. | Cabot F, Kankariya VP, Ruggeri M, Yoo SH, Vaddavalli PK, Parel JM, Kymionis GD. High-resolution optical coherence tomography-guided donor tissue preparation for descemet membrane endothelial keratoplasty using the reverse big bubble technique. Cornea. 2014;33:428-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Lu CD, Kraus MF, Potsaid B, Liu JJ, Choi W, Jayaraman V, Cable AE, Hornegger J, Duker JS, Fujimoto JG. Handheld ultrahigh speed swept source optical coherence tomography instrument using a MEMS scanning mirror. Biomed Opt Express. 2013;5:293-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 42. | Grulkowski I, Liu JJ, Potsaid B, Jayaraman V, Lu CD, Jiang J, Cable AE, Duker JS, Fujimoto JG. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers. Biomed Opt Express. 2012;3:2733-2751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 43. | Jung W, Kim J, Jeon M, Chaney EJ, Stewart CN, Boppart SA. Handheld optical coherence tomography scanner for primary care diagnostics. IEEE Trans Biomed Eng. 2011;58:741-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |